Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6917
Peer-review started: February 19, 2016
First decision: March 31, 2016
Revised: April 4, 2016
Accepted: April 15, 2016
Article in press: April 15, 2016
Published online: August 14, 2016
Processing time: 168 Days and 21.5 Hours
AIM: To assess anti-migration potential of six biliary covered self-expandable metal stents (C-SEMSs) by using a newly designed phantom model.
METHODS: In the phantom model, the stent was placed in differently sized holes in a silicone wall and retracted with a retraction robot. Resistance force to migration (RFM) was measured by a force gauge on the stent end. Radial force (RF) was measured with a RF measurement machine. Measured flare structure variables were the outer diameter, height, and taper angle of the flare (ODF, HF, and TAF, respectively). Correlations between RFM and RF or flare variables were analyzed using a linear correlated model.
RESULTS: Out of the six stents, five stents were braided, the other was laser-cut. The RF and RFM of each stent were expressed as the average of five replicate measurements. For all six stents, RFM and RF decreased as the hole diameter increased. For all six stents, RFM and RF correlated strongly when the stent had not fully expanded. This correlation was not observed in the five braided stents excluding the laser cut stent. For all six stents, there was a strong correlation between RFM and TAF when the stent fully expanded. For the five braided stents, RFM after full stent expansion correlated strongly with all three stent flare structure variables (ODF, HF, and TAF). The laser-cut C-SEMS had higher RFMs than the braided C-SEMSs regardless of expansion state.
CONCLUSION: RF was an important anti-migration property when the C-SEMS did not fully expand. Once fully expanded, stent flare structure variables plays an important role in anti-migration.
Core tip: Ability of prevention of migration is very important to improve the results of covered self-expandable metal stents (C-SEMSs) for biliary stricture. This study aims to assess the anti-migration potential of six C-SEMSs by using a newly designed phantom model which allows the resistance force to migration (RFM) measurement of the stents. We found that RFM and radial force correlated strongly when the stent had not fully expanded. Once fully expanded, stent flare structure variables affected the anti-migration property of the stent. We concluded that several stent properties, including radial force and flare structure should be considered when selecting C-SEMS.
- Citation: Minaga K, Kitano M, Imai H, Harwani Y, Yamao K, Kamata K, Miyata T, Omoto S, Kadosaka K, Sakurai T, Nishida N, Kudo M. Evaluation of anti-migration properties of biliary covered self-expandable metal stents. World J Gastroenterol 2016; 22(30): 6917-6924
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6917.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6917
Endoscopic biliary stent placement in patients with biliary malignancy plays a pivotal role in relieving obstructive jaundice[1-8]. The biliary stents used to palliate malignant biliary obstruction should have a long patency duration. Since the self-expandable metal stent (SEMS) has an especially long patency, it is widely recognized to be an effective standard biliary endoprosthesis[9-12]. Covered SEMSs (C-SEMSs) have also been developed to prevent tumor in growth through the stent mesh. One disadvantage of C-SEMSs is that they are more prone to migration than uncovered SEMSs[13-17]. As a result, biliary C-SEMS have been provided with anti-migration mechanical properties, including higher radial force (RF), an anchoring flap, and specific stent flare structures[18-20]. A recent study comparing covered and uncovered SEMSs showed that neither of the stents with uncovered flare ends and relatively low axial force did not migrate[21]. Moreover, the C-SEMS associated with a significantly longer stent patency duration and longer patient survival time without stent dysfunction. Thus, anti-migration systems may not only prevent C-SEMS migration, they may also prevent stent dysfunction and prolong patency.
Of the various anti-migration properties, RF may be particularly important in preventing SEMS migration. RF is the radially outward expanding force that maintains the luminal patency at the stricture once the SEMS is deployed. Other anti-migration features, including stent structures, covering material, and flare structures may also contribute to the anti-migration potential of the stent. The present study was conducted to determine the contributions of RF and other anti-migration features of six currently commercially available C-SEMSs in Japan. The resistance force to retraction in the axial direction is denoted in this study as the resistance force to migration (RFM) and was measured by using a phantom model of biliary stricture.
Table 1 lists the six commercially available C-SEMSs in Japan and their structures, stent materials, cover materials, and manufacturers. The six stents had four types of structures: braided with cross wire (Wallflex), braided with hook wire (ComVi and SUPREMO stents), braided with both hook and cross wires (BONA and HANARO stents), and a laser-cut structure (ZEO stent). Thus, there were five braided and one laser-cut stents. The stents were made of either nitinol (all except Wallflex) or platinum-cored nitinol (Wallflex) and their cover membranes were composed of silicone (Wallflex, BONA, HANARO, and SUPREMO), expanded polytetrafluoroethylene (ComVi), or polyurethane (ZEO). All stents were 10 mm in outer diameter and 80 mm in length.
| C-SEMS | Structure | Stent material | Cover material | ODF (mm) | HF (mm) | TAF (degree) | Manufacturer |
| Wallflex stent (fully covered) | Braided | Platinum-cored nitinol | Silicone | 11.8 | 1.9 | 11.3 | Boston scientific |
| Cross wire | |||||||
| BONA stent | Braided | Nitinol | Silicone | 13.4 | 3.2 | 21.8 | Standard Sci Tech |
| Cross and hook wire | |||||||
| HANARO stent | Braided | Nitinol | Silicone | 13.4 | 3.2 | 16.7 | M.I. Tech |
| Cross and hook wire | |||||||
| ComVi stent | Braided | Nitinol | e-PTFE | 9.7 | 0 | 0 | Taewoong |
| Hook wire | |||||||
| SUPREMO stent | Braided | Nitinol | Silicone | 12.2 | 2 | 16.7 | Taewoong |
| Hook wire | |||||||
| ZEO stent | Laser-cut | Nitinol | Polyurethane | 11.1 | 1.9 | 26.6 | Zeon |
A phantom model of inducing biliary SEMS migration was created by using a retraction robot, a 3-mm thick silicone wall (Shin-etsu chemical, Tokyo, Japan), and a force gauge (Model DPRS5T, Imada, Tokyo, Japan). The metal stents were fixed into a round hole in the silicone wall that had a diameter of 6, 8, or 10 mm. A force gauge was fixed to the distal end of the stent. The phantom model was placed in a box and the temperature was maintained at 37 °C by injecting heated air. During the experiments, the distal end of the stent (with the attached force gauge) was retracted at a speed of 1 mm/s. by using the retraction robot (Figure 1). The force of the resistance of the stent to the retraction (i.e., RFM) was measured from the time stent retraction started until the time the distal end of the stent was dislocated from the silicone wall. The maximum value of resistance force during retraction was used for analysis.
RF was measured as described previously[18]. Thus, the RF measurement machine (Model RTA310, Blockwise Engineering, Tempe, AZ, United States) was placed in a box where the temperature was maintained at 37 °C by injecting heated air. The SEMS sample in its fully expanded state was placed in the cylindrical space of the machine and the cylinder was contracted, thereby causing the SEMS to shrink to its minimum size (2 mm). The force on the cylinder was then reversed so that the SEMS expanded automatically until it achieved its fully expanded state. The force that expanded the SEMS (i.e., the RF) was continuously recorded by a force gauge that was deployed inside the cylinder. The force required to expand the SEMS to an outer diameter of 6, 8, and 10 mm was recorded and used for analysis.
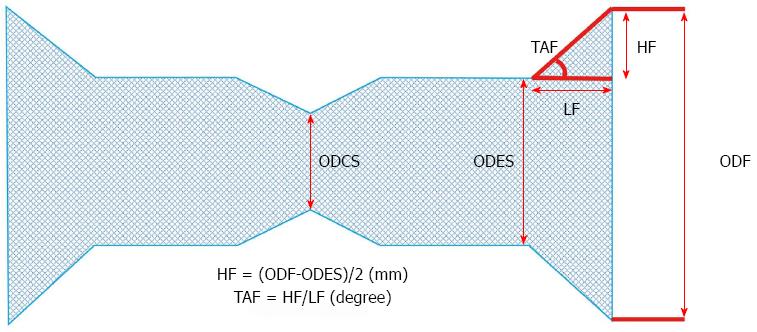
Stent structure was expressed as outer diameter of the fully expanded stent (ODES; mm), outer diameter of the compressed stent (ODCS; mm), outer diameter of the flare (ODF; mm), height of the flare [HF = (ODF-ODES)/2; mm], length of the flare (LF; mm), and taper angle of the flare (TAF = HF/LF; degree) (Figure 2). The three key stent structure variables were ODF, HF, and TAF.
The RF and RFM of each stent were expressed as the average of five replicate measurements. The correlations between RFM and RF or stent flare structure variables were analyzed by simple linear regression and correlation analysis, and expressed by correlation coefficient (r). For these correlation analyses, either all six stents or only the five braided stents were included. A correlation was deemed to be strong if r≥ 0.7. All statistical analyses were performed by using the statistical software SAS 9.4. (SAS Institute Inc., Cary, NC, United States).
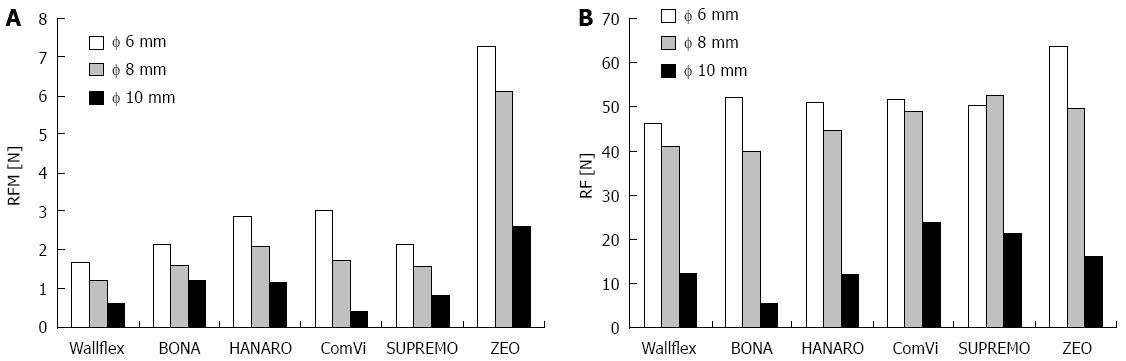
Figure 3A shows the RFM measurement results when the hole of the wall (ODCS) was 6, 8, and 10 mm in diameter. For all six stents, the RFM dropped as the hole diameter (ODCS) increased. The laser-cut ZEO stent had the highest RFM at all three hole diameters (6, 8, and 10 mm ODCS). Figure 3B shows the RF measurement results during expansion, namely, at the times when the ODCS was 6, 8, and 10 mm. For all six stents, the RF decreased as the ODCS increased. At the ODCS of 6 mm, the laser-cut ZEO stent had the highest RF. At the ODCSs of 8 and 10 mm, the braided stents SUPREMO and ComVi had the highest RFs, followed by the laser-cut ZEO stent. Table 1 shows the stent flare structure variables (ODF, HF, and TAF) of the stents. The BONA and HANARO stents both had the highest ODFs and HFs. The laser-cut ZEO stent had the highest TAF. Of the five braided stents, BONA had the highest TAF.
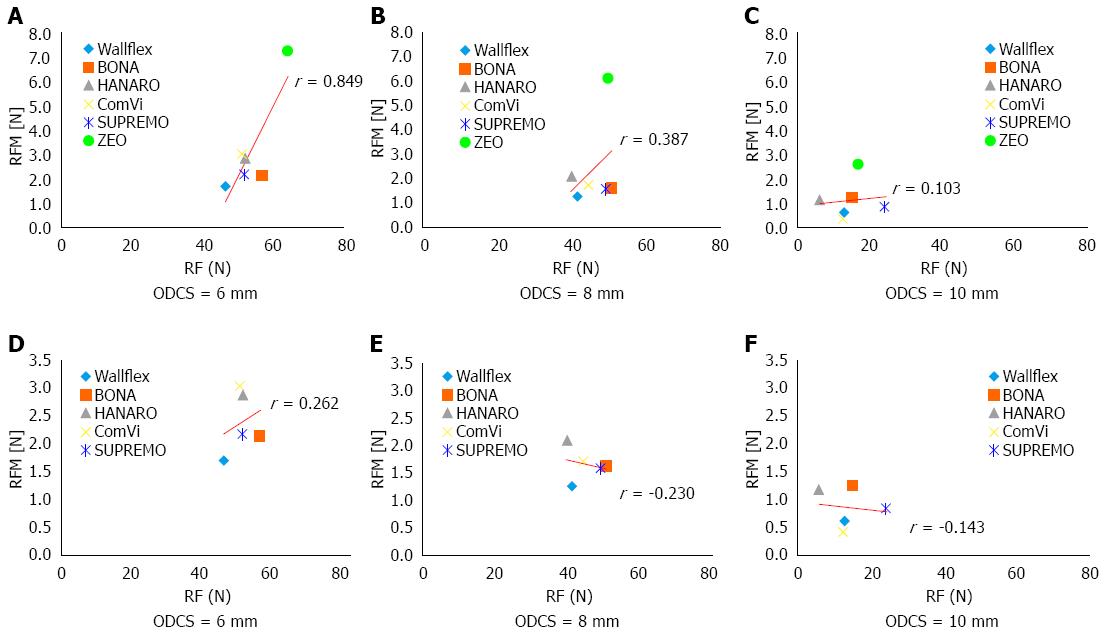
For all six C-SEMSs, the coefficients for the correlation between RFM and RF when the ODCS was 6, 8, and 10 mm were 0.849, 0.387, and 0.103, respectively (Figure 4A-C). Thus, the correlation was only strong when the ODCS was 6 mm. When excluding the laser cut stent, the correlation coefficients between RFM and RF when the ODCS was 6 mm, 8 mm and 10 mm were 0.262, -0.230 and -0.143, respectively (Figure 4D-F). Thus, the RFM of the five braided stents did not correlate strongly with RF regardless of the ODCS.
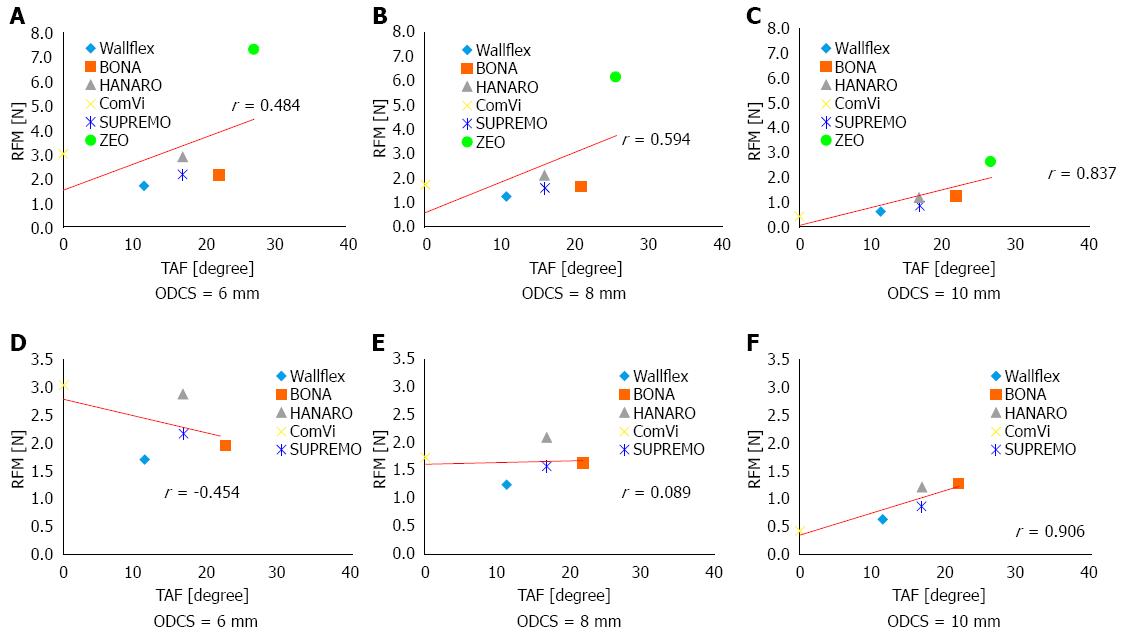
For all six SEMSs, the coefficients for the correlation between RFM and ODF when the ODCS was 6, 8, and 10 mm were -0.359, -0.257, and 0.098, respectively. The correlation coefficients between RFM and HF were -0.359, -0.020, and -0.134, respectively. The correlation coefficients between RFM and TAF were 0.484, 0.594, and 0.837 mm, respectively (Figure 5A-C). Thus, for all six stents, RFM did not correlate well with ODF and HF. It correlated better with TAF but only achieved a strong correlation when the ODCS was 10 mm.
When excluding the laser cut stent, the coefficients for the correlation between RFM and ODF when the ODCS was 6 mm, 8 mm and 10 mm were -0.301, 0.271, and 0.952, respectively. The correlation coefficients between RFM and HF were -0.327, 0.242, and 0.943, respectively. The correlation coefficients between RFM and TAF were -0.454, 0.089, and 0.906, respectively (Figure 5D-F). Thus, for the braided stents, RFM correlated strongly with all stent structure variables when the ODCS was 10 mm.
Thus, overall, when all six stents were considered, their RFM correlated strongly with RF at 6 mm and TAF at 10 mm. Other variables did not correlate with RFM. By contrast, when only the braided stents were considered, their RFM did not correlate with RF but did correlate with all three flare variables at 10 mm.
Although C-SEMSs tend to show a higher rate of stent migration than uncovered SEMSs[13-17], they associate with significantly longer patency than uncovered SEMSs in some reports[13,21,22]. To reduce their migration, C-SEMSs have been equipped with various anti-migration mechanical properties, namely, different stent frameworks, membrane materials, higher RF, different flare structures, and the presence of an anchoring flap[18-20]. However, to the best of our knowledge, only three studies have examined whether the anti-migration features of biliary SEMSs actually prevent migration[19,20,23]. When Park et al[19] compared the anti-migration effects of two types of SEMSs, they concluded that the anchoring design was superior to the flare-end design. Isayama et al[20] designed a novel C-SEMS with flare and bank structures and reported the low rate of stent migration of this C-SEMS. Recently, Nakai et al[23] suggested that if RF is low C-SEMSs can easily slip through the biliary stricture. Their study revealed that RF but not axial force was associated with C-SEMS migration in patients with distal malignant biliary obstruction due to pancreatic cancer[23]. In the present study, we assessed the anti-migration potential of six C-SEMSs by using a new phantom model to measure RFM.
We found several key relationships between RFM and stent variables. First, for all six stents, RFM correlated strongly with RF only when the stent did not fully expand (i.e., the ODCS was 6 mm) (r = 0.849); this was not observed when the stent fully expanded (i.e., the ODCS was 10 mm). It was also not observed when the five braided stents were examined. Recent article indicated that low RF was one of the significant risk factors for early C-SEMS migration[23]. Considering this clinical outcome, RF may remain playing a pivotal role to prevent migration of the stent, when the stent does not fully expand due to compression by a growing tumor.
Second, for all six stents, there was a strong correlation between RFM and TAF when the stent fully expanded (r = 0.837); a correlation with RFM was not observed for the other two flare variables (ODF and HF). However, when only the five braided stents were assessed, they showed strong correlations between RFM and all three flare variables at 10 mm expansion (ODF, r = 0.952; HF, r = 0.943; TAF, r = 0.906).
When Isayama et al[18] measured the RF of 14 different SEMSs, including five C-SEMSs, they found that the RF vs diameter curves exhibited two characteristics. In addition, the values during expansion were different from those during contraction. We hypothesized that the expansion process is most appropriate for measuring the anti-migration potential of stents in the setting of biliary stricture. Therefore, we used the values of RF during expansion at different outer diameters (i.e., when the ODCS was 6, 8, and 10 mm). Regarding our six stents, RF only correlated closely with RFM when the stent did not fully expand (6 mm). This suggests that RF may play an important role in preventing migration during stent deployment until full expansion is achieved.
The present study also showed that when the stent did not fully expand (6 mm), the laser-cut C-SEMS had a higher RF than any of the five braided C-SEMSs. However, the laser-cut C-SEMS also had a higher RFM than the braided SEMSs at all three ODCSs (6, 8, and 10 mm). These observations suggest that the high RFM of the laser-cut C-SEMS was caused not only by its high RF but also by its other properties. In other words, the scaly framework of this laser-cut stent may have contributed to its anti-migration potential.
When all stents were considered, a close correlation between RFM and the stent flare property TAF was observed when the stent fully expanded; this correlation was not observed when the stent did not fully expand or for the other two stent flare properties. However, when the laser-cut C-SEMS was excluded from the analysis, extremely close correlations between RFM and all three stent flare properties was observed. These results indicate that an effective stent flare structure, particularly a high TAF, is very important for preventing the migration of the stent after deployment and full expansion.
The present study has some limitations. First, this phantom model may not reflect the real clinical situation of biliary strictures. In the real clinical situation, stent migration may be caused by several factors, including duodenum motility and bile flow. By contrast, our phantom model only measures the resistance of the stents to mechanical retraction from a silicone wall. Second, we could not assess the mechanical properties of C-SEMS other than RFM, RF and flare structure in our analysis. Other properties such as axial force may associate with anti-migration potential. However, axial force occurs only in a tortuous bile duct. As the stent was not bent in our phantom model, relationship between axial force and RFM could not be assessed. Third, only one laser-cut C-SEMS was used in the experiments. Other SEMSs should be evaluated in terms of their anti-migration potential by using this phantom model.
In conclusion, RF plays an important role in anti-migration when the C-SEMS has not yet fully expanded. However, in the fully expanded state, stent flare structure variables may strongly affect the anti-migration property of the stent. The laser-cut stent appears to have an extremely high resistance to migration whether or not the stent fully expands. Thus, several stent properties, including RF, flare structure, and stent framework should be considered when selecting C-SEMS for biliary stricture.
We thank Mr. Chimyon Gon, MT from Research and Development Center, Zeon Corporation for technical assistance regarding the radial force and resistance force to migration measurements.
Biliary covered self-expandable metal stents (C-SEMSs) tend to migrate more frequently than uncovered SEMSs. Mechanical anti-migration properties of C-SEMS such as the stent frame work, flare structure and radial force (RF) may prevent this. However, to our knowledge, only three studies have examined whether the anti-migration features of biliary SEMSs actually prevent migration
Of the various anti-migration properties, RF may be particularly important in preventing SEMS migration. RF is the radially outward expanding force that maintains the luminal patency at the stricture once the SEMS is deployed. Other anti-migration features, including stent structures, covering material, and flare structures may also contribute to the anti-migration potential of the stent.
This is the first study to assess the anti-migration potential of biliary C-SEMSs by using a phantom model which enabled to measure the resistance force to migration (RFM) of the stents. According to the results of our study, RF was an important anti-migration property when the C-SEMS did not fully expand. Once fully expanded, stent flare structure variables may strongly affect the anti-migration property of the stent.
The results of this study suggest that several stent properties, including RF, flare structure, and stent framework should be considered when selecting C-SEMS for biliary stricture to reduce stent-related complications. This study highlights the problem of C-SEMS migration faced by clinicians and provides useful information for stent selection in future clinical practice.
RFM in this study denotes the resistance force of the stents to retraction in the axial direction and this force was measured by using a phantom model of biliary stricture.
In this in vitro study, the authors evaluated resistance force against migration and its correlations with RF and the flare structure of six commonly-used C-SEMSs. This study highlights the problem of C-SEMS migration faced by clinicians and provides useful information for stent selection in future clinical practice.
| 1. | Soehendra N, Reynders-Frederix V. Palliative bile duct drainage - a new endoscopic method of introducing a transpapillary drain. Endoscopy. 1980;12:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 248] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Huibregtse K, Tytgat GN. Palliative treatment of obstructive jaundice by transpapillary introduction of large bore bile duct endoprosthesis. Gut. 1982;23:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Walta DC, Fausel CS, Brant B. Endoscopic biliary stents and obstructive jaundice. Am J Surg. 1987;153:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 456] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Huibregtse K, Cheng J, Coene PP, Fockens P, Tytgat GN. Endoscopic placement of expandable metal stents for biliary strictures--a preliminary report on experience with 33 patients. Endoscopy. 1989;21:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Irving JD, Adam A, Dick R, Dondelinger RF, Lunderquist A, Roche A. Gianturco expandable metallic biliary stents: results of a European clinical trial. Radiology. 1989;172:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 132] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Lammer J, Klein GE, Kleinert R, Hausegger K, Einspieler R. Obstructive jaundice: use of expandable metal endoprosthesis for biliary drainage. Work in progress. Radiology. 1990;177:789-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 559] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 702] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 10. | Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 359] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Lammer J, Hausegger KA, Flückiger F, Winkelbauer FW, Wildling R, Klein GE, Thurnher SA, Havelec L. Common bile duct obstruction due to malignancy: treatment with plastic versus metal stents. Radiology. 1996;201:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Prat F, Chapat O, Ducot B, Ponchon T, Pelletier G, Fritsch J, Choury AD, Buffet C. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 307] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 466] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 14. | Park DH, Kim MH, Choi JS, Lee SS, Seo DW, Kim JH, Han J, Kim JC, Choi EK, Lee SK. Covered versus uncovered wallstent for malignant extrahepatic biliary obstruction: a cohort comparative analysis. Clin Gastroenterol Hepatol. 2006;4:790-796. [PubMed] |
| 15. | Kullman E, Frozanpor F, Söderlund C, Linder S, Sandström P, Lindhoff-Larsson A, Toth E, Lindell G, Jonas E, Freedman J. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010;72:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Telford JJ, Carr-Locke DL, Baron TH, Poneros JM, Bounds BC, Kelsey PB, Schapiro RH, Huang CS, Lichtenstein DR, Jacobson BC. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010;72:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Lee JH, Krishna SG, Singh A, Ladha HS, Slack RS, Ramireddy S, Raju GS, Davila M, Ross WA. Comparison of the utility of covered metal stents versus uncovered metal stents in the management of malignant biliary strictures in 749 patients. Gastrointest Endosc. 2013;78:312-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Isayama H, Nakai Y, Toyokawa Y, Togawa O, Gon C, Ito Y, Yashima Y, Yagioka H, Kogure H, Sasaki T. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointest Endosc. 2009;70:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Park DH, Lee SS, Lee TH, Ryu CH, Kim HJ, Seo DW, Park SH, Lee SK, Kim MH, Kim SJ. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc. 2011;73:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Isayama H, Kawakubo K, Nakai Y, Inoue K, Gon C, Matsubara S, Kogure H, Ito Y, Tsujino T, Mizuno S. A novel, fully covered laser-cut nitinol stent with antimigration properties for nonresectable distal malignant biliary obstruction: a multicenter feasibility study. Gut Liver. 2013;7:725-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Kitano M, Yamashita Y, Tanaka K, Konishi H, Yazumi S, Nakai Y, Nishiyama O, Uehara H, Mitoro A, Sanuki T. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol. 2013;108:1713-1722. [PubMed] |
| 22. | Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321-327.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Nakai Y, Isayama H, Kogure H, Hamada T, Togawa O, Ito Y, Matsubara S, Arizumi T, Yagioka H, Mizuno S. Risk factors for covered metallic stent migration in patients with distal malignant biliary obstruction due to pancreatic cancer. J Gastroenterol Hepatol. 2014;29:1744-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hu B S- Editor: Gong ZM L- Editor: A E- Editor: Ma S