Published online Sep 28, 2016. doi: 10.3748/wjg.v22.i36.8070
Peer-review started: March 26, 2016
First decision: May 12, 2016
Revised: June 25, 2016
Accepted: August 1, 2016
Article in press: August 1, 2016
Published online: September 28, 2016
Processing time: 184 Days and 9.2 Hours
Colorectal cancer (CRC) is a major cause of cancer-related mortality and morbidity worldwide. While improved treatments have enhanced overall patient outcome, disease burden encompassing quality of life, cost of care, and patient survival has seen little benefit. Consequently, additional advances in CRC treatments remain important, with an emphasis on preventative measures. Guanylyl cyclase C (GUCY2C), a transmembrane receptor expressed on intestinal epithelial cells, plays an important role in orchestrating intestinal homeostatic mechanisms. These effects are mediated by the endogenous hormones guanylin (GUCA2A) and uroguanylin (GUCA2B), which bind and activate GUCY2C to regulate proliferation, metabolism and barrier function in intestine. Recent studies have demonstrated a link between GUCY2C silencing and intestinal dysfunction, including tumorigenesis. Indeed, GUCY2C silencing by the near universal loss of its paracrine hormone ligands increases colon cancer susceptibility in animals and humans. GUCY2C’s role as a tumor suppressor has opened the door to a new paradigm for CRC prevention by hormone replacement therapy using synthetic hormone analogs, such as the FDA-approved oral GUCY2C ligand linaclotide (Linzess™). Here we review the known contributions of the GUCY2C signaling axis to CRC, and relate them to a novel clinical strategy targeting tumor chemoprevention.
Core tip: Guanylyl cyclase C (GUCY2C) is a tissue-specific transmembrane receptor found in apical membranes of intestinal enterocytes that drives intestinal homeostatic mechanisms and opposes colorectal tumorigenesis. The receptor’s endogenous hormone ligands, guanylin and uroguanylin, are the most commonly lost gene products in colorectal cancer, making GUCY2C a potential therapeutic target for tumor prevention through hormone replacement therapy.
- Citation: Pattison AM, Merlino DJ, Blomain ES, Waldman SA. Guanylyl cyclase C signaling axis and colon cancer prevention. World J Gastroenterol 2016; 22(36): 8070-8077
- URL: https://www.wjgnet.com/1007-9327/full/v22/i36/8070.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i36.8070
Colorectal cancer (CRC) is the fourth most common cancer in the United States and the second-leading cause of cancer-associated death[1,2]. It is estimated that approximately one million new cases of CRC will occur in 2016, and approximately 600000 people will succumb to the disease worldwide[2,3]. CRC incidence and mortality rates have experienced a continuous decline over the past few decades, specifically due to marginal improvements in treatment[4], awareness of CRC risk-factors[3], and routine early detection screening[5]. However, the high prevalence of adenomatous polyp diagnoses despite the growth in screening awareness have led to persistently high morbidity and mortality rates for CRC, demonstrating the continual clinical need for improved CRC prevention and treatment strategies. Currently, CRC prognosis is dependent upon disease stage, with primary treatment for localized disease resulting in complete surgical resection of the tumor. Although surgery has contributed to a 5 year survival rate of greater than 65%, disease reoccurrence has been reported in nearly 50% of CRC patients[6,7]. With nearly one fourth of patients presenting with metastatic disease, it is crucial to develop highly effective therapies targeted towards earlier prevention and treatment that slows disease initiation and progression[4,8].
Colorectal tumorigenesis is widely accepted as a disease of both sporadic and inherited genomic instability resulting in three forms of CRC: (1) microsatellite instability (MSI); (2) chromosomal instability [CIN; i.e., the activation of oncogenes (K-ras) and inactivation of tumor suppressors (adenomatous polyposis coli, APC; p53)]; and (3) chromosomal translocations[9-12]. Apart from oncogenomic risk factors leading to CRC, recent work suggests that loss of the guanylyl cyclase C (GUCY2C) signaling cascade contributes to CRC susceptibility[13-15]. In an effect preserved across species, CRC is associated with a near universal loss of the GUCY2C hormone ligands guanylin and uroguanylin, disrupting intestinal homeostasis and initiating disease progression[14,16-21]. While the processes by which these hormone ligands are suppressed in CRC remain unknown, they are of great interest to better understand mechanisms of tumorigenesis. This emerging role of GUCY2C as a tumor suppressor in the intestine, whose loss of signaling is the result of a hormone deficiency, provides a unique opportunity to reactivate GUCY2C signaling through hormone replacement therapy[15]. This review highlights recent insights in the GUCY2C-hormone signaling axis, its relation to the previously defined hallmarks of CRC, and its potential as a preventative treatment option.
Sporadic CRC arises predominantly through a characteristic accumulation of acquired somatic mutations over decades, leading to cellular transformation, subsequent adenoma formation, and ultimately malignancy[9,22,23]. Hereditary CRC, despite comprising a only 15%-30% of all CRC cases, have helped provide insight into this stepwise accumulation of mutations implicated in the pathogenesis of sporadic CRC[24].
Almost all inherited colorectal syndromes are categorized either as familial adenomatous polyposis (FAP) or hereditary nonpolyposis CRC (HNPCC)[25]. In FAP, individuals possess an inactivating mutation in the adenomatous polyposis coli (APC) gene. Over time, sporadic mutations to the remaining APC gene result in cells with complete loss of APC function. These cells then expand to generate adenomas, some of which then progress to malignant adenocarcinoma. The APC protein functions as an essential regulatory element in the canonical Wnt signaling pathway, preventing the accumulation of oncogenic β-catenin by promoting its degradation. In the absence of functional APC, β-catenin accumulates and translocates to the nucleus, where it acts as a cofactor for the oncogenic heterodimer transcription factor TCF/LEF[26]. This heterodimer increases transcription of genes involved in cell proliferation and growth, including the proto-oncogenes c-MYC and CCND1 (cyclin D1)[26]. The loss of functional APC is a key event for adenoma initiation, as it occurs early in the morphogenesis model of CRC[22].
Alternatively, HNPCC arises from mutations to a protein complex responsible for DNA mismatch repair (MMR)[24,27]. This complex, which is conserved across biological kingdoms, recognizes and binds mismatched DNA, then excises and repairs the error. Mutations in MMR genes, like MSH2 and MLH1, increase the accumulation of additional DNA mutations, leading to increased genomic instability, particularly in microsatellites[27]. Although mutations in MMR genes are relatively uncommon in sporadic CRC, MSI still occurs in approximately 10%-15% of cases, indicating that genomic instability and inadequate DNA repair play important roles in CRC development[25,27].
GUCY2C is one of several mammalian transmembrane guanylyl cyclase receptors that react to extracellular signals present in the microenvironment[16,28]. GUCY2C is predominantly expressed on apical brush border membranes of intestinal enterocytes, transducing extracellular peptide signals in the gut lumen into intracellular signaling cascades required for normal intestinal physiology[16,29]. GUCY2C was originally identified as an orphan receptor for the bacterial heat-stable enterotoxin, STa, produced by a number of enteric bacteria including enterotoxigenic Escherichia coli (ETEC)[30]. STa functions as a GUCY2C agonist, inducing a signaling cascade that causes excessive fluid and electrolyte secretion into the intestinal lumen, which manifests clinically as enterotoxigenic “traveler’s” diarrhea[31]. Interaction of STa with the GUCY2C extracellular ligand-binding domain activates its cytoplasmic catalytic domain, driving the conversion of GTP to cyclic guanosine monophosphate (cGMP)[16,28,29]. Intracellular cGMP then operates as a second messenger for downstream signaling, specifically activating cGMP-dependent protein kinase II, which then phosphorylates and activates the cystic fibrosis conductance regulator (CFTR). Activation of CFTR induces chloride secretion into the intestinal lumen, generating an electrochemical gradient that drives sodium into the gut lumen. Combined with cGMP-induced inhibition of the sodium-hydrogen exchanger (NHE3), CFTR activation elevates extracellular solute concentration to generate an osmotic gradient resulting in fluid accumulation in the lumen[16,28,29].
To date, two novel mutations in GUCY2C that affect gastrointestinal motility have been identified. The first, an autosomal dominant “gain of function” mutation in a Norwegian family, reflected a non-synonymous mutation resulting in the substitution of serine for isoleucine at residue 840 of the GUCY2C catalytic domain. This mutation increased ligand-dependent cGMP production which manifested clinically as chronic diarrhea and increased susceptibility to inflammatory bowel disease (IBD)[28,32,33]. Separately, two autosomal recessive inactivating GUCY2C mutations were discovered in two Bedouin families which reduced GUCY2C function leading to neonatal meconium ileus[28,34].
Exogenous STa is a molecular mimic of two endogenous peptide ligands, which also function as GUCY2C agonists. These ligands, guanylin (GUCA2A) and uroguanylin (GUCA2B), both expressed in gut epithelial cells[15,35,36], act locally as autocrine and paracrine hormones to regulate GUCY2C signaling and fluid and electrolyte homeostasis[28,31]. Additionally, uroguanylin acts as an endocrine hormone, secreted into the systemic circulation postprandially to activate hypothalamic GUCY2C and induce satiety[37-39]. Although GUCY2C signaling is utilized by bacteria to induce pathogenic diarrhea, several important characteristics differentiate endogenous guanylin and uroguanylin from exogenous STa. First, guanylin and uroguanylin have 10- to 100-fold lower affinities for GUCY2C than STa. Further, unlike STa, which contains three disulfide bonds, guanylin and uroguanylin contain only two disulfide bonds, increasing their susceptibility to proteolytic degradation in the gut lumen in comparison to STs[16,35].
The cellular sources of these intestinal peptides in both rodents and humans have been explored. Seminal studies utilizing custom antibodies described guanylin protein expression as confined to mature goblet cells throughout the rat small intestine and colon, as well as the columnar epithelial cells of the colon[40]. These data were supported by Brenna et al[41], which utilized in situ hybridization to identify guanylin mRNA expression in rat and human goblet cells and colonocytes. Guanylin mRNA also was enriched in both the rat and human duodenum, however cell-specific guanylin expression differed between species[41]. Immunohistochemistry first identified uroguanylin protein expression in rat proximal small intestine, with enrichment in enterochromaffin cells (EC)[42]. In contrast, in situ hybridization experiments by Brenna et al[41] did not detect uroguanylin mRNA expression in cells co-expressing CHGA, a marker for EC, in either rat or human intestine. Although this study further supported localized expression of uroguanylin in rat and human proximal small intestine, specifically the duodenum, the precise cellular origin of uroguanylin remains incompletely defined.
In addition to its role in the regulation of fluid and electrolyte balance, GUCY2C also serves a protective role against colorectal tumorigenesis[43,44]. The mammalian intestinal tract is lined with differentiated columnar cells derived from progenitor cells that differentiate during their progression along the crypt-villus unit[45,46]. The inner lining of the intestine is one of the most rapidly renewing tissues in the human body, with the entire epithelial surface turning over every 4-5 d[47]. Consequently, the intestine must coordinate a delicate balance between cell proliferation, differentiation, migration and apoptosis to prevent tumorigenesis[46-48]. GUCY2C, expressed in all cells lining the small intestine and colon, helps maintain this delicate balance. In mice lacking GUCY2C (Gucy2c-/-) this disrupted homeostasis manifests as an increase in proliferative cells, poorly developed differentiated compartments, accelerated cell cycle, and increased crypt depth, ultimately increasing the risk of tumorigenesis[31].
These observations suggest that events that impair GUCY2C signaling, such as loss of receptor or hormone expression, should similarly increase the risk of tumorigenesis, and this is indeed the case. In that context, several conditions associated with CRC, such as IBD[32,49,50] and obesity, silence GUCY2C signaling prior to overt tumorigenesis, re-enforcing the possibility that silenced GUCY2C mediates the pathogenesis of tumor initiation and progression[51,52]. Importantly, in both of these cases, GUCY2C silencing is mediated by impaired ligand expression rather than loss of GUCY2C, providing a potential therapeutic target in CRC. Conversely, epidemiological observations revealed that areas of endemic ETEC, in which inhabitants are chronically colonized with STa-producing organisms, have lower rates of CRC than geographic regions free of ETEC infections[16]. These observations, coupled with the observation that guanylin and uroguanylin are universally lost along the CRC pathophysiological continuum, strongly suggest a role for GUCY2C as a tumor suppressor[33,43,53].
Chronic intestinal inflammation, such as in IBD, is a well-known risk factor for CRC[32,49,50]. Gucy2c-/- mice demonstrate increased susceptibility to dextran sodium sulfate (DSS)-induced colitis[21], 2,4,6-trinitrobenzene sulfonic acid-induced colitis[52], as well as lipopolysaccharide-induced intestinal injury[54], while transgenic overexpression of guanylin (Guca2a+) renders mice resistant to DSS-induced colitis[21]. Mechanistically, this effect appears to be mediated at least in part by maintaining intestinal epithelial integrity and barrier function[21,48,49,54,55], since Gucy2c-/- mice have reduced expression of proteins associated with tight-junction integral proteins, such as occludin, claudin 2, claudin 4, and JAM-A[21,54]. Further, humans with IBD demonstrate significant decreases in guanylin, uroguanylin, and GUCY2C mRNA[51,52]. Additionally, loss of these proteins has been linked with increased severity of IBD in patients[51]. Together, these data suggest that GUCY2C signaling protects against chronic inflammation, and may play a critical role in inflammation-induced CRC.
In addition to its anti-inflammatory effect, GUCY2C also helps maintain genomic integrity in the intestine, in both genetic (ApcMin/+) as well as carcinogen [azoxymethane (AOM)]-induced mouse models of CRC[18]. In both models, Gucy2c-/- mice exhibited higher rates of tumorigenesis compared to mice with intact GUCY2C signaling[18]. In addition, impaired GUCY2C signaling resulted in increased aberrant crypt foci, the earliest precursors to CRC, when treated with the mutagen MNU compared to Gucy2c+/+ mice[17]. Because both of these models rely on DNA damage to induce tumor formation, these findings suggest that GUCY2C signaling improves genomic stability. Indeed this was the case, as Gucy2c-/- mice displayed increased DNA damage, measured by γ-H2AX, as well as an 8-fold increase in tumors with loss of heterozygosity of the Apc gene in ApcMin mice[18]. Taken together, these data demonstrate disruption of GUCY2C signaling is associated with genomic instability in the intestine that potentiates tumor initiation and growth.
The regenerating epithelium requires a reservoir of cells undergoing continuous cycles of proliferation in order to preserve intestinal functions[56], while tight control of this proliferation is required in order to oppose tumorigenesis. Loss of GUCY2C function leads to dysregulated cell cycle progression[17,18], as well as expansion of the proliferative compartment[20]. Further, Gucy2c-/- mice have upregulated expression or activation of oncogenes that promote proliferation, such as cyclin D1, pRb, β-catenin, and phosphorylated AKT (pAKT), as well as a decreased expression of tumor suppressors such as p27 and p21[17,20]. In CRC cells, silencing GUCY2C promotes pAKT-mediated secretion of tumor growth factor beta (TGF-β), resulting in increased hepatocyte growth factor expression by submucosal fibroblasts, which feeds back to produce epithelial cMET signaling, an important driver of CRC proliferation[57].
Loss of GUCY2C signaling is also associated with metabolic reprogramming to a more glycolytic phenotype, an effect seen in many tumor types. For example, silencing GUCY2C increases expression of proteins associated with glucose transport (glucose transporter 1), glycolysis (hexokinase II), fatty acid synthesis (acetylCoA carboxylase) and lactate production, all of which are characteristic of the Warburg metabolic phenotype[20]. Mechanistically, these effects appear to be mediated by GUCY2C-mediated inhibition of AKT, as constitutively active AKT eliminated the effects of GUCY2C activation and promoted a metabolic phenotype synonymous with the Warburg effect[20].
The enhanced cell cycle progression, metabolic reprogramming and increased oncogenic signaling in Gucy2c-/- mice also exists in human colon tumors[12]. Importantly, loss of guanylin and uroguanylin occurs early in the progression of CRC, as would be expected in tumors mediated by impaired GUCY2C signaling. In fact, guanylin loss frequently accompanies APC loss in pre-malignant adenomas[58]. While guanylin and uroguanylin expression is lost by nearly all colorectal tumors, the precise mechanisms mediating that loss, and the association of those mechanisms with the different etiologies of CRC (e.g., APC mutation, MSI, CIN) remain to be defined. Nevertheless, accumulated evidence suggests a paradigm whereby loss of GUCY2C signaling represents a pivotal step in the progression of most colorectal tumors which, in turn, provides a potential therapeutic target for cancer prevention and/or treatment by GUCY2C hormone ligand replacement therapy.
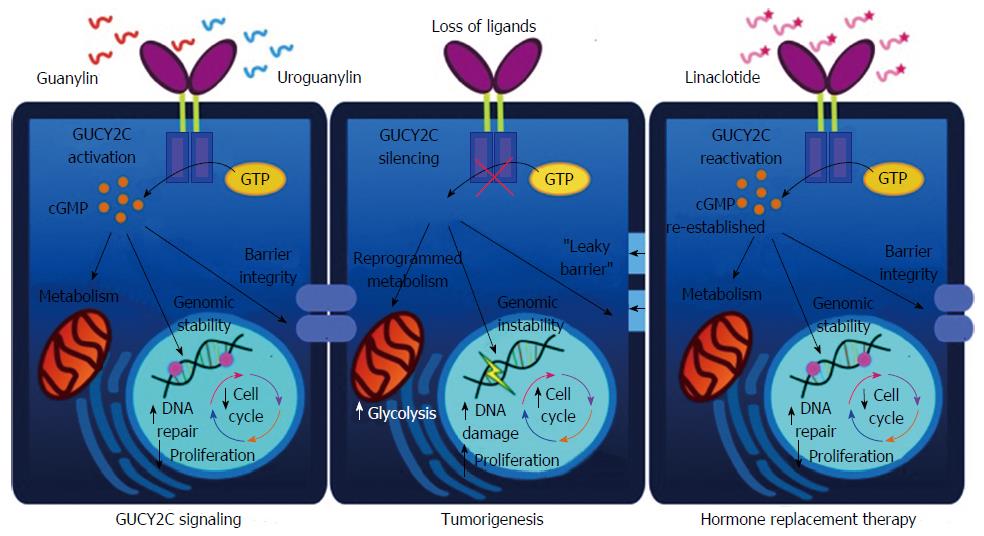
Hormone deficiencies underlie a number of debilitating human diseases, including diabetes, thyroid disorders, and more recently CRC[13]: Guanylin is significantly decreased (100- to 1000 fold) in nearly 90% of all CRC tumors colorectal tumors when compared to adjacent normal mucosa[43]. The observation of a loss of guanylin expression in human colorectal tumors coupled with the potentiation of tumorigenesis observed in GUCY2C knockout mice supports a hypothesis suggesting that CRC initiates as a disease of hormone insufficiency and offers the possibility of a new paradigm for CRC treatment[13,44,59]. Importantly, although loss of the GUCY2C ligands guanylin and uroguanylin occur early in tumor development, GUCY2C receptor expression persists in CRC[60-62]. This suggests that the receptor is latent and that signaling can be reconstituted by ligand supplementation similar to other diseases of hormone deficiency in which reactivation of signaling using synthetic ligand analogs have been therapeutic (Figure 1).
The feasibility of this prevention paradigm is underscored by the development and FDA-approval of linaclotide (Lizness™). Leveraging GUCY2C’s established secretory function, linaclotide was developed as an oral therapeutic for patients with constipation-type irritable bowel syndrome[32,63]. Linaclotide is a synthetic 14-amino acid peptide that shares structural homology with the guanylin peptide family[64]. Linaclotide’s mechanism of action mimics the canonical GUCY2C signaling pathway by binding and activating the mucosal receptor to produce cGMP, supporting its utilization as a clinical therapeutic to re-establish the GUCY2C signaling axis that is lost in CRC[65]. The use of linaclotide to prevent CRC also has been implicated in a number of studies examining the protective functions of GUCY2C signaling. In human CRC cell lines and mice, treatment with ST and cGMP derivatives reduced the rate of cell division by increasing endogenous cell cycle inhibitors and decreasing cell cycle drivers previously discussed[17-21,35]. ST treatment also improved the intestinal hyper-permeability found in Gucy2c-/- mice, a phenotype associated with loss of barrier function and susceptibility to intestinal injury, by reconstituting the expression of several tight junction integral proteins[21,54]. Moreover, oral uroguanylin reduced polyp formation in ApcMin/+ mice[66]. Interestingly, a more recent study investigating the molecular mechanisms associated with diet-induced obesity observed that wild-type mice on a high-fat or high-calorie diet had increased levels of tumorigenesis to the same extent observed in Gucy2c-/- mice[19]. These mice exhibited diet-induced guanylin loss that recapitulated the silencing of GUCY2C found in colorectal tumors as these mice showed elevated p-AKT, cyclin D1, and β-catenin[19]. This study used a mouse model of intestinal transgenic guanylin expression (VilGuca2a+) to significantly reduce colon tumorigenesis induced by high-fat diet and AOM[19], providing a genetic proof of concept for guanylin replacement in the prevention of CRC. In this context, oral administration of GUCY2C ligands has the capacity to prevent the molecular pathology associated with CRC, thus allowing for its translatability into the clinic.
CRC is widely accepted as disease of acquired sequential mutations leading to genomic instability and loss of epithelial integrity. However, the initiating events of epithelial transformation that give rise to the driving genetic mutations of CRC remain elusive. The GUCY2C signaling axis is thought to contribute to tumor initiation as its universal silencing by loss of its hormone ligands results in increased DNA damage, increased cell cycle progression, AKT activation, and deregulation of metabolic processes, which invariably potentiates tumor growth. The loss of GUCY2C hormones highlights CRC as a potential disease of paracrine hormone-insufficiency. Direct targeting of the GUCY2C pathway by hormone replacement therapy might restore homeostatic mechanisms required for normal intestinal function and potentially prevent CRC. The feasibility of hormone replacement therapy in the prevention and treatment of CRC is underscored by the development of the FDA-approved GUCY2C ligand linaclotide (Linzess™), which can be orally administered to patients to restore GUCY2C activation. Other GUCY2C-specific peptide compounds currently are being developed, which will broaden the horizons of hormone replacement therapy for a number of gastrointestinal diseases, and could impact the way CRC is treated in the future. Thus, the paracrine-hormone hypothesis proposes a shift in paradigm for CRC development from irreversible genetic mutations to reversible signaling mechanisms that can be managed through oral hormone replacement therapy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cormier RT, Zhu YL S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 13040] [Article Influence: 1304.0] [Reference Citation Analysis (2)] |
| 2. | Malvezzi M, Carioli G, Bertuccio P, Rosso T, Boffetta P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann Oncol. 2016;27:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (4)] |
| 3. | Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1420] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 4. | Pai SG, Fuloria J. Novel therapeutic agents in the treatment of metastatic colorectal cancer. World J Gastrointest Oncol. 2016;8:99-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kolligs FT. [Colorectal cancer: prevention and early detection]. Dtsch Med Wochenschr. 2015;140:1425-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Taylor DP, Burt RW, Williams MS, Haug PJ, Cannon-Albright LA. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology. 2010;138:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Goldberg RM, Rothenberg ML, Van Cutsem E, Benson AB, Blanke CD, Diasio RB, Grothey A, Lenz HJ, Meropol NJ, Ramanathan RK. The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist. 2007;12:38-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1298] [Article Influence: 86.5] [Reference Citation Analysis (2)] |
| 10. | Sameer AS. Colorectal cancer: molecular mutations and polymorphisms. Front Oncol. 2013;3:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev. 2004;23:11-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknæs M, Hektoen M, Lind GE, Lothe RA. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 714] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 13. | Pitari GM, Li P, Lin JE, Zuzga D, Gibbons AV, Snook AE, Schulz S, Waldman SA. The paracrine hormone hypothesis of colorectal cancer. Clin Pharmacol Ther. 2007;82:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Li P, Lin JE, Schulz S, Pitari GM, Waldman SA. Can colorectal cancer be prevented or treated by oral hormone replacement therapy? Curr Mol Pharmacol. 2009;2:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Blomain ES, Lin JE, Kraft CL, Trela UT, Rock JM, Aing AS, Snook AE, Waldman SA. Translating colorectal cancer prevention through the guanylyl cyclase C signaling axis. Expert Rev Clin Pharmacol. 2013;6:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Basu N, Arshad N, Visweswariah SS. Receptor guanylyl cyclase C (GC-C): regulation and signal transduction. Mol Cell Biochem. 2010;334:67-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Basu N, Saha S, Khan I, Ramachandra SG, Visweswariah SS. Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21. J Biol Chem. 2014;289:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, Baran AA, Siracusa LD, Pitari GM, Waldman SA. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007;133:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Lin JE, Colon-Gonzalez F, Blomain E, Kim GW, Aing A, Stoecker B, Rock J, Snook AE, Zhan T, Hyslop TM. Obesity-Induced Colorectal Cancer Is Driven by Caloric Silencing of the Guanylin-GUCY2C Paracrine Signaling Axis. Cancer Res. 2016;76:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Lin JE, Li P, Snook AE, Schulz S, Dasgupta A, Hyslop TM, Gibbons AV, Marszlowicz G, Pitari GM, Waldman SA. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 2010;138:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, Garcia AV, Valentino MA, Hyslop T, Schulz S. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One. 2012;7:e31686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992;70:1727-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 817] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 24. | Guanti G, Bukvić N. Hereditary colorectal cancer syndromes. Acta Chir Iugosl. 2000;47:23-25. [PubMed] |
| 25. | Mundade R, Imperiale TF, Prabhu L, Loehrer PJ, Lu T. Genetic pathways, prevention, and treatment of sporadic colorectal cancer. Oncoscience. 2014;1:400-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Gerlach JP, Emmink BL, Nojima H, Kranenburg O, Maurice MM. Wnt signalling induces accumulation of phosphorylated β-catenin in two distinct cytosolic complexes. Open Biol. 2014;4:140120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Haraldsdottir S, Roth R, Pearlman R, Hampel H, Arnold CA, Frankel WL. Mismatch repair deficiency concordance between primary colorectal cancer and corresponding metastasis. Fam Cancer. 2016;15:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Brierley SM. Guanylate cyclase-C receptor activation: unexpected biology. Curr Opin Pharmacol. 2012;12:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Arshad N, Visweswariah SS. The multiple and enigmatic roles of guanylyl cyclase C in intestinal homeostasis. FEBS Lett. 2012;586:2835-2840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Schulz S, Green CK, Yuen PS, Garbers DL. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990;63:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 443] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 31. | Steinbrecher KA, Wowk SA, Rudolph JA, Witte DP, Cohen MB. Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation. Am J Pathol. 2002;161:2169-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Pitari GM. Pharmacology and clinical potential of guanylyl cyclase C agonists in the treatment of ulcerative colitis. Drug Des Devel Ther. 2013;7:351-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, Håvik B, Tønder SL, Levy SE, Brackman D. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | Romi H, Cohen I, Landau D, Alkrinawi S, Yerushalmi B, Hershkovitz R, Newman-Heiman N, Cutting GR, Ofir R, Sivan S. Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C. Am J Hum Genet. 2012;90:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Lima AA, Fonteles MC. From Escherichia coli heat-stable enterotoxin to mammalian endogenous guanylin hormones. Braz J Med Biol Res. 2014;47:179-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Ikpa PT, Sleddens HF, Steinbrecher KA, Peppelenbosch MP, de Jonge HR, Smits R, Bijvelds MJ. Guanylin and uroguanylin are produced by mouse intestinal epithelial cells of columnar and secretory lineage. Histochem Cell Biol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Seeley RJ, Tschöp MH. Uroguanylin: how the gut got another satiety hormone. J Clin Invest. 2011;121:3384-3386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, Magee MS, Hyslop T, Schulz S, Waldman SA. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011;121:3578-3588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Kim GW, Lin JE, Snook AE, Aing AS, Merlino DJ, Li P, Waldman SA. Calorie-induced ER stress suppresses uroguanylin satiety signaling in diet-induced obesity. Nutr Diabetes. 2016;6:e211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Li Z, Taylor-Blake B, Light AR, Goy MF. Guanylin, an endogenous ligand for C-type guanylate cyclase, is produced by goblet cells in the rat intestine. Gastroenterology. 1995;109:1863-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Brenna Ø, Furnes MW, Munkvold B, Kidd M, Sandvik AK, Gustafsson BI. Cellular localization of guanylin and uroguanylin mRNAs in human and rat duodenal and colonic mucosa. Cell Tissue Res. 2016;365:331-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Perkins A, Goy MF, Li Z. Uroguanylin is expressed by enterochromaffin cells in the rat gastrointestinal tract. Gastroenterology. 1997;113:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Wilson C, Lin JE, Li P, Snook AE, Gong J, Sato T, Liu C, Girondo MA, Rui H, Hyslop T. The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:2328-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Fajardo AM, Piazza GA, Tinsley HN. The role of cyclic nucleotide signaling pathways in cancer: targets for prevention and treatment. Cancers (Basel). 2014;6:436-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 45. | Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 988] [Article Influence: 76.0] [Reference Citation Analysis (1)] |
| 46. | Vermeulen L, Snippert HJ. Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer. 2014;14:468-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 47. | Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 532] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 48. | Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1682] [Cited by in RCA: 2254] [Article Influence: 187.8] [Reference Citation Analysis (4)] |
| 49. | Steinbrecher KA, Harmel-Laws E, Garin-Laflam MP, Mann EA, Bezerra LD, Hogan SP, Cohen MB. Murine guanylate cyclase C regulates colonic injury and inflammation. J Immunol. 2011;186:7205-7214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22:4794-4801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 285] [Cited by in RCA: 352] [Article Influence: 35.2] [Reference Citation Analysis (4)] |
| 51. | Lan D, Niu J, Miao J, Dong X, Wang H, Yang G, Wang K, Miao Y. Expression of guanylate cyclase-C, guanylin, and uroguanylin is downregulated proportionally to the ulcerative colitis disease activity index. Sci Rep. 2016;6:25034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Brenna Ø, Bruland T, Furnes MW, Granlund Av, Drozdov I, Emgård J, Brønstad G, Kidd M, Sandvik AK, Gustafsson BI. The guanylate cyclase-C signaling pathway is down-regulated in inflammatory bowel disease. Scand J Gastroenterol. 2015;50:1241-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Cohen MB, Hawkins JA, Witte DP. Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Lab Invest. 1998;78:101-108. [PubMed] |
| 54. | Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One. 2011;6:e16139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg. 2010;2:61-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 137] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 56. | Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 933] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 57. | Gibbons AV, Lin JE, Kim GW, Marszalowicz GP, Li P, Stoecker BA, Blomain ES, Rattan S, Snook AE, Schulz S. Intestinal GUCY2C prevents TGF-β secretion coordinating desmoplasia and hyperproliferation in colorectal cancer. Cancer Res. 2013;73:6654-6666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, Cohen MB. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem Biophys Res Commun. 2000;273:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Bryant AP, Busby RW, Bartolini WP, Cordero EA, Hannig G, Kessler MM, Pierce CM, Solinga RM, Tobin JV, Mahajan-Miklos S. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci. 2010;86:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 60. | Cagir B, Gelmann A, Park J, Fava T, Tankelevitch A, Bittner EW, Weaver EJ, Palazzo JP, Weinberg D, Fry RD. Guanylyl cyclase C messenger RNA is a biomarker for recurrent stage II colorectal cancer. Ann Intern Med. 1999;131:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Carrithers SL, Barber MT, Biswas S, Parkinson SJ, Park PK, Goldstein SD, Waldman SA. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc Natl Acad Sci USA. 1996;93:14827-14832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Waldman SA, Cagir B, Rakinic J, Fry RD, Goldstein SD, Isenberg G, Barber M, Biswas S, Minimo C, Palazzo J. Use of guanylyl cyclase C for detecting micrometastases in lymph nodes of patients with colon cancer. Dis Colon Rectum. 1998;41:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Johnston JM, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, O’Dea C, Baird M, Lembo AJ. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology. 2010;139:1877-1886.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 64. | Busby RW, Bryant AP, Bartolini WP, Cordero EA, Hannig G, Kessler MM, Mahajan-Miklos S, Pierce CM, Solinga RM, Sun LJ. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol. 2010;649:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 65. | Tchernychev B, Ge P, Kessler MM, Solinga RM, Wachtel D, Tobin JV, Thomas SR, Lunte CE, Fretzen A, Hannig G. MRP4 Modulation of the Guanylate Cyclase-C/cGMP Pathway: Effects on Linaclotide-Induced Electrolyte Secretion and cGMP Efflux. J Pharmacol Exp Ther. 2015;355:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000;60:5151-5157. [PubMed] |