Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6851
Peer-review started: March 23, 2016
First decision: May 27, 2016
Revised: June 1, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: August 14, 2016
Processing time: 134 Days and 10.3 Hours
Although the current standard treatment for hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) is sorafenib, many previous studies have established the need for a reliable local modality for PVTT control, which is a major cause of liver function deterioration and metastasis. Additionally, there is growing evidence for the prognostic significance of PVTT classification according to the location of tumor thrombosis. Favorable outcomes can be obtained by applying local modalities, including surgery or transarterial chemoembolization, especially in second-order or distal branch PVTT. Rapid control of PVTT could maintain or improve liver function and reduce intrahepatic as well as distant metastasis. Radiotherapy (RT) is one of the main locoregional treatment modalities in oncologic fields, but has rarely been used in HCC because of concerns regarding hepatic toxicity. However, with the development of advanced techniques, RT has been increasingly applied in HCC management. Randomized studies have yet to definitively prove the benefit of RT, but several comparative studies have justified the application of RT in HCC. The value of RT is especially noticeable in HCC with PVTT; several prospective and retrospective studies have reported favorable outcomes, including a 40% to 60% objective response rate and median overall survival of 15 mo to 20 mo in responders. In this review, we evaluate the role of RT as an alternative local modality in HCC with PVTT.
Core tip: The optimal management of portal vein tumor thrombosis (PVTT), which can induce liver function deterioration and act as a source of metastasis, in patients with hepatocellular carcinoma (HCC) remains unclear. With growing evidence for the prognostic significance of PVTT classification and promising outcomes of local modalities in selected patients, the need for a reliable local modality of control is becoming increasingly apparent. In this review, the outcomes of radiotherapy as an alternative local modality for PVTT control in HCC are presented.
- Citation: Yu JI, Park HC. Radiotherapy as valid modality for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2016; 22(30): 6851-6863
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6851.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6851
Hepatocellular carcinoma (HCC) is the fifth most common cancer and second leading cause of cancer mortality worldwide[1,2]. Despite remarkable improvements in the management of HCC, including antiviral agents[3-5], adaptation of magnetic resonance imaging[6] and major advances in local treatment modalities[7-9], this disease remains a concerning health issue. The major issue in HCC management is that approximately 70% of newly diagnosed patients are not candidates for curative local treatment modalities[10]; in particular, portal vein tumor thrombosis (PVTT), which is a common complication of HCC, is frequently an obstacle to the application of such modalities[11].
PVTT is known to be present in approximately 30% to 40% of cases of advanced HCC[10-12]. The association between PVTT and dismal outcomes has been confirmed by many studies[13,14]. Because of the role of PVTT as the most powerful prognosticator in HCC, curative local modalities are not recommended for HCC with PVTT in the Barcelona Clinic Liver Cancer (BCLC) staging and treatment system[15,16]. Nevertheless, local treatment modalities including surgical resection, trans-arterial chemoembolization (TACE), and radiotherapy (RT) are commonly applied in HCC with PVTT to obtain early and durable local control[17,18].
RT is a key locoregional modality in oncology[19]. It is minimally invasive and, unlike surgery, is not associated with pain or a long recovery period. In cancer patients with a relatively high risk of recurrence at other sites, as in those with oligo-metastatic disease, RT is generally preferred for obtaining local control over invasive surgical resection in order to avoid missing the optimal timing for systemic management[20]. However, RT has played a very limited role in past management of HCC, and is usually administered for palliation of symptoms related to extrahepatic metastasis, rather than for control of the primary liver tumor[21]. The source of this limitation is the radiosensitive nature of the cirrhotic non-tumorous liver and related concern regarding the maintenance of clinical liver function, which is a main aim in HCC management[22].
With the development of improved RT techniques and radiobiology, RT has recently been increasingly applied in HCC management. Although there is no definitive evidence for the role of RT, superior outcomes obtained by including RT in HCC management have been reported in several meta-analyses as well as many prospective and retrospective studies[23-35]. Based on these favorable clinical outcomes of innovative RT techniques, liver-directed RT has been recommended for HCC patients who are not candidates for curative loco-regional modalities in the guidelines from the Korean Liver Cancer Study Group[36] and the National Comprehensive Cancer Network[37]. However, even though the use of RT in unresectable HCC is gradually increasing worldwide as a result of these recommendations[38], RT is still not recommended by the BCLC system, which is the most widely used set of HCC management guidelines[16].
In this review, we introduce the current status of RT in HCC and recently reported outcomes and discuss why the addition of RT is beneficial in HCC patients with PVTT.
PVTT is present at diagnosis in approximately 30% to 40% of patients with advanced HCC[11,12], and is one of the most important poor prognostic factors of HCC, with a reported median overall survival (OS) of 2.7 mo to 4.0 mo[12,39].
There are several explanations for how PVTT results in dismal clinical outcomes. PVTT itself disturbs the portal blood supply in the normal liver and can therefore cause deterioration of liver function crucial to HCC management[40]. Furthermore, local tumor progression can accelerate the deterioration of liver function. In addition, PVTT might act as a distributor of intrahepatic and extrahepatic metastasis[41,42]. These possible unfavorable roles of PVTT were observed in our previous study evaluating the relationship between PVTT response and clinical outcomes in HCC patients with PVTT who were treated with TACE followed by RT[43]. Elevation of Child-Pugh scores representing liver function deterioration was significantly more frequent in the non-responders than the responders of treatment. Moreover, the rates of intrahepatic and extrahepatic metastasis were significantly lower in responders.
Because of its well-known role as the most powerful unfavorable prognosticator in HCC, the BCLC system does not recommend curative local modalities for patients with HCC with PVTT[16]. Based on its survival benefit in two large prospective randomized phase III trials[44,45], sorafenib, an oral multikinase inhibitor of platelet-derived growth factor receptor, vascular endothelial growth factor receptor and Raf, is the only standard treatment in these patients. However, physicians hesitate to use sorafenib in advanced but localized disease because of the disappointing objective response rate of 2% to 5% and extremely short median time to progression of 2.8 mo found in a study conducted in the Asia-Pacific region[46]. A more reliable local modality that can rapidly control vascular invasion to the portal vein and thus maintain liver function while eliminating the source of metastasis is needed to improve clinical outcomes.
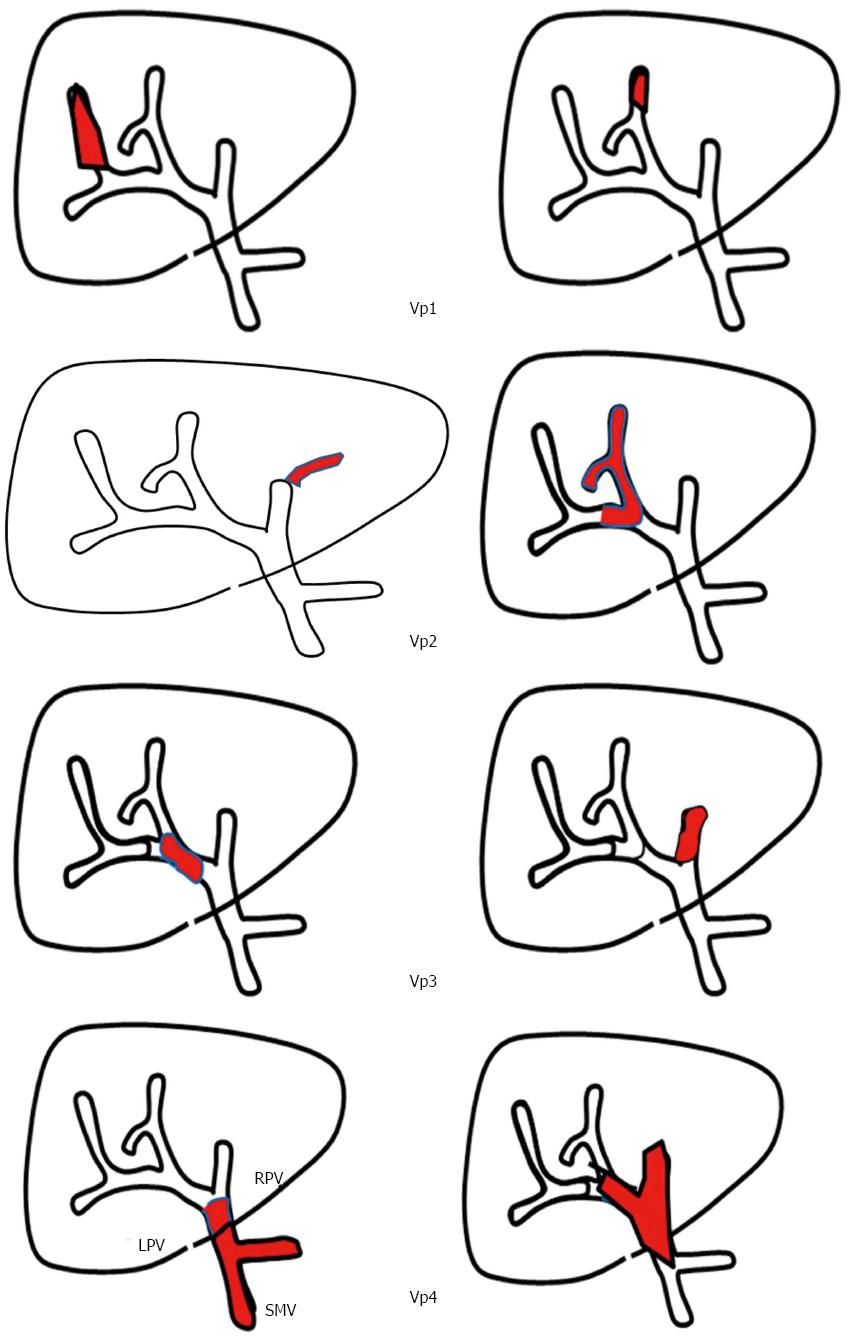
Furthermore, there is a wide spectrum of vascular invasion clinical manifestations of PVTT, and prognoses are clearly separated according to the extent of PVTT[47-50]. PVTT can be classified into four categories as suggested by the Liver Cancer Study Group of Japan[51] (Figure 1): the presence of tumor thrombus distal to the second-order branches of the portal vein is defined as Vp1, in the second-order branches of the portal vein as Vp2, in the first-order branches of the portal vein as Vp3, and in the main trunk or both first-order branches of the portal vein as Vp4. Although definitive evidence is lacking, customized treatments for HCC patients with PVTT according to PVTT classification combined with other recognized factors, such as liver function, performance status, tumor size and number, should be explored.
The importance of local treatment modalities in the management of HCC cannot be overstated. Immediate methods of tumor elimination, such as surgical resection including liver transplantation (LT) and radiofrequency ablation (RFA), can prevent reductions in liver function by preventing tumor encroachment into the surrounding liver and removing the source of intra and/or extrahepatic metastasis[52-55]. Long-term survival is achieved in approximately 60% to 70% of HCC patients treated with curative local modalities, but recurrence eventually occurs in more than half of patients, with the exception of those who undergo LT. However, the majority of patients are not candidates for curative local modalities, mainly due to tumor burden, and PVTT is a major obstacle to the application of curative local modalities[10-12,39].
Notable survival rates of 20% to 40% 5 years after surgical resection were reported by the Liver Cancer Study Group of Japan, mainly in HCC patients with minor class Vp1 or Vp2PVTT[56,57]. Although selection bias is inherent in those studies, the results indicate that a proportion of patients might benefit from early elimination of the tumor and PVTT. Furthermore, some cases of long-term survival were reported after surgical resection of HCC with Vp3 or Vp4 PVTT[58,59].
As an alternative local modality with proven efficacy, TACE is widely used in cases of large multinodular HCC[60-62]. However, a complete response is rarely obtained and the duration of local control is unsatisfactory, especially for large tumors[59]. Furthermore, because of the rationale for TACE, which is selective tumor embolization through the arterial blood supply of HCC, in contrast to the dual supply of the surrounding normal liver, TACE had previously been contraindicated in HCC with PVTT. Recent studies, however, have supported that TACE can be safely administered in these patients because of collateral circulation[63,64]. In clinical practice, the super-selective method of TACE, with or without another modality, is one of the most frequently used treatments in HCC patients with PVTT[48,65].
Surgical outcomes are generally reported to be superior to TACE in HCC with PVTT. Liu et al[66] reported superior outcomes of surgical resection compared to TACE in 108 pairs of matched patients selected using propensity score analysis. It is very hard to directly compare clinical outcomes between surgical resection and TACE because of the clear differences in patient and tumor characteristics, including liver function, performance status, and the extent of disease. Nevertheless, local control could enhance clinical outcomes in HCC with PVTT.
Historically, RT was not used in HCC management, especially for primary lesion of the liver. The main reason for the avoidance of RT in HCC was the low tolerance of liver tissue to RT exposure and concerns regarding radiation-induced liver disease (RILD), which typically presents as anicteric hepatomegaly and ascites 2-3 mo after RT[22]. The pathology of radiation-induced liver damage shows changes similar to veno-occlusive disease, including endothelial swelling, terminal venule occlusion and sinusoidal congestion[67]. RILD is a major concern in liver RT, because there is no established treatment and some patients will die of liver failure, although a few patients may recover[68]. Recently, the incidence of classic RILD has decreased, but non-classic RILD (which appears with jaundice and marked elevation of serum transaminase) is still problematic in the application of RT in HCC[69].
Because the treatment options for RILD are very limited, most investigations have focused on identifying predictive factors of RILD development[69-74]. Factors related to RT planning, such as RT dose and liver volume, have been of particular interest. Mean liver dose was demonstrated by Dawson et al[71] to be a risk factor of RILD, and V20 Gy was suggested by Liang et al[74] to be a dosimetric predictor. Kim et al[72] reported that grade 2 or higher radiation-induced hepatic toxicity was significantly related to the percentage of total normal liver volume exceeding 30 Gy irradiation. Our group reported that V30 Gy was a significant risk factor of Child-Pugh score elevation of two points or greater[69]. There is also a possibility of a higher incidence of liver function deterioration after RT in HCC combined PVTT than in the other, but the dosimetric factors of liver are more important, generally[69,72].
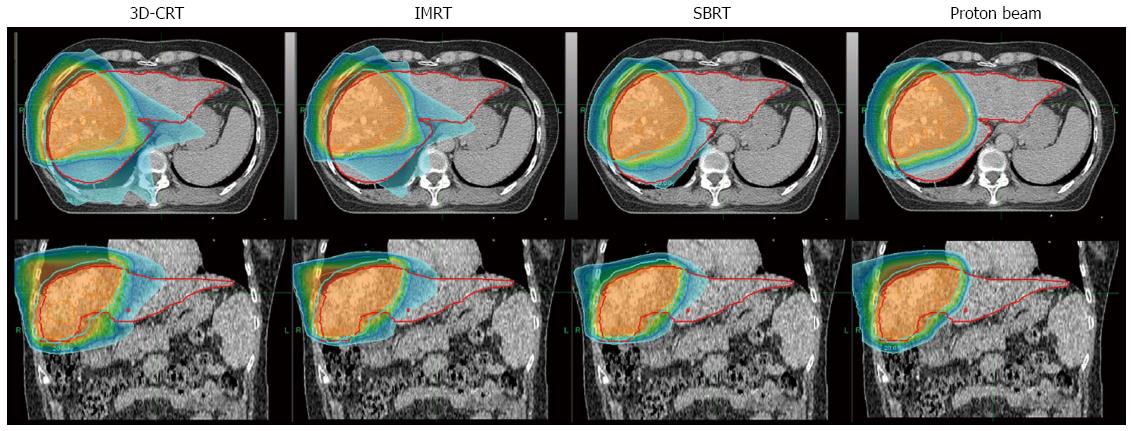
With better information regarding RILD development and the application of computed tomography (CT) to RT planning, higher RT doses for local tumor control can be administered with an acceptable RILD risk level[73,75-77]. Further innovative RT technologies, including three-dimensional conformal RT (3D-CRT)[78], intensity-modulated RT (IMRT)[79], stereotactic body ablative RT (SABR)[80] and particle beam therapies[81], have been introduced and actively applied in HCC management (Figure 2). Additionally, image-guided RT[82], which is used as a supportive/supplementary method with other precision RT techniques, is also standard RT practice. These biological and technical developments have enabled delivery of higher-dose RT with improved precision and better conformation and without an increased probability of normal tissue complications. With these rapid developments in techniques and radiobiology, RT has become an accepted tool in the management of HCC[77].
Although evidence from well-designed randomized trials on the role of RT is still needed, several comparative studies have justified the application of RT in HCC management. The results of three systematic meta-analyses of randomized and non-randomized trials are displayed in Table 1[24,25,30]. In all three reports, the addition of RT to TACE showed a significant survival benefit over TACE alone, with an OR of 1.91 to 2.75 for 3-year OS rates. The complete response (CR) rate was also significantly higher in the TACE plus RT group with an OR of 2.58 to 2.73, and a similar effect was observed in the PVTT subgroup (OR = 2.38)[30].
Several comparative reports presented the efficacy of RT compared to other treatment modalities in HCC, and a summary of these studies is displayed in Table 2[23,26-28,31,33-35,83]. In the retrospective cohort study of Eun et al[83], RT showed a clear survival benefit in advanced HCC over best supportive care (median OS 45.9 mo vs 4.8 mo, P < 0.001). Yoon et al[35] compared concurrent chemoradiotherapy (CCRT) and other treatment modalities, including surgery, RFA and TACE, in locally advanced HCC using a propensity score matching technique and showed that CCRT yielded significantly longer-term OS than other modalities (median OS 11.4 mo vs 6.6 mo, P = 0.02).
| Ref. | Subject | Study aim | n | 1 yr PFS | OS | P value |
| Eun et al[83] | BCLC C/D | RT vs BSC | 29 vs 18 | - | 45.9 vs 4.8 | < 0.001 |
| Yoon et al[35] | Locally advanced | CCRT vs others | 106 vs 106 | - | 11.4 vs 6.6 | 0.02 |
| Cho et al[27] | BCLC C | TACE + RT vs sorafenib | 67 vs 49 | - | 14.1 vs 3.3 | < 0.001 |
| Nakazawa et al[28] | HCC with PVTT | RT vs sorafenib | 28 vs 28 | - | 10.9 vs 4.8 | 0.002 |
| Li et al[33] | HCC with PVTT | TACE + RT vs TACE | 108 vs 108 | - | 10.9 vs 4.1 | < 0.001 |
| Guo et al[23] | Large HCC | TACE + RT vs TACE | 89 vs 76 | 1-yr (%) 64.0 vs 39.9 | < 0.001 | |
| Tang et al[26] | HCC with PVTT | RT vs surgery | 185 vs 186 | 32.3% vs 42.2% | 1-yr (%) 51.6 vs 40.1 | 0.03 |
| Shiozawa et al[31] | ≤ 5-cm solitary HCC | SABR vs RFA | 35 vs 38 | 86.1% vs 85.6% | 1-yr (%) 95.2 vs 100 | 0.08 |
| Wahl et al[34] | Inoperable localized | SABR vs RFA | 63 vs 161 | LC 97.4% vs 83.6% | 1-yr (%) 74.1 vs 69.6 | NS |
Two studies compared RT with or without TACE to sorafenib as the current standard treatment in patients with BCLC stage C disease including PVTT, and both showed a positive effect of RT on survival[27,28]. Moreover, TACE with RT also led to a significant survival advantage in HCC with PVTT in a comparative study using propensity score matching[33]. Cho et al[27] reported that OS was significantly higher in the RT group in a propensity score-matched cohort (median OS 8.9 mo vs 3.1 mo, P < 0.001), as well as in all cohorts (median OS 14.1 mo vs 3.3 mo, P < 0.001). Tang et al[26] also compared surgical resection to RT in 371 patients with resectable HCC with PVTT and reported a median 2.3-mo survival advantage with RT when compared to resection (P = 0.03), with comparable progression-free survival.
The popularity of SABR, also known as stereotactic body RT, has recently increased in RT for HCC[29,31,32,34,84]. Two studies compared SABR and RFA, which is one of standard local modalities, and both showed that local control with SABR was comparable or even higher for larger tumors (≥ 2 cm) with similar OS in early HCC[31,34]. Although differences in characteristics originating from the retrospective design were compensated for in these studies, the results should be interpreted with caution.
The basis of modern RT is 3D-CRT, which involves conformal radiation delivery based on 3-D anatomical information and dose distribution using CT simulation[85]. More innovative RT techniques, which will be introduced in the following sections, are based on 3D-CRT planning. IMRT, for example, makes it possible to deliver an even more conformal RT dose using non-uniform inverse-planned intensity-modulated beams[79,86]. Several techniques, such as tomotherapy and arc-therapy, are classified as IMRT. Theoretically, a dose increased from that used with 3D-CRT could be safely delivered in HCC using these approaches.
There are numerous reports regarding 3D-CRT with or without TACE for locally advanced HCC with Vp3-4 PVTT, and most of these studies yielded a favorable objective response rate of 40% to 60% and promising OS of 8 mo to 11 mo[87-94]. Representative studies of RT with or without TACE are summarized in Table 3. Although there are some discrepancies in clinical outcomes that are probably related to differences in patient and tumor characteristics, a greater than 40% objective response rate was obtained in all studies. In addition, excellent median survival outcomes of more than 12 mo are also reported in treatment responders.
| Ref. | Year | Design | Treatment | No. | OR | Median survival, mo | P value | |
| OR (+) | OR (-) | |||||||
| Tazawa et al[90] | 2001 | Retrospective | TACE + RT | 24 | 50.0% | 9.7 | 3.8 | < 0.001 |
| Yamada et al[91] | 2003 | Prospective | TACE + RT | 19 | 57.9% | 15.4 | 4.6 | 0.16 |
| Kim et al[87] | 2005 | Retrospective | RT | 59 | 45.8% | 10.7 | 5.3 | 0.05 |
| Nakazawa et al[89] | 2007 | Retrospective | RT | 32 | 48.0% | 13.8 | 7.0 | 0.01 |
| Zeng et al[94] | 2008 | Retrospective | RT | 136 | 57.6% | 19.5 (CR) | 7.2 (SD) | < 0.001 |
| 10.2 (PR) | 3.5 (PD) | |||||||
| Yu et al[93] | 2011 | Retrospective | RT ± TACE | 281 | 53.8% | 22.0 | 5.0 | < 0.001 |
| Yoon et al[92] | 2012 | Retrospective | TACE + RT | 412 | 39.6% | 19.4 | 7.0 | < 0.001 |
| Kim et al[88] | 2014 | Retrospective | TACE + RT | 59 | 51.0% | NR | 7.0 | < 0.001 |
Although there is no clear definition of SABR, it is generally defined as extremely conformal and accurate delivery of a larger single fraction size with relatively little fractionation, such as one to five fractions[95,96]. Recently, SABR has been one of the most enthusiastically applied and best-studied areas of RT application, especially in the treatment of HCC[31,34,80,84,97-100]. The number of prospective SABR study protocols for subjects with HCC with or without PVTT registered on Clinicaltrials.gov has dramatically increased since 2008.
Kang et al[101] reported an objective response of greater than 85% with median OS of 12 mo to 15 mo in a retrospective study of SABR with or without TACE involving 101 patients with HCC with PVTT. Prospective phase I and II trials of more than 100 HCC patients with tumor thrombosis showed a 1-year local control rate of 87% (95%CI: 79%-93%) after SABR and a 2-year local control rate of more than 50%, with median OS of 17.0 mo (95%CI: 10.4-21.3 mo)[84]. Based on the favorable results of these large prospective trials, SABR is now recommended for unresectable HCC in the National Comprehensive Cancer Network guidelines[37] and a randomized phase III prospective trial evaluating the addition of SABR to the current standard treatment of sorafenib in locally advanced HCC including PVTT is ongoing[102].
Based mainly on the superior radiation dose distribution, the application of particle beam RT, mainly consisting of proton or carbon ions, is increasing[103-109]. In addition, particle beams show a relative insensitivity to hypoxia, which is the main reason for the resistance of cancer cells to other RT techniques, and these theoretically enhanced biological effects of particle beam RT have been confirmed in some studies[106]. Use of particle beam therapy in HCC has attracted a great deal of attention because of its exceptional ability to spare normal organs including the surrounding liver. Favorable outcomes of particle beam therapy in HCC have been reported by several groups, and a meta-analysis demonstrated that particle beam therapy enhanced survival outcomes as well as reducing toxicity when compared with 3D-CRT and SABR[110,111].
Sugahara et al[112] reported a median OS of 22 months with 91% local control and without grade III or higher late toxicity after proton beam RT in 35 HCC patients with PVTT. Lee et al[113] reported a median OS of 13.2 mo with a 55.6% objective response rate without severe toxicity in HCC with PVTT.
Although the application of carbon ion specifically to HCC with PVTT has not yet been documented, several reports reported promising outcomes of 90% to 100% local control and 22% to 35% 5-year OS in HCC patients including combined with PVTT. These results were similar to those of proton beam RT. Komatsu et al[114] reported that carbon ion and proton therapies for HCC show comparable local control (93%, carbon ion; 90.2%, proton) and 5-year survival rates (36.3%, carbon ion; 38%, proton).
As mentioned above, the wide variety of clinical outcomes of PVTT reflects not only the extent of PVTT, but also other factors[13,14,39,47-50]. Previously, our group reported a prognostic model in HCC with PVTT treated with RT that clearly stratified patient survival outcomes based on other validated prognostic models of HCC[93]. In addition, our prognostic model was validated by another independent data set from a multicenter cohort[115].
A comparative study evaluating surgical resection vs conformal RT combined with TACE for resectable HCC with PVTT including more than 50% Vp3 or Vp4 cases showed a significant survival advantage in the TACE and RT group vs the surgical group (median 12.3 mo vs 10.0 mo, P = 0.03)[26]. In our unpublished analysis comparing surgical resection vs TACE followed by RT in HCC with Vp1/2 PVTT, the OS of patients that underwent surgical resection was slightly higher in cases with small tumor size (< 3 cm), solitary tumor and low α-fetoprotein level (< 200 ng/mL), although statistical significance was not reached.
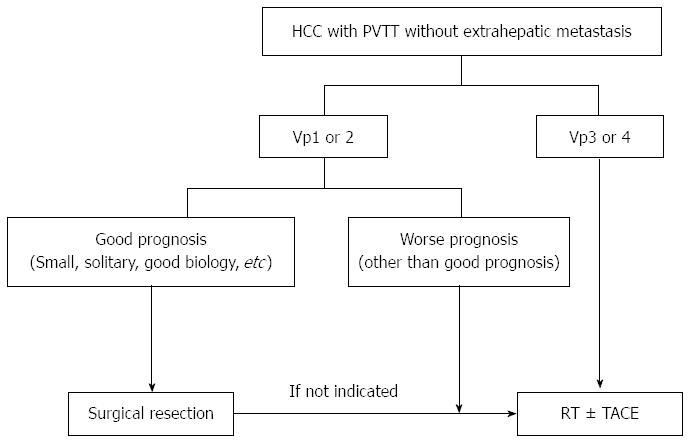
The detrimental role of PVTT as an obstacle to liver function maintenance and a source of metastasis indicates that valid local modalities based on PVTT extent, clinical liver function and tumor extent are urgently needed to improve clinical outcomes for HCC. Suggested local modalities for control of HCC with PVTT according to PVTT classification and/or disease extent are displayed in Figure 3, although prospective studies are still needed on the role of these modalities in the treatment of HCC. Large, prospective, randomized, controlled studies evaluating local treatment modalities and survival outcomes compared to the current standard treatment of sorafenib should be performed.
Although RT has shown promising results including favorable treatment response and survival rates in HCC with PVTT, treatment decisions regarding RT should be made cautiously because of several remaining limitations[116].
Liver function is one of the most important factors determining the method and purpose of HCC management[16], and is a key concern when considering RT. In patients with a Child-Pugh score ≥ 8, liver-directed RT is not generally recommended. Thus, there have been few studies in which RT was applied in those patients[36,37]. Culleton et al[117] reported a prospective study of SABR outcomes in patients with Child-Pugh class B or C HCC, and significantly lower survival was detected in patients with a Child-Pugh score ≥ 8 (9.9 mo in those with scores of 7 vs 2.8 mo with scores of 8 or higher, P = 0.01). In another prospective phase I study of SABR for HCC, 3 of 11 patients with Child-Pugh class B disease developed grade III hepatic toxicity, while none of the 17 patients with Child-Pugh class A disease did[98]. A significantly higher incidence of grade II or higher liver toxicity in patients with Child-Pugh class B disease was reaffirmed by a large retrospective SABR study (36.0% vs 11.9% of Child-Pugh class A patients)[118].
Another important obstacle to RT application in HCC is the radiation susceptibility of the bowel, including the stomach and duodenum. The positive correlation between the incidence of symptomatic bowel toxicity and RT dose/bowel volume has been confirmed in several studies[119-121]. Our group also reported that liver function deterioration is related to a higher incidence of symptomatic bowel toxicity[122].
Before applying RT in HCC, these unresolved obstacles need to be considered. Although promising outcomes were achieved in HCC patients with poor liver function (Child-Pugh class C) or adjacent to the bowel in small studies using particle beam RT[123-125], in these high-risk patients the use of RT should be carefully restricted or limited to prospective clinical trials.
While acceptable local control using RT in HCC has been reported, especially with SABR, frequent intrahepatic recurrence remains an unresolved issue[88,126-128]. Several studies have suggested that RT may induce intrahepatic metastasis via viral reactivation[129,130] and/or expression of vascular endothelial growth factor[131]. With the development and optimal application of antiviral agents and targeted agents like sorafenib, intrahepatic recurrence might be minimized after RT[3-5,131,132]. Further studies on combination treatment with RT are needed.
Considering the role of PVTT in liver function deterioration and metastasis, a reliable local modality for early control of PVTT is urgently needed. Because there is considerable variation in patient prognosis, customized application of local modalities including surgical resection and TACE should be based on the extent of the main mass and/or PVTT. With recent technological advances, RT could be an effective local modality for HCC control, especially for patients with HCC combined with PVTT.
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4300] [Article Influence: 226.3] [Reference Citation Analysis (2)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 13047] [Article Influence: 1304.7] [Reference Citation Analysis (3)] |
| 3. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 788] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 4. | Sun P, Yang X, He RQ, Hu QG, Song ZF, Xiong J, Zheng QC. Antiviral therapy after curative treatment of hepatitis B/C virus-related hepatocellular carcinoma: A systematic review of randomized trials. Hepatol Res. 2014;44:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, Guo W, Zhang H, Wang H, Cheng S. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31:3647-3655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 6. | Willatt JM, Hussain HK, Adusumilli S, Marrero JA. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247:311-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Ishikawa T, Abe S, Hoshii A, Yamada Y, Iiduka A, Nemoto T, Takeda K, Yoshida T. Cone-Beam Computed Tomography Correlates with Conventional Helical Computed Tomography in Evaluation of Lipiodol Accumulation in HCC after Chemoembolization. PLoS One. 2016;11:e0145546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Toshikuni N, Takuma Y, Tomokuni J, Yamamoto H. Planning Sonography Using Real-time Virtual Sonography and Contrast-enhanced Sonography for Radiofrequency Ablation of Inconspicuous Hepatocellular Carcinoma Nodules. Hepatogastroenterology. 2015;62:661-666. [PubMed] |
| 9. | Zou Q, Li J, Wu D, Yan Z, Wan X, Wang K, Shi L, Lau WY, Wu M, Shen F. Nomograms for Pre-operative and Post-operative Prediction of Long-Term Survival of Patients Who Underwent Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Ann Surg Oncol. 2016;23:2618-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Sinn DH, Gwak GY, Cho J, Paik SW, Yoo BC. Comparison of clinical manifestations and outcomes between hepatitis B virus- and hepatitis C virus-related hepatocellular carcinoma: analysis of a nationwide cohort. PLoS One. 2014;9:e112184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Cheung TK, Lai CL, Wong BC, Fung J, Yuen MF. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther. 2006;24:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 921] [Article Influence: 34.1] [Reference Citation Analysis (2)] |
| 13. | Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi H, Yamamoto Y, Ichikawa S, Hasebe A, Yano M, Miyamoto Y. Risk factors for death in 224 cases of hepatocellular carcinoma after transcatheter arterial chemoembolization. Hepatogastroenterology. 2009;56:213-217. [PubMed] |
| 14. | Zhou L, Rui JA, Wang SB, Chen SG, Qu Q, Chi TY, Wei X, Han K, Zhang N, Zhao HT. Outcomes and prognostic factors of cirrhotic patients with hepatocellular carcinoma after radical major hepatectomy. World J Surg. 2007;31:1782-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4520] [Article Influence: 215.2] [Reference Citation Analysis (4)] |
| 16. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3630] [Article Influence: 259.3] [Reference Citation Analysis (12)] |
| 17. | Kalogeridi MA, Zygogianni A, Kyrgias G, Kouvaris J, Chatziioannou S, Kelekis N, Kouloulias V. Role of radiotherapy in the management of hepatocellular carcinoma: A systematic review. World J Hepatol. 2015;7:101-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (2)] |
| 18. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 553] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 19. | Yamoah K, Showalter TN, Ohri N. Radiation Therapy Intensification for Solid Tumors: A Systematic Review of Randomized Trials. Int J Radiat Oncol Biol Phys. 2015;93:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Yoo GS, Yu JI, Park W, Huh SJ, Choi DH. Prognostic factors in breast cancer with extracranial oligometastases and the appropriate role of radiation therapy. Radiat Oncol J. 2015;33:301-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Lee E, Kim TG, Park HC, Yu JI, Lim do H, Nam H, Lee H, Lee JH. Clinical outcomes of stereotactic body radiotherapy for spinal metastases from hepatocellular carcinoma. Radiat Oncol J. 2015;33:217-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation Hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200-208. [PubMed] |
| 23. | Guo WJ, Yu EX, Liu LM, Li J, Chen Z, Lin JH, Meng ZQ, Feng Y. Comparison between chemoembolization combined with radiotherapy and chemoembolization alone for large hepatocellular carcinoma. World J Gastroenterol. 2003;9:1697-1701. [PubMed] |
| 24. | Meng MB, Cui YL, Lu Y, She B, Chen Y, Guan YS, Zhang RM. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2009;92:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Liao M, Huang J, Zhang T, Wu H. Transarterial chemoembolization in combination with local therapies for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e68453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Tang QH, Li AJ, Yang GM, Lai EC, Zhou WP, Jiang ZH, Lau WY, Wu MC. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study. World J Surg. 2013;37:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 27. | Cho JY, Paik YH, Park HC, Yu JI, Sohn W, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW. The feasibility of combined transcatheter arterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma. Liver Int. 2014;34:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Nakazawa T, Hidaka H, Shibuya A, Okuwaki Y, Tanaka Y, Takada J, Minamino T, Watanabe M, Kokubu S, Koizumi W. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Huertas A, Baumann AS, Saunier-Kubs F, Salleron J, Oldrini G, Croisé-Laurent V, Barraud H, Ayav A, Bronowicki JP, Peiffert D. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol. 2015;115:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Huo YR, Eslick GD. Transcatheter Arterial Chemoembolization Plus Radiotherapy Compared With Chemoembolization Alone for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2015;1:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 31. | Shiozawa K, Watanabe M, Ikehara T, Matsukiyo Y, Kogame M, Kishimoto Y, Okubo Y, Makino H, Tsukamoto N, Igarashi Y. Comparison of percutaneous radiofrequency ablation and CyberKnife(®) for initial solitary hepatocellular carcinoma: A pilot study. World J Gastroenterol. 2015;21:13490-13499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Bae SH, Kim MS, Jang WI, Kay CS, Kim W, Kim ES, Kim JH, Kim JH, Yang KM, Lee KC. Practical patterns for stereotactic body radiotherapy to hepatocellular carcinoma in Korea: a survey of the Korean Stereotactic Radiosurgery Group. Jpn J Clin Oncol. 2016;46:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Li XL, Guo WX, Hong XD, Yang L, Wang K, Shi J, Li N, Wu MC, Cheng SQ. Efficacy of the treatment of transarterial chemoembolization combined with radiotherapy for hepatocellular carcinoma with portal vein tumor thrombus: A propensity score analysis. Hepatol Res. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol. 2016;34:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 35. | Yoon HI, Song KJ, Lee IJ, Kim do Y, Han KH, Seong J. Clinical Benefit of Hepatic Arterial Infusion Concurrent Chemoradiotherapy in Locally Advanced Hepatocellular Carcinoma: A Propensity Score Matching Analysis. Cancer Res Treat. 2016;48:190-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC). 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015;9:267-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 37. | National Comprehensive Cancer Network. NCCN guidelines in Oncology. 2014; Available from: https://www.sogou.com/link?url=hedJjaC291MhLETz0L7Ycj5knvQG5V7O&query=National Comprehensive Cancer Network. |
| 38. | Kondo Y, Kimura O, Shimosegawa T. Radiation therapy has been shown to be adaptable for various stages of hepatocellular carcinoma. World J Gastroenterol. 2015;21:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Schöniger-Hekele M, Müller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut. 2001;48:103-109. [PubMed] |
| 40. | Fimognari FL, Violi F. Portal vein thrombosis in liver cirrhosis. Intern Emerg Med. 2008;3:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Addario L, Tritto G, Cavaglià E, Amodio F, Giannelli E, Di Costanzo GG. Preserved liver function, portal thrombosis and absence of oesophageal varices are risk factors for metastasis of hepatocellular carcinoma. Dig Liver Dis. 2011;43:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Toyosaka A, Okamoto E, Mitsunobu M, Oriyama T, Nakao N, Miura K. Pathologic and radiographic studies of intrahepatic metastasis in hepatocellular carcinoma; the role of efferent vessels. HPB Surg. 1996;10:97-103; discussion 103-104. [PubMed] |
| 43. | Yu JI, Park JW, Park HC, Yoon SM, Lim do H, Lee JH, Lee HC, Kim SW, Kim JH. Clinical impact of combined transarterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: An external validation study. Radiother Oncol. 2016;118:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4742] [Article Influence: 263.4] [Reference Citation Analysis (0)] |
| 45. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10537] [Article Influence: 585.4] [Reference Citation Analysis (9)] |
| 46. | Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 670] [Article Influence: 47.9] [Reference Citation Analysis (1)] |
| 47. | Seol SW, Yu JI, Park HC, Lim do H, Oh D, Noh JM, Cho WK, Paik SW. Treatment outcome of hepatic re-irradiation in patients with hepatocellular carcinoma. Radiat Oncol J. 2015;33:276-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Sinn DH, Cho JY, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Different survival of Barcelona clinic liver cancer stage C hepatocellular carcinoma patients by the extent of portal vein invasion and the type of extrahepatic spread. PLoS One. 2015;10:e0124434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Ye JZ, Zhang YQ, Ye HH, Bai T, Ma L, Xiang BD, Li LQ. Appropriate treatment strategies improve survival of hepatocellular carcinoma patients with portal vein tumor thrombus. World J Gastroenterol. 2014;20:17141-17147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, Cai M, Shan H. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology. 2014;272:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 51. | The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989;19:98-129. [PubMed] |
| 52. | Hsu CY, Huang YH, Chiou YY, Su CW, Lin HC, Lee RC, Chiang JH, Huo TI, Lee FY, Lee SD. Comparison of radiofrequency ablation and transarterial chemoembolization for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Liver Transpl. 2011;17:556-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5397] [Article Influence: 179.9] [Reference Citation Analysis (7)] |
| 54. | Yuan BH, Yuan WP, Li RH, Xiang BD, Gong WF, Li LQ, Zhong JH. Propensity score-based comparison of hepatic resection and transarterial chemoembolization for patients with advanced hepatocellular carcinoma. Tumour Biol. 2016;37:2435-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Zhu SL, Zhong JH, Ke Y, Ma L, You XM, Li LQ. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J Gastroenterol. 2015;21:9630-9637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 326] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 57. | Ikai I, Itai Y, Okita K, Omata M, Kojiro M, Kobayashi K, Nakanuma Y, Futagawa S, Makuuchi M, Yamaoka Y. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21-29. [PubMed] |
| 58. | Ban D, Shimada K, Yamamoto Y, Nara S, Esaki M, Sakamoto Y, Kosuge T. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg. 2009;13:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 60. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2303] [Article Influence: 100.1] [Reference Citation Analysis (1)] |
| 61. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2655] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 62. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2009] [Article Influence: 83.7] [Reference Citation Analysis (2)] |
| 63. | Kim JH, Yoon HK, Kim SY, Kim KM, Ko GY, Gwon DI, Sung KB. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. 2007;18:1517-1526; quiz 1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, Ryoo BY, Kang YK, Lee D, Kim KM. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320-329.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Liu PH, Lee YH, Hsia CY, Hsu CY, Huang YH, Chiou YY, Lin HC, Huo TI. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014;21:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | da Silveira EB, Jeffers L, Schiff ER. Diagnostic laparoscopy in radiation-induced liver disease. Gastrointest Endosc. 2002;55:432-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Benson R, Madan R, Kilambi R, Chander S. Radiation induced liver disease: A clinical update. J Egypt Natl Canc Inst. 2016;28:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 69. | Yu JI, Park HC, Lim do H, Park WY. Predictive factors for Child-Pugh score elevation in hepatocellular carcinoma patients treated with conformal radiation therapy: dose-volume histogram analysis. Tumori. 2013;99:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 70. | Cheng JC, Wu JK, Huang CM, Liu HS, Huang DY, Cheng SH, Tsai SY, Jian JJ, Lin YM, Cheng TI. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156-162. [PubMed] |
| 71. | Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810-821. [PubMed] |
| 72. | Kim TH, Kim DY, Park JW, Kim SH, Choi JI, Kim HB, Lee WJ, Park SJ, Hong EK, Kim CM. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 73. | Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 494] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 74. | Liang SX, Huang XB, Zhu XD, Zhang WD, Cai L, Huang HZ, Li YF, Chen L, Liu MZ. Dosimetric predictor identification for radiation-induced liver disease after hypofractionated conformal radiotherapy for primary liver carcinoma patients with Child-Pugh Grade A cirrhosis. Radiother Oncol. 2011;98:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Dawson LA. Overview: Where does radiation therapy fit in the spectrum of liver cancer local-regional therapies? Semin Radiat Oncol. 2011;21:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Lee IJ, Seong J. Radiotherapeutic strategies in the management of hepatocellular carcinoma. Oncology. 2011;81 Suppl 1:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Med J. 2009;50:601-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150-155. [PubMed] |
| 79. | Song JH, Son SH, Kay CS, Jang HS. Reducing the probability of radiation-induced hepatic toxicity by changing the treatment modality from helical tomotherapy to fixed-beam intensity-modulated radiotherapy. Oncotarget. 2015;6:33952-33960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Paik EK, Kim MS, Choi CW, Jang WI, Lee SH, Choi SH, Kim KB, Lee DH. Dosimetric comparison of volumetric modulated arc therapy with robotic stereotactic radiation therapy in hepatocellular carcinoma. Radiat Oncol J. 2015;33:233-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Chung K, Han Y, Kim J, Ahn SH, Ju SG, Jung SH, Chung Y, Cho S, Jo K, Shin EH. The first private-hospital based proton therapy center in Korea; status of the Proton Therapy Center at Samsung Medical Center. Radiat Oncol J. 2015;33:337-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Dawson LA, Jaffray DA. Advances in image-guided radiation therapy. J Clin Oncol. 2007;25:938-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 83. | Eun HS, Kim MJ, Kim HJ, Ko KH, Moon HS, Lee ES, Kim SH, Lee HY, Lee BS. The retrospective cohort study for survival rate in patients with advanced hepatocellular carcinoma receiving radiotherapy or palliative care. Korean J Hepatol. 2011;17:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 611] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 85. | Zou LQ, Zhang BL, Chang Q, Zhu FP, Li YY, Wei YQ, Guan YS. 3D conformal radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2014;20:17227-17234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 86. | Yoon HI, Lee IJ, Han KH, Seong J. Improved oncologic outcomes with image-guided intensity-modulated radiation therapy using helical tomotherapy in locally advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014;140:1595-1605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 87. | Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 88. | Kim SW, Oh D, Park HC, Lim do H, Shin SW, Cho SK, Gwak GY, Choi MS, Paik YH, Paik SW. Transcatheter arterial chemoembolization and radiation therapy for treatment-naïve patients with locally advanced hepatocellular carcinoma. Radiat Oncol J. 2014;32:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Nakazawa T, Adachi S, Kitano M, Isobe Y, Kokubu S, Hidaka H, Ono K, Okuwaki Y, Watanabe M, Shibuya A. Potential prognostic benefits of radiotherapy as an initial treatment for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels. Oncology. 2007;73:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, Miyasaka Y, Nagayama K, Enomoto N, Sato C. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660-665. [PubMed] |
| 91. | Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, Matsumoto S, Soejima T, Sugimura K. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113-119. [PubMed] |
| 92. | Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 93. | Yu JI, Park HC, Lim DH, Park W, Yoo BC, Paik SW, Koh KC, Lee JH. Prognostic index for portal vein tumor thrombosis in patients with hepatocellular carcinoma treated with radiation therapy. J Korean Med Sci. 2011;26:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 94. | Zeng ZC, Fan J, Tang ZY, Zhou J, Wang JH, Wang BL, Guo W. Prognostic factors for patients with hepatocellular carcinoma with macroscopic portal vein or inferior vena cava tumor thrombi receiving external-beam radiation therapy. Cancer Sci. 2008;99:2510-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 95. | Kim MS, Kim W, Park IH, Kim HJ, Lee E, Jung JH, Cho LC, Song CW. Radiobiological mechanisms of stereotactic body radiation therapy and stereotactic radiation surgery. Radiat Oncol J. 2015;33:265-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 96. | Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, Teh BS, McGarry RC, Cardenes HR, Timmerman RD. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 97. | Barry A, Knox JJ, Wei AC, Dawson LA. Can Stereotactic Body Radiotherapy Effectively Treat Hepatocellular Carcinoma? J Clin Oncol. 2016;34:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 99. | Lu XJ, Dong J, Ji LJ, Xiao LX, Ling CQ, Zhou J. Tolerability and efficacy of gamma knife radiosurgery on hepatocellular carcinoma with portal vein tumor thrombosis. Oncotarget. 2016;7:3614-3622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Yoon SM, Lim YS, Park MJ, Kim SY, Cho B, Shim JH, Kim KM, Lee HC, Chung YH, Lee YS. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One. 2013;8:e79854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 101. | Kang J, Nie Q, DU R, Zhang L, Zhang J, Li Q, Li J, Qi W. Stereotactic body radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Mol Clin Oncol. 2014;2:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 102. | Dawson LA. Randomized Phase III Study of Sorafenib versus Stereotactic Body Radiation Therapy followed by Sorafenib in Hepatocellular Carcinoma. : Radiation Therapy Oncology Group 2013; . |
| 103. | Abei M, Okumura T, Fukuda K, Hashimoto T, Araki M, Ishige K, Hyodo I, Kanemoto A, Numajiri H, Mizumoto M. A phase I study on combined therapy with proton-beam radiotherapy and in situ tumor vaccination for locally advanced recurrent hepatocellular carcinoma. Radiat Oncol. 2013;8:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127:S189-S193. [PubMed] |
| 105. | Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053-3059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 106. | Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 466] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 107. | Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, Shoda J, Thono E, Tsuboi K, Tokuuye K. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 108. | Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, Nagase M, Nihei K, Ogino T. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23:1839-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (6)] |
| 109. | Nakayama H, Sugahara S, Tokita M, Fukuda K, Mizumoto M, Abei M, Shoda J, Sakurai H, Tsuboi K, Tokuuye K. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115:5499-5506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 110. | Dionisi F, Ben-Josef E. The use of proton therapy in the treatment of gastrointestinal cancers: liver. Cancer J. 2014;20:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Qi WX, Fu S, Zhang Q, Guo XM. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2015;114:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 112. | Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tsuboi K. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 113. | Lee SU, Park JW, Kim TH, Kim YJ, Woo SM, Koh YH, Lee WJ, Park SJ, Kim DY, Kim CM. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014;190:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 114. | Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, Hori Y, Hishikawa Y, Ku Y, Murakami M. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011;117:4890-4904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 115. | Yu JI, Yoon SM, Park HC, Kim JH, Kim TH, Park JW, Seong J, Lee IJ, Jang HS, Kay CS. Multicenter validation study of a prognostic index for portal vein tumor thrombosis in hepatocellular carcinoma. Cancer Res Treat. 2014;46:348-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 116. | Yu JI, Park HC. Considerations for radiation therapy in hepatocellular carcinoma: the radiation oncologists’ perspective. Dig Dis. 2014;32:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, Ringash J, Dawson LA. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 118. | Jung J, Yoon SM, Kim SY, Cho B, Park JH, Kim SS, Song SY, Lee SW, Ahn SD, Choi EK. Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol. 2013;8:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 119. | Chon YE, Seong J, Kim BK, Cha J, Kim SU, Park JY, Ahn SH, Han KH, Chon CY, Shin SK. Gastroduodenal complications after concurrent chemoradiation therapy in patients with hepatocellular carcinoma: endoscopic findings and risk factors. Int J Radiat Oncol Biol Phys. 2011;81:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 120. | Kim H, Lim DH, Paik SW, Yoo BC, Koh KG, Lee JH, Choi MS, Park W, Park HC, Huh SJ. Predictive factors of gastroduodenal toxicity in cirrhotic patients after three-dimensional conformal radiotherapy for hepatocellular carcinoma. Radiother Oncol. 2009;93:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 121. | Yoon H, Oh D, Park HC, Kang SW, Han Y, Lim DH, Paik SW. Predictive factors for gastroduodenal toxicity based on endoscopy following radiotherapy in patients with hepatocellular carcinoma. Strahlenther Onkol. 2013;189:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Yu JI, Cho JY, Park HC, Lim do H, Gwak GY, Paik SW. Child-Pugh score maintenance in cirrhotic hepatocellular carcinoma patients after radiotherapy: aspects of gastroduodenal complications. Tumori. 2014;100:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 123. | Hata M, Tokuuye K, Sugahara S, Fukumitsu N, Hashimoto T, Ohnishi K, Nemoto K, Ohara K, Matsuzaki Y, Akine Y. Proton beam therapy for hepatocellular carcinoma patients with severe cirrhosis. Strahlenther Onkol. 2006;182:713-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 124. | Imada H, Kato H, Yasuda S, Yamada S, Yanagi T, Kishimoto R, Kandatsu S, Mizoe JE, Kamada T, Yokosuka O. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother Oncol. 2010;96:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 125. | Nakayama H, Sugahara S, Fukuda K, Abei M, Shoda J, Sakurai H, Tsuboi K, Matsuzaki Y, Tokuuye K. Proton beam therapy for hepatocellular carcinoma located adjacent to the alimentary tract. Int J Radiat Oncol Biol Phys. 2011;80:992-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 126. | Choi C, Koom WS, Kim TH, Yoon SM, Kim JH, Lee HS, Nam TK, Seong J. A prospective phase 2 multicenter study for the efficacy of radiation therapy following incomplete transarterial chemoembolization in unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;90:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 127. | Oh D, Lim DH, Park HC, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC, Lim HK, Lee WJ. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol. 2010;33:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 128. | Yu JI, Park HC, Lim do H, Kim CJ, Oh D, Yoo BC, Paik SW, Kho KC, Lee JH. Scheduled interval trans-catheter arterial chemoembolization followed by radiation therapy in patients with unresectable hepatocellular carcinoma. J Korean Med Sci. 2012;27:736-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 129. | Jang JW, Kwon JH, You CR, Kim JD, Woo HY, Bae SH, Choi JY, Yoon SK, Chung KW. Risk of HBV reactivation according to viral status and treatment intensity in patients with hepatocellular carcinoma. Antivir Ther. 2011;16:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 130. | Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Suh YG, Lee EJ, Cha H, Yang SH, Seong J. Prognostic values of vascular endothelial growth factor and matrix metalloproteinase-2 in hepatocellular carcinoma after radiotherapy. Dig Dis. 2014;32:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 132. | Horgan AM, Dawson LA, Swaminath A, Knox JJ. Sorafenib and radiation therapy for the treatment of advanced hepatocellular carcinoma. J Gastrointest Cancer. 2012;43:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Tomizawa M, Zhang Q S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S