Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6757
Peer-review started: March 10, 2016
First decision: May 12, 2016
Revised: May 28, 2016
Accepted: June 28, 2016
Article in press: June 28, 2016
Published online: August 14, 2016
Processing time: 148 Days and 22.1 Hours
Bile acids (BAs) are essential for the absorption of lipids. BA synthesis is inhibited through intestinal farnesoid X receptor (FXR) activity. BA sequestration is known to influence BA metabolism and control serum lipid concentrations. Animal data has demonstrated a regulatory role for the FXR in triglyceride metabolism. FXR inhibits hepatic lipogenesis by inhibiting the expression of sterol regulatory element binding protein 1c via small heterodimer primer activity. Conversely, FXR promotes free fatty acids oxidation by inducing the expression of peroxisome proliferator-activated receptor α. FXR can reduce the expression of microsomal triglyceride transfer protein, which regulates the assembly of very low-density lipoproteins (VLDL). FXR activation in turn promotes the clearance of circulating triglycerides by inducing apolipoprotein C-II, very low-density lipoproteins receptor (VLDL-R) and the expression of Syndecan-1 together with the repression of apolipoprotein C-III, which increases lipoprotein lipase activity. There is currently minimal clinical data on triglyceride metabolism in patients with bile acid diarrhoea (BAD). Emerging data suggests that a third of patients with BAD have hypertriglyceridemia. Further research is required to establish the risk of hypertriglyceridaemia in patients with BAD and elicit the mechanisms behind this, allowing for targeted treatment.
Core tip: Bile acids are essential for the absorption of dietary lipids. The farnesoid X receptor (FXR) has a crucial role in triglyceride metabolism through regulating hepatic de novo lipogenesis and modulating free fatty acids oxidation and triglyceride clearance. There is a reported interruption of the metabolism of triglycerides in patients with bile acid diarrhoea (BAD) with a third of patients suffering with hypertriglyceridemia. Emerging treatments for BAD such as therapeutic imitation of the FXR may aid in alleviating symptoms and improving triglyceride levels.
- Citation: Sagar NM, McFarlane M, Nwokolo C, Bardhan KD, Arasaradnam RP. Mechanisms of triglyceride metabolism in patients with bile acid diarrhea. World J Gastroenterol 2016; 22(30): 6757-6763
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6757.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6757
Bile acids (BAs) are essential for the absorption of lipids - hallmark of the modern diet. Bile acid diarrhoea (BAD) is commonly overlooked in the differential diagnosis of chronic diarrhoea despite it being potentially extremely debilitating for the patient with impact on daily activities due to urgency, increased bowel frequency and the fear of incontinence. Primary bile acid diarrhea (PBAD; formerly known as type 11) is most common with secondary BAD (due to ileal resection, post cholecystectomy etc. formerly known as type 1 and 111 respectively). Since the 1960s, bile acid sequestration has been recognised to influence bile acid metabolism and control serum lipid concentrations however the underlying mechanisms connecting lipid metabolism and bile acids have only started to be recognized more recently[1].
The formation of primary BAs, cholic acid and chenodeoxycholic acid (CDCA), involves hydroxylation of cholesterol catalyzed by the cytochrome P450 enzyme cholesterol 7α-hydroxylase (CYP7A1) resulting in a relative deficiency of hepatic microsomal cholesterol. This is ensued by upregulation of low-density lipoproteins (LDL) receptor expression and activity to harvest cholesterol from the systemic circulation resulting in reduced plasma LDL-cholesterol levels[2]. This mechanism has been reproduced by bile acid sequestrants (BAS) and ileal resection causing an interruption of the enterohepatic circulation of BAs resulting in a hypocholesterolaemic effect and hypertriglyceridemia through increased secretion of triglyceride rich very low-density lipoproteins (VLDL) particles from the liver[3-6]. Resin-bound primary BAs (which have been mixed with BAS resins such as Cholestyramine) become inaccessible for microbial biotransformation into secondary BAs in the intestine which in turn results in a relative reduced proportion of secondary BAs, promoting BA synthesis from cholesterol to restore the BA pool.
BAs emulsify dietary lipids, which are then packaged into chylomicrons by the enterocytes and released into the lymphatic vessels. Chylomicrons are composed of phospholipids, cholesterol esters, triglycerides and apolipoprotein B-48 but only achieve maturation after acquiring Apolipoproteins C-II and E from circulating high-density lipoproteins (HDLs)[7]. The triglycerides are then hydrolysed in the capillaries of adipose and muscle tissue by lipoprotein lipase (LPL) into fatty acids. The residual chylomicrons (which still have a small amount of triglycerides) are finally taken up by the liver and used for the synthesis of VLDLs. Once released into the bloodstream, VLDLs also obtain Apolipoproteins C-II and E from circulating HDLs and release free fatty acids (FFAs) to muscle and adipose tissues. The liver then removes the VLDLs (mainly the larger, triglyceride-rich ones) from the bloodstream. VLDLs may also become LDLs[8].
Changes in expression of the LDL receptor cannot be used to account for the increase in triglyceride levels observed with interruption of the enterohepatic circulation of BAs. Animal data has demonstrated a regulatory role for the farnesoid X-activated receptor (FXR) in triglyceride metabolism. The FXR, a member of the nuclear hormone receptor family, is expressed in hepatocytes and ileal enterocytes. BAs are agonists of the FXR therefore once the ileal FXR is activated, there is release of fibroblast growth factor 19 (FGF-19 in humans, FGF-15 in mice) into the portal circulation which then binds to fibroblast growth factor 4 (FGF-4) in hepatocytes. Through hepatic FXR, this results in downregulation of CYP7A1, which consequently inhibits the classical BA synthetic pathway by induction of small heterodimer primer (SHP) activity, thus inhibiting the conversion of cholesterol to BAs[9]. SHP mediates a signaling cascade to impede the action of liver X receptor (LXR) resulting in an inhibition of the expression of sterol regulatory element binding protein 1c (SREBP-1c) whose expression is otherwise induced by LXR interacting with liver receptor homologue 1. SREBP-1c is an essential transcription factor that regulates hepatic triglyceride synthesis by inducing enzymes such as fatty acid synthase, which are involved in lipogenesis[10].
Other postulated mechanisms may account for the triglyceride-lowering effect of FXR activation. Activation of FXR modulates FFA oxidation and triglyceride clearance[11]. Incubation of human hepatoma HepG2 cells with FXR ligands (CDCA) and agonists demonstrated the induction of expression of peroxisome proliferator-activated receptor α (PPARα) and its target genes to promote FFA oxidation[12]. PPARα is thought to play a critical role in mediating triglyceride metabolism. Fibrates activate PPARα, which lowers hepatic apoC-III production and increases LPL mediated lipolysis. This results in increased catabolism of triglyceride-rich particles and reduced secretion of VLDLs causing hypotriglyceridaemia[13]. Microsomal triglyceride transfer protein (MTP) is critical for lipoprotein synthesis and secretion in the liver and small intestine by participating in the transfer of triglycerides to newly synthesised apolipoprotein B (apo-B). Hepatocyte nuclear factor-4 (HNF-4) has been demonstrated to regulate MTP gene expression through elevated HNF-1 levels. Hep G2 cells cultured with CDCA, a ligand for FXR, exhibited increased expression of SHP, which suppresses HNF-4 activity and reduces mRNA levels for MTP and apo-B thus reducing the secretion of VLDL[14]. Syndecan-1 (SDC1), a heparin sulphate, is involved in the binding, internalisation and degradation of low-density lipoprotein. It is inducted by FXR ligands therefore the administration of CDCA results in increased expression of SDC1 causing a hypotriglyceridaemic effect[15]. Clearance of triglyceride-rich lipoproteins may be induced by CDCA causing an increased expression of the VLDL receptor[16].
A mouse study demonstrated that the administration of synthetic FXR agonists or FGF-15 in mice lacking the apical ileal sodium dependant bile acid transporter (ASBT) gene, a sodium-dependant BA transporter responsible for reabsorbing > 95% of BAs in the ileum, resulted in a decrease in serum triglyceride and cholesterol levels. There was also a 50% reduction in the bile pool size, which was thought to account for the changes seen in lipid metabolism through eliciting a compensatory increase in BA synthesis, which augments the hepatic requirement for cholesterol[17]. Another murine study revealed that pharmacological inhibition of this ileal sodium-dependant BA transporter resulted in reduced plasma triglyceride levels[18].
It has been demonstrated that activation of the FXR by ligands such as cholic acid results in lower plasma triglyceride levels by influencing LPL activity through induction of apolipoprotein C-II expression[19]. BA synthetic activators of the FXR have also exhibited a decrease in triglyceride levels through the repression of apolipoprotein C-III, a cofactor for LPL, which mediates triglyceride hydrolysis[20]. Elevated triglyceride concentrations have been demonstrated in patients with monogenic familial hypertriglyceridaemia who were found to display a defect in ileal BA absorption[21]. Individuals with decreased BA synthesis secondary to a CYP7A1 deficiency have also shown raised triglyceride levels[22]. These clinical observations exhibit an important direct relationship between triglyceride metabolism and BAs.
Despite an increased knowledge of the possible physiological mechanisms underlying triglyceride metabolism in patients with BAD, there is minimal clinical data available to support this occurrence. A study of twenty-four patients with an ileoanal anastomosis (IAA) and ileal pouch, which may disrupt the absorption of bile acids, demonstrated reduced serum total and LDL cholesterol, and LDL and HDL triglyceride levels but similar VLDL triglyceride levels to the control group. Their triglyceride:cholesterol ratio was higher. It was thought that reduced hepatic cholesterol content was secondary to cholesterol malabsorption in these patients with an inadequate increase in cholesterol synthesis, so that biliary cholesterol secretion deteriorated with reduced cholesterol remaining for VLDL production[23].
Another study in 12 patients with an IAA and ileal pouch revealed that the malabsorption of BAs resulted in a significant decrease in plasma concentrations of total cholesterol, LDL cholesterol and apolipoprotein B. Plasma concentrations of total triglycerides and VLDL triglycerides increased significantly[24].
A more recent study in 47 patients with primary BAD who had a Selenium-75-homophoric acid taurine (SeHCAT) scan retention value of less than 15% demonstrated raised triglycerides in 13 (27.7%) of these patients. Raised FGF-19 levels were associated with higher serum triglycerides. There was a significant negative correlation seen between triglycerides and SeHCAT retention with patients exhibiting high triglycerides having significant lower SeHCAT retention values. Furthermore, triglycerides were positively correlated with age and body mass index (BMI)[25].
The few studies that are available are of very small numbers and are detailed in Table 1.
| Study | Type of BAD | Patients | Results |
| Hakala et al[23] | Secondary | 24 patients with IAA + ileal pouch | ↓ LDL chol |
| 1997 | 20 controls | ↓ LDL + HDL triglycerides | |
| Akerlund et al[24] | Secondary | 12 patients with IAA + ileal pouch | ↓ LDL chol |
| 1994 | ↑ VLDL triglycerides | ||
| Walters et al[32] | Primary | 47 patients with BAD | ↑ Triglycerides in 13/47 (27.7%) |
| 2015 | 59 controls | ||
| This study | Total | 67 patients | ↑ Triglycerides in 25/67 (37.3%) |
| 2015 | Primary | 48 patients | 18/48 with PBAD (37.5%) |
| Secondary | 19 patients | 7/19 with SBAD (36.8%) |
In our series, 25 of the 67 patients studied were found to have raised triglyceride levels (> 1.9 mmol/L). 19/25 (76%) had PBAD, with 6/25 (24%) having SBAD. Of the 25 patients with raised triglyceride levels, 17 (68.0%) patients had their BMI recorded. All 17 (100%) patients had a BMI above the normal range with 11/17 (64.7%) being classified as overweight and 6/17 (35.3%) as obese. Co-morbidity was present in 14/25 (56.0%) patients with raised triglyceride levels.
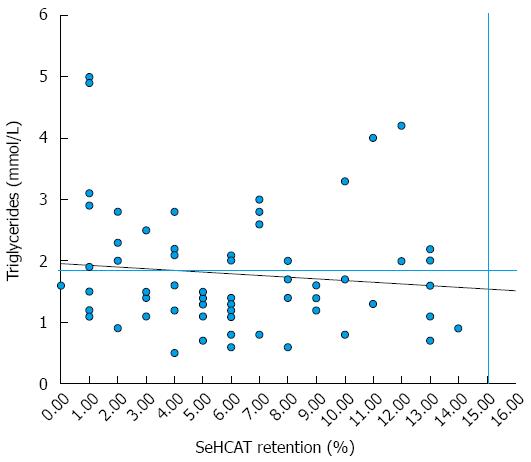
Over a third of patients (37%) in our series with BAD have hypertriglyceridemia, with similar ratios among the PBAD and SBAD groups. Of these patients, most had severe BAD (48%) and were all found to be either overweight or obese (BMI > 30). Other high-risk groups identified in patients with hypertriglyceridemia were those diagnosed with PBAD (76%), BMIs indicative of > 30 (100%) and the presence of co-morbidity (56%). The relationship between triglyceride levels (cut off 1.9 mmol/L) and SeHCAT 7 d retention values in the 67 patients are shown in Figure 1.
The mechanisms underlying the high incidence of hypertriglyceridemia demonstrated in clinical studies detailed in Table 1 remains uncertain with most literature pointing to a variety of mechanisms favouring reduced triglyceride levels in these patients. The mixed triglyceride results exhibited in patients with BAD may be a reflection of the differing genetic variation (e.g., polymorphisms of SREBP-1) in components of the signaling pathway seen in this disease group. One possible theory is that the vast majority of our patients were diagnosed with primary BAD, which may indicate that this is secondary to interruption of the enterohepatic circulation of BAs via a physiological mechanism - as noted in our series. This may be due to impaired responsiveness/activation of the FXR to BAs, defective feedback inhibition of BA synthesis by FGF-19 or mutations in the ASBT gene. Expression of FGF-19 in ileal biopsies has been associated with transcription of ASBT in patients with primary BAD but this correlation was found to be stronger in the control group[25].
Pattni et al[26] demonstrated the median value of serum FGF-19 in the group of patients with primary BAD was 65% lower of the value in the control group who also had chronic diarrhoea but normal SeHCAT retention value. Lower FGF-19 levels were also found in patients with severe BAD (SeHCAT retention value 0-5%) and increased levels of BAs as well as in the obese patients with primary BAD.
Decreased serum FGF-19 levels have been demonstrated in patients with obesity[27]. In addition to this, microRNA (miR-34a) has been found to be elevated in obesity. miRs function as negative gene regulators and inhibit and/or destabilize target mRNAs. After a meal, FXR induces the expression of FGF-19, which then binds to FGF-4 and its coreceptor β-Klotho (βKL), triggering activation of cellular kinases to mediate postpradial responses. miR-34a has a role in regulating β-Klotho expression therefore raised levels in obesity attenuated hepatic FGF-19 signaling by directly targeting βKL[28]. Taken together, these findings would suggest hypertriglyceridemia is more prevalent in patients diagnosed with primary BAD, severe BAD (SehCAT retention < 5%) and being obese.
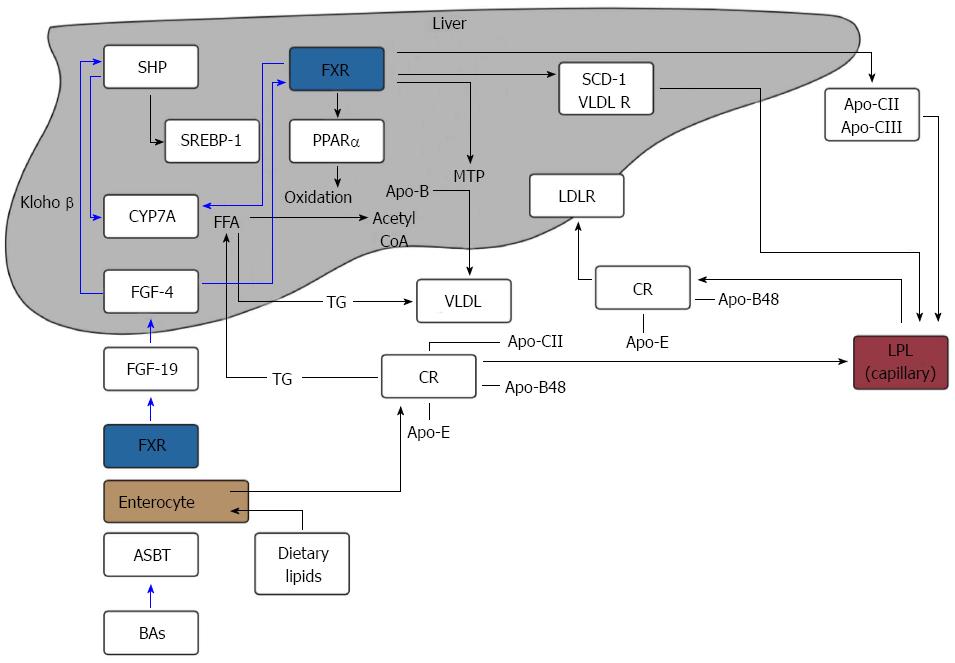
It is unclear whether dietary factors influence bile salt metabolism. In rats, a fat-free diet induced a reduction in BA synthesis likely secondary to a slower recirculation of BAs via the enterohepatic circulation. Further supplementation of cholesterol and saturated fatty acids increased BA synthesis causing an increase in the production of LDL-cholesterol[29]. In humans, ingestion of low and high-fat diets were associated with a 23% and 28% lower synthesis rate of total BAs respectively demonstrating that conversion of cholesterol to bile salts is affected by dietary fat intake[30]. These findings suggest that dietary fat intake may affect BA metabolism and therefore account for the higher levels of hypertriglyceridemia demonstrated in patients with obesity in although the mechanisms behind this remain undefined. An overview of FXR action on BA synthesis and triglycerides metabolism is shown in Figure 2.
FXR is expressed by hepatocytes and ileal enterocytes. BAs, agonists of the FXR, are re-absorbed via the ASBT in the ileum, releasing FGF-19 (hormone) into the portal circulation. FGF-19 binds to FGF-4 in hepatocytes and down-regulates CYP7A1 via klotho β and inhibition of SHP. This impedes BA synthesis.
FXR inhibits hepatic lipogenesis by inhibiting the expression of SREBP-1c via SHP. Conversely, FXR promotes FFA oxidation by inducing the expression of PPARα. FXR can reduce the expression of microsomal MTP, which regulates the assembly of VLDL. FXR activation promotes the clearance of circulating triglycerides by inducing Apo-CII, VLDL-R and the expression of SDC-1 together with the repression of Apo-CIII, which increases LPL activity.
Chylomicrons (CRs) containing Apo-CII, Apo-E and Apo-B48, lose Apo-CII through hydrolysis by LPL and are then taken up by the liver and used for the synthesis of VLDLs. VLDLs follow the same process of CRs once released into the bloodstream and release FFA to adipose and muscle tissues after LPL activation.
FXR plays a crucial role in triglyceride metabolism by regulating hepatic de novo lipogenesis and modulating FFA oxidation and triglyceride clearance. Clinical data supports the interrupted metabolism of triglycerides in patients with BAD. We have also identified risk groups with specific phenotypic patterns of BAD who are particularly vulnerable to this effect. Recognising the specific high-risk groups demonstrated in this study of patients with raised triglyceride levels would permit earlier diagnosis and management of hypertriglyceridemia, which is a strong predictor for coronary heart disease[31].
Further studies linking triglyceride levels with FGF-19 measurement would help in determining the role of FGF signaling in those with BAD. Therapeutic imitation of the FXR signaling pathway in inhibiting hepatic lipogenesis has already been demonstrated in a recent proof-of-concept study investigating the effects of obeticholic acid in patients with primary BAD. This FXR agonist showed an increase in median fasting FGF-19 levels with an improvement in symptoms of diarrhoea and triglyceride levels[32]. Thus emerging treatments for BAD may not only alleviate symptoms but also tackle underlying pathological disturbances in this case hypertriglyceridaemia.
The authors would like to thank all the patients from University Hospitals Coventry and Warwickshire (UHCW NHS) Trust who participated in the study. The authors would also like to acknowledge the BRET (Bardhan Research Education Trust) charity, which has supported funding for materials and equipment costs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| 1. | Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32 Suppl 2:S237-S245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1269] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 3. | Insull W. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J. 2006;99:257-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Shepherd J, Packard CJ, Bicker S, Lawrie TD, Morgan HG. Cholestyramine promotes receptor-mediated low-density-lipoprotein catabolism. N Engl J Med. 1980;302:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 257] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Angelin B, Einarsson K, Hellström K, Leijd B. Effects of cholestyramine and chenodeoxycholic acid on the metabolism of endogenous triglyceride in hyperlipoproteinemia. J Lipid Res. 1978;19:1017-1024. [PubMed] |
| 6. | Beil U, Crouse JR, Einarsson K, Grundy SM. Effects of interruption of the enterohepatic circulation of bile acids on the transport of very low density-lipoprotein triglycerides. Metabolism. 1982;31:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Stellaard F, Sackmann M, Sauerbruch T, Paumgartner G. Simultaneous determination of cholic acid and chenodeoxycholic acid pool sizes and fractional turnover rates in human serum using 13C-labeled bile acids. J Lipid Res. 1984;25:1313-1319. [PubMed] |
| 8. | Ponziani FR, Pecere S, Gasbarrini A, Ojetti V. Physiology and pathophysiology of liver lipid metabolism. Expert Rev Gastroenterol Hepatol. 2015;9:1055-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Camilleri M. Advances in understanding of bile acid diarrhea. Expert Rev Gastroenterol Hepatol. 2014;8:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 609] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 11. | Jiao Y, Lu Y, Li XY. Farnesoid X receptor: a master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol Sin. 2015;36:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 12. | Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 374] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 13. | Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1182] [Cited by in RCA: 1233] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 14. | Hirokane H, Nakahara M, Tachibana S, Shimizu M, Sato R. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4. J Biol Chem. 2004;279:45685-45692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Anisfeld AM, Kast-Woelbern HR, Meyer ME, Jones SA, Zhang Y, Williams KJ, Willson T, Edwards PA. Syndecan-1 expression is regulated in an isoform-specific manner by the farnesoid-X receptor. J Biol Chem. 2003;278:20420-20428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Sirvent A, Claudel T, Martin G, Brozek J, Kosykh V, Darteil R, Hum DW, Fruchart JC, Staels B. The farnesoid X receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 2004;566:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Jung D, Inagaki T, Gerard RD, Dawson PA, Kliewer SA, Mangelsdorf DJ, Moschetta A. FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J Lipid Res. 2007;48:2693-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Lundåsen T, Andersson EM, Snaith M, Lindmark H, Lundberg J, Östlund-Lindqvist AM, Angelin B, Rudling M. Inhibition of intestinal bile acid transporter Slc10a2 improves triglyceride metabolism and normalizes elevated plasma glucose levels in mice. PLoS One. 2012;7:e37787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Kast HR, Nguyen CM, Sinal CJ, Jones SA, Laffitte BA, Reue K, Gonzalez FJ, Willson TM, Edwards PA. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol. 2001;15:1720-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J, Fruchart JC, Gonzalez FJ, Staels B. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Angelin B, Hershon KS, Brunzell JD. Bile acid metabolism in hereditary forms of hypertriglyceridemia: evidence for an increased synthesis rate in monogenic familial hypertriglyceridemia. Proc Natl Acad Sci USA. 1987;84:5434-5438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 373] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Hakala K, Vuoristo M, Luukkonen P, Järvinen HJ, Miettinen TA. Impaired absorption of cholesterol and bile acids in patients with an ileoanal anastomosis. Gut. 1997;41:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Akerlund JE, Björkhem I, Angelin B, Liljeqvist L, Einarsson K. Apparent selective bile acid malabsorption as a consequence of ileal exclusion: effects on bile acid, cholesterol, and lipoprotein metabolism. Gut. 1994;35:1116-1120. [PubMed] |
| 25. | Johnston IM, Nolan JD, Pattni SS, Appleby RN, Zhang JH, Kennie SL, Madhan GK, Jameie-Oskooei S, Pathmasrirengam S, Lin J. Characterizing Factors Associated With Differences in FGF19 Blood Levels and Synthesis in Patients With Primary Bile Acid Diarrhea. Am J Gastroenterol. 2016;111:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Pattni SS, Brydon WG, Dew T, Johnston IM, Nolan JD, Srinivas M, Basumani P, Bardhan KD, Walters JR. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther. 2013;38:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 293] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 28. | Fu T, Choi SE, Kim DH, Seok S, Suino-Powell KM, Xu HE, Kemper JK. Aberrantly elevated microRNA-34a in obesity attenuates hepatic responses to FGF19 by targeting a membrane coreceptor β-Klotho. Proc Natl Acad Sci USA. 2012;109:16137-16142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Bertolotti M, Spady DK, Dietschy JM. Regulation of hepatic cholesterol metabolism in the rat in vivo: effect of a synthetic fat-free diet on sterol synthesis and low-density lipoprotein transport. Biochim Biophys Acta. 1995;1255:293-300. [PubMed] |
| 30. | Bisschop PH, Bandsma RH, Stellaard F, ter Harmsel A, Meijer AJ, Sauerwein HP, Kuipers F, Romijn JA. Low-fat, high-carbohydrate and high-fat, low-carbohydrate diets decrease primary bile acid synthesis in humans. Am J Clin Nutr. 2004;79:570-576. [PubMed] |
| 31. | Cullen P. Evidence that triglycerides are an independent coronary heart disease risk factor. Am J Cardiol. 2000;86:943-949. [PubMed] |
| 32. | Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Slomiany BL S- Editor: Qi Y L- Editor: A E- Editor: Ma S