Published online Aug 28, 2016. doi: 10.3748/wjg.v22.i32.7383
Peer-review started: March 27, 2016
First decision: May 12, 2016
Revised: May 24, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: August 28, 2016
Processing time: 150 Days and 11.8 Hours
We describe a rare case of an 81-year-old man who presented with severe epigastralgia. A chest radiograph showed massive free gas bilaterally in the diaphragmatic spaces. Computed tomography (CT) scan also showed massive free gas in the peritoneal cavity with portal venous gas. We used a wait-and-see approach and carefully considered surgery again when the time was appropriate. The patient received conservative therapy with fasting, an intravenous infusion of antibiotics, and nasogastric intubation. The patient soon recovered and was able to start eating meals 4 d after treatment; thus, surgical intervention was avoided. Thereafter, colonoscopy examination showed pneumatosis cystoides intestinalis in the ascending colon. On retrospective review, CT scan demonstrated sporadic air-filled cysts in the ascending colon. The present case taught us a lesson: the presence of massive intraabdominal free gas with portal venous gas does not necessarily require surgical intervention. Pneumatosis cystoides intestinalis should be considered as a potential causative factor of free gas with portal venous gas when making the differential diagnosis.
Core tip: We describe a rare case of an 81-year-old man with pneumatosis cystoides intestinalis (PCI). PCI is characterized by free gas in the submucosal or subserosal layer of the gastrointestinal tract, and its etiology is unknown. The patient presented with massive intraabdominal free gas and portal venous gas (PVG) due to PCI, and he was successfully treated without surgical intervention. When clinicians encounter free gas with PVG, PCI should be considered.
- Citation: Furihata T, Furihata M, Ishikawa K, Kosaka M, Satoh N, Kubota K. Does massive intraabdominal free gas require surgical intervention? World J Gastroenterol 2016; 22(32): 7383-7388
- URL: https://www.wjgnet.com/1007-9327/full/v22/i32/7383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i32.7383
Pneumatosis cystoides intestinalis (PCI) is a rare clinical entity with an unknown etiology, and it is characterized by free gas in the submucosal or subserosal layer of the gastrointestinal tract[1-3]. PCI has been associated with various underlying etiologies to explain the pathogenic mechanisms causally involved in the accumulation of intramural gas. There are four main theories: (1) the mechanical theory; (2) pulmonary theory; (3) bacterial theory; and (4) chemical theory or nutrition deficiency theory. Currently, chemotherapy, hormonal therapy, steroids, immunosuppression, and connective tissue disease have been reported as causative factors of PCI[2,4-8]. Yet, the exact theory has not been determined. Symptoms usually stem from a secondary underlying disease, including abdominal discomfort, diarrhea, constipation, rectal bleeding, tenesmus, or weight loss. About 3% of patients with PCI develop complications, including tension pneumoperitoneum, intestinal volvulus, obstruction, hemorrhage, and intestinal perforation[9]. One study reported a higher rate of complications with PCI (16.3%), including intestinal obstruction (51.3%), intestinal perforation (35.9%), atypical hyperplasia and canceration (10.2%), and intussusceptions and intestinal necrosis (2.6%)[2]. The most important issue for physicians is recognizing the entity of PCI so that they do not misdiagnose or mismanage it as another disease such as a malignancy or polyposis. It is also important to differentiate the harmless type from the life-threatening type, for which immediate surgery is required[8]. Thus, the conditions of patients with PCI can be confusing, and the decision to treat PCI should be carefully made in consideration of the risks. We herein emphasize how all physicians in the field of gastroenterology can recognize PCI by describing a patient who presented with massive intraabdominal free gas and portal venous gas (PVG) who was successfully treated without surgical intervention.
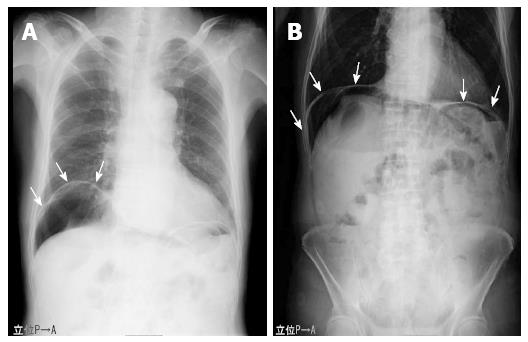
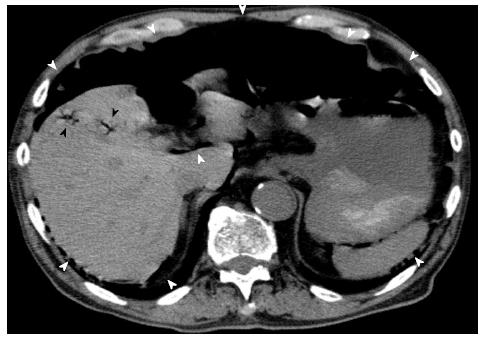
An 81-year-old man who presented at our emergency department with acute severe epigastralgia, periumbilical pain, and progressive abdominal distention was admitted to the Department of General Surgery at our institution. The patient visited our institution for an old myocardial and cerebral infarction, hypertension, and chronic obstipation, and he received a variety of medications. He had a mildly increased body temperature (37.2 °C). His cramping but persistent pain started abruptly. Physical examination showed severe epigastric tenderness and distension without any peritoneal signs. His facial appearance expressed agony, and he presented with excessive sweating. His blood pressure slightly decreased to 12.7/8.65 kPa, which was his usual hypertensive state, and his pulse rate was 61 beats/min. Regarding the hematological parameters, the white blood cell count and C-reactive protein level were mildly increased (10, 500/μL and 0.91 mg/dL, respectively). Serum levels of blood urea nitrogen and creatinine were also increased (26.3 mg/dL and 1.29 mg/dL, respectively), and the levels were slightly different compared to those of his blood samples collected during his normal state (18.5 mg/dL and 0.89 mg/dL, respectively). On radiologic examination, a chest and abdominal plain radiograph showed massive free gas bilaterally in the subdiaphragmatic spaces (Figure 1). Computed tomography (CT) scan also showed that massive free gas was widespread bilaterally under the diaphragm. Moreover, PVG was identified, but ascites was not (Figure 2). Consequently, we performed arterial blood gas analysis, which showed no abnormality (pH = 7.46). First, we considered perforation of the digestive tract. Then, we assumed that the pathological digestive tract would have been accompanied by irreversible ischemia; however, we could not establish a definitive preoperative diagnosis. We used a wait-and-see approach, because the physical examinations did not show any peritoneal signs, although radiologic examinations showed marked findings. Consequently, he received conservative therapy with fasting, an intravenous infusion of antibiotics, and nasogastric intubation. The patient was relieved of the symptoms, and he was permitted to eat food orally 4 d after treatment. The patient has been doing well since hospital discharge.
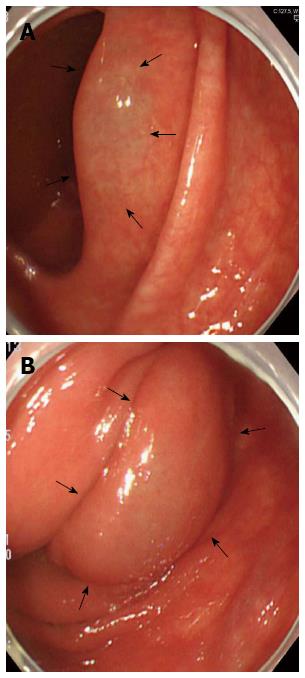
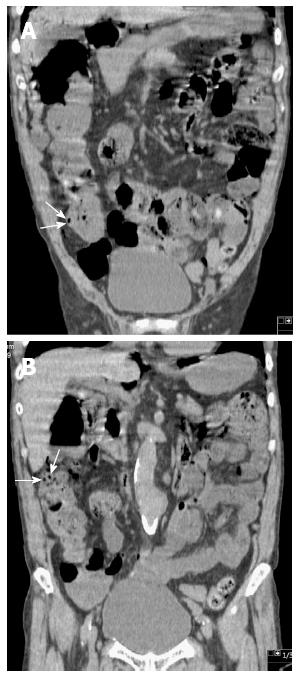
Subsequently, the patient underwent upper endoscopy and total colonoscopy 1 mo after hospital discharge. Upper endoscopy findings showed no abnormality, whereas total colonoscopy results demonstrated a sporadic, round, and smooth-surfaced elevated lesion mimicking a submucosal tumor in the ascending colon (Figure 3). On retrospective review, coronal sections on CT showed sporadic PCI in the ascending colon (Figure 4). The CT findings matched those of total colonoscopy. Thus, we diagnosed the patient’s condition as PCI on follow-up examinations. PCI in the present case was retrospectively diagnosed as secondary PCI, because the patient had an underlying disease of chronic obstipation.
The most important point of the present case is whether surgical intervention is indicated when intraabdominal free gas or PVG is observed. It is normal for clinicians to fear digestive tract perforation, as it can be fatal. PCI is one of the factors of pneumoperitoneum, but it has not been well recognized by clinicians such as gastroenterologists, surgeons, endoscopists, and radiologists. The term “pneumatosis intestinalis” was first described as primary PCI by Duo Vernoi while observing autopsy specimens in 1730[1]. Subsequently, Mayer used the term “pneumatosis cystoides intestinalis” to describe its occurrence in hogs in 1825, and the first well-documented case in humans was reported by Bang in 1876[10]. Recently, Koss reviewed cases with PCI and reported that approximately 85% of all cases of PCI were classified as secondary PCI, which results from other underlying diseases[11]. In the remaining 15% of cases, PCI was idiopathic or primary. Morris et al[12] reported that the incidence of PCI was 46% in the colon, followed by 27% in the small intestine, 7% in the colon and small intestine combined, 5% in the stomach, and 15% in other parts of the gastrointestinal tract. The most common localization of gas was in the submucosa (69.9%)[2]. On the basis of autopsy studies, its incidence in the general population has been estimated as three per 10000 individuals[13,14]. Its recognition has not been widespread in terms of frequency.
PVG is also a radiological sign of serious underlying gastrointestinal pathology. The mortality for PVG ranges between 75% and 90%. The most common cause of PVG is acute mesenteric ischemia. Although hepatic PVG helps distinguish between benign and life-threatening PCI, it may also occur with or without PCI due to nonischemic conditions. Mesenteric abscess formation, portomesenteric thrombophlebitis, sepsis, abdominal trauma, severe enteritis, cholangitis, chronic cholecystitis, pancreatitis, inflammatory bowel disease, diverticulitis, and gastrointestinal surgery or liver transplantation are considered causative factors of PVG[14,15]. The association between PVG and PCI seems to have been noted first in the article by Wolfe and Evans in 1955[15,16]. They reported gas in the portal veins of six living infants, three of whom had associated pneumatosis of the bowel. Khalil et al[13] reported that the combination of PCI and PVG is associated with bowel ischemia in about 70% of cases[13,17,18]. Sooby et al[15] classified 88 cases of PCI with PVG into three distinct subgroups: mechanical, ischemic, and benign. They also reported that of 88 patients with PCI, 19 were diagnosed as having benign PCI, including 6 patients with both PI and PVG[14]. Considering these cases, PCI may be one of the factors that induce PVG, which can also be fatal. Conversely, PCI and PVG are associated with each other, and they can occur together in various non-ischemic conditions that are not associated with an unfavorable outcome[13,14,18-20]. Even if life-threatening conditions of intraabdominal free gas, PCI, and PVG occur simultaneously, patients’ conditions may not necessarily be serious. Thus, deepening the knowledge of these conditions is essential for clinicians (Table 1). In the present case, the mechanisms of these life-threatening conditions were connected, and the following hypotheses have been made. First, secondary PCI probably occurred due to mucosal disruption caused by chronic obstipation. Second, increased gas in the serosal or subserosal layer is partially transported via the mesenteric drainage veins to the hepatic portal veins. Sequentially, ruptures of serosal or subserosal cysts cause pneumoperitoneum. Therefore, physicians must determine whether it is best for patients to undergo surgical intervention. Considering these life-threatening conditions, the most important diagnostic information is patients’ vital signs and complaints, and physical examination of the abdomen.
| Pulmonary disease | Drugs | Infectious disease |
| Asthma | Alpha-glucosidase inhibitor | AIDS |
| Chronic bronchitis | Corticosteroids | Whipple’ disease |
| Chronic obstructive pulmonary disease | Lactulose | Candida albicans |
| Positive end-expiratory pressure | Sorbitol | Clostridia |
| Pulmonary fibrosis | Chemotherapy agents | Escherichia coli |
| Gastrointestinal disorders | Gefitinib, Sorafenib, Cetuximab, | Mycobacterium tuberculosis |
| Pyloric stenosis | Sunitinib, imatinib, 5-FU, etc. | Intestinal parasites |
| Pyloric ulcer disease | Choral hydrate | Virus |
| Peptic ulcers | Caustic agents | Cytomegalovirus, Rotavirus, |
| Bowel obstruction | Mechanical causes | Adenovirus, Varicella zoster virus |
| Rupture of jejunal diverticula | Endoscopy | Autoimmune disease |
| Inflammatory bowel disease | Billiary stent perforation, Sclerotherapy | Scleroderma |
| Crohn’s disease, ulcerative colitis | Barium enema | Lupus variants |
| Adynamic ileus | Operation | Polymyositis |
| Appendicitis | Jejunoileal bypass, Jejunostomy tubes, | Dermatomyositis |
| Toxic megacolon | Post-surgical anastomosis | Sarcoidosis |
| Volvulus | Organ transplantation | Polyarteritis nodosa |
| Carcinoma | Cardiac, Bone marrow, Kidney, Liver, | Vascular disease |
| Hirschsprung disease | Lung, Graft versus host disease | Mesenteric vascular disease |
| Celiac sprue | Trauma | Intestinal infarction and ischemia |
| Enteritis and colitis | Intestinal ischemia | |
| Diverticulitis | Intestinal strangulation | |
| Emphysematous gastritis | Collagen vascular disease | |
| Bowel necrosis |
In general, surgical indications of PCI include cases suggestive of inconvertible intestinal obstruction or perforation, or patients with precancerous conditions[13,21]. Wu et al[2] mentioned that these complications associated with PCI occur in approximately 16.3% of cases. Hence, radiologic investigations are important for diagnosing PCI. Particularly, multidetector CT is clearly beneficial, because it enables clinicians to make a more confident diagnosis of PCI by reformations in the coronal, sagittal, and axial planes[3,14]. However, radiologic or endoscopic examination does not necessarily show typical findings, which means that patients with PCI cannot be diagnosed definitively, as shown in the present case. We reached the definite diagnosis retrospectively by reformation of CT in the coronal plane.
We were able to avoid surgical intervention in the present case because of the following important factors: (1) the patient did not show any abdominal peritoneal signs; (2) arterial blood gas measurement did not show metabolic acidosis; (3) radiological examinations demonstrated that there was a considerable amount of free gas bilaterally in the subdiaphragmatic spaces together with PVG, but no ascites was observed; and (4) despite mild renal dysfunction, urine output was obtained, and the patient had stable vital signs. It was a bold decision to avoid surgical intervention, because the patient would have required prompt action if perforation or ischemia of the digestive tract perforation had occurred. Interestingly, nine of 27 patients with PCI died for an overall mortality rate of 33%. Eleven patients observed without surgery had a mortality rate of 18%, whereas those who underwent surgery had a mortality rate of 44%. The remaining nine patients who improved without surgery did not manifest any clinical signs that would have prompted surgery[22]. Therefore, it seems that some patients with PCI can be successfully managed with conservative treatment. However, 37% to 75% of patients with PVG have bowel infarction, and 10 of 12 patients with both PCI and PVG died within 48 h[8]. The long-term prognosis of PCI is unknown, and a long-term follow-up study is required to evaluate this further.
Thus, complementary evaluations such as blood gas analysis can be helpful. Knechtle et al[22] advocated the classification of clinical and laboratory values, including the assessment of arterial blood gas (pH, HCO3-), so that they could predict the occurrence of ischemic bowel and the patient’s outcome. Khalil et al[13] propounded a decision-making algorithm after PCI is diagnosed. The proposal makes sense, because the checklists include a variety of essential criteria for diagnosing PCI, including physical conditions, physical examinations, and medical history intake. Obtaining the patient’s medical history of comorbidities such as pulmonary disease, gastrointestinal disease, autoimmune disease, infectious disease, and vascular disease, as well as possible iatrogenic causes and the use of drugs is very important for cases of secondary PCI. The lack of knowledge may lead to misdiagnosis, which causes the patient to undergo unnecessary operations. As previously described, the fact that CT did not show ascites was favorable, because ascites often indicate ischemia or perforation of the gastrointestinal tract. Briefly, if CT demonstrates intraabdominal free gas with PVG in the absence of ascites, PCI is a possible diagnosis.
In conclusion, massive intraabdominal free gas with PVG does not always require surgical intervention. Therefore, the decision to perform surgery should be made on the basis of the knowledge of PCI. When clinicians encounter free gas with PVG in the abdomen, PCI should be considered when making the differential diagnosis.
We thank Yuki Matsuhashi, Department of Endocrinology and Metabolism, and Hirosaki University Graduate School of Medicine for their assistance in making the tables and figures.
An 81-year-old man presented with severe epigastralgia.
Physical examination showed severe epigastric tenderness and distension without any peritoneal signs.
Regarding the hematological parameters, the white blood cell count and C-reactive protein level were mildly increased (10.500/μL and 0.91 mg/dL, respectively). Serum levels of blood urea nitrogen and creatinine were also increased (26.3 mg/dL and 1.29 mg/dL, respectively). Arterial blood gas analysis showed no abnormality (PH = 7.46).
Computed tomography scan showed that massive free gas was widespread bilaterally under the diaphragm. Moreover, portal venous gas was identified, but ascites was not observed.
There was no specimen to make a pathological diagnosis.
The authors used a wait-and-see approach, because the physical examinations did not show any peritoneal signs, although radiologic examinations showed marked findings. Consequently, he received conservative therapy with fasting, an intravenous infusion of antibiotics, and nasogastric intubation. We were able to avoid surgical intervention.
Pneumatosis cystoides intestinalis (PCI) is a rare entity. In addition, there are few reports of patients with PCI successfully treated without surgical intervention.
PCI is a rare clinical entity with an unknown etiology, and it is characterized by free gas in the submucosal or subserosal layer of the gastrointestinal tract.
The present case taught us the following lesson: the presence of intraabdominal free gas with portal venous gas (PVG) does not necessarily require surgical intervention. When gastroenterologists encounter free gas with PVG, PCI should be considered.
It is a case report of a patient with abdominal pain that was proved to be caused by PCI, and was managed conservatively. It is very interesting.
| 1. | Du Vernoy JG. Aer intestinorum tam sub extima quam intima tunica inclusus: observationes anatomicae: comment. Acad Scient Petropol. 1730;5:213-225. |
| 2. | Wu LL, Yang YS, Dou Y, Liu QS. A systematic analysis of pneumatosis cystoids intestinalis. World J Gastroenterol. 2013;19:4973-4978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 123] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Ivanović A, Kovač J, Mašulović D, Stefanović A, Jakšić E, Saranović D. Education and imaging. Gastrointestinal: the role of multidetector computer tomography in diagnosis of pneumatosis cystoides intestinalis. J Gastroenterol Hepatol. 2012;27:182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Groninger E, Hulscher JB, Timmer B, Tamminga RY, Broens PM. Free air intraperitoneally during chemotherapy for acute lymphoblastic leukemia: consider pneumatosis cystoides intestinalis. J Pediatr Hematol Oncol. 2010;32:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 5. | Vendryes C, Hunter CJ, Harlan SR, Ford HR, Stein J, Pierce JR. Pneumatosis intestinalis after laparoscopic appendectomy: case report and review of the literature. J Pediatr Surg. 2011;46:e21-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Balbir-Gurman A, Brook OR, Chermesh I, Braun-Moscovici Y. Pneumatosis cystoides intestinalis in scleroderma-related conditions. Intern Med J. 2012;42:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Korhonen K, Lovvorn HN, Koyama T, Koehler E, Calder C, Manes B, Evans M, Bruce K, Ho RH, Domm J. Incidence, risk factors, and outcome of pneumatosis intestinalis in pediatric stem cell transplant recipients. Pediatr Blood Cancer. 2012;58:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Heng Y, Schuffler MD, Haggitt RC, Rohrmann CA. Pneumatosis intestinalis: a review. Am J Gastroenterol. 1995;90:1747-1758. [PubMed] |
| 9. | Arikanoglu Z, Aygen E, Camci C, Akbulut S, Basbug M, Dogru O, Cetinkaya Z, Kirkil C. Pneumatosis cystoides intestinalis: a single center experience. World J Gastroenterol. 2012;18:453-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | KOSS LG. Abdominal gas cysts (pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53:523-549. [PubMed] |
| 12. | Morris MS, Gee AC, Cho SD, Limbaugh K, Underwood S, Ham B, Schreiber MA. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195:679-682; discussion 682-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 13. | Khalil PN, Huber-Wagner S, Ladurner R, Kleespies A, Siebeck M, Mutschler W, Hallfeldt K, Kanz KG. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res. 2009;14:231-239. [PubMed] |
| 14. | Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol. 2007;188:1604-1613. [PubMed] |
| 15. | Sooby P, Harshen R, Joarder R. An unusual triad of pneumatosis intestinalis, portal venous gas and pneumoperitoneum in an asymptomatic patient. J Surg Case Rep. 2015;2015:pii rjv035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wolfe JN, Evans WA. Gas in the portal veins of the liver in infants; a roentgenographic demonstration with postmortem anatomical correlation. Am J Roentgenol Radium Ther Nucl Med. 1955;74:486-488. [PubMed] |
| 17. | Paran H, Epstein T, Gutman M, Shapiro Feinberg M, Zissin R. Mesenteric and portal vein gas: computerized tomography findings and clinical significance. Dig Surg. 2003;20:127-132. [PubMed] |
| 18. | Wiesner W, Mortelé KJ, Glickman JN, Ji H, Ros PR. Portal-venous gas unrelated to mesenteric ischemia. Eur Radiol. 2002;12:1432-1437. [PubMed] |
| 19. | Wiesner W, Mortelé KJ, Glickman JN, Ji H, Ros PR. Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol. 2001;177:1319-1323. [PubMed] |
| 21. | Zhang H, Jun SL, Brennan TV. Pneumatosis intestinalis: not always a surgical indication. Case Rep Surg. 2012;2012:719713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Misiakos EP S- Editor: Ma YJ L- Editor: A E- Editor: Ma S