Published online Sep 21, 2016. doi: 10.3748/wjg.v22.i35.8050
Peer-review started: June 5, 2016
First decision: June 20, 2016
Revised: July 21, 2016
Accepted: August 10, 2016
Article in press: August 10, 2016
Published online: September 21, 2016
Processing time: 102 Days and 18.7 Hours
To gather data on the antiviral efficacy and safety of second generation direct acting antiviral (DAA) treatment with respect to sustained virological response (SVR) 12 wk after conclusion of treatment, and to determine predictors of SVR12 in this setting.
Two hundred and sixty patients treated with SOF combination partners PR (n = 51), R (n = 10), SMV (n = 30), DCV (n = 81), LDV (n = 73), or 3D (n = 15). 144/260 were pre-treated, 89/260 had liver cirrhosis, 56/260 had portal hypertension with platelets < 100/nL, 25/260 had a MELD score ≥ 10 and 17/260 were post-liver transplantation patients. 194/260 had HCV GT1, 44/260 HCV GT3.
Two hundred and forty/256 (93.7%) patients achieved SVR12 (mITT); 4/260 were lost to follow-up. SVR12 rates for subgroups were: 92% for SOF/DCV, 93% for each SOF/SMV, SOF/PR, 94% for SOF/LDV, 100% for 3D, 94% for pretreated, 87% for liver cirrhosis, 82% for patients with platelets < 100/nL, 88% post-liver transplantation, 95% for GT1a, 93% for GT1b, 90% for GT3, 100% for GT2, 4, and 6. 12 patients suffered from relapse, 6 prematurely discontinued treatment, of which 4 died. Negative predictors of SVR12 were a platelet count < 100/nL, MELD score ≥ 10 (P < 0.0001), liver cirrhosis (P = 0.005) at baseline. In Interferon-free treatment GT3 had significantly lower SVR rates than GT1 (P = 0.016). Side effects were mild.
Excellent SVR12 rates and the favorable side-effect profile of DAA-combination therapy can be well translated into “real-world”. Patients with advanced liver disease, signs of portal hypertension, especially with platelets < 100/nL and patients with GT3 are in special need for further research efforts to overcome comparatively higher rates of virological failure.
Core tip: From 2014 on the second wave of direct acting antiviral agents was available for treatment of chronic hepatitis C infection. Due to the more heterogeneous character of patients in the “real world”, the therapeutic performance of these new drugs outside randomized clinical trials is of interest. Therefore, in this monocentric retrospective cohort study, we analyzed the efficacy, safety, and predictors of sustained virological response 12 for treatment with combinations of second generation direct acting antivirals in a “real-world” setting.
- Citation: Werner CR, Schwarz JM, Egetemeyr DP, Beck R, Malek NP, Lauer UM, Berg CP. Second-generation direct-acting-antiviral hepatitis C virus treatment: Efficacy, safety, and predictors of SVR12. World J Gastroenterol 2016; 22(35): 8050-8059
- URL: https://www.wjgnet.com/1007-9327/full/v22/i35/8050.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i35.8050
Chronic infection with Hepatitis C virus (HCV) is still one of the main causes for liver disease with a prevalence of 0.2%-2% in Western countries, while worldwide about 80 million people are threatened by HCV[1-4]. After many years of just moderate sustained viral response (SVR) rates of around 50%-60% through all genotypes (GT) under Interferon (IFN)-based treatment regimens[5-9], in 2011 the first direct acting antiviral compounds (DAA), the protease inhibitors (PI) Telaprevir and Boceprevir had been approved for treatment of HCV GT1[10-14]. Beyond that, promising SVR results obtained in the clinical trials were shown to be achieved also in “real-world” settings[15,16]. However, treatment with first generation DAA was only subject to GT1 patients, and antiviral potency was counteracted by aggravated side-effects. For the second wave of DAA, diverse drug classes were developed: (1) polymerase-; (2) NS5A-, as well as (3) new protease inhibitors (PI). From early 2014 on, consecutively the first-in-class polymerase inhibitor Sofosbuvir (SOF), a second wave PI Simeprevir (SMV), the first-in-class NS5A inhibitor Daclatasvir (DCV), and another NS5A inhibitor Ledipasvir (LDV), were approved. Accordingly, for the first time, IFN-free treatments consisting of combinations of these DAA, with or without Ribavirin (R), were possible, showing impressive SVR rates and a superior side effect profile. From 2014 until 2015, SOF constituted the “backbone” of most combination treatments: in combination with R alone (SOF/R), with pegylated Interferon and R (SOF/PR), or combined with SMV (SOF/SMV), DCV (SOF/DCV), or LDV (SOF/LDV) with or without R. In 2015, this “monopoly” was tackled by the fixed combination of Dasabuvir, a non-nucleosidic polymerase inhibitor, with Ombitasvir, and Paritaprevir/r (3D).
Now, after more than two years since the approval of SOF and its combination partners, and a year after approval of the 3D regimen, we here summarize our experiences with these combination treatments being obtained in the “real-world” setting of a tertiary center. This retrospective analysis was conducted to gather data on the antiviral efficacy and safety of second generation DAA treatment with respect to sustained virological response 12 wk after conclusion of treatment (SVR12), and to determine predictors of SVR12 in this setting.
The clinical characteristics of our retrospective cohort are presented in Table 1. The study cohort includes all 260 consecutive patients, who were treated at our center with a DAA containing therapy between January 2014 and December 2015. Treatment decisions were based on antiviral activity against GT of respective DAA according to approval, severity of liver disease, comorbidities, approval of DAA at time-point of treatment start, and economic reasons.
| Demographics | ||
| n | 260 | |
| Age | (yr)1 | 53 (44-60) |
| Sex | Male/Female n (%) | 157 (60)/103 (40) |
| Baseline viral characteristics | ||
| Genotype 1a/1b/1x | n (%)/n (%)/n (%) | 76 (29)/115 (44)/3(1) |
| Genotype 2/3 | n (%)/n (%) | 8 (3)/44 (17) |
| Genotype 4/5/6 | n (%)/n (%) | 11 (4)/1 (0.4)/2 (0.8) |
| Baseline viral load | (IU/mL)1 | 1.33 Mio. (414.750-3.4 Mio.) |
| Baseline viral load ≥ 800.000 IU/mL | n (%) | 169 (65) |
| Baseline viral load ≥ 6 Mio IU/mL | n (%) | 28 (11) |
| Special populations | ||
| LCi2 | n (%) | 89 (34) |
| Low platelets ( ≤ 100/nL) | n (%) | 56 (22) |
| Post Liver Transplantation | n (%) | 17 (7) |
| Patients ≥ 60 yr | n (%) | 72 (28) |
| Patients with MELD score ≥ 10 | n (%) | 25 (10) |
| Treatment history | ||
| Treatment experienced | n (%) | 144 (55) |
| Treatment regimens [thereof liver cirrhosis n (%)] | ||
| SOF PR | n (%)/LCi n (%) | 51 (20)/6 (12) |
| SOF R | n (%)/LCi n (%) | 10 (4)/4 (40) |
| SOF SMV | n (%)/LCi n (%)/R n (%) | 30 (12)/16 (53)/15 (19) |
| SOF DCV | n (%)/LCi n (%)/R n (%) | 81 (31)/42 (52)/12 (40) |
| SOF LDV | n (%)/LCi n (%)/R n (%) | 73 (28)/17 (23)/16 (22) |
| 3D | n (%)/LCi n (%)/R n (%) | 15 (6)/4 (27)/6 (40) |
| Baseline clinical chemistry | ||
| WBC | (/μL)1 | 5935 (4683-7670) |
| Hemoglobin | (g/dL)1 | 14.5 (13.1-15.6) |
| Platelets | (thousand/μL)1 | 174 (113-228) |
| Creatinine | (mg/dL)1 | 0.7 (0.6-0.8) |
| Total Bilirubin | (mg/dL)1 | 0.7 (0.5-1.0) |
| INR | INR1 | 1 (1-1) |
| ALT | IU/l1 | 67 (44-105) |
If possible, IFN-free treatments were favored. Treatment of patients presenting hepatic impairment was postponed, if possible, until IFN-free regimens were available. 17 patients were treated in the context of post-liver transplantation, two of these with cholestatic recurrence of HCV after liver transplantation who were treated with SOF/DCV in a compassionate use program. Following the dates of approval of the different DAA, patients were treated with SOF/R or SOF/PR respectively, when being started with treatment in early 2014, until later on additionally a SOF/SMV combination became available. From autumn 2014 on, patients were, if possible, treated with SOF/DCV, and again later on this year, patients could be treated also with SOF/LDV. From Early 2015 on, patients could also be treated with the 3D regimen. Proportions of different treatment regimens are shown in Table 1.
Baseline clinical chemistry is shown in Table 1. 13/260 patients had leukopenia (leukocyte count < 3000/μL), 56/260 patients presented with thrombopenia (platelet count < 100/nL). Transaminases were elevated in 223/260 patients (ALT > 35 IU/mL). Data of albumin levels were available only on an occasional basis.
Diagnosis of liver cirrhosis was based upon liver histology, Fibroscan (> 12.5 kPa), or clinical diagnosis (e.g., ascites, esophageal varices, distinct sonographic signs of portal hypertension or liver cirrhosis). For assessment of severity of liver disease, we calculated the MELD score. In this retrospective analysis, Child Turcotte Pugh score or other assessment scores for severity of liver disease could not be calculated due to lack of data[17,18]. For retrospective identification of patients with possible portal hypertension, a threshold of 100 platelets/nL was assumed.
For virological analyses Roche CobasAmpliprep/Roche CobasTaqMan [Roche Diagnostics GmbH, Mannheim, Germany; lower limit of quantification (LLOQ) 15 IU/mL] was used.
The institutional review board of the Medical Faculty of the University of Tübingen approved this retrospective analysis and waived the need for written informed consent because of the anonymous evaluation of patient data from patient records.
Data were statistically analyzed using Microsoft Office Excel, SPSS, and Graph Pad Prism. 4/260 patients were lost to follow-up. These patients were excluded from analysis [modified intention to treat analysis (mITT)].
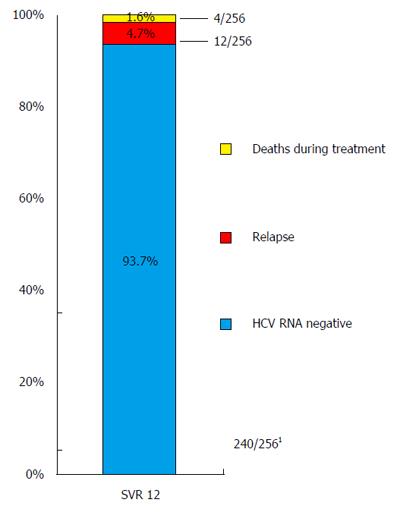
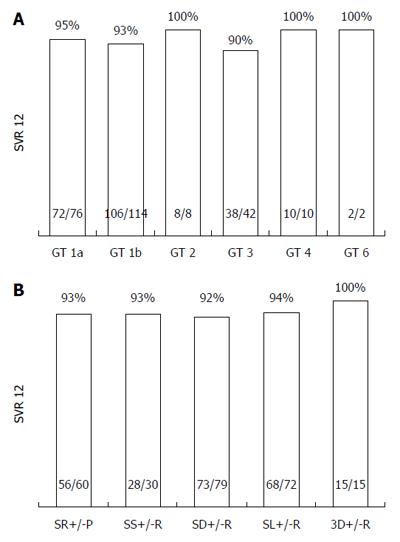
The overall SVR12 rate is shown in Figure 1 (93.7%; 240/256 patients mITT; of those, 2/256 patients discontinued treatment prematurely, but achieved SVR 12; 4/260 patients were lost to follow-up and were excluded from analysis. SVR12 rates according to GT, and diverse treatment regimens are shown in Figure 2. Additionally, SVR12 rates according to several baseline characteristics (sex, age, cirrhosis status, platelet count, MELD score, viral load, and treatment experienced patients) and early viral kinetics are shown in Table 2. SVR12 rates in different GT (1a, 1b, 2, 3, 4, and 6) ranged from 90%-100%. One additional patient with HCV GT 5 achieved SVR 12. Depending on different treatment regimens, SVR12 rates ranged from 92%-100%. Special subgroups of patients known to be “hard-to-treat” (patients post-liver transplantation (n = 17)], patients with liver cirrhosis (n = 89), patients of older age (≥ 60 years; n = 72), and treatment experienced patients (n = 144) achieved highly acceptable SVR12 rates in the range of 88%-95%. However, patients with low platelet count at baseline (< 100/nL; n = 56), and patients with a MELD score ≥ 10 at baseline (n = 25) achieved SVR12 rates of 82%, and 72%, respectively.
| SVR 12 (n/N; %)2 | Univariate analysis | Multivariate analysis | |||
| Odds ratio (95%CI) | Wald P value | Odds ratio (95%CI) | Wald P value | ||
| Viral kinetics | |||||
| RVR | 143/152; 94% | ||||
| vs Non-RVR | 83/89; 93% | 1.149 (0.395, 3.341) | 0.799 | 0.728 | |
| Baseline demographic parameters | |||||
| Fibrosis | |||||
| Liver Cirrhosis | 76/87; 87% | ||||
| vs no Liver Cirrhosis | 164/169; 97% | 0.211 (0.071, 0.627) | 0.005 | 0.290 | |
| Transplant Status | |||||
| LTx | 15/17; 88% | ||||
| vs no LTx | 225/239; 94% | 0.467 (0.097, 2.246) | 0.467 | 0.972 | |
| Sex | |||||
| Male | 140/153; 92% | ||||
| vs female | 100/103; 97% | 0.329 (0.091, 1.184) | 0.089 | 0.282 | |
| Age | |||||
| Patients ≥ 60 yr | 68/71; 96% | ||||
| vs < 60 yr | 172/185; 93% | 0.999 (0.959, 1.04) | 0.948 | 0.078 | |
| Baseline viral load | |||||
| High viral load (> 6 Mio IU/mL) | 28/28; 100% | ||||
| vs low viral load ( ≤ 6 Mio IU/mL) | 212/228; 93% | NA | 0.998 | 0.251 | |
| Genotype | |||||
| 1 | 180/192; 93% | ||||
| vs 3 | 38/42; 90% | 1.606 (0.510, 5.060) | 0.418 | 0.424 | |
| Genotype1 | |||||
| 1 IFN-free, per protocol | 160/164/; 98% | ||||
| vs 3 IFN-free, per protocol | 16/20/; 80% | 5.000 (1.355, 18.45) | 0.016 | NA | |
| Baseline platelet count | |||||
| Platelets ≤ 100/nL | 45/55; 82% | ||||
| vs > 100/nL | 195/201; 97% | 0.138 (0.048, 0.401) | < 0.0001 | 0.24 (0.072, 0.88) | 0.031 |
| Baseline MELD ≥ 10 | |||||
| MELD ≥ 10 | 18/25; 72% | ||||
| vs MELD < 10 | 222/231; 96% | 0.104 (0.035, 0.313) | < 0.0001 | 0.117 (0.037, 0.373) | < 0.0001 |
| Previous treatment response | |||||
| Pre-treated | 133/142; 94% | ||||
| vs treatment-naive | 107/114; 94% | 0.967 (0.349, 2.681) | 0.948 | 0.457 | |
For evaluation of predictors of SVR12, see Table 2. Presence of liver cirrhosis (P = 0.005), a platelet count < 100/nL (P < 0.0001), and a MELD score ≥ 10 (P < 0.0001) at baseline were significant negative predictors of SVR12 in our study cohort (univariate analysis). Multivariate analysis identified a platelet count < 100/nL (P = 0.031), and a MELD score ≥ 10 (P < 0.0001) at baseline as independent predictors for achievement of a diminished SVR12 rate (see Table 2 for details).
With respect to GT, in a “per-protocol” subgroup analysis of GT1 and GT3 patients strictly treated with IFN-free protocols, GT3 patients had a significantly lower SVR12 rate than GT1 patients (P = 0.016, univariate analysis).
However, in our cohort, treatment experience, sex, age, baseline viral load, and early virological kinetics were found not to be significant as predictors of SVR12.
Virological failure was a rare event. No primary non-response or virological breakthrough occurred in our cohort. In addition, only 12 of the 256 patients (4.7%, mITT) suffered from virological relapse during follow-up (see Table 3 for baseline characteristics, treatment details, and resistance analysis of respective patients). One patient (No. 9 in Table 3) exhibiting a liver cirrhosis with Child-Turcotte-Pugh (CTP) stadium A suffered from relapse after SOF/PR. In this patient liver function deteriorated upon relapse to CTP stadium C, and therefore the patient had to be listed for liver transplantation (at a MELD score of 28). After having achieved an active listing status, treatment was initiated with SOF/DCV for 24 wk, leading to SVR12. During treatment, the liver function restored to CTP stadium A, and thus, the patient could be de-listed for liver transplantation again.
| Age (yr) | Sex | BL viral load, IU/mL | GT | LCi | BL MELD | Previous treatment | Current treatment | Treatment duration | Cause for premature discontinuation | Outcome | RAV NS3 protease | RAV NS5A | RAV NS5B polymerase | |
| Patients, who died during treatment, or discontinued treatment prematurely | ||||||||||||||
| No. 1 | 63 | male | 1.41 Mio | 1b | Yes | 12 | PR | SOF/SMV | 12 | Sepsis | death | |||
| No. 2 | 66 | male | 1.69 Mio | 1b | Yes, HTx | 14 | - | SOF/LDV | 5 | Intracranial bleeding | death | |||
| No. 3 | 55 | male | 56200 | 1b | Yes, LTx | 24 | TVR/PR | SOF/SMV/R | 14 | Re-LTx, sepsis | death | |||
| No. 4 | 49 | male | 157000 | 1a | Yes, | 14 | PR | SOF/LDV/R | 8 | Right-heart failure | death | |||
| No. 5 | 79 | female | 5.85 Mio | 1b | Yes | 6 | TVR/PR | SOF/SMV | 10 | Back-pain | SVR 24 | |||
| No. 6 | 55 | male | 396000 | 1a | Yes, TIPSS | 10 | PR | SOF/DCV | 16 | Hepatic encephalopathy | SVR 24 | |||
| Patients with relapse after end of treatment | ||||||||||||||
| No. 7 | 54 | male | 10300 | 1a | Yes | 8 | PR | SOF/PR | 12 | NA | NA | NA | ||
| No. 8 | 63 | male | 321000 | 1b | - | 6 | - | SOF/PR | 12 | NA | NA | NA | ||
| No. 9 | 43 | female | 408000 | 1a | Yes | 8 | - | SOF/PR | 12 | NA | NA | NA | ||
| No. 10 | 40 | male | 2.74 Mio | 1b | - | 6 | PR | SOF/PR | 12 | NA | NA | NA | ||
| No. 11 | 48 | male | 4.89 Mio | 3a | No, LTx | 11 | PR | SOF/DCV/R | 24 | F43F/L | A30K | - | ||
| No. 12 | 56 | female | 1.38 Mio | 3a | Yes | 7 | PR | SOF/DCV | 24 | - | Y93H | S282S/T | ||
| No. 13 | 38 | male | 3.39 Mio | 1b | - | 6 | - | SOF/DCV | 12 | Q80K | L31M, Y93H | - | ||
| No. 14 | 52 | male | 2.47 Mio | 3a | Yes | 10 | - | SOF/DCV/R | 24 | - | Y93H | - | ||
| No. 15 | 55 | female | 3.03 Mio | 1a | - | 6 | TVR/PR | SOF/DCV | 12 | T54S, R155K | L31M/L | - | ||
| No. 16 | 49 | male | 8340 | 3 | Yes | 6 | - | SOF/LDV/R | 24 | - | A30S | - | ||
| No. 17 | 52 | male | 239000 | 1b | Yes | 6 | - | SOF/LDV/R | 12 | T54S, V55I, Q80K | Q30E | - | ||
| No. 18 | 57 | male | 137 (on SOF/R) | 1a | Yes, HCC | 11 | SOF/R | SOF/DCV | 12 | - | L31M, P58S, Y93C | - | ||
For GT1, 8/194 patients (4%) suffered from virological relapse, for GT3 a rate of 3/41 (9%) was found. All GT3 patients with relapse had advanced liver disease (cirrhosis or post-liver transplantation), while the GT1 cohort of relapsers was more heterogeneous. This emphasizes the new paradigm of GT3 (with advanced liver disease) being one of the harder-to-treat GT in the era of DAA (possibly due to an unintended “misfit design” of DAA with respect to this distinct genotype). In the group of IFN-free treated patients, the difference in SVR12 rates between GT1 and 3 was significant (P = 0.016, univariate analysis; Table 2).
In all patients with virological relapse after IFN-free DAA treatment resistance analysis was performed: Patients receiving a NS5A inhibitor always revealed NS5A-specific resistance associated variants (RAV) after relapse. Only one patient was detected with a NS5B-polymerase RAV (see Table 3 for details).
Altogether, 6 out of 256 (2.3%) patients discontinued treatment prematurely (see Table 3 for baseline characteristics, treatment details, and outcome). Four of those patients died during or shortly after discontinuation of treatment: two patients developed sepsis, one patient died from right-sided heart failure, and one patient died from cerebral hemorrhage. All these patients suffered from decompensated liver disease at baseline, two of those additionally were on immunosuppression due to prior organ transplantation (liver, and heart, respectively). Notably, these numbers are much too small to calculate significant associations between the respective causes of death and the DAA-class which had been used for HCV treatment, but the mere fact that 4 of our patients died emphasizes the need for treatment of those patients in an experienced tertiary center. However, these patients would have never been treated at time of IFN-containing regimens.
One patient discontinued due to back-pain after 10 wk of treatment, without contacting our center; nevertheless, this patient was found later on of having achieved SVR 24. Another patient with HCV GT1a, liver cirrhosis, and a transjugular portosystemic shunt (TIPSS) was discontinued after 4 mo of treatment due to several episodes of hepatic encephalopathy. Nevertheless, this patient also achieved SVR 24.
One hundred and forty-seven/237 (62%) patients with data available for side effects reported side effects during treatment (depicted in detail in Table 4). In general, side effects were mild in intensity, thus, just one patient discontinued treatment prematurely due to a self-reported intolerance (i.e., back-pain; Table 3). Overall, fatigue, headache, bone pain or joint pain, and myalgia were the most frequently reported side effects. As expected, the rate of side effects was found to differ significantly between patients with IFN-containing regimens versus patients with IFN-free treatment.
| Side effects | Overall1 | PEG-IFN co-treatment2 | IFN-free treatment3 | P value | |||
| Fatigue | 93 | 39.2% | 26 | 54.2% | 67 | 35.4% | 0.020 |
| Cephalgia | 52 | 21.9% | 9 | 18.8% | 43 | 22.8% | 0.697 |
| Bone/joint pain/myalgia | 38 | 16.0% | 12 | 25.0% | 26 | 13.8% | 0.077 |
| Nausea/vomiting | 38 | 16.0% | 14 | 29.2% | 24 | 12.7% | 0.008 |
| 0.163 | |||||||
| Insomnia | 28 | 11.8% | 16 | 33.3% | 12 | 6.3% | < 0.0001 |
| Vertigo | 16 | 6.8% | 6 | 12.5% | 10 | 5.3% | 0.102 |
| Flu-like symptoms | 14 | 5.9% | 12 | 25.0% | 2 | 1.1% | < 0.0001 |
| Abdominal discomfort/pain | 17 | 7.2% | 3 | 6.3% | 15 | 7.9% | 1.000 |
| Pruritus | 18 | 7.6% | 4 | 8.3% | 13 | 6.9% | 0.755 |
| Diarrhea | 13 | 5.5% | 7 | 14.6% | 6 | 3.2% | 0.006 |
| Any rash | 14 | 5.9% | 7 | 14.6% | 7 | 3.7% | 0.010 |
| Anorexia | 9 | 3.8% | 4 | 8.3% | 5 | 2.6% | 0.085 |
| Nervousness | 7 | 3.0% | 4 | 8.3% | 3 | 1.6% | 0.033 |
| Depression/fear | 5 | 2.1% | 2 | 4.2% | 3 | 1.6% | 0.267 |
| Dyspnoea | 7 | 3.0% | 4 | 8.3% | 3 | 1.6% | 0.030 |
| Concentration weakness | 4 | 1.7% | 2 | 4.2% | 2 | 1.1% | 0.183 |
| Visual changes | 4 | 1.7% | 1 | 2.1% | 3 | 1.6% | 1.000 |
| Loss of hair | 4 | 1.7% | 1 | 2.1% | 3 | 1.6% | 1.000 |
| Tachycardia/palpitations | 2 | 0.8% | 2 | 4.3% | 0 | 0.0% | 0.040 |
| Meteorism | 3 | 1.3% | 0 | 0.0% | 3 | 1.6% | 1.000 |
| Aggression | 2 | 0.8% | 2 | 4.2% | 0 | 0.0% | 0.040 |
| Fever/chills | 1 | 0.4% | 1 | 2.1% | 0 | 0.0% | 0.200 |
| Attacks of sweating | 1 | 0.4% | 1 | 2.1% | 0 | 0.0% | 0.200 |
| Gingivitis | 1 | 0.4% | 0 | 0.0% | 1 | 0.5% | 1.000 |
| Cough | 1 | 0.4% | 1 | 2.1% | 0 | 0.0% | 0.200 |
| Neurological symptoms | 1 | 0.4% | 0 | 0.0% | 1 | 0.7% | 1.000 |
Hospital admissions were required during treatment with DAA-based regimens in 22 (8.6%) patients. Some of those patients had to be hospitalized twice or more. Reasons for hospitalization were: infections (n = 8; 4%), hepatic decompensation (n = 3; 1.5%), cardiovascular disease (n = 3), cholestatic hepatitis after liver transplantation (n = 2), treatment of hepatocellular carcinoma (n = 2), lumbago, vomitus, and diabetes insipidus, right upper quadrant pain (n = 1, each). Of those hospitalized, 4 patients deceased: Two patients died from infectious complications, and two patients died from cardiovascular disease (right-heart failure, intracerebral bleeding, respectively; see above, and Table 3 for details).
One hundred and ten patients received R as part of their combination treatment. Of those, 5 patients had to reduce R dosage due to anemia (4.5%), and 2 due to renal impairment. 2 patients had to completely withdraw R, one due to pruritus, and the other due to hemolysis, respectively.
This “real-world” monocentric retrospective cohort study analyzing safety, efficacy, and predictors of SVR12 of second generation DAA treatment shows impressive overall SVR rates (93.7%).
Due to the relatively small number of patients and the retrospective character of this study a comparison between subgroups according to DAA combination partners is only of limited significance.
Furthermore, due to the lesser tolerability and presumed lower activity of SOF/PR in patients with advanced liver disease, this treatment a priori was reserved in our hands to patients showing up with a well-compensated liver function only.
The results in our heterogeneous cohort, containing meaningful fractions of hard-to-treat patients (liver cirrhosis, portal hypertension, post-liver transplantation) are similar to SVR rates of so far published study trials: our cohort of PR or R co-treated patients achieved a SVR rate of 93%, while in the NEUTRINO trial the cohort treated with SOF/PR achieved a SVR rate in previously untreated patients of 90%[19], and the VA-real world cohort achieved SVR12 in 66.8%-79%[20].
In other “real-world” analyses with SMV ± R as combination partners of SOF, SVR rates of 74.1%-84.2% were achieved[20-22]. In the OPTIMIST study, a phase III trial, a SVR12 rate of 97% in non-cirrhotic patients[23], while in the OPTIMIST-2 study treating cirrhotic patients with SOF, SMV ± R, SVR 12 in 83% of patients was achieved[24]. However, in our cohort, with more than half of patients being patients with liver cirrhosis, we achieved a SVR rate of 93% with this combination of drugs.
For patients with SOF, DCV ± R as combination partners, SVR rates of 86%-93% have been reported[25]. In our cohort, we were in line with those results and could achieve a SVR rate of 92%, including two patients with recurrent cholestatic Hepatitis C post-liver transplantation, one of those being a non-responder to a prior Telaprevir triple therapy being undertaken post-liver transplantation, and both decompensated at the beginning of treatment.
In previously treated and untreated patients with HCV, a combination of LDV ± R, and SOF led to SVR rates of 94%-99%[26-28], which is in line with results of our cohort, in which we could register a SVR12 rate of 94%, while in another real-world analysis, SVR rates of 91.3%-92% were achieved[29].
For our small group of 3D regimen-receiving patients, we could achieve SVR in 100%, while in the Phase III trials SVR rates of 91.8%-99.5% were observed[30,31].
Thus, altogether, favorable SVR rates achieved in the randomized controlled clinical trials could be translated into the “real-world”, and importantly, in our cohort; even extra hard-to-treat groups of patients exhibited favorable treatment outcomes (SVR12 post-liver transplantation: 88%, SVR12 in cirrhotic patients with platelets < 100/nL: 82%), which exceed results with former treatment options by far[15,32].
Nevertheless, patients with liver cirrhosis show significantly lower response rates (87% with liver cirrhosis, 97% without; P = 0.005), and especially those with advanced portal hypertension (platelets < 100/nL), or high MELD score (≥ 10) show significantly lower SVR12 rates than patients without (P < 0.0001, uni- and multivariate analysis). These findings were also observed in other real world studies with low platelets, low albumin and liver cirrhosis as negative predictors of SVR in larger cohorts[21,33,34]. Thus, this subgroup of patients still resembles a group of patients in the “catch 22”-situation of being in the greatest need of treatment while showing the lowest response rates. Moreover, the whole drug class of HCV protease inhibitors (represented by SMV, and Paritaprevir/r) is not recommendeded for those patients (CTP B “plus”) due to hepatotoxicity. Therefore, new strategies are needed to tackle this problem, e.g., by implementation of screening programs to identify patients infected with HCV at an early stage of their liver disease, and more importantly by development of more potent agents in the near future. However, 4 patients were lost in our cohort. Even if not associated with anti-HCV medication, this emphasizes, that treatment of patients with advanced liver disease should remain in the hands of experienced tertiary centers even in times of “easy” treatment with second generation DAA.
In our cohort, results of early viral kinetics had no impact on prediction of SVR12, thus costly “in-between” measurements of HCV viral load possibly are expendable.
Previous treatment with PR may not play a role any more in treatment decisions, as in our cohort previous treatment was not identified as a negative predictor of SVR, as it was in the HCV-TARGET cohort[21].
Most probably due to the favorable tolerability and low-toxicity profile of second generation DAA, and the omission of pegylated Interferon, now also senior patients benefit from SOF-based DAA treatment on the same scale as younger patients do[21].
Another subgroup of patients in need of further research efforts seems to be the one with GT3: Even though we could achieve a favorable overall SVR rate of 90%, at least in IFN-free treatment regimens, the SVR rates in a “per-protocol” analysis are significantly lower in GT3 patients than in GT1 (80% in GT3, 98% in GT1, respectively, P = 0.016; univariate analysis), again with a special negative focus on patients with GT3 and concomitant advanced liver disease. Moreover, means are limited with respect to treatment of GT3 due to the insufficient antiviral activity of protease- and NS5A-inhibitors (except DCV) in this GT. However, new pangenotypic NS5A inhibitors like Velpatasvir or upcoming new pangenotypic protease inhibitors hopefully close that gap in the near future.
However, since DAA treatment forms RAVs in the viriom of patients, pretreatment with DAA of any generation has to be considered more and more in future treatment attempts after any prior exposure to DAA. All patients in our cohort, who suffered from virological relapse showed RAVs at time-point of relapse. Especially NS5A-RAVs are frequent due to the low resistance barrier of NS5A-inhibitors, as exemplified also in our “real-word” cohort. Since NS5A-RAVs lead to just minimal impairment of viral “fitness”, unfortunately they are detectable for a long time in exposed patients[35]. While for some RAVs (like NS5A L31M, Y93H) clear associations between existence of RAV and virological failure exist, for others (like NS5A A30S) this association is not well established[35]. This may lead to confusion in case of a future re-treatment, if minor RAVs have been detected, and even more in case of a baseline test in treatment naïve patients. However, as the population of patients with relapse after DAA treatment grows, the need for controlled trials with new DAA-combinations (e.g., pangenotypic protease inhibitor) for those patients to address this problem is obvious. Therefore, after virological relapse we recommend resistance testing for individualized adjustment of future DAA therapies.
In our retrospective analysis excellent SVR12 rates of second generation DAA could be translated from the large study trials into “real-world” scenarios. Patients with advanced liver disease, signs of portal hypertension, especially with platelets < 100/nL, or MELD ≥ 10, and patients with GT3 are at relatively higher risk to suffer from virological failure and development of resistance associated variants after exposure to DAA. To overcome this unsolved problem, further research efforts are needed.
From 2014 on, successively the second wave of direct acting antiviral agents (Sofosbuvir, Daclatasvir, Simeprevir, Ledipasvir, Dasabuvir, Ombitasvir, Paritaprevir/r) was available for treatment of chronic hepatitis C infection. In randomized clinical trials, superb rates of sustained virological response (SVR) 12 could be achieved.
Due to the more heterogeneous character of patients in the “real world”, the therapeutic performance of these new drugs outside randomized clinical trials is of interest. Therefore, in this monocentric retrospective cohort study, we analyzed the efficacy, safety, and predictors of SVR 12 for treatment with combinations of second generation direct acting antivirals in a “real-world” setting.
In this retrospective study, similar SVR rates could be achieved compared to randomized clinical trials. However, certain subgroups of patients have significantly lower viral response rates: Significant negative predictors of SVR12 were a platelet count < 100/nL, a MELD score ≥ 10 (both P < 0.0001), liver cirrhosis at baseline (P = 0.005). Moreover, in Interferon-free treatment patients with HCV genotype 3 had significantly lower SVR rates than patients with HCV genotype 1 (P = 0.016). In the future, these subgroups of patients should be more in the focus of research efforts to overcome lower rates of SVR.
Current retrospective analysis shows that excellent SVR12 rates and the favorable side-effect profile of direct acting antiviral-combination therapy can be well translated into “real-world”.
Good level-study to be ameliorated in the presentation of characteristics of cirrhotic patients that are an important part of the studied population.
| 1. | Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 941] [Article Influence: 55.4] [Reference Citation Analysis (1)] |
| 2. | Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1938] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 3. | Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 309] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 4. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1381] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 5. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4753] [Article Influence: 198.0] [Reference Citation Analysis (1)] |
| 6. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] |
| 7. | Morisco F, Granata R, Stroffolini T, Guarino M, Donnarumma L, Gaeta L, Loperto I, Gentile I, Auriemma F, Caporaso N. Sustained virological response: a milestone in the treatment of chronic hepatitis C. World J Gastroenterol. 2013;19:2793-2798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Rosina F, Tosti ME, Borghesio E, Masocco M, Mele A, Coppola C, Milella M, Borgia G, Andreone P, Koch M. Pegylated interferon α plus ribavirin for the treatment of chronic hepatitis C: a multicentre independent study supported by the Italian Drug Agency. Dig Liver Dis. 2014;46:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Braks RE, Ganne-Carrie N, Fontaine H, Paries J, Grando-Lemaire V, Beaugrand M, Pol S, Trinchet JC. Effect of sustained virological response on long-term clinical outcome in 113 patients with compensated hepatitis C-related cirrhosis treated by interferon alpha and ribavirin. World J Gastroenterol. 2007;13:5648-5653. [PubMed] |
| 10. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1865] [Article Influence: 124.3] [Reference Citation Analysis (4)] |
| 11. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 601] [Article Influence: 40.1] [Reference Citation Analysis (1)] |
| 12. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1214] [Article Influence: 80.9] [Reference Citation Analysis (4)] |
| 13. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1984] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 14. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1312] [Article Influence: 87.5] [Reference Citation Analysis (2)] |
| 15. | Werner CR, Franz C, Egetemeyr DP, Beck R, Malek NP, Lauer UM, Berg CP. First-generation protease inhibitor-triple therapy: SVR 24, safety, and predictors of response in a large single center cohort. Virol J. 2015;12:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Werner CR, Franz C, Egetemeyr DP, Janke-Maier P, Malek NP, Lauer UM, Berg CP. Efficacy and safety of telaprevir (TVR) triple therapy in a ‘real-life’ cohort of 102 patients with HCV genotype 1: interim analysis after 24 weeks of treatment. J Viral Hepat. 2014;21:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Tarantino G, Citro V, Conca P, Riccio A, Tarantino M, Capone D, Cirillo M, Lobello R, Iaccarino V. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol. 2009;9:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Tarantino G, Citro V, Esposito P, Giaquinto S, de Leone A, Milan G, Tripodi FS, Cirillo M, Lobello R. Blood ammonia levels in liver cirrhosis: a clue for the presence of portosystemic collateral veins. BMC Gastroenterol. 2009;9:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1330] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 20. | Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Effectiveness of sofosbuvir-based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. Veterans. Aliment Pharmacol Ther. 2015;42:559-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 21. | Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, Morelli G, Darling JM, Feld JJ, Brown RS. Effectiveness of Simeprevir Plus Sofosbuvir, With or Without Ribavirin, in Real-World Patients With HCV Genotype 1 Infection. Gastroenterology. 2016;150:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 22. | Pillai AA, Wedd J, Norvell JP, Parekh S, Cheng N, Young N, Spivey JR, Ford R. Simeprevir and Sofosbuvir (SMV-SOF) for 12 Weeks for the Treatment of Chronic Hepatitis C Genotype 1 Infection: A Real World (Transplant) Hepatology Practice Experience. Am J Gastroenterol. 2016;111:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Kwo P, Gitlin N, Nahass R, Bernstein D, Etzkorn K, Rojter S, Schiff E, Davis M, Ruane P, Younes Z. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016;64:370-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, Godofsky E, Herring RW, Poleynard G, Sheikh A. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2). Hepatology. 2016;64:360-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 914] [Article Influence: 76.2] [Reference Citation Analysis (1)] |
| 26. | Wilder JM, Jeffers LJ, Ravendhran N, Shiffman ML, Poulos J, Sulkowski MS, Gitlin N, Workowski K, Zhu Y, Yang JC. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: A retrospective analysis of phase 3 data. Hepatology. 2016;63:437-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1376] [Article Influence: 114.7] [Reference Citation Analysis (1)] |
| 28. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1066] [Article Influence: 88.8] [Reference Citation Analysis (1)] |
| 29. | Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 30. | Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 31. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 685] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 32. | Werner CR, Egetemeyr DP, Nadalin S, Königsrainer A, Malek NP, Lauer UM, Berg CP. Treatment of recurrent genotype 1 hepatitis C post-liver transplantation: single center experience with telaprevir-based triple therapy. Z Gastroenterol. 2014;52:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 33. | Terrault N, Zeuzem S, Di Bisceglie AM, Lim JK, Pockros P, Frazier L, Kuo A, Lok AS, Shiffman M, Fried MW. Treatment Outcomes With 8, 12, and 24 Week Regimens of Ledipasvir/ Sofosbuvir for the Treatment of Hepatitis C Infection: Analysis of a Multicenter Prospective, Observational Study Abstract #94. Hepatology. 2015;62:67A. |
| 34. | Deterding K, Höner Zu Siederdissen C, Port K, Solbach P, Sollik L, Kirschner J, Mix C, Cornberg J, Worzala D, Mix H. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment Pharmacol Ther. 2015;42:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016;64:486-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Pischke S, Tarantino G S- Editor: Qi Y L- Editor: A E- Editor: Ma S