Published online Oct 14, 2016. doi: 10.3748/wjg.v22.i38.8558
Peer-review started: June 4, 2016
First decision: July 12, 2016
Revised: July 27, 2016
Accepted: August 23, 2016
Article in press: August 23, 2016
Published online: October 14, 2016
Processing time: 137 Days and 13.9 Hours
To determine if our health system’s integrated model reflects sustained virologic response (SVR) outcomes similar to those in clinical trial data, maximizes adherence, and averts drug interactions.
Subjects with chronic hepatitis C had their medical records reviewed from November 1st, 2014 through March 1st, 2016. Patients eligible for treatment were entered into an integrated care model therapy algorithm. The primary outcome was SVR12 based on intention to treat (ITT) analysis. Inclusion criteria consisted of both treatment naïve and experienced patients over the age of 18 who were at least twelve weeks post-therapy completion with any genotype (GT) or METAVIR score. Secondary outcomes included adherence, adverse events, and number of drug interaction interventions.
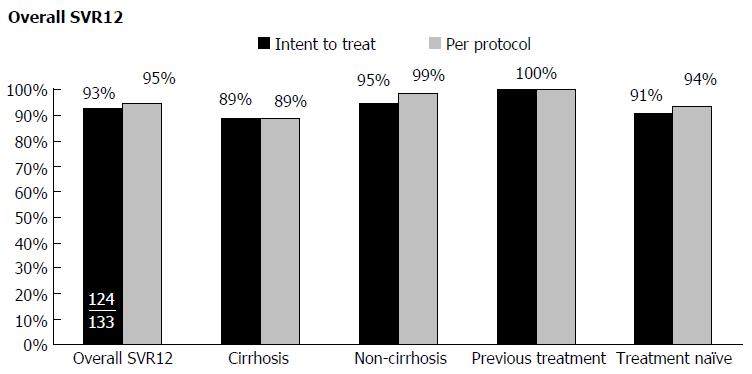
At the time of analysis, 133 patients had reached twelve weeks post therapy with ITT. In the ITT analysis 70 patients were GT 1a, 26 GT 1b, 23 could not be differentiated between GT 1a or 1b, 8 GT 2, 4 GT 3, and 2 patients with multiple genotypes. The ITT treatment regimens consisted of 97 sofosbuvir (SOF)/ledipasvir (LDV), 8 SOF/LDV and ribavirin (RBV), 7 SOF and Simeprevir (SMV), 6 3D and RBV, 1 3D, 11 SOF and RBV, and 1 SOF, peg interferon alpha, and RBV. The overall SVR12 rate was 93% in the ITT analysis with a total of 6 patients relapsing. In patients with cirrhosis, 89% obtained SVR12. All 33 patients who were previous treatment failures achieved SVR12. Drug-drug interactions were identified in 56.4% of our patient population, 69 of which required interventions made by the pharmacist. The most common side effects were fatigue (41.4%), headache (28.6%), nausea (18.1%), and diarrhea (8.3%). No serious adverse effects were reported.
Dean Health System’s integrated care model successfully managed patients being treated for hepatitis C virus (HCV). The integrated care model demonstrates high SVR rates amongst patients with different levels of fibrosis, genotypes, and HCV treatment history.
Core tip: There are new effective options for treating hepatitis C virus. To maximize their effectiveness our health system developed an innovative integrated care model to manage these patients. Through our original therapy algorithm we were able to closely monitor patients from time of insurance approval to the time of obtaining a sustained virologic response (SVR). This real world retrospective study analyses our patient’s SVR rate, adherence, and interventions made by the patient care team. Additionally it will provide a model for other systems to improve their care coordination and response with direct acting antiviral treatment.
- Citation: Levin JM, Dabirshahsahebi S, Bauer M, Huckins E. Retrospective analysis of hepatitis C infected patients treated through an integrated care model. World J Gastroenterol 2016; 22(38): 8558-8567
- URL: https://www.wjgnet.com/1007-9327/full/v22/i38/8558.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i38.8558
Globally, an estimated 185 million people are chronically infected with hepatitis C virus (HCV) infection with about 3.5 million individuals living with chronic HCV in the United States period HCV infection is associated with sizable morbidity and mortality with over 350000 deaths annually[1-3]. Long term effects of chronic HCV can lead to complications such as liver cirrhosis, hepatocellular carcinoma (HCC), and end-stage liver disease requiring liver transplantation[4]. Management of chronic HCV with antiviral therapy is aimed at halting disease progression, preventing cirrhosis decompensation, reducing the risk of HCC, and limiting extrahepatic complications of the infection. The goal of antiviral therapy is to eradicate HCV RNA. Historically, clinical trials of HCV treatment regimens have used a sustained virologic response (SVR) as the primary efficacy endpoint[5]. SVR is defined as undetectable HCV RNA levels 12 wk post-treatment (SVR 12)[6].
Treatment of HCV is evolving and treatment success is often based on the severity of liver fibrosis, presence of cirrhosis, previous treatment failure, and genotype (GT). For years, peg interferon alpha (PEG-IFN)/ribavirin (RBV) combination was the only treatment option. An improved understanding of the HCV genome has led to the development of multiple direct-acting antivirals (DAAs) targeted at specific proteins of the virus, resulting in the disruption of viral replication[7]. New DAAs for HCV are categorized into classes shown in Table 1, defined by their mechanism of action.
| Class (targeting non-structural proteins) | Examples |
| NS3/4A protease inhibitors | |
| First generation | telaprevir, boceprevir |
| Second generation | grazoprevir1, paritaprevir2, simeprevir |
| NS5A inhibitors | ledipasvir1, ombitasvir2, daclatasvir, elbasvir1 |
| NS5B RNA-dependent RNA polymerase inhibitors | |
| NS5B nucleoside polymerase inhibitors | sofosbuvir |
| NS5B non-nucleoside polymerase inhibitors | dasabuvir1 |
Since DAAs target critical steps of HCV replication, selection of resistant mutants is inevitable with monotherapy[9,10]. Combining HCV medications without overlapping resistance patterns, effectively shuts down viral replication which for many patients results in clearance of the virus from the liver. Available guidelines for the therapeutic management of HCV infection include the American Association for the Study of Liver Diseases in conjunction with the Infectious Diseases Society of America AASLD/IDSA, the European Association for the Study of the Liver, the United Kingdom consensus guidelines and the World Health Organization[3,11-13].
Usage of DAAs has been complicated by the high cost of therapy. However, there is also high utilization and costs for the health care system associated with treating HCV-related complications. Achievement of SVR has implications beyond those of viral eradication including improved long-term clinical outcomes, economic benefits and improved health-related quality of life[14]. Achievement of an SVR with DAAs can reduce the risk of advanced liver disease, liver transplant, and liver-related death. Research has shown that the cost associated with liver-related tests, outpatient drugs, and hospitalizations can be significantly lower for patients who achieved SVR than for those without SVR[15].
DAAs have shown remarkable cure rates with SVR12 of 90%-100% in clinical trials[16,17]. A number of phase 3 trials of patients with chronic HCV-GT1 have achieved very high SVR12 rates with different DAA drug combinations. In the ION-2 clinical trial, Ledipasvir (LDV)/Sofosbuvir (SOF) ± RBV was used for 12 or 24 wk in treatment-experienced HCV-GT1 patients with or without cirrhosis[18]. In the OPTIMIST-1 trial, Simeprevir (SMV)/SOF combination was used for 8 and 12 wk in HCV-GT1 treatment-naïve and -experienced patients without cirrhosis[19]. In TURQUOISE-II, the combination of ombitasvir/paritaprevir/ritonavir and dasabuvir (3D) + RBV was used for 12 or 24 wk in HCV-GT1 treatment naïve and experienced patients and compensated cirrhosis[20]. The most common adverse events in all four trials were fatigue, headache, and nausea.
In addition to high SVR12 rates with DAAs, durability of SVR and the long term virologic and clinical outcomes with DAA-only regimens have been demonstrated. Data from one of two 3-year registries showed 99.7% (5414/5433) of patients maintaining SVR with 0.3% (19/5433) having emergent virus in the SVR registry[21]. Viral emergence occurred by week 96 in all patients.
In addition to antiviral therapies, general measures in the management of patients with chronic HCV are as follows: psychological counseling, symptom management by dose adjustment of medications, and emphasizing the importance of adherence[5]. The efficacy of DAA therapy is directly proportional to the adherence of these agents. HCV cure rates in real practice are often less than what is seen in highly monitored and controlled clinical trials. Often, there is a decrease in efficacy in intention-to-treat (ITT) real world data due to higher loss to follow up, non-adherence, and insurance barriers. Traditional disconnected models between the physician and pharmacy have demonstrated diminished adherence, ineffective drug interaction management, and lower SVR outcomes compared to those seen in the clinical trials. In order to maximize the benefits of these high cost medications, our health system created an integrated care model between the clinic and pharmacy to maximize the benefits of DAA, minimize potential for drug-drug interactions, provide side effect management, and increase adherence. The purpose of this study is to determine if our health system’s integrated model reflects SVR outcomes similar to those seen in clinical trial data, maximize adherence, and avert drug interactions that can impact efficacy. Our hypothesis is that patients treated through our integrated care model will demonstrate SVR rates similar to those seen in the studies based on their associated treatment status and stage of fibrosis. Additionally we anticipate the results of the study to demonstrate an increased number of drug interaction interventions and decreased number of required office visits.
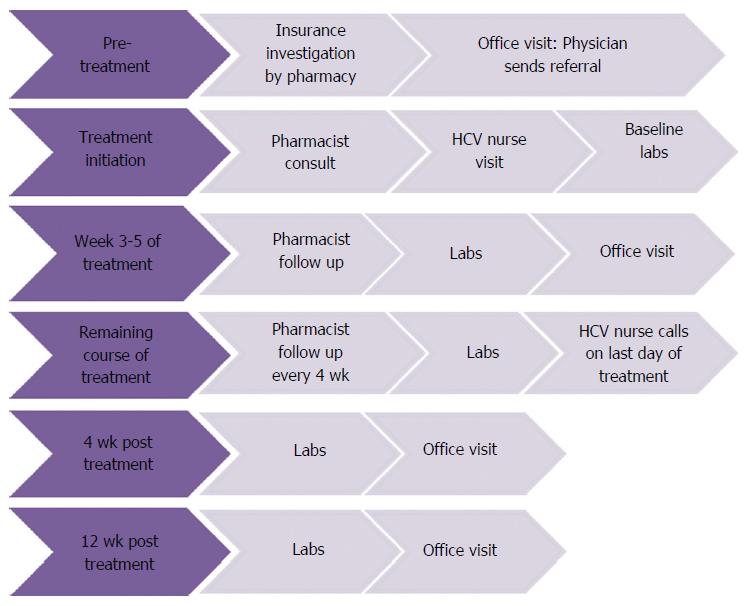
This retrospective review was conducted at Dean Clinic based in Wisconsin, United States. Patient electronic medical records were reviewed from November 1st, 2014 through March 1st, 2016. Treatment was determined by the ordering physician with recommendations made by the pharmacist based on AASLD/IDSA Guidelines. All therapies were given at FDA approved doses. HCV treatment was managed by a multidisciplinary care team comprised of an infectious disease physician, HCV nurse, and a specialty pharmacist. Patients were referred to pharmacy as treatment candidates by an infectious disease physician. Once referred to pharmacy, patients underwent insurance benefits verification and treatment authorization was submitted to the patients’ insurances. Patients that were approved through their insurance followed the integrated therapy algorithm (Figure 1). The initial screening step in the therapy algorithm was for patients’ medication lists, laboratory values, and fibrosis measures to be reviewed by the specialty pharmacist. All drug-drug interactions were addressed by the pharmacist and recommendations were relayed to the infectious disease physician and the physician who prescribed the interacting non-HCV medication. The patients were subsequently set up for an antiviral treatment education session where they spoke with a pharmacist and an HCV nurse educator. At the education session the patient was given a therapy calendar with dates for scheduled laboratory tests and appointments with the infectious disease physician. Proper laboratory measurements were performed at baseline and during the course of treatment based on treatment regimen. Throughout the course of treatment there were regular follow-ups scheduled by the pharmacist in addition to an office visit with the infectious disease physician at week 4 of treatment. During the pharmacist follow ups, patients were assessed for adherence, changes to their medications, and side effect management. At the end of therapy the patients were contacted by the nurse and established with post-treatment follow ups. A per protocol (PP) analysis looked at patients that started treatment with a Dean Clinic physician, completed the entire course of therapy, and were able to have an HCV viral load drawn at 12 wk post therapy. The primary outcome was SVR12 based on ITT analysis. Secondary outcomes included adherence, adverse events, and number of drug interaction interventions. Inclusion criteria consisted of both treatment naïve and experienced patients over the age of 18 who were at least twelve weeks post-therapy completion with any GT or METAVIR score. Adverse events were patient-reported during the course of treatment. Adherence rates were monitored using a patient-reported tablet count that was recorded during patient follow ups with the pharmacist as well as the scheduled last day of treatment.
At the time of analysis, 133 patients had reached twelve weeks post hyphen therapy with ITT and 130 patients with PP analyses. Baseline demographics are reported in Table 2. In the ITT analysis, 70 patients were GT 1a, 26 GT 1b, 23 could not be differentiated between GT 1a or 1b, 8 GT 2, and 4 GT 3. Two patients in the undifferentiated GT 1 group had an infection with a second GT. One patient was GT 1 and 2 and the other patient was GT 1 and 4. Two patients in the ITT analysis were lost to follow up after treatment completion. Another patient passed away from unrelated causes after achieving SVR4. A total of 33 (24.8%) patients had undergone previous treatments for hepatitis C. The ITT treatment regimens consisted of 97 SOF/LDV, 8 SOF/LDV and RBV, 7 SOF and SMV, 6 3D and RBV, 1 3D, 11 SOF and RBV, and 1 SOF, PEG-IFN, and RBV.
| Total patients | 133 (100) |
| Median Age | 58 |
| Male | 89 (66.9) |
| Cirrhosis | 47 (35.3) |
| Treatment Experienced | 33 (24.8) |
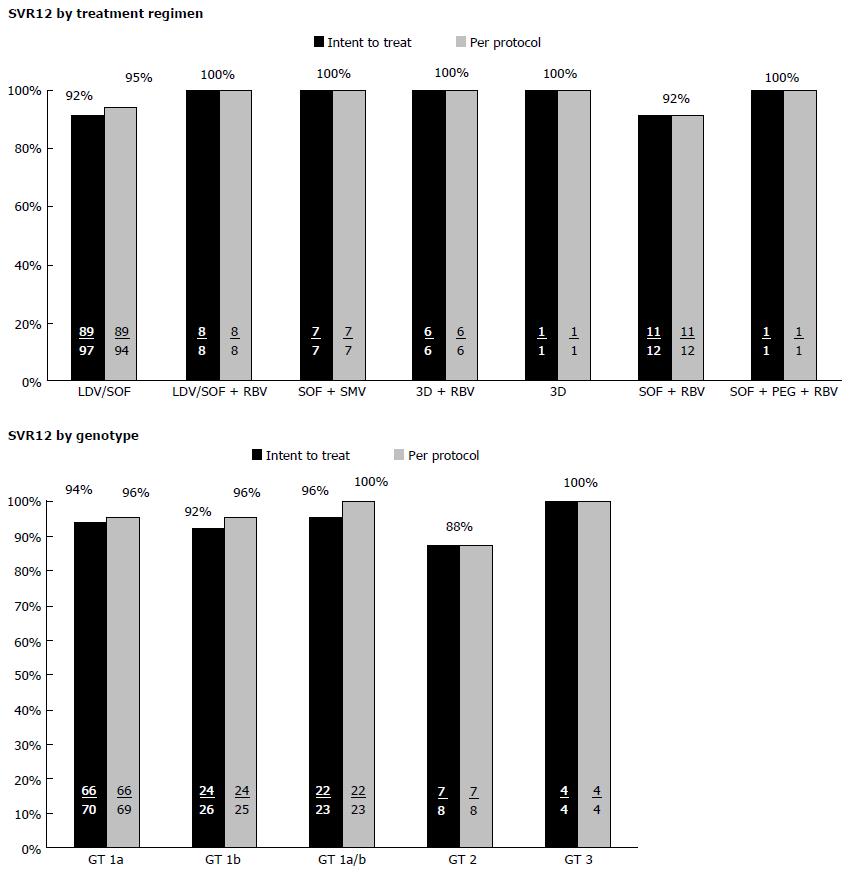
The overall SVR12 rate was 93% and 95% in patients who had completed the ITT and PP analysis, respectively (Figure 2). SVR12 rates were 89% and 95% for cirrhotic and non-cirrhotic patients, respectively. All treatment-experienced patients (100%) achieved SVR12 in both ITT and PP analyses. Treatment naïve patients with or without cirrhosis achieved an SVR12 rate of 94% in the PP analysis. Efficacy varied based on specific treatment regimens and genotypes (Figure 3). Further analysis was done on the patients who relapsed (n = 6). One of the patients that relapsed had GT 1b with underlying cirrhosis. The patient was treated with LDV/SOF for twelve weeks, and had break in therapy of 5 d due to insurance coverage termination. A second patient with GT 1a HCV who relapsed was treated with LDV/SOF for twelve weeks and had advanced cirrhosis and HCC. A third relapse was seen in a GT 1a cirrhotic African American patient co-infected with HIV, on efavirenz/tenofivir/emtricitabine and was being treated for HCV with LDV/SOF for twelve weeks concomitantly. A fourth GT 1a relapsed patient with cirrhosis was treated with LDV/SOF for twelve weeks and reported reusing diabetic supplies to test blood glucose during the course of treatment. A fifth patient who relapsed had GT 2 without cirrhosis, was treatment-naïve, and was treated with SOF and RBV for twelve weeks with no additional reported variables. The sixth patient relapse case was GT 1a with cirrhosis with no additional reported variables.
The majority of patients demonstrated adverse effects; however, no patients discontinued DAA therapy prematurely due to adverse effects. The majority of the side effects reported were fatigue (41%) or headache (28.6%), most of which were mild to moderate in severity. A full list of adverse effects with a prevalence greater than 5% is reported in Table 3.
| Adverse events > 5% | n (%) |
| Fatigue | 58 (41.4) |
| Headache | 38 (28.6) |
| Nausea | 24 (18.1) |
| Diarrhea | 11 (8.3) |
| Dyspepsia | 7 (5.3) |
| Anemia | 7 (5.3) |
Drug-drug Interactions were identified in 56.4% of our patient population, 69 of which required interventions made by the pharmacist. The most prevalent drug-drug interaction intervention was dosing of proton pump inhibitors (PPIs) with LDV regimens (28.6%). The recommendation was made to discontinue or decrease the dose of the PPI to 20 mg omeprazole equivalent to be taken at the exact same time as the LDV/SOF. If this was not achievable, the patient was not a candidate for this therapy. Additional stomach pH related drug-drug interaction interventions included histamine 2 receptor antagonists (5.3%) or short acting antacids (9.8%). Other medication interventions (< 5%) included drug-drug interactions with phenobarbital, phenytoin, carbamazepine, milk thistle, St. John’s wort, fluoxetine, clonazepam, amlodipine, and inhaled corticosteroids.
A total of 79.1% of patients had adherence rates that were 100% on the treatment algorithm. There were 17.1% of patients that missed three or less doses. One patient (0.8%) had more than 3 doses missed. Additionally, 2.4% of patients were lost to follow up after treatment without a documented adherence rate.
HCV treatment guidelines emphasize the importance of addressing adherence, adverse effects, and drug interactions with HCV regimens as clinically indicated. However, no specific recommendations are made regarding follow-up methods. Thus, effective real-world care models need to be identified for the newer DAA therapies to ensure the best HCV treatment outcomes are achieved in real-world practice settings. Our study describes an integrated multidisciplinary care team model with SVR12 rates comparable to those seen in controlled clinical trial settings. Overall SVR12 among patients in the current study was 93% in the ITT cohort and 95% in the PP cohort. Among patients with cirrhosis our SVR12 rates remained high at 89% for both PP and ITT cohorts, despite this patient population generally being more difficult to treat. Another patient population that achieved notably high SVR12 rates in our study was the treatment-experienced cohort with a 100% SVR12 rate for PP and ITT analyses. This cohort achieved a higher SVR12 rate compared to our treatment-naïve patients of which 91% in the ITT cohort and 94% in the PP cohort achieved SVR12. This was an unexpected finding we cannot explain. This was additionally unexpected because more patients in the treatment-experienced cohort were cirrhotic compared to the treatment-naïve cohort (57.6% and 28.9% cirrhotic, respectively).
SVR12 achievement rates were similar to clinical trial results based on the specific treatment regimen as well. Patients who completed LDV/SOF regimens achieved 95% SVR12 PP (92% ITT) in our study. The ION-1 study included GT 1 treatment-naïve patients with or without cirrhosis treated with a fixed-dose combination of LDV/SOF with or without RBV[16]. SVR12 rates were 99% with LDV/SOF. ION-2 included GT 1 treatment-experienced patients with or without cirrhosis treated with a fixed-dose combination of LDV/SOF with or without RBV[18]. SVR12 rates were 96% with LDV/SOF. The addition of RBV did not significantly impact SVR12 rates in our study or in ION-1 or -2; SVR12 remained high.
The SMV/SOF regimen resulted in 100% SVR12 PP (100% ITT) in our study patients. OPTIMIST-1 and OPTIMIST-2 investigated SMV/SOF among GT 1 treatment-naïve and treatment-experienced patients[19,22]. Patients without cirrhosis were included in OPTIMIST-1 and the resulting SVR12 was 97%. OPTIMIST-2 included patients with cirrhosis and the SVR12 was 84%. Although only seven patients total received the SMV/SOF regimen among our study patients, five out of the seven were cirrhotic. Our SVR12 rates of 100% were unexpectedly higher than those seen in the OPTIMIST trials.
Patients who completed the 3D plus RBV regimen achieved 100% SVR12 PP (100% ITT) in our study. Two of the six patients were cirrhotic. The SAPPHIRE I and SAPPHIRE II clinical trials included patients that were treatment-naïve and treatment-experienced, respectively, without cirrhosis treated with 3D plus RBV for 12 wk. SVR12 was 96% for both studies[17,23]. In the TURQUOISE II trial, 92% of treatment-naïve or experienced patients with cirrhosis who received 3D plus RBV for 12 wk achieved SVR12[20].
Compared to other real-world analyses of newer DAA treatments, our response rates are either higher than or similar to other studies, demonstrating the effectiveness of our model. A real-world analysis of treatment-naïve or experienced patients with HCV GT 1 with or without cirrhosis was conducted on patients in the HCV-TARGET cohort treated with SMV/SOF with or without RBV[24]. The overall SVR12 rate for SMV/SOF without RBV was 85%, which was lower than the SVR12 of 100% (PP and ITT) seen in our study for patients who were treated with the SMV/SOF regimen.
A real-world study from Israel included treatment-naïve or experienced HCV GT 1 patients with stage 3 or 4 fibrosis treated with 3D with or without RBV. Amongst the patients who completed therapy and retested 12 wk after completion, SVR12 rates were 97.8%[25]. Seven patients in our study received treatment with 3D plus RBV and only two were cirrhotic. Our SVR12 rates with this regimen were 100% for both PP and ITT analyses.
Another real-world effectiveness study from a large integrated health care system in the United States enrolled patients with GT 1 infection and receiving LDV/SOF with or without RBV. Patients were treatment-naïve or experienced and both cirrhotic and noncirrhotic. SVR12 for LDV/SOF was 93% in the ITT analysis[26]. The overall SVR12 in our study for patients treated with LDV/SOF was similar at 92% in the ITT analysis. The addition of RBV did not significantly impact SVR12 rates in either study.
Six patients in our study relapsed. One patient with GT 1b and underlying cirrhosis may have relapsed due to a 5-d break in therapy, another with GT 1a and cirrhosis was due to reinfection from reusing diabetes supplies, and one patient with GT 1a and cirrhosis relapsed for unknown reasons. The other three cases warrant further discussion. The GT 1a infected patient with advanced cirrhosis and HCC treated with LDV/SOF for 12 wk in the current study would also have been treated with RBV if evidence from the SOLAR-1 and SOLAR-2 Phase 2 trials were available at the time of treatment course selection, which may have prevented the relapse. SOLAR-1 and SOLAR-2 enrolled patients with HCV GT 1 or 4 with cirrhosis and moderate to severe hepatic impairment (Child-Pugh class B and C) with and without a history of previous liver transplant[27,28]. Patients were treated with 12 or 24 wk of a fixed-dose combination of LDV/SOF once daily plus RBV. SVR12 was 87% in non-transplant patients treated for 12 wk in SOLAR-1. In SOLAR-2, SVR12 was approximately 86% after 12 wk of treatment in non-transplant patients with GT 1.
The African American patient with GT 1a, cirrhosis, and HIV coinfection relapsed after 12 wk of treatment with LDV/SOF for an undermined reason. A recent study, ION-4, enrolled patients with HCV GT 1 or 4 coinfected with HIV-1. All patients received a 12-wk, fixed-dose combination of LDV/SOF for their HCV treatment regimen[29]. Thirty-four percent of patients in this study were black. Black patients had a lower SVR12 rate than other races (90% vs 99%, P < 0.001). Of note, 10 of the 335 patients in ION-4 relapsed and all were black. Seven of the relapsed patients had the TT allele in the gene encoding IL28B and 8 were receiving efavirenz as part of their HIV treatment regimen. Black race and presence of the TT allele were both significantly associated with relapse in ION-4. Among black patients in ION-4, 13% relapsed if they were also taking efavirenz and only 4% relapsed if they were taking other antiretroviral regimens. However, the difference was not found to be significant. It is possible that the patient in our case possesses the TT allele; however, we did not test patients in our study for the presence of this allele. Concomitantly taking efavirenz could have provoked the relapse in our patient, even though the role efavirenz plays in reduced effectiveness of HCV treatment remains unclear.
The non-cirrhotic, treatment-naive patient with GT2 who relapsed after being treated with 12 wk of SOF and RBV was somewhat surprising to us. The VALENCE trial confirmed that this same regimen is 96.7% effective in naïve, non-cirrhotic patients with GT2[30]. We cannot provide an explanation for why this particular patient relapsed.
In our study, 130 patients completed the analysis PP and 133 were in the ITT analysis. The high percentage of PP patients represents a high engagement between patient and clinical staff monitoring in our model. Furthermore, in our model, a high percentage (79.1%) of patients were 100% adherent on their treatment regimen and only one patient missed more than three doses. Other real-world studies looking at adherence demonstrated about 14% of patients were non-adherent to their treatment regimen and 18% had gaps in therapy of greater than 14 d[31]. A second study reported that 89.3% of patients completed treatment and 9% were non-adherent to therapy in a real-world setting[25].
The specialty pharmacist in our model identified drug interactions in 56.4% of patients. Sixty-nine drug interaction interventions were made with the most prevalent intervention being PPI dosing changes. Overall, drugs to lower gastric pH accounted for about 44% of all drug interaction interventions made. A study from Europe of drug-drug interactions identified that between 12%-19% of patients being treated for HCV were taking a drug that was contraindicated with one or more drugs in their HCV treatment regimen[32]. This same study showed that 29%-39% of patients were on two or more drugs that were either contraindicated or required additional monitoring or dose reduction with their HCV regimen. Similarly to our study, a high percentage (27%-38%) of interacting drugs in the Marra et al[32] study were drugs that target the gastrointestinal tract. The frequency and severity of drug-drug interactions with HCV therapies supports the workflow in our model where a specialty pharmacist consistently screened all patients for drug interactions.
Adverse reactions reported by our patients were consistent with those reported in DAA clinical trials and real-world experience with fatigue, headache, and nausea being the most common[24]. No serious adverse events were recorded. Drop-out rates due to adverse effects tend to be low with the newer generation DAAs, but no patients discontinued treatment for this reason in our study. One possible reason for this may be due to close follow-up by the pharmacist on adverse effects and management strategies.
Our study had some notable limitations. A major limitation is the lack of a control group to allow a statistical comparison of the effectiveness of our integrated model compared to a non-integrated model. Only qualitative comparisons to clinical trial data and other real-world data could be made. A second limitation is that this is a single-center study and results may not be generalizable to patient populations with different demographics. The population at our site is primarily Caucasian and insured. A third limitation is that the methodology of fibrosis determination was not standardized in our protocol. A fourth limitation was that adherence was self-reported by patients via tablet counts. There are inherent limitations with using patient-reported information in a study. The high adherence rates reported in our study likely reflected reality as shown by the high rates of SVR12 in our patients.
The results of this study have demonstrated the need to continue to manage patients using the integrated care model in our current practice. However, the limitations of this study have showed that future research is needed to find causation for patients that relapsed on DAAs. In the scope of our practice, follow up studies will be pursued to assess the impact of adherence and how new technology may assist in increasing adherence to therapy. Additionally, future studies at our practice will analyze if there is correlation of NS5A resistance associated variants and treatment efficacy in our patient population. Furthermore, additional focus will be put on the financial savings that the integrated care model has on the system and the patient.
In conclusion, there is a scarcity of published trials that describe real-world integrated care models for successful treatment of patients with the newer DAA HCV therapies. Dean Health System’s integrated care model helped successfully manage the patients being treated for HCV. The results of our study demonstrated favorable outcomes despite not being able to statistically compare across other studies. The integrated care model demonstrates high SVR rates amongst patients with different levels of fibrosis, genotypes, and HCV treatment history. The integrated care model assisted in catching and evading potential drug interactions that may have impacted treatment efficacy and tolerability. Overall, the evidence from this retrospective analysis demonstrates the benefits and value of treating HCV patients in an integrated care delivery model.
New direct-acting antivirals (DAAs) for hepatitis C virus (HCV) infected patients have produced sustained sustained virologic response (SVR) rates of 90%-100% in clinical trials. The efficacy of DAA therapy is directly proportional to the adherence to these agents. Traditional disconnected models between the provider and pharmacy have demonstrated diminished adherence, ineffective drug interaction management, and lower SVR outcomes compared to those seen in the clinical trials. In order to maximize the benefits of these high cost medications, our health system created an integrated care model between the clinic and pharmacy to maximize benefits of DAA therapy.
The adherence to DAA therapy and their efficacy are typically lower in less monitored environments models as shown in a few prior real world reports compared to controlled clinical trials. The results of the present study suggest our health system’s integrated model reflects SVR outcomes similar to those in clinical trial data.
In this study, the model was a useful tool for improving adherence rates and achieving 100% SVR12 for cirrhotic and non-cirrhotic patients on 3D plus RBV regimen. In previous 3D plus RBV clinical trials cirrhotic and non-cirrhotic patients treated showed lower SVR12 rates of up to 96%. Compared to other real-world analyses of newer DAA treatments, the response rates were either higher than or similar to other studies, demonstrating the effectiveness of this model.
The authors integrated care model between clinic staff and pharmacy helped better manage the HCV patients. This model demonstrated high cure rates, maximized adherence, and aversion of a high volume of potential drug interactions that may have impacted treatment efficacy and tolerability. Future research is needed to find causation for patients that relapsed on DAAs and follow-up studies will be pursued to assess the impact of adherence and how new technology may assist in increasing adherence to therapy.
Integrated care model: Worldwide trend in health care reforms and new organizational arrangements focusing on more systematic coordination and integrated forms of care provision. Integrated care may be seen as a response to the fragmented delivery of health services. Medication adherence: the extent to which patients take medications as prescribed by their health care providers.
This manuscript was very interesting.
| 1. | Holtzman D. traveler’s health. Chapter 3. Infectious diseases related to travel. Hepatitis C. last updated July 10. 2015; Available from: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/hepatitis-c. |
| 2. | Gish RG, Cohen CA, Block JM, Brosgart CL, Block TM, Clary R, Le LT, Ninburg MH, Sandt L, Kowdley KV. Data supporting updating estimates of the prevalence of chronic hepatitis B and C in the United States. Hepatology. 2015;62:1339-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Stasi C, Silvestri C, Voller F, Cipriani F. The epidemiological changes of HCV and HBV infections in the era of new antiviral therapies and the anti-HBV vaccine. J Infect Public Health. 2016;9:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1857] [Article Influence: 92.9] [Reference Citation Analysis (1)] |
| 5. | Chopra S, Pockris P. Overview of the management of chronic hepatitis C virus infection. Available from: http://www-uptodate-com.ezproxy.library.wisc.edu/contents/overview-of-the-management-of-chronic-hepatitis-c-virus-infection?source=preview&search=hepatitisc&language=en-US&anchor=H1&selectedTitle=1~150#H1. |
| 6. | Yoshida EM, Sulkowski MS, Gane EJ, Herring RW, Ratziu V, Ding X, Wang J, Chuang SM, Ma J, McNally J. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Pockros P. Direct-acting antivirals for the treatment of hepatitis C virus infection. Available from: http://www-uptodate-com.ezproxy.library.wisc.edu/contents/direct-acting-antivirals-for-the-treatment-of-hepatitis-c-virus-infection?source=machineLearning&search=directactingantivirals&selectedTitle=1~150§ionRank=5&anchor=H1818005026#H1818005026. |
| 8. | Poordad F, Dieterich D. Treating hepatitis C: current standard of care and emerging direct-acting antiviral agents. J Viral Hepat. 2012;19:449-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Vachon ML, Dieterich DT. The era of direct-acting antivirals has begun: the beginning of the end for HCV? Semin Liver Dis. 2011;31:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Kieffer TL, Kwong AD, Picchio GR. Viral resistance to specifically targeted antiviral therapies for hepatitis C (STAT-Cs). J Antimicrob Chemother. 2010;65:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Recommendations for Testing, Managing, and Treating Hepatitis C. Joint panel from the American Association of the Study of Liver Diseases and the Infectious Diseases Society of America. Available from: http://www.hcvguidelines.org. |
| 12. | European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 911] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 13. | Miller MH, Agarwal K, Austin A, Brown A, Barclay ST, Dundas P, Dusheiko GM, Foster GR, Fox R, Hayes PC. Review article: 2014 UK consensus guidelines - hepatitis C management and direct-acting anti-viral therapy. Aliment Pharmacol Ther. 2014;39:1363-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis. 2015;15:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Manos MM, Darbinian J, Rubin J, Ray GT, Shvachko V, Denis B, Velez F, Quesenberry C. The effect of hepatitis C treatment response on medical costs: a longitudinal analysis in an integrated care setting. J Manag Care Pharm. 2013;19:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1376] [Article Influence: 114.7] [Reference Citation Analysis (1)] |
| 17. | Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 18. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1066] [Article Influence: 88.8] [Reference Citation Analysis (1)] |
| 19. | Kwo P, Gitlin N, Nahass R, Bernstein D, Etzkorn K, Rojter S, Schiff E, Davis M, Ruane P, Younes Z. Simeprevir plus sofosbuvir (12 and 8 weeks) in HCV genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016;64:370-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 20. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 685] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 21. | Lawitz EJ, Ruane P, Stedman C, Foster G, Hyland RH, Coogan S, Moody S, Dvory-Sobol H, Knox SJ, Brainard DM, Abergel A, Agarwal K, Younes Z, Schwabe C. Long-term follow-up of patients with chronic HCV infection following treatment with direct acting antiviral regimens: maintenance of SVR, persistence of resistance mutations and clinical outcomes. Paper presented at the European Association for the Study of the Liver meeting; 2016 Apr 15; Barcelona, Spain. Abstract FRI-166. . |
| 22. | Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, Godofsky E, Herring RW, Poleynard G, Sheikh A. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2). Hepatology. 2016;64:360-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 23. | Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, Sulkowski MS, Wedemeyer H, Tam E, Desmond P. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 445] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 24. | Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, Morelli G, Darling JM, Feld JJ, Brown RS. Effectiveness of Simeprevir Plus Sofosbuvir, With or Without Ribavirin, in Real-World Patients With HCV Genotype 1 Infection. Gastroenterology. 2016;150:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Cohen M, Kahan NR, Waitman D, Tur-Kaspa R. An interim analysis of a national program for treating hepatitis c with novel antiviral agents. The European Association for the Study of the Liver, The International Liver Congress 2016; Barcelona, Spain. . |
| 26. | Lai J, Witt M, Witt D. Real-word effectiveness of 8, 12 and 24 weeks ledipasvir (LDV)/sofosbuvir (SOF)-based therapy for hepatitis c virus (HCV) genotype 1: analysis in a large integrated health care system. The European Association for the Study of the Liver, The International Liver Congress 2016; Barcelona, Spain. . |
| 27. | Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Fried MW, Terrault NA, O’Leary JG, Vargas HE. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 637] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 28. | Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 358] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 29. | Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, Marks K, Luetkemeyer A, Baden RP, Sax PE. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 384] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 30. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 637] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 31. | Kamble PS, Walker DR, Marx S, Harvey R, Uribe CL, Bunniran S, Collins J. Adherence and discontinuation rates of sofosbuvir-based regimens: modeling real world experience in a large managed care organization. Paper presented at: The American Association for the Study of Liver Diseases Liver Meeting; 2015 Nov 15; San Francisco, CA. Abstract 1050. . |
| 32. | Marra F, Leber W, Barclay ST, Christensen S, Ouzan D, Oules V, McMahon PS, Kostev K, Ansolabehere X. High prevalence of co-morbidities and complex polypharmacy with drug-drug interaction (DDI) potential in patients with chronic Hepatitis C (CHC). Consistent findings from large primary care databases in the United Kingdom, Germany, and France. Paper presented at: The American Association for the Study of Liver Diseases Liver Meeting; 2015 Nov 15; San Francisco, CA. Abstract 1052. . |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Sargsyants N S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF