Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.933
Peer-review started: May 12, 2015
First decision: September 9, 2015
Revised: September 28, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: January 21, 2016
Processing time: 261 Days and 10.5 Hours
Vitamin D deficiency has been associated with a wide range of diseases and multiple forms of cancer including breast, colon, and prostate cancers. Relatively recent work has demonstrated vitamin D to be critical in immune function and therefore important in inflammatory diseases such as inflammatory bowel disease (IBD). Because vitamin D deficiency or insufficiency is increasingly prevalent around the world, with an estimated 30%-50% of children and adults at risk for vitamin D deficiency worldwide, it could have a significant impact on IBD. Epidemiologic studies suggest that low serum vitamin D levels are a risk factor for IBD and colon cancer, and vitamin D supplementation is associated with decreased colitis disease activity and/or alleviated symptoms. Patients diagnosed with IBD have a higher incidence of colorectal cancer than the general population, which supports the notion that inflammation plays a key role in cancer development and underscores the importance of understanding how vitamin D influences inflammation and its cancer-promoting effects. In addition to human epidemiological data, studies utilizing mouse models of colitis have shown that vitamin D is beneficial in preventing or ameliorating inflammation and clinical disease. The precise role of vitamin D on colitis is unknown; however, vitamin D regulates immune cell trafficking and differentiation, gut barrier function and antimicrobial peptide synthesis, all of which may be protective from IBD and colon cancer. Here we focus on effects of vitamin D on inflammation and inflammation-associated colon cancer and discuss the potential use of vitamin D for protection and treatment of IBD and colon cancer.
Core tip: Vitamin D is inversely related to inflammation-associated diseases such as inflammatory bowel disease (IBD) and colon cancer. As vitamin D deficiency and insufficiency are prevalent worldwide, it can significantly impact risk and progression of these diseases. Here we provide an overview of human epidemiologic and animal studies linking vitamin D, IBD and colon cancer. We also review potential mechanisms of vitamin D action that were elucidated by using animal models. Finally, we address current knowledge gaps in this field of research to help determine the potential benefits and risks of using vitamin D to prevent and/or treat IBD and cancer.
- Citation: Meeker S, Seamons A, Maggio-Price L, Paik J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J Gastroenterol 2016; 22(3): 933-948
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.933
Vitamin D is primarily known for its role in regulation of bone metabolism by controlling intestinal calcium absorption[1-3] and bone remodeling[4], the importance of which is demonstrated by prolonged vitamin D deficiency resulting in delayed growth and rickets in children[1] as well as osteoporosis and osteopenia in adults[5]. In addition to its role in bone metabolism, it has become increasingly apparent that vitamin D participates in a variety of other physiological functions, including immune responses. Consequently, vitamin D deficiency is also associated with a wide range of diseases including: asthma[6], multiple sclerosis[7], rheumatoid arthritis[8], type 1 diabetes[9,10], heart disease[11,12], depression[13,14], and tuberculosis[15]. For our interests, epidemiologic studies link low serum vitamin D levels with an increased risk of developing inflammatory bowel disease (IBD) and colon cancer[16-22]. As vitamin D deficiency or insufficiency is increasingly prevalent around the world, with estimates of 30%-50% of children and adults at risk of vitamin D deficiency worldwide[3], it could significantly influence incidence and progression of IBD and colon cancer. In this paper, we will review current evidence linking vitamin D, IBD, and colitis-associated colon cancer (CAC) and summarize the potential benefits and risks of using vitamin D to prevent or treat these diseases. Several reviews have been published recently concerning vitamin D and the immune system[18,23-26] and cancer[19,27,28].
IBD is a group of diseases characterized as chronic remittent or progressive inflammation of the gastrointestinal tract. The two primary conditions in humans are Crohn’s disease (CD) and ulcerative colitis (UC), and the prevalence of both conditions have been increasing in western countries over the past 50 years with CD affecting 50-200/100000 people and UC affecting 120-200/100000 people per year[29]. Though the precise etiology underlying IBD remains unclear, dysregulation of the mucosal immune system in response to enteric antigens is believed to be one of the initiators of the chronic inflammation[30]. Patients diagnosed with IBD are at increased risk for developing CAC compared to the general population, especially if the colitis is not well controlled[30-32]. Inflammation is believed to play a role in the development of CAC through promotion of angiogenesis, tumor-promoting cytokine production, tumor cell invasive behavior, cellular proliferation and alterations in immune responses[33]. Treatments to limit inflammation may be beneficial in reducing the incidence of CAC in these high-risk populations[34]. Because vitamin D has been shown to alter many of the pathways involved in inflammation as well as tumorigenesis, the potential to use vitamin D supplementation as an adjunct therapy in IBD patients is being explored[35-38].
We have recently shown that dietary vitamin D supplementation provides protection against IBD and subsequent inflammation-induced colon cancer in the Smad3-/- mouse model of CAC[39]. Our data suggest that the chemoprotective effect of vitamin D is likely due to decreased colitis prior to tumor development. Our findings are consistent with other studies demonstrating association between vitamin D supplementation and decreased colitis, disease activity and/or alleviated symptoms of IBD in both mice[39-42] and humans[21,36,37]. Additionally, both our work and others have demonstrated that vitamin D decreases the incidence of dysplasia, a precursor to cancer, in models of IBD-associated colon cancer[39,42,43]. These data support the notion that vitamin D may be a beneficial adjunct therapy for IBD and CAC. Because there is currently no cure for IBD, patients typically require long-term immunomodulatory drug therapy to manage their symptoms with substantial economic costs over their lifetime[44]. The use of vitamin D as a treatment in patients with IBD would be simple and inexpensive to implement. However, the specific mechanisms through which vitamin D ameliorates colitis remain unknown though vitamin D has been shown to regulate immune cell trafficking and differentiation, gut barrier function, and antimicrobial peptide synthesis, all of which may play a role in mediating protection from disease[45-47]. Thus, additional studies are needed to determine the patient populations that might benefit from vitamin D therapy.
To explain mechanisms by which vitamin D might influence IBD and colon cancer, we will briefly review the metabolism and actions of vitamin D. More thorough reviews on this topic can be found in recent publications[3,48].
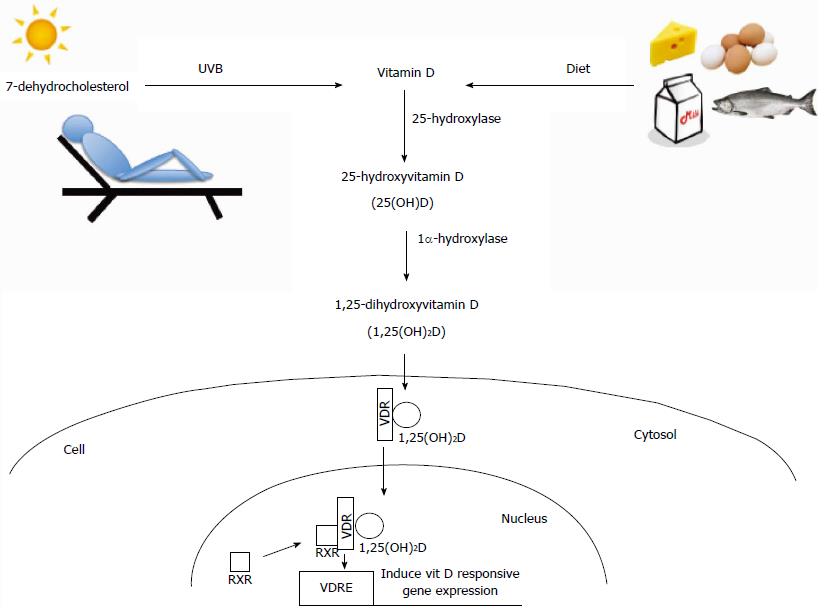
Humans obtain the majority of their vitamin D from exposure to sunlight as UVB radiation is able to convert 7-dehydrocholesterol to vitamin D3 (cholecalciferol) in the skin (Figure 1). Diet, however, becomes an important means of obtaining vitamin D in individuals with limited exposure to sunlight, such as might occur due to insufficient exposure to natural sunlight because of residing at higher latitudes or limited amounts of time spent outdoors, or increased protection against sun exposure through clothing and/or sunscreen. Therefore, inadequate intake of foods rich in vitamin D such as oily fish, beef liver, and fortified milk products can significantly impact vitamin D status in some individuals[2,49].
Vitamin D, synthesized in the skin or taken up from the diet, undergoes two hydroxylation steps to become biologically active 1,25-dihydroxyvitamin D (1,25(OH)2D) (Figure 1). The first hydroxylation step occurs in the liver where a 25-hydroxylase (Cyp2R1 and possibly other enzymes) produces 25-hydroxyvitamin D (25(OH)D)[50,51]. This is the main circulating form of vitamin D and is most often used as an indicator of nutritional vitamin D status, both in humans and animals, as it is highly stable and has a long half-life[3]. The majority of 25-hydroxyvitamin D circulates in blood bound to either vitamin D binding protein (DBP; 85%-90% bound) or albumin (10%-15% bound) with < 1% unbound as free vitamin D[45]. The second hydroxylation step occurs primarily in the kidney by 1α-hydroxylase (Cyp27b1) to produce active 1,25-dihydroxyvitamin D (1,25(OH)2D) (Figure 1). The active form of vitamin D can also be measured in the serum, although its concentrations do not reflect nutritional vitamin D status as the half-life is relatively short (4-20 h) and its production is highly regulated by serum levels of calcium and phosphorus as well as activities of parathyroid hormone and fibroblast-like growth factor-23[3]. Both 25(OH)D and 1,25(OH)2D can also be inactivated through further hydroxylation by 24-hydroxylase (Cyp24a1), which is induced by vitamin D signaling in almost all target tissues of vitamin D[3]. More detailed information on the synthesis, uptake, and metabolism of vitamin D is reviewed in other recent publications[3,52]. Although the majority of vitamin D hydroxylation occurs in the liver (25-hydroxylation) and kidney (1α-hydroxylation), other tissues, including intestinal epithelial cells and immune cells (T and B cells, macrophages, dendritic cells), express vitamin D hydroxylase enzymes (Cyp27b1, Cyp24a1)[53,54], suggesting that these cells can regulate levels of the active hormone locally.
1,25(OH)2D elicits its effects through binding to the vitamin D receptor (VDR), a transcription factor for genes with promoters containing vitamin D response elements (VDREs)[55] (Figure 1). VDR bound by 1,25(OH)2D can recruit transcription machinery to activate a plethora of down-stream genes. Through chromatin precipitation parallel DNA sequencing studies (CHIP-seq) more than 2776 VDR binding sites have been identified throughout the human genome[56]. Interestingly, this study noted that VDR binding sites were significantly enriched near genes known to be associated with autoimmune disease and cancer, including PTPN2, a gene that has been implicated in CD and UC due to its important role in maintaining epithelial barrier function[56]. This suggests that expression of these genes may be decreased in people with inadequate levels of vitamin D due to reduced VDR ligand. More in-depth reviews on VDR and gene expression have been previously published[57-59].
Multiple VDR polymorphisms have been reported in humans and are associated with various diseases including IBD, cancer, asthma and obesity[60-62]. However, many of these polymorphisms occur in non-coding regions of the VDR gene and appear to have no direct consequence to the VDR protein itself, except for one polymorphism where the transcription start site is changed resulting in a shorter and more active VDR[60]. More frequently, VDR gene polymorphisms affect VDR transcription due to changes in the promoter region or 3’UTR, but the functional consequence(s) of these changes is not well defined[60]. Loss of function mutations in the VDR result in vitamin D deficiency symptoms including rickets[63]. When mutations affect VDR’s binding to its ligand, 1,25(OH)2D, symptoms are similar to vitamin D nutritional deficiency. However, when mutations affect VDR’s binding to DNA (via VDREs), alopecia can occur in addition to symptoms of vitamin D deficiency, suggesting that VDR has ligand-independent functions[63]. These types of mutations have been replicated in murine models[64,65], and studies using these animal models revealed that VDR can actively suppress down-stream genes when bound to VDREs without its ligand. Thus, mice with VDR mutations affecting ligand binding but maintaining DNA binding ability have more severe skeletal defects than mice with vitamin D deficiency or mice with VDR null mutations[64]. The mutated VDR, VDRgem, does not respond to endogenous VDR ligand 1,25(OH)2D but can be activated by synthetic analogs, gemini ligands. Hence, the VDRgem model may be useful in deciphering ligand-dependent and -independent roles of VDR[64]. For our interests, this mouse model could be used to determine if ligand-independent VDR function influences immune responses and thus the development of IBD/CAC and to develop drugs to target these specific functions[64].
It has been observed that the incidence of IBD is higher in people residing at northern latitudes compared to those at more southern latitudes both in the United States[66] and in Europe[67-69], suggesting that limited exposure to UVB and resultant decreased vitamin D levels may influence risk of IBD. In addition, lower UVB exposure and vitamin D deficiency has been associated with onset of IBD[16], increased clinical disease activity[70-73], greater rates of hospitalization, prolonged hospitalization and an increased need for bowel surgery in patients with IBD[74,75], and risk of malignant transformation[22]. Along similar lines, results from a questionnaire assessing the overall quality of life of IBD patients (141 CD and 79 UC) noted that increased serum vitamin D levels correlated with increased health-related quality of life in both CD and UC patients during winter/spring months[76]. Interestingly, VDR expression was significantly decreased in intestinal epithelial cells from IBD patients compared to healthy controls. In addition, intestinal VDR expression was lower in patients with active CD compared to those with quiescent disease despite one cohort of patients studied having “adequate” serum levels of vitamin D[77], suggesting that epithelial responses to vitamin D may be diminished during active IBD and this may not be altered by increasing serum vitamin D alone.
Similar to the findings in IBD patients, epidemiologic data demonstrate a significant north/south gradient associated with colon cancer with increasing incidence and mortality associated with reduced exposure to natural light[17,20,78]. Prospective cohort studies have demonstrated an inverse association between serum vitamin D levels [25(OH)D] and cancer development (colon, breast and prostate)[28,79,80]. Interestingly, Ananthakrishnan et al[22] demonstrated that within a cohort of 2809 IBD patients almost a third of the patients were vitamin D deficient. Over an eleven-year follow up period, 7% of those patients went on to develop cancer. Strikingly, the patients that went on to develop cancer had significantly lower vitamin D levels than those who did not develop cancer. It was estimated that for every 1 ng/mL increase in plasma 25(OH)D concentration, there was a 6% reduction in colorectal cancer risk[22].
Although there is a strong inverse association between serum levels of 25(OH)D and the risk for IBD/CAC, there is no consensus about what the adequate serum vitamin D concentration should be to prevent these diseases. Historically, adequate levels of vitamin D were defined based on maintenance of calcium homeostasis without causing bone disease. However, as our knowledge of the impacts of vitamin D on human health and disease become broader, experts have sought to re-define the levels of vitamin D to provide the most protective health benefits. Unfortunately, this is much more difficult to determine as “health benefit” is hard to define. According to the Institute of Medicine, vitamin D sufficiency was determined to be ≥ 50 nmol/L serum 25(OH)D, while serum vitamin D levels of 25-50 nmol/L were classified as insufficient and < 25 nmol/L deficient[52]. In contrast, the Endocrine Society defined vitamin D sufficiency as ≥ 75 nmol/L serum 25(OH)D and deficiency as < 50 nmol/L[81]. At this time, further research investigating how varied serum levels of vitamin D are related to health and disease are needed in order to better define “adequate” serum vitamin D levels.
Though the epidemiologic data suggest a link between circulating vitamin D levels and disease outcome, these studies cannot address the question as to whether vitamin D deficiency is a cause of or sequela of the disease. For these questions, animal models have been useful to address mechanisms through which vitamin D influences inflammation and cancer development.
Animal models are an important and necessary tool for studying the effects of vitamin D deficiency and/or supplementation on IBD and cancer. Mouse models permit modulation of vitamin D levels while controlling for other environmental and genetic factors that may influence disease development. Tables 1 and 2 provide summaries of animal models of IBD (Table 1) and CAC (Table 2) in which the role of vitamin D with regard to disease has been addressed. Mouse models of IBD and CAC have been categorized into vitamin D supplementation, vitamin D deficiency, and animals with genetically altered VDR. Categories have been further broken down to highlight studies that show amelioration vs exacerbation of disease. In the following section, we briefly discuss some of the important mechanisms through which vitamin D impacts IBD and CAC using the models outlined in Tables 1 and 2.
| Strain | Model | Vitamin D metabolite/analog | Dose, route | Treatment window and duration | Serum vit D measure | Outcome | Ref. |
| Vitamin D Supplementation | |||||||
| Protection | |||||||
| Il10-/- | Spontaneous | 1,25(OH)2D3 and D3 | 0.005 μg 1,25(OH)2D3/d or 5 μg D3/d, diet | 3 wk of age; maintained throughout study | ND | Ameliorated IBD | [40] |
| 0.2 μg 1,25(OH)2D3/d, diet | At the first sign of IBD (diarrhea), lasted for 2 wk | Effective at blocking the progression and ameliorating the symptoms in established disease | |||||
| Il10-/- | Spontaneous | 1,25(OH)2D3 | 20 ng/d, diet | 4 wk of age; maintained throughout study | ND | Significantly reduced spontaneous colitis. More effective protection with combined treatment with calcium | [113] |
| C57BL/6 | DSS (0.5-3.5%), 5 d | 1,25(OH)2D3 | 50 ng/d, diet or 10 ng, intrarectally | Diet given 1 wk prior to DSS treatment; maintained throughout study. Intrarectal administration given 1 d prior to DSS; given every other day | ND | Decreased DSS-induced colitis. Intrarectal administration more effective than dietary delivery | [106] |
| Balb/c | DSS (3%), 7 d | ZK191784 (1,25(OH)2D3 analog) | 100 μg/kg per day, orally | 3 d prior or at the start of DSS treatment; given daily | ND | Significantly decreased DSS-induced colitis | [115] |
| C57BL/6 | DSS (3%), 7 d | 1,25(OH)2D3 | 0.5 μg/kg, IP | 1 d prior to DSS treatment; given daily up to 11 d | ND | Decreased inflammation and tissue damage following DSS treatment; Increased weight loss likely due to hypercalcemia | [41] |
| C57BL/6 | DSS (2%), 7 d | 1,25(OH)2D3 | 0.2 μg/25 g BW/d, oral gavage | After DSS treatment | ND | Decreased clinical disease, colonic inflammation and intestinal permeability following DSS treatment | [117] |
| Cyp27b1-/- and Vdr-/- | DSS (3.5%), 5 d | 1,25(OH)2D3 | 50 ng/d, diet | 2 wk prior to DSS treatment; maintained throughout study | ND | Cyp27b1-/- mice more susceptible to DSS-induced colitis; supplementation with 1,25(OH)2D3 partially ameliorated disease | [91] |
| C57BL/6 | DSS (3%), 7 d | NA | Adoptive transfer of CYP27B1 over-expressing monocytes | Adoptive transfer on 5th day of DSS treatment | ND | CD11b+/Gr1+ monocytes overexpressing CYP27B1 trafficked to the inflamed colon and ameliorated DSS-induced colitis | [114] |
| C57BL/6 | DSS (2%), 5 d of treatment followed by 2 d of regular water throughout study | 1,25(OH)2D3 | 0.2 μg/25 g BW/d, oral gavage | 2 wk after initiation of DSS treatment; maintained throughout study | ND | Vitamin D decreased clinical disease and colonic inflammation following DSS treatment. Vitamin D treatment significantly decreased mononuclear cell infiltrates present in the spleen and mesenteric lymph node following DSS | [139] |
| Smad3-/- | Helicobacter bilis | D3 | 1000 IU or 5000 IU per kg diet, diet | 2 wk prior to H. bilis infection; maintained throughout study | 25(OH)D | Higher vitamin D significantly decrease colonic inflammation | [39] |
| Balb/c | TNBS (100 mg/kg) | 1,25(OH)2D3 | 0.2 μg/kg, IP | Given 2 h before and 3 d post (acute) or 3, 4 and 5 d post TNBS (interventional) | ND | Significantly reduced colitis; greater protection with concurrent treatment with dexamethasone | [140] |
| C57BL/6 | TNBS (100 mg/kg) | Paracalitriol (1,25(OH)2D3 analog) | 0.5 μg/kg, IP | Given 30 min before and 1, 3 and 5 d post TNBS | ND | Ameliorated TNBS colitis and protected against epithelial barrier disruption | [116] |
| Neutral | |||||||
| Il10-/- | Spontaneous colitis | D3 | 25 IU or 5000 IU per kg diet, diet | Before conception; maintained throughout study | 25(OH)D | No significant difference in inflammation score | [109] |
| Rag-/- | Adoptive transfer of Il10-/- T cells + piroxicam (7 d) | D3 | 1000 IU or 5000 IU per kg diet, diet | After peroxicam treatment ends; for 12 d | 1,25(OH)2D, 25(OH)D | Vitamin D supplementation during active colitis did not alter colonic inflammation; increased trabecular bone deterioration | [108] |
| Exacerbation | |||||||
| C57BL/6 | Citrobacter rodentium | 1,25(OH)2D3 | 0.5 μg/kg, IP | 1 d prior to treatment; daily | ND | Increased colonic ulceration and bacterial burdens compared to controls | [41] |
| Vitamin D Deficiency | |||||||
| Il10-/- | Spontaneous | NA | Vit D deficient diet | Maintain throughout study | ND | Increased mortality and more rapid disease development compared to vitamin D sufficient animals | [40] |
| C57BL/6 | DSS (2.5%), 6 d | NA | Vit D deficient diet | 6 wk prior to DSS treatment; maintained throughout study | 25(OH)D | Exacerbated DSS colitis and increased bacterial loads within the colonic tissue | [105] |
| Il10-/- | AOM + DSS (2%), 7 d | NA | Vit D deficient diet | 3 wk of age; maintained throughout study | 25(OH)D | Increased mortality and disease severity in AOM/DSS colitis | [141] |
| Smad3-/- | Helicobacter bilis | NA | Vit D deficient diet | 2 wk prior to infection; maintain throughout study | 25(OH)D | Did not alter IBD development or severity | [39] |
| Il10-/- | DSS (2%), 9 days or E. coli | NA | Vit D deficient diet | Maintain throughout experiment | 1,25OH)2D and 25(OH)D | Decreased survival and increased intestinal permeability following DSS treatment; increased susceptibility to infection with invasive E. coli | [103] |
| Other (genetically altered VDR) | |||||||
| hVDR Tg | TNBS, DSS, and adoptive T cell transfer | NA | NA | NA | ND | Overexpression of VDR in the intestinal epithelial cells protected against TNBS, DSS and adoptive T cell transfer -induced colitis | [77] |
| hVDR Tg Il10-/- | Spontaneous | NA | NA | NA | ND | Overexpression of VDR in the intestinal epithelial cells protected against spontaneous disease in Il10-/- mice | [142] |
| Vdr-/- | DSS (0.5%-3.5%), 5 d | NA | NA | NA | ND | Increased sensitivity, mortality and disease severity in response to DSS | [107] |
| Vdr-/- | DSS (2%-2.5%), 7 d | NA | NA | NA | ND | More sensitive to DSS inducted colitis resulting in severe mucosal ulcerations, impaired mucosal wound healing, decreased epithelial barrier function and increased mortality | [102] |
| Vdr-/- | Salmonella typhimurium | NA | NA | NA | ND | Administration of probiotics (Lactobacillus rhamnosus and Lactobacillus plantarum) increased VDR protein expression in colon epithelial cells. Probiotic administration protected against Salmonella-induced colitis in wild type but not Vdr-/- mice | [138] |
| VDRΔIEC | DSS (5%), 7 d | NA | NA | NA | ND | Loss of intestinal epithelial VDR exacerbated DSS colitis, altered paneth cell development and changed autophagy gene expression | [137] |
| Vdr-/- and Il10-/-/Vdr-/- | Adoptive T Cell Transfer and Spontaneous | NA | NA | NA | ND | Il10-/-/Vdr-/- mice developed more severe IBD and increased mortality compared to Il10-/- mice. Adoptive transfer of Vdr-/- CD4 T cells into Rag-/- mice induced severe IBD | [143] |
| Il10-/-/Vdr-/- | Spontaneous | NA | NA | NA | ND | Il10-/-/Vdr-/- mice developed more severe IBD compared to IL10-/- mice | [106] |
| Il10-/-/Vdr-/- | Spontaneous and Adoptive T cell Transfer | NA | NA | NA | ND | Adoptive transfer of Il10-/-/Vdr-/- CD4 T cells into Rag-/- mice induced severe colitis. Loss of VDR results in decreased T cell homing to the gut and decreased CD4/CD8αα intraepithelial lymphocytes | [89] |
| Vdr-/- Il10-/-/Vdr-/-, and Rag-/- | Adoptive T Cell Transfer | NA | NA | NA | ND | Adoptive transfer of Il10-/-/Vdr-/- CD8 T cells into Rag-/- mice induces severe colitis. Vdr-/- CD8 T cells exacerbate CD4/CD45RBhigh cell-induced colitis | [144] |
| Strain | Model | Vitamin D metabolite/analog | Dose, route | Treatment window and duration | Serum vit D measure | Outcome | Ref. |
| Vitamin D Supplementation | |||||||
| A/J | AOM + DSS (3%), 7 d | Ro26-2198 (analog) | 0.01 μg/kg per day, subcutaneous pump | 1 wk prior to AOM | ND | Delayed onset of clinical colitis, decreased cellular proliferation and decreased dysplasia following AOM/DSS | [43] |
| CF1 | AOM + DSS (2.5%) × 7 d | D3, 25(OH)D, 1,25(OH)2D3 and 1,25(OH)2D5 | 500 μg D3/kg diet , - 500 μg 25(OH)D/kg diet, 2.5 μg 1,25(OH)2D3/kg diet and 25 μg 1,25(OH)2D5/kg diet | 1 wk prior to AOM + DSS treatment and maintained throughout study | ND | D3, 25(OH)D, and 1,25(OH)2D5 significantly decreased tumor incidence. 1,25(OH)2D3 induced significant weight loss compared to other treatments; not included in final analysis | [118] |
| C57BL/6 | AOM + DSS (2%), 4 d × 3 cycles | D3 | 100, 400, 1000, 2500 or 5000 IU/kg diet, diet | 2.5 wk prior to AOM + DSS treatment | 25(OH)D | Vitamin D supplementation significantly decreased dysplasia in a dose dependent manner | [42] |
| Smad3-/- | Helicobacter bilis | D3 | 1000 IU or 5000 IU/kg diet, diet | 1 wk prior to infection; maintain throughout study | 25(OH)D | Higher vitamin D significantly decreased tumor incidence | [39] |
| Vitamin D Deficiency | |||||||
| Smad3-/- | Helicobacter bilis | NA | Vit D deficient diet | 2 wk prior to infection; maintain throughout study | 25(OH)D | No change in dysplasia score or tumor incidence | [39] |
| Other (VDR Knockout) | |||||||
| Vdr-/- | AOM + DSS (1.5%), 5 d × 3 cycles | NA | NA | NA | ND | Increased inflammation and tumor burdens; increased activation of EGFR and ErbB2 signaling | [145] |
One mechanism through which vitamin D may impact IBD development and progression is through altering immune responses[18,26,82,83]. Multiple studies have demonstrated that T cells are both direct and indirect targets of vitamin D[84-87]. CD4+ effector T cells that lack VDR or CYP27B1 express higher concentrations of the inflammatory cytokines interleukin (IL)-17 and interferon gamma (IFNγ), proliferate more rapidly, and induce more severe colitis following adoptive transfer into naïve recipient mice, compared to WT cells. In addition, Vdr-/- mice fail to develop iNKT and CD8αα T cell populations, both of which are important regulatory T cell subsets necessary in the resolution of inflammation[88-90]. Treatment of T cells or mice with active vitamin D both in vitro and in vivo results in decreased proliferation of both CD4+ and CD8+ subsets as well as decreased secretion of IL-2 and IFNγ, resulting in decreased Th1 and Th17 responses and an overall dampening of inflammation[85,88]. In addition to affecting T cells directly, vitamin D alters inflammation by affecting antigen-presenting cells. Macrophages and dendritic cells treated with vitamin D have decreased secretion of proinflammatory cytokines following activation[91,92]. In addition, vitamin D treatment modulates dendritic cell differentiation and maturation: vitamin D-treated dendritic cells express fewer maturation markers including CD80, CD86, and MHCII and secrete less IL-12. This results in decreased dendritic cell-mediated activation and proliferation of T cells and an increase in T cells with regulatory phenotypes[92-94]. The effects of vitamin D on NK cell responses are understudied considering the role these cells have been shown to have in autoimmune disease[95] as well as their role in interacting with dendritic and T cells and modulating their responses[96]. Vitamin D has been shown to enhance cytotoxic function of human NK cell lines[97,98] and may also impair NK cell development and inflammatory cytokine expression in vitro[99].
Another mechanism through which vitamin D may protect against IBD is by improving intestinal epithelial barrier function. Patients with CD have increased intestinal barrier permeability which has been associated with inflammation and dysbiosis[100] as it results in increased exposure of the immune system to intestinal microbiota. In vitro studies demonstrate that vitamin D signaling is imperative for the maintenance of epithelial barrier integrity by increasing the expression of tight junction proteins including Occludin, Zo-1, Zo-2, Vinculin, and Claudin, and altering transepithelial resistance[101-104]. Interestingly, Kong et al[102] have demonstrated that mice lacking VDR have decreased transepithelial electric resistance and disruption of the epithelial cell tight junctions resulting in an increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Conversely, Liu et al[77] have demonstrated that overexpression of VDR within the colonic epithelial cells is protective in several different mouse models of colitis, including DSS and the adoptive transfer of T cells into naïve mice, by preserving epithelial tight junctions and attenuating epithelial cell apoptosis thus preserving the gut epithelial barrier function.
In agreement with the epidemiologic human data, vitamin D deficiency exacerbates IBD in mouse models. Mice fed a vitamin D deficient diet are more susceptible to DSS-induced colitis compared to those fed a vitamin D sufficient diet[105]. Similarly, vitamin D deficient Il10-/- mice, which develop colitis in response to enteric flora, develop more severe disease compared to vitamin D sufficient animals[106]. In addition, mice lacking VDR or CYP27B1 develop more severe IBD following treatment with DSS compared to wild type controls[84,91,102], again supporting the epidemiologic evidence that vitamin D deficiency may play a role in IBD development and progression. Conversely, vitamin D supplementation ameliorates symptoms of colitis in vitamin D deficient animals[77,107]. However, in mice, vitamin D deficiency does not always worsen IBD and CAC. While increased dietary vitamin D protected Smad3-/- mice from developing IBD and CAC induced by Helicobacter, a vitamin D-deficient diet did not increase colitis and CAC in these mice compared to a control group fed normal levels of dietary vitamin D[39]. Mice fed a vitamin D deficient diet had significantly lower levels of serum 25(OH)D compared to controls. Interestingly, while high vitamin D diet suppressed early inflammation, the vitamin D deficient diet did not cause more severe inflammation compared to controls, suggesting that high levels of vitamin D may inhibit inflammation but lack of vitamin D does not accelerate or exacerbate the process. Another possible explanation for this observation is that the severity of the deficiency may be important to disease susceptibility in this mouse model: in this study, although serum levels of vitamin D were significantly decreased at the time of disease initiation, mice were not completely vitamin D deficient as they had only been on diet for two weeks prior to infection with Helicobacter.
Despite the majority of studies showing a strong inverse association between vitamin D levels and IBD, studies that use vitamin D to treat IBD have as of yet, yielded variable results (Table 1). Larmonier et al[108] used supplemental dietary vitamin D to ameliorate IBD in an adoptive T cell transfer model of colitis. In this study, animals were placed on increased dietary vitamin D supplementation after the onset of colitis but there was no difference in colitis or expression in proinflammatory cytokines in vitamin D supplemented animals[108]. In a study performed by Glenn et al[109], Il10-/- mice were fed either 25 IU or 5000 IU vitamin D per kg diet in the food throughout life until 3 mo of age, when the animals were necropsied. While animals fed low levels of dietary vitamin D had decreased VDR expression in proximal colon, there were no differences in incidence or severity of IBD nor were there differences in gene expression by microarray between the groups[109]. Ryz et al[41] demonstrated that while daily treatment with active vitamin D was sufficient to protect against DSS-induced colitis, it exacerbated colitis induced by infection with Citrobacter rodentium. Our group showed protection with supplemental vitamin D using a bacterially-induced colitis model. In Smad3-/- mice, increased dietary vitamin D resulted in decreased inflammation, dysplasia, and colon cancer incidence[39]. We also demonstrated dietary supplementation of vitamin D was a convenient and effective way to improve vitamin D nutritional status as evidenced by a significant increase in serum vitamin D in mice fed increased vitamin D compared to those fed control diet. Importantly, we showed that dietary vitamin D levels that were 5-10 times that of control diet levels did not alter serum calcium levels. These studies as well as others are summarized in Table 1.
One of the conundrums of vitamin D therapy has been how to achieve effective concentrations of the active form of vitamin D within the target tissues without inducing hypercalcemia and vitamin D toxicity. Although effective as an anti-inflammatory or anti-tumor agent, administering metabolically active 1,25(OH)2D3 to animals and humans has resulted in hypercalcemia[40,110-113]. Interestingly, Li et al[114] attempted to avoid vitamin D toxicity by genetically engineering intestinal macrophages to upregulate expression of Cyp27b1 (1α-hydroxylase) specifically in the intestinal tissue following an inflammatory trigger. Using this strategy, they were able to protect mice against DSS-induced colitis and decrease the expression of proinflammatory cytokines without altering serum calcium levels by localizing the active vitamin D to the colon[114]. Other investigators including Strauch et al[115] and Zhu et al[116] have administered 1,25(OH)2D analogs and shown protection against IBD in mouse models while avoiding hypercalcemic effects of active vitamin D.
Chronic inflammation is believed to play a key role in carcinogenesis[33]. Links between inflammation and cancer have been observed in colon cancer in human patients with IBD[30] as well as in liver, pancreatic, stomach, esophageal, and prostate cancers[33]. As discussed above, both human and animal studies suggest that vitamin D can be beneficial in preventing or ameliorating inflammation and clinical disease in IBD[40,41,117]; however, few of these studies further evaluate how these changes in inflammation impact subsequent tumor development. Our laboratory has demonstrated that increased dietary vitamin D significantly decreases the incidence and severity of IBD in Smad3-/- mice, which resulted in significant decreases in resultant tumor formation[39]. Consistent with these results, other investigators have demonstrated that increased concentrations of dietary vitamin D are protective against preneoplastic lesions in another model of inflammation-associated cancer, DSS/azoxymethane (AOM), in a dose-dependent manner[42,118]. Results of animal studies performed evaluating the effects of vitamin D on CAC are summarized in Table 2. Based on our data and that of others, we believe that vitamin D is protective against inflammation-associated cancer at least in part by altering key inflammatory pathways and dampening inflammation that precedes cancer development. However, we have not yet determined whether vitamin D is protective against CAC after the onset of inflammation in Smad3-/- mice, an important question to address, and one that can be addressed, using this animal model. While these studies suggest that vitamin D supplementation may be a useful therapy in a subset of patients with IBD and/or CAC, further research is needed to understand the mechanisms through which vitamin D is working in order to better predict the timing of when patients should be treated with supplemental vitamin D and which populations would benefit the most. In addition, research of vitamin D analogs that do not cause hypercalcemia would be useful in the treatment of IBD/CAC without significant vitamin D toxicity. Lu et al[119] and Klampfer et al[120] provide additional comprehensive reviews of vitamin D signaling pathways and how they might be involved in carcinogenesis.
Aberrant immune responses to intestinal microbiota have been implicated in the etiology of IBD in humans[121]. Similarly, the role of gut bacteria in IBD development is well documented in animal models of IBD[122,123]. While no specific bacteria have been identified as a causative pathogen for IBD, general dysbiosis[119,124-126] as well as an overall decrease in microbial diversity[22,127] has been observed in IBD patients compared to healthy controls. In addition, UC patients had lower microbial diversity during active disease periods compared to periods of disease remission[47,127], demonstrating the fluidity of the microbial composition even within the same individual. Studies using twin pairs discordant for IBD also show that microbial diversity is reduced in twins with IBD compared to healthy counterparts[128]. Interestingly, the twin pairs discordant for UC had not only lower microbial diversity but also reduced host gene transcripts in colonic mucosa compared to healthy twin pairs, suggesting that cross-talk between host and microbiota might also be reduced in UC patients[128]. Antibiotics are part of the treatment armamentarium for IBD patients with active and/or fistulizing disease[36], indicating that manipulation of the microbiome can affect the disease process. In addition, studies are underway to examine the benefit of fecal transplantation for therapy and/or cure of IBD[23,27,37,48,129].
The gut microbiome is not only strongly linked to IBD, but also linked to the development of colon cancer in humans and mice[130]. The microbiota of colorectal cancer patients has been found to be enriched in bacteria that reflect an overall pro-inflammatory configuration[131]. Although the association between an altered gut microbiome and IBD appears to be strong, it is currently unknown whether this is a cause or a consequence of the disease. Recently, the bacterial driver-passenger model has been proposed by Tjalsma et al[132] as a hypothesis that could explain microbial changes observed during colon tumorigenesis. In this model, specific bacterial populations with pro-carcinogenic features (driver bacteria) contribute to tumor initiation through the induction of epithelial cell damage, DNA damage, and inciting of inflammation. Then as the tumor progresses, other bacterial populations (passenger bacteria) gain growth advantage with changes in the tumor microenvironment, slowly replacing the “driver” bacteria. Candela et al[131] delve further into the potential role of gut microbiota in colon cancer carcinogenesis in their recent review.
Recent studies indicate that diet plays a role in regulating the microbiome[128] and that the interaction between the gut microbiota and diet may significantly affect IBD development[133,134]. However, mechanistic studies evaluating the impact of individual dietary nutrients on disease and microbiota are not feasible to perform in human populations and therefore, animal models are crucial for answering these questions.
As vitamin D plays a significant role in host immune responses, it has the potential to influence the gut microbiome. Although the role of vitamin D in shaping the microbiome has not been explored, studies indicate several mechanisms through which vitamin D may influence microbiota. Vitamin D can affect both immune cells and colonic epithelial cells; via modulating immune cell differentiation and maturation, colon barrier function, and the secretion of antimicrobial peptides, mucins, and cytokines, all of which have the potential to modulate the gut bacteria[91,101,135]. Vitamin D deficient mice have decreased expression of Angiogenin-4 (an antimicrobial peptide) within the colon and consequently have an increased bacterial load with decreases in Lactobacillus species and increases in Clostridium and Bacteroides species within the colon, suggesting that vitamin D deficiency may impair microbial homeostasis[105,136]. Similarly, mice lacking VDR in the colonic epithelial cells have increased bacterial loads within the colonic mucosa, an increased concentration of Bacteroides fragilis, a bacterial species that has been associated with IBD in humans, and an increased susceptibility to colitis[137]. In addition, mice lacking the ability to respond to vitamin D (due to lack of VDR or CYP27B1) have distinct differences in their gut microbiome compared to wild-type littermates with intact vitamin D signaling[91,137], demonstrating that host responses to vitamin D impact the microbiome. Conversely, Wu et al[138] have demonstrated that specific gut bacteria such as Lactobacillus rhamnosus and Lactobacillus plantarum, two bacteria commonly found in probiotics, are associated with increased VDR expression in colon epithelial cells and ameliorate colitis in a VDR-dependent manner. Interestingly, when the VDR mutant animals are co-housed with wild type animals, the wild type mice exhibit an increase in susceptibility to DSS colitis, indicating that the susceptibility-inducing microbiota is transferrable and directly influences clinical disease[137].
While these studies suggest that vitamin D signaling can influence the microbiome and vice versa, further studies are needed to better understand specifically how vitamin D is altering the composition of the microbiome and what role this could play in disease development. Recent technical advancements in germ-free animal research and microbiome research provide opportunities to address whether microbiota play a causative role on IBD/CAC as well as whether nutrients such as vitamin D can influence disease by modulating the microbiome.
Both human epidemiologic and animal studies suggest that vitamin D plays a role in the development of inflammatory bowel disease as well as colon cancer[16-22]. While vitamin D has been shown to regulate immune cell trafficking and differentiation, cellular proliferation, gut barrier function and antimicrobial peptide synthesis[45-47], the specific mechanisms through which vitamin D ameliorates colitis remain unknown. The majority of evidence demonstrates protective links between vitamin D, colitis, and colitis-associated colon cancer, although studies attempting to demonstrate the usefulness of vitamin D as a therapy for these diseases in animal models have yielded mixed results, likely due to our lack of complete understanding of the model systems as well as the role of vitamin D in inflammation and gut homeostasis. As the use of vitamin D as a treatment in patients with IBD would be simple and inexpensive to implement, it is important that we continue to gain additional insights into its mechanisms of action to determine which patient populations may best benefit from this potential adjunctive therapy.
| 1. | Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9399] [Cited by in RCA: 9579] [Article Influence: 504.2] [Reference Citation Analysis (1)] |
| 2. | Holick MF. Vitamin D: Physiology, molecular biology, and clinical applications. 2nd ed. New York: Humana Press 2010; . |
| 3. | Feldman D, Pike JW, Adams JS. Vitamin D. 3rd ed. Amsterdam, Boston: Academic Press 2011; . |
| 4. | Eisman JA, Bouillon R. Vitamin D: direct effects of vitamin D metabolites on bone: lessons from genetically modified mice. Bonekey Rep. 2014;3:499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Holick MF. Optimal vitamin D status for the prevention and treatment of osteoporosis. Drugs Aging. 2007;24:1017-1029. [PubMed] |
| 6. | Brown SD, Calvert HH, Fitzpatrick AM. Vitamin D and asthma. Dermatoendocrinol. 2012;4:137-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 7. | Hewer S, Lucas R, van der Mei I, Taylor BV. Vitamin D and multiple sclerosis. J Clin Neurosci. 2013;20:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 8. | Kostoglou-Athanassiou I, Athanassiou P, Lyraki A, Raftakis I, Antoniadis C. Vitamin D and rheumatoid arthritis. Ther Adv Endocrinol Metab. 2012;3:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823-825. [PubMed] |
| 10. | Lee S, Clark SA, Gill RK, Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology. 1994;134:1602-1610. [PubMed] |
| 11. | Correia LC, Sodré F, Garcia G, Sabino M, Brito M, Kalil F, Barreto B, Lima JC, Noya-Rabelo MM. Relation of severe deficiency of vitamin D to cardiovascular mortality during acute coronary syndromes. Am J Cardiol. 2013;111:324-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Brøndum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. 2012;32:2794-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Milaneschi Y, Hoogendijk W, Lips P, Heijboer AC, Schoevers R, van Hemert AM, Beekman AT, Smit JH, Penninx BW. The association between low vitamin D and depressive disorders. Mol Psychiatry. 2014;19:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 559] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 15. | Sato S, Tanino Y, Saito J, Nikaido T, Inokoshi Y, Fukuhara A, Fukuhara N, Wang X, Ishida T, Munakata M. Relationship between 25-hydroxyvitamin D levels and treatment course of pulmonary tuberculosis. Respir Investig. 2012;50:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 16. | Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 17. | Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 18. | Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine. 2010;77:552-557. [PubMed] |
| 19. | Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684-700. [PubMed] |
| 20. | Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227-231. [PubMed] |
| 21. | Mouli VP, Ananthakrishnan AN. Review article: vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;39:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Ananthakrishnan AN, Cheng SC, Cai T, Cagan A, Gainer VS, Szolovits P, Shaw SY, Churchill S, Karlson EW, Murphy SN. Association between reduced plasma 25-hydroxy vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Wöbke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol. 2014;5:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39:365-379, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 346] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 25. | Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321:103-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 718] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 27. | Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 969] [Article Influence: 80.8] [Reference Citation Analysis (2)] |
| 28. | Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 301] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 29. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1600] [Article Influence: 106.7] [Reference Citation Analysis (3)] |
| 30. | Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 31. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 877] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 32. | Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 33. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [PubMed] |
| 34. | Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 603] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 35. | Zator ZA, Cantu SM, Konijeti GG, Nguyen DD, Sauk J, Yajnik V, Ananthakrishnan AN. Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-α therapy in inflammatory bowel diseases. JPEN J Parenter Enteral Nutr. 2014;38:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Yang L, Weaver V, Smith JP, Bingaman S, Hartman TJ, Cantorna MT. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clin Transl Gastroenterol. 2013;4:e33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 37. | Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 38. | Raftery T, O’Sullivan M. Optimal vitamin D levels in Crohn’s disease: a review. Proc Nutr Soc. 2015;74:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Meeker S, Seamons A, Paik J, Treuting PM, Brabb T, Grady WM, Maggio-Price L. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 2014;74:4398-4408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 40. | Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648-2652. [PubMed] |
| 41. | Ryz NR, Patterson SJ, Zhang Y, Ma C, Huang T, Bhinder G, Wu X, Chan J, Glesby A, Sham HP. Active vitamin D (1,25-dihydroxyvitamin D3) increases host susceptibility to Citrobacter rodentium by suppressing mucosal Th17 responses. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1299-G1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 42. | Hummel DM, Thiem U, Höbaus J, Mesteri I, Gober L, Stremnitzer C, Graça J, Obermayer-Pietsch B, Kallay E. Prevention of preneoplastic lesions by dietary vitamin D in a mouse model of colorectal carcinogenesis. J Steroid Biochem Mol Biol. 2013;136:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Fichera A, Little N, Dougherty U, Mustafi R, Cerda S, Li YC, Delgado J, Arora A, Campbell LK, Joseph L. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J Surg Res. 2007;142:239-245. [PubMed] |
| 44. | Wan GJ, Kozma CM, Slaton TL, Olson WH, Feagan BG. Inflammatory bowel disease: healthcare costs for patients who are adherent or non-adherent with infliximab therapy. J Med Econ. 2014;17:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Garg M, Lubel JS, Sparrow MP, Holt SG, Gibson PR. Review article: vitamin D and inflammatory bowel disease--established concepts and future directions. Aliment Pharmacol Ther. 2012;36:324-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 46. | Neuman MG, Nanau RM. Inflammatory bowel disease: role of diet, microbiota, life style. Transl Res. 2012;160:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 47. | Cantorna MT. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc. 2010;69:286-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 48. | Carlberg C, Molnár F. Vitamin D receptor signaling and its therapeutic implications: Genome-wide and structural view. Can J Physiol Pharmacol. 2015;93:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165-1168. [PubMed] |
| 50. | Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J Biol Chem. 2003;278:38084-38093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 51. | Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci USA. 2013;110:15650-15655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 52. | Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14:938-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 53. | Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 1223] [Article Influence: 101.9] [Reference Citation Analysis (1)] |
| 54. | Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1184] [Cited by in RCA: 1107] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 55. | Dimitrov V, Salehi-Tabar R, An BS, White JH. Non-classical mechanisms of transcriptional regulation by the vitamin D receptor: insights into calcium homeostasis, immune system regulation and cancer chemoprevention. J Steroid Biochem Mol Biol. 2014;144 Pt A:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 684] [Cited by in RCA: 634] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 57. | Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2:203-216. [PubMed] |
| 58. | Carlberg C, Dunlop TW. The impact of chromatin organization of vitamin D target genes. Anticancer Res. 2006;26:2637-2645. [PubMed] |
| 59. | Carlberg C, Molnár F. Detailed molecular understanding of agonistic and antagonistic vitamin D receptor ligands. Curr Top Med Chem. 2006;6:1243-1253. [PubMed] |
| 60. | Poon AH, Gong L, Brasch-Andersen C, Litonjua AA, Raby BA, Hamid Q, Laprise C, Weiss ST, Altman RB, Klein TE. Very important pharmacogene summary for VDR. Pharmacogenet Genomics. 2012;22:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000;47:211-214. [PubMed] |
| 62. | Bentley RW, Keown D, Merriman TR, Raj Krishnan M, Gearry RB, Barclay ML, Roberts RL, Day AS. Vitamin D receptor gene polymorphism associated with inflammatory bowel disease in New Zealand males. Aliment Pharmacol Ther. 2011;33:855-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 63. | Malloy PJ, Tasic V, Taha D, Tütüncüler F, Ying GS, Yin LK, Wang J, Feldman D. Vitamin D receptor mutations in patients with hereditary 1,25-dihydroxyvitamin D-resistant rickets. Mol Genet Metab. 2014;111:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 64. | Huet T, Laverny G, Ciesielski F, Molnár F, Ramamoorthy TG, Belorusova AY, Antony P, Potier N, Metzger D, Moras D. A vitamin D receptor selectively activated by gemini analogs reveals ligand dependent and independent effects. Cell Rep. 2015;10:516-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA. 1997;94:9831-9835. [PubMed] |
| 66. | Khalili H, Talasaz AH, Salarifar M. Serum vitamin D concentration status and its correlation with early biomarkers of remodeling following acute myocardial infarction. Clin Res Cardiol. 2012;101:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996;39:690-697. [PubMed] |
| 68. | Schultz M, Butt AG. Is the north to south gradient in inflammatory bowel disease a global phenomenon? Expert Rev Gastroenterol Hepatol. 2012;6:445-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Burisch J, Jess T, Martinato M, Lakatos PL. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 763] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 70. | Jørgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF. Active Crohn’s disease is associated with low vitamin D levels. J Crohns Colitis. 2013;7:e407-e413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (2)] |
| 71. | Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, Issa M. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 72. | Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn’s disease: association with nutrition and disease activity. Gut. 1985;26:1197-1203. [PubMed] |
| 73. | Torki M, Gholamrezaei A, Mirbagher L, Danesh M, Kheiri S, Emami MH. Vitamin D Deficiency Associated with Disease Activity in Patients with Inflammatory Bowel Diseases. Dig Dis Sci. 2015;60:3085-3091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, Chen P, Szolovits P, Xia Z, De Jager PL. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1921-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 75. | Limketkai BN, Bayless TM, Brant SR, Hutfless SM. Lower regional and temporal ultraviolet exposure is associated with increased rates and severity of inflammatory bowel disease hospitalisation. Aliment Pharmacol Ther. 2014;40:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Hlavaty T, Krajcovicova A, Koller T, Toth J, Nevidanska M, Huorka M, Payer J. Higher vitamin D serum concentration increases health related quality of life in patients with inflammatory bowel diseases. World J Gastroenterol. 2014;20:15787-15796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 77. | Liu W, Chen Y, Golan MA, Annunziata ML, Du J, Dougherty U, Kong J, Musch M, Huang Y, Pekow J. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123:3983-3996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 78. | Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 685] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 79. | Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, Giovannucci EL. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502-1508. [PubMed] |
| 80. | Giovannucci E. Vitamin D and cancer incidence in the Harvard cohorts. Ann Epidemiol. 2009;19:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 81. | Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6974] [Cited by in RCA: 7045] [Article Influence: 469.7] [Reference Citation Analysis (1)] |
| 82. | Bruce D, Ooi JH, Yu S, Cantorna MT. Vitamin D and host resistance to infection? Putting the cart in front of the horse. Exp Biol Med (Maywood). 2010;235:921-927. [PubMed] |
| 83. | Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011;23:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 84. | Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood). 2014;239:1524-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 85. | Cantorna MT, Waddell A. The vitamin D receptor turns off chronically activated T cells. Ann N Y Acad Sci. 2014;1317:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 86. | Ooi JH, Chen J, Cantorna MT. Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors? Mol Aspects Med. 2012;33:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 87. | Khoo AL, Joosten I, Michels M, Woestenenk R, Preijers F, He XH, Netea MG, van der Ven AJ, Koenen HJ. 1,25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology. 2011;134:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 88. | Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin d receptor and T cell function. Front Immunol. 2013;4:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 89. | Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci USA. 2008;105:20834-20839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 90. | Cantorna MT, Zhao J, Yang L. Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proc Nutr Soc. 2012;71:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143:1679-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 92. | Bartels LE, Jørgensen SP, Bendix M, Hvas CL, Agnholt J, Agger R, Dahlerup JF. 25-Hydroxy vitamin D3 modulates dendritic cell phenotype and function in Crohn’s disease. Inflammopharmacology. 2013;21:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 93. | Lyakh LA, Sanford M, Chekol S, Young HA, Roberts AB. TGF-beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J Immunol. 2005;174:2061-2070. [PubMed] |
| 94. | Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Z, Kaur S, Raza K, Sansom DM. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189:5155-5164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 95. | Flodström-Tullberg M, Bryceson YT, Shi FD, Höglund P, Ljunggren HG. Natural killer cells in human autoimmunity. Curr Opin Immunol. 2009;21:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 96. | Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol. 2015;15:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 384] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 97. | Al-Jaderi Z, Maghazachi AA. Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins (Basel). 2013;5:1932-1947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 98. | Balogh G, de Boland AR, Boland R, Barja P. Effect of 1,25(OH)(2)-vitamin D(3) on the activation of natural killer cells: role of protein kinase C and extracellular calcium. Exp Mol Pathol. 1999;67:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Weeres MA, Robien K, Ahn YO, Neulen ML, Bergerson R, Miller JS, Verneris MR. The effects of 1,25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells. J Immunol. 2014;193:3456-3462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 100. | Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 483] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 101. | Zhang YG, Wu S, Sun J. Vitamin D, Vitamin D Receptor, and Tissue Barriers. Tissue Barriers. 2013;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 102. | Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208-G216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 515] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 103. | Assa A, Vong L, Pinnell LJ, Rautava J, Avitzur N, Johnson-Henry KC, Sherman PM. Vitamin D deficiency predisposes to adherent-invasive Escherichia coli-induced barrier dysfunction and experimental colonic injury. Inflamm Bowel Dis. 2015;21:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 104. | Chen SW, Wang PY, Zhu J, Chen GW, Zhang JL, Chen ZY, Zuo S, Liu YC, Pan YS. Protective effect of 1,25-dihydroxyvitamin d3 on lipopolysaccharide-induced intestinal epithelial tight junction injury in caco-2 cell monolayers. Inflammation. 2015;38:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 105. | Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423-2432. [PubMed] |
| 106. | Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 107. | Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Larmonier CB, McFadden RM, Hill FM, Schreiner R, Ramalingam R, Besselsen DG, Ghishan FK, Kiela PR. High vitamin D3 diet administered during active colitis negatively affects bone metabolism in an adoptive T cell transfer model. Am J Physiol Gastrointest Liver Physiol. 2013;305:G35-G46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 109. | Glenn AJ, Fielding KA, Chen J, Comelli EM, Ward WE. Long-term vitamin D3 supplementation does not prevent colonic inflammation or modulate bone health in IL-10 knockout mice at young adulthood. Nutrients. 2014;6:3847-3862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Akhter J, Chen X, Bowrey P, Bolton EJ, Morris DL. Vitamin D3 analog, EB1089, inhibits growth of subcutaneous xenografts of the human colon cancer cell line, LoVo, in a nude mouse model. Dis Colon Rectum. 1997;40:317-321. [PubMed] |
| 112. | Huerta S, Irwin RW, Heber D, Go VL, Koeffler HP, Uskokovic MR, Harris DM. 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) Mouse. Cancer Res. 2002;62:741-746. [PubMed] |
| 113. | Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 114. | Li B, Baylink DJ, Walter MH, Lau KH, Meng X, Wang J, Cherkas A, Tang X, Qin X. Targeted 25-hydroxyvitamin D3 1α-hydroxylase adoptive gene therapy ameliorates dss-induced colitis without causing hypercalcemia in mice. Mol Ther. 2015;23:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Strauch UG, Obermeier F, Grunwald N, Dunger N, Rath HC, Schölmerich J, Steinmeyer A, Zügel U, Herfarth HH. Calcitriol analog ZK191784 ameliorates acute and chronic dextran sodium sulfate-induced colitis by modulation of intestinal dendritic cell numbers and phenotype. World J Gastroenterol. 2007;13:6529-6537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 116. | Zhu T, Liu TJ, Shi YY, Zhao Q. Vitamin D/VDR signaling pathway ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis by inhibiting intestinal epithelial apoptosis. Int J Mol Med. 2015;35:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 117. | Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 118. | Murillo G, Nagpal V, Tiwari N, Benya RV, Mehta RG. Actions of vitamin D are mediated by the TLR4 pathway in inflammation-induced colon cancer. J Steroid Biochem Mol Biol. 2010;121:403-407. [PubMed] |
| 119. | Lu R, Wu S, Xia Y, Sun J. The Vitamin D Receptor, Inflammatory Bowel Diseases, and Colon Cancer. Curr Colorectal Cancer Rep. 2012;8:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 120. | Klampfer L. Vitamin D and colon cancer. World J Gastrointest Oncol. 2014;6:430-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 121. | Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 356] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (11)] |
| 122. | Hernández GA, Appleyard CB. Bacterial load in animal models of acute and chronic ‘reactivated’ colitis. Digestion. 2003;67:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1735] [Article Influence: 123.9] [Reference Citation Analysis (1)] |
| 124. | Lepage P, Häsler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Doré J, Raedler A. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 125. | Paik J, Fierce Y, Treuting PM, Brabb T, Maggio-Price L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a-/- male mice. J Nutr. 2013;143:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 126. | Craven B, Zaric V, Martin A, Mureau C, Egan LJ. Effect of genetic deletion or pharmacological antagonism of tumor necrosis factor alpha on colitis-associated carcinogenesis in mice. Inflamm Bowel Dis. 2015;21:485-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Huang DC, Papavasiliou V, Rhim JS, Horst RL, Kremer R. Targeted disruption of the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in ras-transformed keratinocytes demonstrates that locally produced 1alpha,25-dihydroxyvitamin D3 suppresses growth and induces differentiation in an autocrine fashion. Mol Cancer Res. 2002;1:56-67. [PubMed] |
| 128. | de Wouters T, Doré J, Lepage P. Does our food (environment) change our gut microbiome (‘in-vironment’): a potential role for inflammatory bowel disease? Dig Dis. 2012;30 Suppl 3:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 129. | Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1452-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 130. | Zhu Q, Gao R, Wu W, Qin H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biol. 2013;34:1285-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 131. | Candela M, Turroni S, Biagi E, Carbonero F, Rampelli S, Fiorentini C, Brigidi P. Inflammation and colorectal cancer, when microbiota-host mutualism breaks. World J Gastroenterol. 2014;20:908-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 132. | Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 133. | Haller D. Nutrigenomics and IBD: the intestinal microbiota at the cross-road between inflammation and metabolism. J Clin Gastroenterol. 2010;44 Suppl 1:S6-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 134. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3506] [Article Influence: 184.5] [Reference Citation Analysis (1)] |
| 135. | Bartley J. Vitamin D: emerging roles in infection and immunity. Expert Rev Anti Infect Ther. 2010;8:1359-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 136. | Jin D, Wu S, Zhang YG, Lu R, Xia Y, Dong H, Sun J. Lack of Vitamin D Receptor Causes Dysbiosis and Changes the Functions of the Murine Intestinal Microbiome. Clin Ther. 2015;37:996-1009.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 137. | Wu S, Zhang YG, Lu R, Xia Y, Zhou D, Petrof EO, Claud EC, Chen D, Chang EB, Carmeliet G. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015;64:1082-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 138. | Wu S, Yoon S, Zhang YG, Lu R, Xia Y, Wan J, Petrof EO, Claud EC, Chen D, Sun J. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G341-G349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 139. | Zhang H, Wu H, Liu L, Li H, Shih DQ, Zhang X. 1,25-dihydroxyvitamin D3 regulates the development of chronic colitis by modulating both T helper (Th)1 and Th17 activation. APMIS. 2015;123:490-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 140. | Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 363] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 141. | Knackstedt RW, Moseley VR, Sun S, Wargovich MJ. Vitamin D receptor and retinoid X receptor α status and vitamin D insufficiency in models of murine colitis. Cancer Prev Res (Phila). 2013;6:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 142. | Golan MA, Liu W, Shi Y, Chen L, Wang J, Liu T, Li YC. Transgenic Expression of Vitamin D Receptor in Gut Epithelial Cells Ameliorates Spontaneous Colitis Caused by Interleukin-10 Deficiency. Dig Dis Sci. 2015;60:1941-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 143. | Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386-2392. [PubMed] [DOI] [Full Text] |
| 144. | Chen J, Bruce D, Cantorna MT. Vitamin D receptor expression controls proliferation of naïve CD8+ T cells and development of CD8 mediated gastrointestinal inflammation. BMC Immunol. 2014;15:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 145. | Dougherty U, Mustafi R, Sadiq F, Almoghrabi A, Mustafi D, Kreisheh M, Sundaramurthy S, Liu W, Konda VJ, Pekow J. The renin-angiotensin system mediates EGF receptor-vitamin d receptor cross-talk in colitis-associated colon cancer. Clin Cancer Res. 2014;20:5848-5859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gassler N, Shehata MM S- Editor: Gong ZM L- Editor: A E- Editor: Liu XM