Published online Oct 7, 2016. doi: 10.3748/wjg.v22.i37.8257
Peer-review started: May 30, 2016
First decision: July 12, 2016
Revised: July 22, 2016
Accepted: August 10, 2016
Article in press: August 10, 2016
Published online: October 7, 2016
Processing time: 123 Days and 13 Hours
Most pancreatic cancers and extrahepatic cholangiocarcinomas are unresectable at the time of diagnosis, and even in case of a resectable cancer, for elderly or patients with coexistent comorbidities, surgery is not an option. Current treatment alternatives in these scenarios are very limited. Biliary stenting with self-expanding metal stents (SEMS) is the mainstay palliative treatment of biliary obstruction due to unresectable pancreatic cancer or cholangiocarcinoma. Nevertheless, more than 50% of SEMS become occluded after 6 mo due to tumour over- and ingrowth, leading to hospital readmissions and reinterventions that significantly impair quality of life. Regimes of chemotherapy or chemoradiotherapy also provide minimal survival benefits. Therefore, novel therapies are eagerly awaited. Radiofrequency (RF) energy causes coagulative necrosis leading to local destruction of the accessed malignant tissue and has an established role in the treatment of malignancies in several solid organs, especially liver cancers. However, pancreatic and extrahepatic biliary cancers are not easily accessed by a percutaneous route, making the procedure dangerous. Over the past five years, the development of dedicated devices compatible with endoscopic instruments has offered a minimally invasive option for RF energy delivery in biliopancreatic cancers. Emerging experience with endoscopic RF ablation (RFA) in this setting has been reported in the literature, but little is known about its feasibility, efficacy and safety. A literature review makes it clear that RFA in biliopancreatic tumours is feasible with high rates of technical success and acceptable safety profile. Although available data suggest a benefit of survival with RFA, there is not enough evidence to draw a firm conclusion about its efficacy. For this reason, prospective randomized trials comparing RFA with standard palliative treatments with quality-of-life and survival endpoints are required. Anecdotal reports have also highlighted a potential curative role of RFA in small pancreatic tumours and benign conditions, such as ductal extension of ampullomas, intrahepatic adenomas or non-tumoural biliary strictures. These newest indications also deserve further examination in larger series of studies.
Core tip: Most pancreatic cancers and extrahepatic cholangiocarcinomas are unresectable at the time of diagnosis. Radiofrequency (RF) energy causes coagulative necrosis leading to local destruction of the accessed malignant tissue. Endoscopic RF has emerged as a novel ablative therapy. In the present study, we aim to review general principles and technical aspects and to evaluate clinical benefits and complications of endoscopy-guided RF in biliary and pancreatic indications based on recent literature.
- Citation: Alvarez-Sánchez MV, Napoléon B. Review of endoscopic radiofrequency in biliopancreatic tumours with emphasis on clinical benefits, controversies and safety. World J Gastroenterol 2016; 22(37): 8257-8270
- URL: https://www.wjgnet.com/1007-9327/full/v22/i37/8257.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i37.8257
Extrahepatic cholangiocarcinomas (CC), including hilar tumours, and pancreatic carcinomas (PC) are aggressive cancers often discovered at an advanced stage for curative surgical resection. Less than 20% of PC and 30% of CC are resectable at the time of diagnosis; moreover, surgery is not always an option in patients with poor functional status or coexisting comorbidities[1-4]. Chemotherapy and radiotherapy provide minimal survival benefits in patients with unresectable locally advanced pancreatic or biliary cancer, and the average survival is measured in months rather than years[5,6]. The need for novel therapies to positively impact survival has led to the development of a variety of local ablative methods, among which radiofrequency (RF) has generated wide interest after its first application for hepatic tumours in the early 1990s[7-9].
RF has been widely used percutaneously or intraoperatively in malignancies of several solid organs, such as the liver, breast, lung and kidney[10]. However, PC is not usually amenable to percutaneous RF treatment because of the difficult visualization of these deeper tumours with the risk of thermal injury of the adjacent duodenum and blood vessels. Intraoperative RF ablation (RFA) provides better visualization and the ability to manipulate nearby structures, but many patients with biliopancreatic cancers are also unfit for surgery. Percutaneous endobiliary RF of extrahepatic CC has been shown to be successful, but percutaneous transhepatic bile duct access is an invasive technique; therefore, endoscopic bile duct access by endoscopic retrograde cholangiopancreatography (ERCP) is usually favoured over the percutaneous approach[11,12]. The development of new over-the-wire and flexible RF probes that can be placed down the working channel of an endoscope or through an endoscopic ultrasonography (EUS) needle has allowed for a minimally invasive approach for delivering RF under endoscopic guidance in pancreatic and extrahepatic biliary cancers[13,14]. Nevertheless, data on safety and efficacy are scarce. In the present study, we aim to review general principles and technical aspects and to evaluate clinical benefits and complications of endoscopy-guided RF in biliary and pancreatic indications based on recent literature.
RFA creates an electrical circuit, either through the body with monopolar probes, between an electrode positioned in the tumour and a grounding pad placed on the skin, or between two interstitial electrodes with bipolar catheters, by using an alternating current with a frequency in the range of radio waves (400-500 kHz). Ions within the tissue try to follow the alternating path of the current, and thus, current flowing through the tissues leads to ion agitation and subsequent frictional heat. Friction heats the surrounding tissues to 50-100 °C causing protein denaturation followed by cell dehydration and coagulative necrosis[10,15,16]. Because of the poor electrical conductivity of tissues, the closest areas to the electrode experience the highest current and temperature, whereas tissues farther away are heated by thermal conduction; in these regions, the heat may not be sufficiently high to cause necrosis[15].
Therefore, a key limitation is the extent of coagulation produced by RF, which is often insufficient to cover the tumour volume[16]. This deficiency is mainly related to the physical consequences of RF. During the desiccation induced by RF, tissues become dehydrated and charred with the loss of ions. Then, current stops leading to a rise in impedance, which limits the volume of tissue successfully ablated[10,15-17]. This roll-off phenomenon may be reduced by using pulsed RF, which allows for the tissue to cool and rehydrate between pulses, with a decrease in impedance enabling larger volumes of thermal destruction. Another strategy involves the use of internally cooled electrodes by circulating water that increases the temperature at the interface tissue electrode, limiting the charring process and allowing for longer lasting current flow[10,16]. However, the extent of thermal injury is also dependent on the length and gauge of the electrode, the temperature generated and the length of RF. The selection of the correct settings in the generator, the optimal application time and the development of bipolar probes and multiple hooked electrodes in an array are other approaches for optimizing the tumour volume ablated[16]. Another cause of incomplete tumour ablation is the heat-sink effect created by the proximity of large vessels to tumours. Vascular flow may dissipate heat and cool the adjacent tissues, preventing the required temperature from being attained, which is particularly important when considering PC[15,16].
In addition to thermal injury, recent studies in animal models have suggested that RF may stimulate systemic antitumor immunity, which acts synergistically in subsequent tumour eradication. RFA generates large amounts of cellular debris that results in increased dendritic cell infiltration, inducing tumour-specific T-cell responses[18]. Another plausible mechanism by which an anti-tumour immunity response can be triggered is the induction of heat shock protein (HSP) expression because hyperthermia has been reported to enhance the immunogenicity of cancer cells concomitantly with the expression of HSP[19].
There are two different categories of complications resulting from RF. The first category corresponds to complications related to thermal therapy and includes a flu-like syndrome that is usually resolved within the first 24 h, post-procedure pain, skin burns at the grounding pad site and thermal injury of surrounding structures[10,20]. During RFA with monopolar probes, a similar amount of energy is generated at the ground and at the electrode surface. The surface area, orientation and material of the pad as well as the electrode to pad distance affect the grounding pad temperature. With small grounding pad areas and high-current RF, deleterious heating effects may be observed at the ground site. Second- and third-degree skin burns are now uncommon owing to the use of large-area foil pads oriented to maximize the leading edge of the ground to safely dissipate heat. The risk of skin burns is also obviated using bipolar probes. Structures adjacent to a tumour may become irreversibly coagulated during the procedure[16,20]. The damage of vessels and the gastrointestinal wall with secondary perforation are the most feared complications. These complications may be avoided by maintaining a 1 cm separation between these structures and the targeted tumour. However, vascular damage is not as common as anticipated due to the protective effect of the heat-sink phenomenon. The second category of complications includes those related to electrode placement[20]. The complications consist mainly of bleeding, infection and tumour seeding and depend on the access route and technique applied to the targeted organ (ERCP or EUS-FNA in the case of CC and PC, respectively).
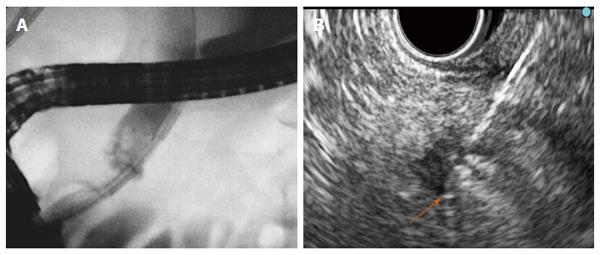
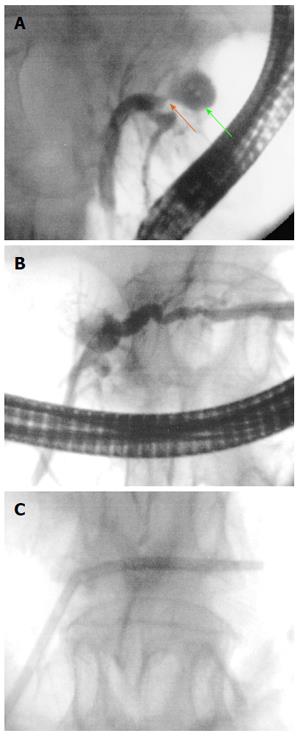
Six different RF probes have been developed that enable endoscopic RF in the pancreas and the bile duct. Two of them are designed to be used over a guide wire during ERCP for biliary strictures (HabibTM EndoHBP and ELRATM), and the other four are used under EUS guidance for pancreatic tumours (HabibTM EUS RFA, Cryotherm probe, EUSRA RF electrode and a 19-gauge EUS-FNA needle) (Figure 1). Among them, the 19-gauge EUS-FNA needle has been used only in liver procedures on animal models, the HabibTM EUS RFA and the EUSRA RF electrodes are monopolar probes and the other three are bipolar. A cooling system is available only with the cryotherm probe, the ELRATM, and the EUSRA RF electrode. Other technical characteristics are presented more extensively elsewhere[21-26].
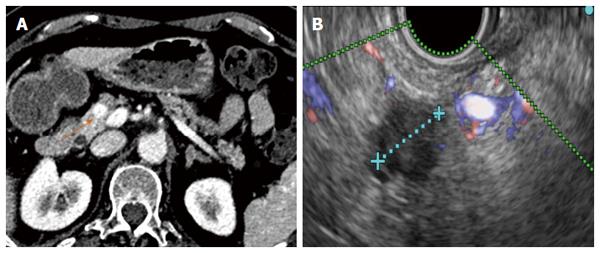
RF ablation of pancreatic tumours is performed by using a convex linear-array echoendoscope. When using the cryotherm or the EUSRA RF electrodes, the probe is passed through the operative channel of the echoendoscope and directly inserted into the target mass. However, with the HabibTM EUS RFA, a 19-gauge EUS needle is first placed into the mass, positioning the needle tip at the far end of the mass. The stylet is then removed, and the RF probe is introduced through the needle. Finally, the needle is withdrawn by 3 cm to avoid direct contact between the metallic needle and the active electrode[27]. Regardless of what needle is used, real-time Doppler imaging is helpful to avoid major vessel injury. The ablation must start at the far end of the lesion, and for large lesions the probe is repositioned along the same trajectory or using a fanning technique to ablate the entire lesion[25,28].
For biliary RF, the biliary tract is cannulated by conventional ERCP, and biliary tree opacification is performed to clearly determine the location of the stricture and to delineate its length and diameter. Although not necessary, a sphincterotomy is usually performed. Depending on the stricture diameter, balloon dilation of the stricture may be required before inserting the RF catheter. The probe is then introduced over the guide until the stricture is reached[29,30]. RF energy is delivered over the selected period, and before moving the probe, a rest period of 1 min is observed to prevent tissues from adhering to the electrodes. Based on the stricture length, several RF applications are performed during the same session, from the proximal margin of the stricture to the distal one, with minimal overlap to reduce the risk of complications. In patients with Klatskin tumours, RF is also applied to more than one stricture (common bile duct, left and/or right hepatic ducts) in the same session. After withdrawing the probe, coagulated tissue debris are removed with balloon sweeps, and a plastic or metal stent is placed to ensure biliary drainage[29,30].
The selected power and time settings in endoscopy-guided biliopancreatic RF vary among the different probes and are those recommended by manufacturers based on the results of preclinical studies in animals and ex vivo human studies
To evaluate the feasibility, clinical efficacy and safety of endoscopy-guided biliopancreatic RF, an electronic search was performed in Pubmed and Embase. The review was restricted to English literature published up to March 2016. The search terms used were “pancreatic cancer” or “pancreatic tumour” or “cholangiocarcinoma” or “biliary cancer” or “biliary stricture” and “radiofrequency ablation”. The reference list of published articles was hand-searched to select original studies reporting on endoscopic RF for biliopancreatic cancers in humans. Because of the paucity of reports on this subject, we aimed to consider all types of evidence available. For this reason, we also included case reports and relevant abstracts reporting on technical feasibility, clinical outcomes or complications of endoscopy-guided biliopancreatic RF. Studies whose patients were included in further larger series were not considered. Indications, technical details, technical success, clinical outcomes, impact on survival, complications and mortality were extracted and further discussed.
Results: A literature search using the terms “pancreatic cancer” or “pancreatic tumour” and “RF ablation” yielded 276 relevant articles from the Pubmed and Embase databases. Of these articles, only seven were suitable and corresponded to four prospective studies, one case series of three patients and two case reports[25,27,28,31-34]. Data extracted from these articles are summarized in Table 1.
| Ref. | n | Indication | Mean size mm (range) | RF device | Thermokinetics | RF sessions | Outcome | Survival (range) | Complications |
| Armellini et al[31], 2005 | 1 | PNET | 20 | 18 G Needle electrode | NA | NA | Complete ablation | - | No complication |
| (STARmed) | |||||||||
| Arcidiacono et al[32], 2012 | 22 | Locally advanced PC | 36 | CTP | 18 W (heating) | NA | Significant volume reduction in 16 patients | 6 mo1 | Early: |
| (23-54) | 650 psi (cooling) | (P = 0.07) | (1-12) | 3 transient abdominal pain | |||||
| 107 (10-360) s | Technical failure in 6 patients | 1 minor duodenal bleeding | |||||||
| Late: | |||||||||
| 2 jaundice | |||||||||
| 1 duodenal stricture | |||||||||
| 1 cystic fluid collection | |||||||||
| Rossi et al[33], 2014 | 1 | PNET | 9 | Habib EUS RFA | 10-15 W | 1 | Complete thermal ablation | - | No complication |
| 360 s | No recurrence | ||||||||
| (34 mo follow-up) | |||||||||
| Weigt et al[34], 2014 | 1 | IPMN | 10 | Habib EndoHBP | 8 W | NA | 2 cm ablation | - | Mild acute pancreatitis |
| (recurrent bleeding) | 90 s | No rebleeding | |||||||
| (10 wk follow-up) | |||||||||
| Pai et al[27], 2015 | 8 | Mucinous cyst (4) | 41 (24-70) | Habib EUS RFA | 5-25 W | 4.5 | - | 2 mild abdominal pain | |
| IPMN (1) | 35 | 90-120 s | (2-7) | 2 cyst resolution | |||||
| Microcystic adenoma (1) | 20 | 4 cyst reduction | |||||||
| PNET (2) | 27 (15-40) | (48 % reduction) | |||||||
| 2 PNET with vascularity change | |||||||||
| Lakhtakia et al[25], 2015 | 3 | Insulinoma | 18 | 18 G Needle electrode | 50 W | NA | No recurrent hypoglycemia | - | No complication |
| (Hypoglycemia) | (14-22) | (STARmed) | 10-15 s | (12 mo follow-up) | |||||
| Song et al[28], 2016 | 6 | Locally advanced PC (4) | 38 | 18 G | 20-50 W | 1.3 | Necrosis at the ablation site | NA | 2 mild abdominal pain |
| Metastatic PC (2) | (30-90) | Needle electrode | 10 s | (1-2) | |||||
| (STARmed) |
Overall, 42 patients underwent EUS-guided RFA, and indications were advanced unresectable PC in 28 patients (2 in the uncinated process, 20 in the head, and 6 in the tail), PNET in 7, mucinous cysts in 4, IPMN in 2 and microcystic adenoma in 1. All patients with a resectable tumour were either unfit for surgery or refused surgery. Among patients with PNET, three corresponded to symptomatic insulinomas (hypoglycemia with recurrent episodes of seizures or syncope and frequent eating with significant weight gain)[27]. One patient with IPMN presented recurrent tumour bleeding through the ampulla[34]. Technical success was achieved in 36 patients (86%), but in 6 patients it was not possible to introduce the CTP inside the tumour. The proposed explanation was that the stiffness due to the duodenal infiltration and desmoplastic reaction prevented the probe insertion[32]. The required number of RF sessions was not specifically reported in the majority of studies. The selected power and application time varied widely, ranging from 5 to 50 W and from 10 to 360 s, respectively, primarily depending on the tumour size.
Following RF treatment, the four symptomatic tumours became asymptomatic. No further bleeding occurred in the patient with IPMN during 10 wk of follow-up. Biochemical improvement was observed in the first 48 h after RF in the three insulinomas, and these patients remained free of symptoms during a 12-mo follow-up[25]. A favourable response was observed in the remaining PNETs, either with complete ablation estimated at 1-month image exam in one case or with a vascularity change with central necrosis in the other two PNETs[27,31,33]. Two mucinous cysts had complete resolution, and the volumes of the other four cysts were nearly halved (48% reduction in volume)[27]. One study in patients with unresectable PC focused on the feasibility and safety of EUS-guided RF, and therefore, survival, the main outcome in this group, was not evaluable[28]. Two of sixteen patients were lost to follow-up, and another one died during hospitalization in another study involving patients with locally advanced PC. The median survival of the remaining 13 patients was 6 mo[32].
There was no procedure-related mortality, and no patient required surgery. Mild early complications were observed in 9 of 36 patients (25%) with successful RF treatment. The most frequent complication, observed in seven patients, was mild abdominal pain that lasted 24 h after treatment and responded to common analgesics. There was one case of mild acute pancreatitis and one case of duodenal bleeding treated endoscopically without the need for blood transfusion[32,34]. One patient had a cystic fluid collection between the pancreas and the left hepatic lobe as a late complication. The collection was asymptomatic and resolved spontaneously[32].
Discussion: Current experience, although preliminary, demonstrates that EUS-guided RF is a feasible treatment. However, technical failure occurred in six cases (14%) when using a cryotherm probe. Results of recent studies involving percutaneous and intraoperative RF suggest that pancreatic RF may be dangerous without additional cooling because of the risk of unintended thermal injury of surrounding structures[35]. The CTP for EUS-guided RF that incorporates a cooling system cannot be inserted through a EUS needle because of its larger diameter and, therefore, it is introduced directly inside the echo endoscope channel. This characteristic, along with the flexible nature of this probe, makes it difficult to enter a hard tumour with a desmoplastic reaction. Technological improvements providing these probes with cutting current, like a needle-knife, or rendering the probes thin enough to be inserted through a EUS needle, may increase the success rate.
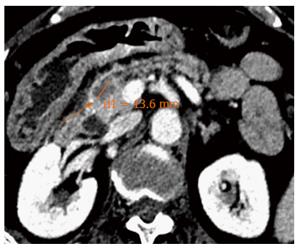
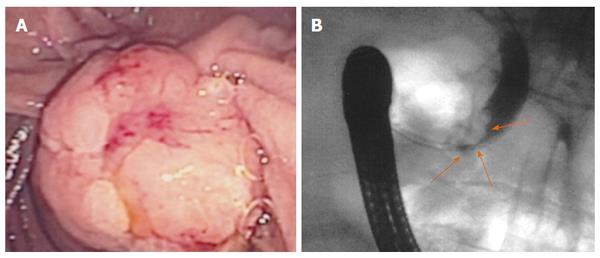
Although the technical feasibility of EUS-guided RF may be accepted, its clinical efficacy is more difficult to affirm because of the scarcity of available experience. The intended effect is primarily palliative, and possible recurrence must be expected, although some authors have suggested that it may be curative for small tumours (Figures 2 and 3). Beneficial effects with immediate relief of symptoms were observed in the four symptomatic tumours, but the follow-up period was very limited. Therefore, it is impossible to predict the recurrence rate, time until symptom reappearance and how many frequent RF sessions would be required. In addition, for locally advanced PC, EUS-guided RF aims to improve quality of life and to prolong survival by means of a cytoreductive effect. However, no study has had survival as a primary endpoint, a quality-of-life assessment has not been performed and there are no randomized studies comparing EUS-guided RF with the standard palliative treatment.
The successful results of RF in hepatic tumours and the need for less invasive alternatives to surgery for pancreatic tumours have prompted to attempt RF in the pancreas. Nevertheless, important biological and anatomical differences between the liver and the pancreas may determine very different safety profiles of RF in the two contexts. First, the pancreas is a highly thermosensitive organ, and thermal injury may lead to serious inflammatory consequences[27]. Second, hepatic tumours are usually surrounded by normal parenchyma, and thermal injury beyond the hepatic tumours does not usually affect important structures, whereas pancreatic tumours often encase vessels and the distal bile duct or are in contact with the gastric or duodenal wall. For this reason, the safety of intraoperative and percutaneous pancreatic RF is still under debate, and frequent and severe complications have been reported[36-38]. In contrast, only mild complications have been described for EUS-guided RF in pancreas, even when RF without additional cooling was applied. No case of severe acute pancreatitis, duodenal perforation, severe gastrointestinal haemorrhage or bile leak have occurred after RF under EUS guidance. Although all patients with a locally advanced cancer in one series had major vessel involvement, no case of portal or splenic thrombosis was reported[32]. One explanation for this low morbidity may be that EUS is the best modality for real-time imaging of the pancreas, minimizing the risk of inadvertent damage of adjacent anatomical structures. Nevertheless, mostly large and advanced tumours have been included in these series, and adjacent normal structures were likely far from the probe. In small benign lesions, other injuries may be observed (Figures 4 and 5). Further prospective series are needed to confirm the low morbidity.
Results: The primary search identified 227 publications. Titles and abstracts were screened for relevance, and 174 records were excluded. After a full-text review of the 53 remaining studies, 25 papers were determined to be eligible for inclusion (Tables 2 and 3)[39-64]. The selected studies comprised eight retrospective series, two prospective trials, seven case reports and eight abstracts. Review of article references yielded one more abstract[39].
| Ref. | n | Indication | Stricture length (mm) | Thermokinetics power - time | RF sessions | Technical success | Stricture diameter before RF (mm) | Stricture diameter after RF (mm) | Stent patency (d) | Median survival (mo) | Complications |
| Pozsár et al[39], 2011 | 5 | Occluded SEMS | 15 | 10 W - 120 s | 2 (1-3) | 100% | 2 | 4.7 | 62 | - | No complication |
| (malignant strictures) | (9-236) | ||||||||||
| Monga et al[40], 2011 | 1 | CC | 15 | 5 W - 120 s | 1 | 100% | - | - | - | - | No complication |
| Steel et al[41], 2011 | 21 | 16 PC | - | 7-10 W | 2 (1- 4) | 100% | 0 (0-1) | 4 (3-6) | 76% | - | 1 hyperamylasemia |
| 6 Klatskin/intrahepatic CC | 120 s | at 90-d FU | 2 cholecystitis | ||||||||
| 1 rigors | |||||||||||
| Yoon et al[42], 2012 | 1 | CHD CC | - | 7 W - 90 s | 2 | 100% | - | - | - | - | No complication |
| Mavrogenis et al[43], 2012 | 1 | Intrahepatic adenoma | - | - | - | 100% | - | - | - | - | - |
| Dzeletovic et al[44], 2012 | 1 | Ampullary adenoma | 10 | 1 | - | 100% | - | - | - | - | CBD stenosis |
| with CBD invasion | (Complete ablation) | ||||||||||
| Sonpal et al[45], 2012 | 1 | Occluded SEMS (Klatskin CC) | - | 8 - 10 W | 2 | 100% | - | - | 90 | - | - |
| 90 s | |||||||||||
| Lewis et al[46], 2012 | 5 | 4 CC | - | 7-10 W | 1 (1-2) | 100% | - | - | - | - | No complication |
| 1 colon met. | 90 s | ||||||||||
| Watson et al[47], 2012 | 3 | 3 Klatskin CC | 2 | 7-10 W | 3 | 6 | No complication | ||||
| 90 s | |||||||||||
| Kallis et al[48], 2015 | 11 | Occluded SEMS: | - | - | 1 | 100% | - | - | 146 | - | No complication |
| 6 PC/3 CC | (1-2) | ||||||||||
| 2 liver met | |||||||||||
| Topazian et al[49], 2013 | 1 | Intrahepatic adenoma | 10 W - 90 s | 1 | 100% | - | - | - | - | Hepatic artery pseudoaneurysm | |
| (Complete ablation) | |||||||||||
| Figueroa-Barojas et al[50], 2013 | 20 | 11 CC/7 PC | 15.2 | 7-10 W | - | 100% | 1.7 | 5.2 | 100% | 5 pain | |
| 1 IPMN/1 Met. | (3.5-33) | 120 s | (0.5-3.4) | (2.6-9) | at 30-d FU | 1 mild pancreatitis and cholecystitis | |||||
| Alis et al[51], 2013 | 10 | CC | 20 | 10 W - 120 s | 3 (3-4) | 100% | 1.5 | 5 | 270 | - | 2 mild pancreatitis |
| (20-35) | (1.5-2) | (4-7) | (180-450) | ||||||||
| Lui et al[52], 2013 | 1 | Occluded SEMS | 10 W - 150 s | 1 | 100% | - | - | 60 | - | No complication | |
| (Klatskin CC) | |||||||||||
| Law et al[53], 2013 | 2 | PC | - | 10 W - 120 s | 1 | 100% | - | - | - | - | - |
| Tal et al[54], 2014 | 12 | 2 intrahepatic CC | - | 8-10 W | 1 | 100% | - | - | - | 8.5 | 3 hemobilia (2 deaths) |
| 8 Klatskin IV CC | 60-90 s | (1-5) | 3 cholangitis | ||||||||
| 2 GB can. | |||||||||||
| 1 gastric Met |
| Ref. | n | Indication | Stricture length (mm) | Thermokinetics power - time | RF sessions | Technical success | Stricture diameter before RF (mm) | Stricture diameter after RF (mm) | Stent patency (d) | Survival (mo) | Complications |
| Hu et al[55], 2014 | 9 | 4 postsurgical | - | 10 W - 90 s | 1 | 100% | - | - | - | - | 2 abdominal pain |
| 3 liver transplant | (5 complete resolution | 2 transient leucocytosis | |||||||||
| 2 chronic inflam | 4 improvement) | 1 mild pancreatitis | |||||||||
| Uppal et al[56], 2014 | 2 | Prehepatic transplant | - | - | - | 100% | - | - | - | 19-35 mo FU | 1 hemobilia |
| 1 LHD-CHD CC | (No malignancy in explant) | ||||||||||
| 1 RHD-CHD CC | |||||||||||
| Strand et al[57], 2014 | 16 | 13 Klatskin CC | - | 7 W - 90 s | - | 100% | - | - | - | 9.6 | Occurrence/month: |
| 1 intrahepatic CC | Stent occlusion 0.06 | ||||||||||
| 2 extrahepatic CC | Stent migration 0.02 | ||||||||||
| Cholangitis 013 | |||||||||||
| Hepatic abscess 0.02 | |||||||||||
| Dolak et al[58], 2014 | 58 | 50 Klatskin CC | - | 10 W - 180 s | 1 | 100% | - | - | 170 | 10.6 | 1 partial liver infarction |
| 4 PC | (1-5) | 5 cholangitis | |||||||||
| 1GB can | 2 cholangiosepsis | ||||||||||
| 1 met | 3 hemobilia | ||||||||||
| 1 HCC | 1 GB empyema | ||||||||||
| 1 HCC and CC | 1 hepatic coma (1 death) | ||||||||||
| 1 left bundle branch block | |||||||||||
| Mukund et al[59], 2014 | 8 | Occluded SEMS: | - | - | 1 | 100% | - | - | 114 | No complication | |
| 4 GB cancer | (1-2) | ||||||||||
| 2 CC/2 PC | |||||||||||
| Mehendiratta et al[60], 2015 | 1 | Ampullary adenoma | - | 7 W - 90 s | - | 100% | - | - | - | - | No complication |
| with CBD invasion | (Complete ablation) | ||||||||||
| Musquer et al[61], 2015 | 1 | Occluded SEMS | - | 10 W - 90 s | - | 100% | - | - | 180 | - | - |
| (CC) | |||||||||||
| Sharaiha et al[62], 2015 | 69 | 45 CC | 14.5 | 8 W - 90 s | 1 | 100% | 2 | 4.9 | 96% | 15 for PC | 1 pancreatitis |
| 19 PC | (3.5-60) | (1-4) | at 30-d FU | 18 for CC | 2 cholecystitis | ||||||
| 1 GB cancer | 1 hemobilia | ||||||||||
| 1 gastric cancer | 3 abdominal pain | ||||||||||
| 3 liver met | |||||||||||
| Laquière et al[63], 2015 | 12 | CC | 19.5 | 10 W - 90 s | 1 | 100% | - | - | - | 12 | 1 sepsis |
| 4 Bismuth I | (10-35) | (1-3) | 1 cholangitis | ||||||||
| 3 Bismuth II | |||||||||||
| 2 Bismuth III | |||||||||||
| 3 Bismuth IV | |||||||||||
| Atar et al[64], 2015 | 21 | Occluded SEMS: | - | 10 W - 90 s | 1 | 100% | - | - | 62% | - | - |
| 11 PC/7 CC | (1-5) | at 90-d FU | |||||||||
| 1 GB can/2 liver met |
Over the last 5 years, a total of 293 patients were reported in the literature to have undergone biliary RF under endoscopic guidance. The indications in the literature were malignant strictures in 232 patients (79%), occluded self-expanding metal stents in 48 (16%), benign non-tumoural strictures in 9 (3%), intrahepatic adenoma in 2 (0.6%) and bile duct ingrowth of ampulloma in the remaining 2 patients (0.6%). In some studies, all malignant strictures corresponded to cholangiocarcinoma, but in others malignant strictures encompassed pancreatic cancer, gallbladder cancer, hepatic carcinoma and metastatic cancers as well. Only one study reported patients with benign non-tumoural strictures and included four postsurgical strictures, three after liver transplant and two chronic inflammatory strictures[55]. The RF probe used in all the reported cases was the HabibTM EndoHBP. The ELRATM electrode has been launched to the market recently, and to date, only one experimental study on animals has been reported[65]. Power and time settings ranged from 5 to 10 W and from 60 to 180 s, respectively; nevertheless, most studies applied RF at 10 W over a period of 90 s. In most studies, patients underwent RFA only once. However, some operators performed RF either twice during the same session with a rest period of 1-2 min or at every ERCP for stent exchange during follow-up.
Technical success with satisfactory placement and deployment of the RF catheter was achieved in all patients. Only in one study was RF not applied in one included patient due to an irretrievable plastic stent with proximal migration. Because RFA was not attempted, we did not consider this case a technical failure[41]. Regarding efficacy, three main outcome measures may be considered: biliary decompression, stent patency and survival. Biliary decompression was possible in all cases but two (99%). In one patient, extensive intrahepatic biliary malignancy prevented successful biliary drainage[41], and in one other, biliary decompression was not achieved, despite successful RFA and endoscopic stenting requiring percutaneous drainage[51]. However, stent patency and survival have not been uniformly described. Only five studies have detailed data about stent patency in patients with malignant strictures treated with RF before placing a self-expanding metal stent (SEMS)[40,50,51,58,62]. In two studies, the mean lengths of stent patency in 10 and 58 patients with malignant strictures were 270 and 170 d (range 180-450 and 63-277)[51,58]. The three other studies reported 96 to 100 %[41,50,62] and 76 % of stent patency at 30 and 90 d[41] of follow-up, respectively. Moreover, RFA of occluded stents achieved 60, 62, 90, 114, 146 and 180 d of mean patency in six studies, and 62% of stents were still patent at 90 d of follow-up after RF for stent occlusion in one abstract report[64]. Six authors evaluated the survival of patients treated with RF before biliary stenting, and it was always longer than 8 mo, ranging from 8.5 to 18 mo[54,57,58,62,63]. As secondary outcomes, differences between luminal diameter before and after RFA, as determined by repeated cholangiography, were recorded in some studies, and a significant increase in diameter was observed immediately after RF treatment in all cases[39,41,50,62].
Two studies compared the stent patency and survival of patients treated with SEMS following RFA with those of patients undergoing biliary stenting with SEMS alone, which represents the conventional practice. These studies were not considered in the present analysis because patients in both cases were included in larger series. In the first study by Sharaiha et al[66] 26 patients, who underwent RF, were matched with 40 patients receiving a SEMS alone. There was no difference in pre- and post-RF stricture diameter (20.4 ± 7.33 vs 23.17 ± 8.07, P = 0.1 and 1.6 ± 0.75 vs 1.38 ± 0.18, P = 0 respectively), mean number of ERCP (2.26 ± 1 vs 1.94 ± 1.27, P = 0.84) or survival (median survival of the groups was 5.9 mo P = 0.87) between the two groups. However, multivariate analysis revealed RF as an independent predictor of survival (HR = 0.9 (0.1-0.76), P = 0.012). The second series by Kallis et al[67] consisted of 23 patients undergoing RF followed by biliary stenting and 46 receiving a SEMS alone. SEMS patency rates between the RF treated group and control group were equivalent (472 d vs 324 d, HR = 1.186, 95%CI: 0.536-2.656, P = 0.669). Median survival in the RFA group was 226 vs 123.5 d in the control group (P = 0.010), and RF was observed to be an independent predictive factor of survival at 90 and 180 d (OR = 21.07, 95%CI: 1.45-306.64, P = 0.026; OR = 4.48, 95%CI: 1.04-19.30, P = 0.044, respectively). Because photodynamic therapy (PDT) has been shown to confer a significant survival advantage compared with biliary stenting, Strand et al[57] aimed to compare RFA with PDT in patients with unresectable CC. Overall survival was similar (9.6 mo vs 7.5 mo respectively, P = 0.799) in patients who underwent RF (n = 16) and in patients receiving PDT (n = 32).
RF was anecdotally used to treat intraductal extension of an ampullary adenoma in two cases and an intrahepatic adenoma in two other patients; all of them were successfully ablated[43,44,49,60]. In addition, nine patients with benign strictures (four post-surgery, three after the liver transplant and two with chronic inflammation) and prior unsuccessful endoscopic treatment underwent RF[55]. All the strictures improved, and complete resolution was observed in more than half.
In some studies, data on complications were not available[43,45,53,57,61,64]. Therefore, among 252 patients, complications occurred in 49 (19 %), and overall mortality was less than 2% (n = 3). Infectious complications, the most frequent adverse event, were reported in 8% (nine cholangitis, five cholecystitis, three cholangiosepsis, two transient leucocytosis, one gallbladder empyema and one patient with rigors). At least two cases of cholecystitis may be explained by tumour encasement of the cystic duct, as shown by CT scan and sepsis before ERCP. Four percent of patients (n = 10) complained of postprocedure abdominal pain well controlled with analgesics, and 2 % suffered mild acute pancreatitis. Haemobilia occurred in 4% (n = 9) and in two cases was fatal[54]. Another case was due to a pseudoaneurysm of the hepatic artery, which was percutaneously thrombosed with thrombin[49]. The pseudoaneurysm was related to RF due to the close temporal relationship with the RF session. Liver infarction was also described in one patient and successfully recovered with conservative treatment[58]. Thermal injury of surrounding vessels was proposed as the hypothetical cause. Finally, hepatic coma with fatal outcome was recorded once[58].
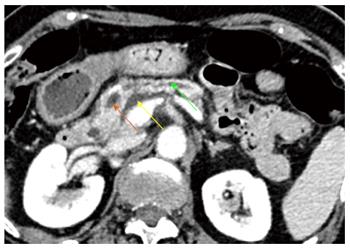
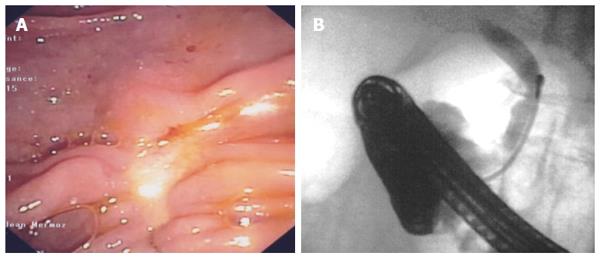
Discussion: SEMS placement is the mainstay palliative treatment of malignant biliary strictures, but more than 50% of SEMS become occluded after 6 mo. It was hypothesized that RF might lengthen stent patency. Therefore, RF has been primarily used as neoadjuvant therapy for malignant strictures prior to inserting a SEMS. Afterwards and in the same line, RF has been applied to treat SEMS occlusion by tumour ingrowth and overgrowth. Anecdotal applications in benign tumoural and non-tumoural strictures have also been reported. Current experience demonstrates that biliary RF under endoscopic guidance is feasible and easy to perform with high technical success rates. Although results seem promising, two studies comparing RF plus SEMS and the conventional palliative treatment with SEMS alone failed to show longer patency of stents after RF. However, the results of these studies suggest a benefit of survival with RF. RF was also compared with PDT, which had been shown to increase stent patency, quality of life and survival, and RF was found to provide a similar survival. Moreover, potential advantages of RF over PDT are the unnecessary limitation of sunlight exposure and its significantly lower cost. Nevertheless, it is still early to draw conclusions about the efficacy of endoscopic biliary RF because most of the available data consist of small retrospective series with high heterogeneity regarding the aetiology of malignant strictures, the power settings selected, the number of sessions, the disease stage and other concomitant therapies, such as chemotherapy. Prospective randomized trials are required to obtain more reliable results about the efficacy of biliary RF and to explore novel indications. The possibility to ablate intraductal extension of ampullary adenomas might reduce the risk of recurrence rate and the need for radical surgery after endoscopic papillectomy (Figures 6 and 7), but only two cases have been reported so far. An ongoing French prospective trial may help to draw conclusions about the interest in RF in this indication. RF may also improve the endoscopic treatment of benign strictures and may even be a rescue treatment for benign strictures refractory to conventional endoscopic treatment.
Although RF has an acceptable safety profile, complications are not uncommon and mortality is not zero. The most frequent adverse outcomes are infectious complications and postprocedure abdominal pain. Abdominal pain is usually self-limited, and the prophylactic pre- and post-procedural use of antibiotics may decrease the risk of infections. Infectious complications, as well as acute pancreatitis, may be primarily attributed to ERCP, but we cannot dismiss the possibility of an increased risk of bacterial translocation after RF and thermal injury of the neighbouring pancreas. The most feared complications at first, such as bile duct or duodenal perforation, were not observed. Preventing biliary fistula was always pursued by inserting a plastic or SEMS after RFA. However, other serious events have been reported, such as liver infarction and fatal haemobilia. Both are believed to be secondary to thermal injury of the hepatic artery. The use of intraductal ultrasonography may help to evaluate the proximity of the hepatic artery to adjust the power settings for more limited energy delivery. This measure is especially relevant for hilar lesions located near liver parenchyma and for strictures without an associated mass.
RF is an ablative modality of treatment that has been recently added to the therapeutic armamentarium of endoscopy. In the setting of unresectable biliopancreatic cancers, in which treatment options are very limited, high expectations are held for this modality. Available experience suggests a beneficial effect on survival with RFA, but current evidence is scarce because most studies have been performed on small and heterogeneous groups of patients using a retrospective design. The safety profile appears to be acceptable, though serious complications have been reported. This finding may be explained in part because the energy settings are not clearly standardized and have been extrapolated from either in vivo animal models with non-tumoural tissues or ex vivo human studies without considering the delayed necrosis and the heat-sink effects in vivo. Prospective randomized controlled trials are awaited to accurately evaluate its efficacy in terms of survival and quality-of-life and to optimize energy parameters in order to reduce the risk of complications. Newest indications such as refractory benign strictures, biliary extension of ampullomas or branch-duct intraductal papillary mucinous neoplasms deserve also further assessment.
| 1. | Moss RA, Lee C. Current and emerging therapies for the treatment of pancreatic cancer. Onco Targets Ther. 2010;3:111-127. [PubMed] |
| 2. | Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Maréchal R, Van Laethem JL, Ducreux M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer. 2016;57:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Brandi G, Venturi M, Pantaleo MA, Ercolani G. Cholangiocarcinoma: Current opinion on clinical practice diagnostic and therapeutic algorithms: A review of the literature and a long-standing experience of a referral center. Dig Liver Dis. 2016;48:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol. 2000;19:156-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105-4112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 637] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 6. | Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11:13-21.e1; quiz e3-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:2267-2278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (2)] |
| 8. | Roque J, Ho SH, Reddy N, Goh KL. Endoscopic ablation therapy for biliopancreatic malignancies. Clin Endosc. 2015;48:15-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267-270. [PubMed] |
| 10. | Shah DR, Green S, Elliot A, McGahan JP, Khatri VP. Current oncologic applications of radiofrequency ablation therapies. World J Gastrointest Oncol. 2013;5:71-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Wu TT, Li HC, Li WM, Ao GK, Lin H, Zheng F, Song JY. Percutaneous Intraluminal Radiofrequency Ablation for Malignant Extrahepatic Biliary Obstruction: A Safe and Feasible Method. Dig Dis Sci. 2015;60:2158-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Weber A, Schmid RM, Prinz C. Diagnostic approaches for cholangiocarcinoma. World J Gastroenterol. 2008;14:4131-4136. [PubMed] |
| 13. | Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392-401. [PubMed] |
| 14. | Itoi T, Isayama H, Sofuni A, Itokawa F, Tamura M, Watanabe Y, Moriyasu F, Kahaleh M, Habib N, Nagao T. Evaluation of effects of a novel endoscopically applied radiofrequency ablation biliary catheter using an ex-vivo pig liver. J Hepatobiliary Pancreat Sci. 2012;19:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 16. | Goldberg SN, Gazelle GS. Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepatogastroenterology. 2001;48:359-367. [PubMed] |
| 17. | Chiou SY, Liu JB, Needleman L. Current status of sonographically guided radiofrequency ablation techniques. J Ultrasound Med. 2007;26:487-499. [PubMed] |
| 18. | Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Teng LS, Jin KT, Han N, Cao J. Radiofrequency ablation, heat shock protein 70 and potential anti-tumor immunity in hepatic and pancreatic cancers: a minireview. Hepatobiliary Pancreat Dis Int. 2010;9:361-365. [PubMed] |
| 20. | Rhim H, Dodd GD, Chintapalli KN, Wood BJ, Dupuy DE, Hvizda JL, Sewell PE, Goldberg SN. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics. 2004;24:41-52. [PubMed] |
| 21. | Zacharoulis D, Lazoura O, Sioka E, Potamianos S, Tzovaras G, Nicholls J, Koukoulis G, Habib N. Habib EndoHPB: a novel endobiliary radiofrequency ablation device. An experimental study. J Invest Surg. 2013;26:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Cho JH, Lee KH, Kim JM, Kim YJ, Lee DH, Jeong S. Safety and Efficacy of a novel endobiliary radiofrequency ablation catheter (ELRA®) in a swine model. Gastrointest Endosc. 2015;81:AB350. |
| 23. | Gaidhane M, Smith I, Ellen K, Gatesman J, Habib N, Foley P, Moskaluk C, Kahaleh M. Endoscopic Ultrasound-Guided Radiofrequency Ablation (EUS-RFA) of the Pancreas in a Porcine Model. Gastroenterol Res Pract. 2012;2012:431451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Carrara S, Arcidiacono PG, Albarello L, Addis A, Enderle MD, Boemo C, Neugebauer A, Campagnol M, Doglioni C, Testoni PA. Endoscopic ultrasound-guided application of a new internally gas-cooled radiofrequency ablation probe in the liver and spleen of an animal model: a preliminary study. Endoscopy. 2008;40:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Lakhtakia S, Ramchandani M, Galasso D, Gupta R, Venugopal S, Kalpala R, Reddy DN. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc. 2016;83:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Varadarajulu S, Jhala NC, Drelichman ER. EUS-guided radiofrequency ablation with a prototype electrode array system in an animal model (with video). Gastrointest Endosc. 2009;70:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 167] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 28. | Song TJ, Seo DW, Lakhtakia S, Reddy N, Oh DW, Park do H, Lee SS, Lee SK, Kim MH. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2016;83:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Rustagi T, Jamidar PA. Intraductal radiofrequency ablation for management of malignant biliary obstruction. Dig Dis Sci. 2014;59:2635-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Mensah ET, Martin J, Topazian M. Radiofrequency ablation for biliary malignancies. Curr Opin Gastroenterol. 2016;32:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Armellini E, Crinò SF, Ballarè M, Occhipinti P. Endoscopic ultrasound-guided radiofrequency ablation of a pancreatic neuroendocrine tumor. Endoscopy. 2015;47 Suppl 1 UCTN:E600-E601. [PubMed] |
| 32. | Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Rossi S, Viera FT, Ghittoni G, Cobianchi L, Rosa LL, Siciliani L, Bortolotto C, Veronese L, Vercelli A, Gallotti A. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Weigt J, Kandulski A, Malfertheiner P. Endoscopic intraductal radiofrequency ablation of remnant intrapapillary mucinous neoplasm with acute hemorrhage after incomplete surgical resection. Endoscopy. 2014;46 Suppl 1 UCTN:E489-E490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14-20. [PubMed] |
| 37. | Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392-395. [PubMed] |
| 38. | Elias D, Baton O, Sideris L, Lasser P, Pocard M. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol. 2004;30:85-87. [PubMed] |
| 39. | Pozsár J, Tarpay A, Burai J, Pap A. Intraductal radiofrequency ablation can restore patency of occluded biliary self-expanding metal stents. Z Gastroenterol. 2011;49:A70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, Reddy DN. Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos). Gastrointest Endosc. 2011;74:935-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 42. | Yoon WJ, Brugge WR. Radiofrequency ablation of malignant biliary obstruction. Gastrointest Endosc. 2012;75:AB116. |
| 43. | Mavrogenis G, Deprez PH, Wallon J, Warzée P. Bile duct adenoma causing recurrent cholangitis: diagnosis and management with targeted Spyglass access and radiofrequency ablation. Endoscopy. 2012;44 Suppl 2 UCTN:E290-E291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Dzeletovic I, Topazian MD, Baron TH. Endoscopic balloon dilation to facilitate treatment of intraductal extension of ampullary adenomas (with video). Gastrointest Endosc. 2012;76:1266-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Sonpal N, Saitta P, Haber G. Maintaining stent patency with radiofrequency ablation and interim plastic stent placement for Klatskin tumors. Am J Gastroenterol. 2012;107:S337. |
| 46. | Lewis J, Mehendiratta V, Korenblit J, Siddiqui AA, Kowalski TE, Loren DE. Safety of an endoscopic bipolar radiofrequency probe in the management of malignant biliary strictures: A single center experience. Gastrointest Endosc. 2012;75:AB388. |
| 47. | Watson J, Habr F. Safety and efficacy of endoscopic radiofrequency ablation in nonresectable cholangiocarcinoma: A case series. Am J Gastroenterol. 2012;107:S78. |
| 48. | Kallis Y, Phillips N, Steel A, Dickinson R, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Radiofrequency ablation for biliary metal stent occlusion: evolution of a novel endoscopic technique and proof of concept. Gastrointest Endosc. 2012;75:AB377. |
| 49. | Topazian M, Levy MJ, Patel S, Charlton MR, Baron TH. Hepatic artery pseudoaneurysm formation following intraductal biliary radiofrequency ablation. Endoscopy. 2013;45 Suppl 2 UCTN:E161-E162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013;2013:910897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Alis H, Sengoz C, Gonenc M, Kalayci MU, Kocatas A. Endobiliary radiofrequency ablation for malignant biliary obstruction. Hepatobiliary Pancreat Dis Int. 2013;12:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Lui KL, Li KK. Intraductal radiofrequency ablation of tumour ingrowth into an uncovered metal stent used for inoperable cholangiocarcinoma. Hong Kong Med J. 2013;19:539-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Law R, Pai M, Baron TH, Habib N. The effects of endobiliary radiofrequency ablation in two patients with pancreatic cancer: Gross and microscopic findings. Gastrointest Interv. 2013;2:124-126. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Tal AO, Vermehren J, Friedrich-Rust M, Bojunga J, Sarrazin C, Zeuzem S, Trojan J, Albert JG. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc. 2014;6:13-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (4)] |
| 55. | Hu B, Gao DJ, Wu J, Wang TT, Yang XM, Ye X. Intraductal radiofrequency ablation for refractory benign biliary stricture: pilot feasibility study. Dig Endosc. 2014;26:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Uppal DS, Northup PG, Argo CK, Pelletier SJ, Maluf DG, Rahma OE, Read PW, Cox DG, Strand DS, Wang Y. Endoscopically-delivered neoadjuvant photodynamic therapy and radiofrequency ablation in patients with unresectable cholangiocarcinoma awaiting liver transplantation: a pilot experience. Gastroenterology. 2015;148:1029. [DOI] [Full Text] |
| 57. | Strand DS, Cosgrove ND, Patrie JT, Cox DG, Bauer TW, Adams RB, Mann JA, Sauer BG, Shami VM, Wang AY. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014;80:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Dolak W, Schreiber F, Schwaighofer H, Gschwantler M, Plieschnegger W, Ziachehabi A, Mayer A, Kramer L, Kopecky A, Schrutka-Kölbl C. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2014;28:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Mukund A, Rajesh S, Arora A, Panda D. Endobiliary RFA and balloon sweep to restore the patency of occluded metallic biliary stents - a feasibility study. J Vasc Interv Radiol. 2014;25:S75. |
| 60. | Mehendiratta V, Desilets DJ. Use of radiofrequency ablation probe for eradication of residual adenoma after ampullectomy. Gastrointest Endosc. 2015;81:1055-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Musquer N, Ménager Tabourel E, Luet D, Caroli-Bosc FX, Métivier Cesbron E. Recanalization of obstructed metallic uncovered biliary stent using endobiliary radiofrequency ablation. Gastrointest Endosc. 2016;83:256-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 62. | Sharaiha RZ, Sethi A, Weaver KR, Gonda TA, Shah RJ, Fukami N, Kedia P, Kumta NA, Clavo CM, Saunders MD. Impact of Radiofrequency Ablation on Malignant Biliary Strictures: Results of a Collaborative Registry. Dig Dis Sci. 2015;60:2164-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 63. | Laquière A, Boustière C, Leblanc S, Penaranda G, Désilets E, Prat F. Safety and feasibility of endoscopic biliary radiofrequency ablation treatment of extrahepatic cholangiocarcinoma. Surg Endosc. 2016;30:1242-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Atar M, Kadayifci A, Forcione DG, Casey B, Kelsey PB, Brugge WR. Efficacy of radiofrequency ablation (RFA) for the management of occluded biliary metal stents. Gastrointest Endosc. 2015;81:AB195. |
| 65. | Cho JH, Lee KH, Kim JM, Kim YJ, Lee DH, Jeong S. Safety and efficacy of a novel radiofrequency ablation catheter (Elra®) in a swine model. Gastrointest Endosc. 2015;81:Su1612. |
| 66. | Sharaiha RZ, Natov N, Glockenberg KS, Widmer J, Gaidhane M, Kahaleh M. Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: is there an added benefit? Dig Dis Sci. 2014;59:3099-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Kallis Y, Phillips N, Steel A, Kaltsidis H, Vlavianos P, Habib N, Westaby D. Analysis of Endoscopic Radiofrequency Ablation of Biliary Malignant Strictures in Pancreatic Cancer Suggests Potential Survival Benefit. Dig Dis Sci. 2015;60:3449-3455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Corrales FJ, Garg P, Gupta C, Slomiany BL S- Editor: Qi Y L- Editor: A E- Editor: Wang CH