Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.906
Peer-review started: June 12, 2015
First decision: October 14, 2015
Revised: November 5, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: January 21, 2016
Processing time: 219 Days and 12.6 Hours
Best known for their anti-resorptive activity in bone, bisphosphonates (BPs) have generated interest as potential antineoplastic agents given their pleiotropic biological effects which include antiproliferative, antiangiogenic and immune-modulating properties. Clinical studies in multiple malignancies suggest that BPs may be active in the prevention or treatment of cancer. Digestive tract malignancies represent a large and heterogeneous disease group, and the activity of BPs in these cancers has not been extensively studied. Recent data showing that some BPs inhibit human epidermal growth factor receptor (HER) signaling highlight a potential therapeutic opportunity in digestive cancers, many of which have alterations in the HER axis. Herein, we review the available evidence providing a rationale for the repurposing of BPs as a therapeutic adjunct in the treatment of digestive malignancies, especially in HER-driven subgroups.
Core tip: Bisphosphonates demonstrate antineoplastic activity in various malignancies but have received little attention in cancers of the digestive tract. We review the preclinical and clinical experience with bisphosphonates in digestive cancers and discuss their potential therapeutic application in this disease group, particularly in the context of recent data on bisphosphonate-induced inhibition of human epidermal growth factor receptor signaling.
- Citation: Ang C, Doyle E, Branch A. Bisphosphonates as potential adjuvants for patients with cancers of the digestive system. World J Gastroenterol 2016; 22(3): 906-916
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/906.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.906
Cancers of the digestive system, including esophagogastric, hepatocellular, pancreatobiliary, small and large bowel carcinomas were projected to comprise about 17% of the 1.6 million new cancer diagnoses made in the United States during 2014[1]. Systemic therapy with cytotoxic chemotherapy and/or molecularly targeted agents is the mainstay of treatment for these cancers when they are at an advanced stage. Despite advances in drug development and improved insights into the molecular pathobiology of these diseases, median survival for most stage IV digestive cancers is less than 12 mo, the exceptions being small bowel and colorectal adenocarcinoma. These sobering facts underscore the chasm between theoretical knowledge and clinical application, and highlight the urgent need for novel therapeutic approaches.
In recent years, there has been a growing recognition that some drugs that are effective in treating one type of disease can be “repurposed” for treatment of an unrelated condition. Repurposing is a particularly attractive option because the therapeutic agents have known safety profiles.
Bisphosphonates (BPs) inhibit osteoclast-induced bone resorption which is a property that underlies their use in the treatment of bone resorption disorders such as osteoporosis and Paget’s disease. In patients with advanced cancer, BPs are used in the supportive management of complications such as hypercalcemia of malignancy, and the prevention of skeletal-related complications in patients with bone metastases. Indications that BPs might have direct antineoplastic effects came from randomized clinical trials of adjuvant estrogen suppression therapy in women with resected breast cancer which revealed that the addition of BPs not only decreased bone density loss but also decreased the risk of contralateral breast cancer and improved disease-free survival[2-4]. The beneficial effects of BPs on clinical outcomes were most pronounced in postmenopausal women in whom systemic estrogen levels are low[4]. Subsequent randomized trials in patients with multiple myeloma and other advanced solid tumors such as lung and prostate cancer provided additional evidence that BPs improve oncologic outcomes including overall survival and prevention of bone metastases[5-10]. In addition, a number of observational studies have reported decreases in risk of breast and colorectal cancer among BP users[11-17]. Collectively, these data suggest that BPs may be clinically active in the prevention as well as treatment of cancer. Studies focusing on the activity of BPs in patients with digestive tract cancers are limited, however.
BPs, especially nitrogen-containing bisphosphonates (NBPs), have antiproliferative, antimotility, pro-apoptotic, antiangiogenic and immunomodulatory properties[5,18,19]. Many of these activities are attributed to inhibition of the mevalonate synthesis pathway by NBPs[20,21]. Recently, NBPs have been shown to bind to and inhibit signaling by the human epidermal growth factor receptor (human EGFR/HER), causing apoptosis in HER-driven cancer cell lines and synergizing with HER tyrosine kinase inhibitors[22,23]. Many digestive cancers have alterations in the HER axis, highlighting an actionable target for NBPs. In this review, we summarize the preclinical and clinical experience with BPs in digestive malignancies and discuss how BPs might be integrated into current treatment strategies.
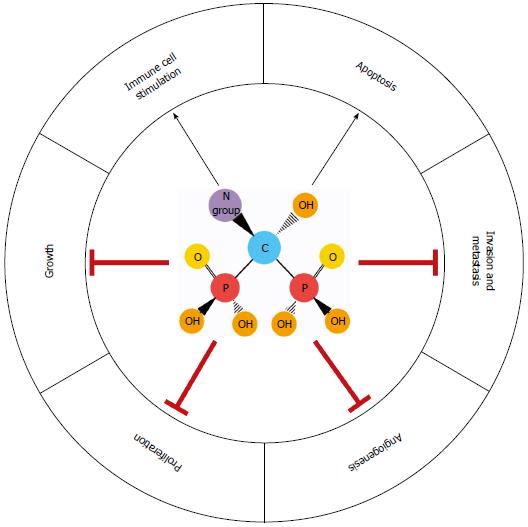
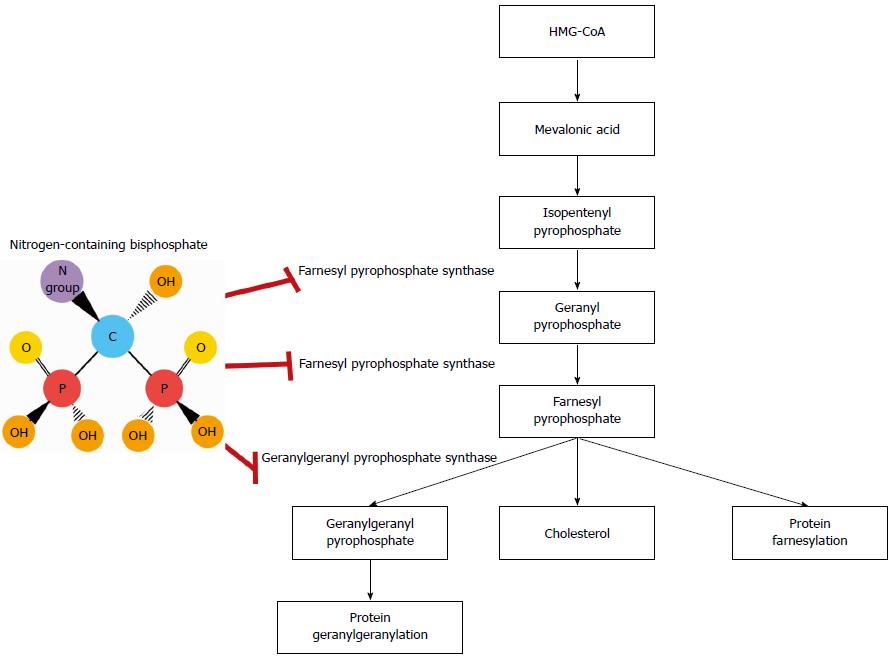
BPs are inorganic pyrophosphate derivatives with a central nonhydrolyzable carbon atom, a hydroxyl group, and two flanking phosphate groups (Figure 1). The chemical structure of BPs confers a strong affinity for the mineral component of bone, which facilitates their uptake by osteoclasts[24]. Bone resorption is inhibited by BPs due to osteoclast growth arrest and apoptosis. The addition of a nitrogen group increases the antiresorptive potency of BPs by up to 10000 fold[21,24]. NBPs currently used in clinical practice include the oral agents alendronate, ibandronate and risedronate, and intravenous formulations such as pamidronate and zoledronic acid (ZA).
The molecular mechanisms of action differ between BPs and NBPs. Early generation BPs, such as etidronate and clodronate, induce osteoclast death by generating cytotoxic ATP analogs, which impair mitochondrial oxygen consumption[25]. As previously mentioned, many of the biological effects of NBPs are attributed to their interactions with the mevalonate synthesis pathway. Among the key components of this pathway are farnesyl pyrophosphate synthase and geranylgeranyl pyrophosphate synthase, which mediate the posttranslational prenylation and activation of small signaling GTPases (e.g., Rab, Rac, Rho, Rap1A and Ras), promoting cell growth, proliferation, migration and survival (Figure 1)[18-21,26,27]. Suppression of the mevalonate synthesis pathway inhibits protein prenylation, arresting these processes in osteoclasts as well as other cell types. In breast cancer cells, ZA inhibits farnesylation of centromere protein-F, preventing assembly of the mitotic spindle apparatus and halting cell cycle progression. The addition of farnesol reverses this process, allowing mitosis to resume[28]. Interestingly, dendritic cells treated with ZA have an enhanced ability to stimulate expansion of γδ T cells, which are cytotoxic against a variety of solid tumor cell lines[29,30]. These events are associated with the accumulation of isopentenyl pyrophosphate, a potent chemoattractant and stimulator of γδ T cells. Increased isopentenyl pyrophosphate also promotes formation of cytotoxic ATP analogs, which disable mitochondrial adenine nuclear translocase, causing apoptosis[31]. Furthermore, tumor cells treated with NBPs show increased sensitivity to γδ T cell-mediated cytotoxicity[32]. BPs also target angiogenesis and cell invasion by countering hypoxia-inducible factor-1α, vascular endothelial growth factor, tumor associated macrophages (TAMs) and matrix metalloproteinases[33-35]. These findings illustrate the pleiotropic effects of BPs on cancer cells and the tumor microenvironment (Figure 2).
A novel mechanism of action of NBPs involving the HER pathway has recently been described. Using protein thermal shift, cell-free kinase assays and computational modeling, NBPs have been shown to bind to the tyrosine kinase domain of HER1/2. Binding leads to global inhibition of HER signaling and decreased viability of HER-driven breast and lung cancer cell lines[22]. The growth inhibitory effects persist despite knockdown of farnesyl pyrophosphate synthase, but are completely abrogated by knockdown of HER, indicating that they are dependent on HER and not the mevalonate synthesis pathway. ZA enhances the antineoplastic efficacy of HER1 tyrosine kinase inhibitor, erlotinib, in lung cancer cells, and inhibits tumor growth and viability in cells that have become erlotinib-resistant[23]. These findings highlight the therapeutic potential of co-targeting HER with both NBPs and anti-HER agents, particularly in patients with HER-driven cancers.
It is important to note the unique toxicities of BPs stemming from their mechanism of action. Osteonecrosis of the jaw is one of the most serious side effects BPs, with a reported incidence ranging from 0.85%-18.6%[36]. The use of BPs for malignant vs benign indications, intravenous vs oral BP formulations, prolonged duration and high cumulative dose of therapy, recent dental procedure, and concurrent therapy (e.g., glucocorticoids, anti-angiogenic agents) are variables that may increase the risk of developing osteonecrosis of the jaw. Atypical femoral fractures are another unusual side effect of BPs, the reported incidence ranging from 0.3 to 11 per 100000 person years[37]. Patients with a prior history of low-energy fracture, glucocorticoid exposure, long duration of BP therapy, pre-existing rheumatoid arthritis or collagen disease and low serum vitamin D levels may be at higher risk. Other reported adverse events include nephrotoxicity, flu-like symptoms, ocular inflammation, atrial fibrillation and hypocalcemia[38].
Studies assessing activity of BPs in esophagogastric cancers are in early stages, but have yielded promising results. In vitro chemosensitivity testing performed on the bone marrow aspirate of a patient with metastatic signet ring gastric adenocarcinoma demonstrated synergy with the combination of gemcitabine, oxaliplatin and ZA[39]. The patient was treated with this combination and experienced a durable complete response that included clearance of cancer cells from the bone marrow. In another study, an alendronate-fluoropyrimidine conjugate showed cytostatic activity in gastric adenocarcinoma cell lines[40]. In esophageal squamous cell carcinoma, high centromere protein F expression has been associated with decreased survival, but confers an increased sensitivity to the combination of ZA and cisplatin[41]. In cells that overexpress centromere protein F, the antiproliferative activity of ZA and cisplatin is synergistic whereas it is additive in cells with low centromere protein F levels. In another study, a synthetic BP analog induced cell cycle arrest, apoptosis and inhibited growth of well, moderate and poorly-differentiated human gastric cancer cell lines in vitro, and in a mouse xenograft model[42]. The induction of apoptosis appeared to be linked to activation of ERK1/2, though activation of MEK and Raf-1 was also observed (Table 1).
| Study Design | Cancer type | Population | Therapy | Main Findings |
| Prospective studies | ||||
| Phase I pilot | Esophagus, gastric | Advanced disease | IMAB362 + ZA +/- IL-2 | Ongoing |
| (NCT01671774) | Claudin 18.2 expression+ | |||
| Phase I | Pancreas | n = 23 with resectable disease | ZA once pre-op, and twice post-op | Median/1 yr/2 yr OS: |
| 18 mo/86%/33% | ||||
| Median/1 yr/2 yr PFS: | ||||
| 12 mo/27%/9% | ||||
| No improvement | ||||
| Phase II | HCC | Advanced disease | Sorafenib + ZA | Ongoing |
| (NCT01259193) | ||||
| Observational studies | ||||
| Restricted open cohort study[15] | Colorectal | Region: Denmark 30505 postmenopausal female BP users matched 1:4 BP non-users | Alendronate | Alendronate associated with decreased incidence (HR = 0.69, 95%CI: 0.6-0.79), risk of death (HR = 0.62, 95%CI: 0.52-0.72) and longer survival (HR = 0.82, 95%CI: 0.7-0.97, P < 0.05)1 |
| Systematic review and meta-analysis[16] | Colorectal | Country: various | Alendronate, pamidronate, etidronate, ibandronate, risedronate, ZA | Significant decrease in cancer incidence (HR = 0.83, 95%CI: 0.76-0.90) |
| 20001 cancer cases | ||||
| 392106 patients total | ||||
| Case control[17] | Colorectal | Country: Israel | Any oral BP (95% alendronate) | BP use > 1 yr associated with significant decrease in cancer risk (RR = 0.5, 95%CI:I 0.35-0.71)2 |
| Postmenopausal women | ||||
| 933 cancer cases matched 1:1 with controls without cancer | ||||
| Case control[46] | Esophagus, gastric | Country: United Kingdom | Any BP except pamidronate and ibandronate | Esophagus cancer risk significantly higher in female BP users than non-users (OR = 1.43, 95%CI: 1.18-1.72)3 |
| 8636 cancer cases matched 1:4 with controls without cancer | Higher risk with alendronate | |||
| No difference in gastric cancer risk | ||||
| Nested case control[47] | Esophagus, gastric, colorectal | Country: United Kingdom | Any oral BP | |
| 15613 cancer cases matched 1:5 with controls without cancer | Rx for BP associated with significant increase in risk of esophagus (RR = 1.3, 95%CI: 1.02-1.66, P = 0.02) but not gastric or colorectal cancer4 | |||
| Matched cohort[48] | Esophagus, gastric | Country: Denmark | Highest risk: ≥ 10 Rx, ≥ 3 yr | |
| History of fracture | Any oral BP | 85 cancer cases total | ||
| 13678 cases who filled BP Rx matched 1:2 with controls who did not fill BP Rx | (alendronate > etidronate > ibandronate, risedronate, clodronate) | BP use associated with significantly decreased risk of esophagus cancer (HR = 0.35, 95%CI: 0.14-0.85, P = 0.02)5 No effect on gastric cancer risk | ||
| Matched cohort[49] | Esophagus, gastric | Country: United Kingdom | Any oral BP | 207 cancer cases total |
| 41826 cases Rx BP matched 1:1 with controls not Rx BP | No increase in risk of esophagus or gastric cancer. Risk not affected by NBP vs non-NBP, duration of use, history of GERD6 | |||
| Nested matched case control[50] | Esophagus | Country: United States | Etidronate, tiludronate, | < 2% of cases and controls filled Rx for BP |
| History of Barrett’s esophagus | alendronate, ibandronate, risedronate | Non-significant association between BP | ||
| 116 with cancer matched 1:6 with controls without cancer | use and esophagus cancer risk (incidence density ratio 0.92, 95%CI: 0.21-4.15)7 | |||
| Nested case control using 2 datasets[51] | Esophagus, gastric, colorectal | Country: United Kingdom 55952 cancer cases matched 1:5 with controls without cancer | Alendronate, etidronate, ibandronate, risedronate | BP use not associated with risk of esophagus or colorectal cancer Short but not long term alendronate associated with increased risk of gastric cancer (OR = 1.91, 95%CI: 1.34-2.72, P < 0.001) in one dataset8 |
| Case control[52] | Esophagus | Country: Taiwan | Alendronate, risedronate, | No relationship between BP use and esophagus cancer risk |
| 16204 cancer cases matched 1:4 with controls without cancer | clodronate, etidronate | Inverse relationship between esophagus cancer risk and BP duration and frequency of use | ||
| Meta-analysis observational data[53] | Esophagus | Country: various 3778 cancer cases 173612 BP users 483797 BP non-users | Ibandronate, etidronate, clodronate, zoledronate, pamidronate, alendronate | No association between BP use and esophagus cancer risk |
| Cohort study[55] | Esophagus, gastric | Country: United States 1.64 million patients > 68 yr old with history of osteoporosis and/or BP use | Any oral BP | No association between BP use and esophagogastric cancer risk9 |
| 2308 cancer cases | ||||
| 624840 BP users | ||||
| Meta-analysis of observational data[56] | Esophagus, gastric, colorectal | Country: various 16662 cancer cases | Any oral BP | No significant association between BP use and overall digestive cancer risk |
| 79379 controls without cancer | ||||
| Meta-analysis of observational data[61] | Colorectal | Country: various 63363 cancer cases 200047 BP users | Any oral BP | No significant change or borderline significant decrease in risk of colorectal cancer |
| 1038526 BP non-users | ||||
| Case series[70] | HCC | Country: Italy n = 15 patients with bone | ZA | Decreased pain score and analgesic requirements |
| metastases, heavily pre-treated | Median OS 10 mo | |||
| Retrospective cohort study[73] | HCC | Country: Japan n = 31 patients with bone metastases treated with radiation, 12 also received ZA | ZA | Significant decrease in 6-mo time to pain progression of radiated (0% vs 34%, P = 0.045) and non-irradiated (20% vs 66%, P = 0.005) bone metastases Significant decrease in 3-mo radiographic progression rate of non-irradiated bone metastases (29% vs 91%, P = 0.009) |
HER-2 overexpression in 15%-20% of gastric and gastroesophageal junction adenocarcinomas highlights a patient subgroup who might be particularly responsive to the antineoplastic effects of BPs. Patients with HER-2-positive disease experience improved response rates and survival outcomes with the addition of the anti-HER-2 antibody, trastuzumab, to chemotherapy with 5-fluorouracil and cisplatin[43]. In light of recent data showing that NBPs bind to and inhibit HER1/2 signaling[22], there is rationale for evaluating combination therapy with ZA, trastuzumab and chemotherapy in HER-2-positive gastric and gastroesophageal junction adenocarcinomas. There are, however, currently no trials assessing the combination of BPs and trastuzumab in these cancers. The only clinical study evaluating BPs in gastroesophageal cancers is a phase I trial of ZA, IL-2 and IMAB 362 in patients with Claudin-18.2-expressing cancers (PILOT trial; NCT01671774).
The potential benefits need to be balanced against potential risks, keeping in mind that BP use in patients with a diagnosis of advanced esophagogastric cancers might be of relatively short duration so side effects could be less of a limitation. Severe esophagitis has been reported in users of oral BPs, especially alendronate[44]. Decreasing the dosing frequency of oral BPs, and use of intravenous BPs like ZA which do not come into direct contact the esophageal mucosa have helped to decrease the incidence of esophagitis[38]. Concerns of a potential carcinogenic effect of BPs were raised with reports of esophageal cancer among relatively short-term users (median duration of exposure 1-2 years) of oral BPs[45]. Results of several population-based studies evaluating esophageal cancer risk among BP users and non-users have been inconsistent. Some studies have reported a significantly increased risk among female and long term users of BPs[46,47], while others have reported a decreased risk[48] or no increase in the risk of esophageal cancer among BP users compared to non-users, including those with a history of Barrett’s esophagus[49-53]. At this time, the FDA has not concluded that oral BPs increase the risk of esophageal cancer, nor does it endorse endoscopic screening of patients taking oral BPs who do not have symptoms of esophagitis[54]. Concerning the risk of gastric cancer, studies have reported either a decreased risk or no association with oral BP use[46,47,50,53,55,56]. Additional studies are needed to clarify the risk/benefit ratio.
NBPs may exert a protective effect on intestinal mucosa. Several observational population-based studies have reported a decreased incidence of colorectal cancer as well as increased post-cancer-diagnosis survival among long-term users of oral NBPs[15-17,57]. Mechanisms underlying the chemoprotective properties of NBPs on the intestine have not been well defined, but direct effects on intestinal epithelial cells as well as the stromal compartment are considered likely possibilities. Macrophage activation by intestinal commensal bacteria can precipitate intestinal epithelial inflammation, genetic abnormalities and malignant transformation. Administration of encapsulated liposomal clodronate was shown to deplete colonic macrophages, inhibit inflammation, Wnt/β-catenin signaling and carcinogenesis in IL-10 knockout mice colonized with Enterococcus faecalis[58].
NBPs demonstrate antineoplastic activity in colorectal cancer. ZA induces apoptosis and decreases growth of colon cancer cells in vitro[59]. ZA also promotes colon cancer cell death through adoptive immunotherapy. Colon cancer stem cells exposed to ZA demonstrate an enhanced capacity to expand and activate s Vγ9Vδ2 T cells and are, in turn, rendered more susceptible to cytolysis by Vγ9Vδ2 T cells[60].
Upregulation of the HER pathway is pathogenic in colorectal cancer. Anti-HER1 monoclonal antibodies cetuximab and panitumumab are useful in the treatment of patients with metastatic colorectal cancer whose tumors lack activating KRAS mutations. Growth of HER-driven colon cancer cells, but not cells with low EGFR expression, is inhibited by NBPs[22]. Since KRAS-mutated colorectal cancers are resistant to the antineoplastic effects of anti-HER1 antibodies, it would be interesting to test whether NBPs sensitize tumors to these agents via dual inhibition of HER and RAS.
Despite the strong preclinical rationale and beneficial effects reported by observational studies, composite data from 6 cohort and 4 case-control studies suggest the preventive effect of BPs on the risk of colorectal cancer, if any, is small[61]. Prospective studies are clearly needed to determine the effect of BPs on colorectal cancer outcomes. While it is unlikely that a randomized study of BPs as chemoprevention will be performed, the utility of BPs as adjuncts to standard therapy in patients diagnosed with colorectal cancer warrants investigation. There are no ongoing clinical trials of BPs in colorectal carcinoma currently posted on ClinicalTrials.gov.
Activating mutations in RAS and HER overexpression are among the most common molecular alterations in pancreas cancer and are actionable targets for NBPs. ZA-induced inhibition of RAS and its dependent downstream signal transduction cascades prevents migration and causes growth suppression and apoptosis of human pancreatic cancer cells in vitro[62,63]. Erlotinib is FDA approved for metastatic pancreas cancer in combination with gemcitabine based on a phase III trial demonstrating a statistically significant, though clinically modest increase (6.24 mo vs 5.91 mo, HR = 0.82, P = 0.038) in overall survival compared to gemcitabine alone[64]. Low doses of gemcitabine and ZA demonstrate synergy in inhibiting pancreatic cancer cell growth, invasion and metastases in vitro and in vivo[65]. Given the enhanced antiproliferative activity observed with the addition of ZA to erlotinib[23], it would be interesting to assess the effects of combining chemotherapy with RAS inhibition and dual HER inhibition using ZA and erlotinib in advanced pancreas cancer.
BPs promote pancreatic cancer cell death through other mechanisms. Pancreatic cancer cells cultured in ZA are significantly more susceptible to γδ T cell cytotoxicity than non-cultured cells[63]. BPs may also improve the radiosensitivity of pancreas cancer. Genes involved in cholesterol synthesis, including farnesyl diphosphate synthase have been implicated in pancreatic cancer radioresistance[66]. Inhibition of farnesyl disphosphate synthase by ZA was shown to radiosensitize pancreatic cancer cells in vitro and in vivo in an allograft mouse model.
In addition to their direct effects on tumor cells, BPs may also act upon the stromal compartment in pancreas cancer. TAMs and myeloid derived suppressor cells promote pancreas cancer cell progression by secreting growth factors and impairing host adaptive immune response. In murine pancreatic cancer models, BPs diminish both of these macrophage populations, causing decreases in tumor growth and neoangiogenesis, increased T cell recruitment and improved survival[67,68].
Results of a phase I clinical trial of perioperative ZA in patients with resectable pancreas cancer were recently reported[69]. Treatment with ZA was safe but did not significantly improve overall survival compared to historical institutional data (18 mo vs 17.7 mo, P = 0.9404), and there was no decrease in granulocyte- myeloid-derived suppressor cells in peripheral blood or bone marrow as had been observed in vitro[69]. Potential explanations for the absence of an observed benefit include small sample size (n = 23) and heterogeneity in the use of adjuvant chemotherapy and/or radiotherapy following surgery.
Several case reports and small single institutional series have reported improvements in symptoms and disease control among hepatocellular carcinoma (HCC) patients treated with NBPs[70-74]. Benefits include alleviation of pain and hypercalcemia from bone metastases and improved survival. A patient with HCC and bone metastases experienced a durable complete response with the combination of ZA and sorafenib that persisted for 12 mo after treatment discontinuation[71]. In hepatoma cells BPs activate pro-apoptotic cascades, induce cell cycle arrest and inhibit signaling pathways responsible cell proliferation, survival, adhesion, motility and differentiation[75-79]. ZA also suppresses HCC progression through its effects on several immune cell populations. TAMs enable angiogenesis and are associated with increased tumor microvessel density and disease recurrence after surgery or radiofrequency ablation in HCC[80,81]. Treatment with sorafenib strongly induces peripheral blood recruitment and tumor infiltration by TAMs, and suppresses IL-12b, which stimulates natural killer cells. The addition of ZA restores IL-12b levels and depletes TAMs, causing tumor shrinkage, decreased angiogenesis and lung metastases in HCC mouse models[82,83]. ZA-induced amplification of cytotoxic γδ T cells also enhances hepatoma cell lysis[84,85]. These observations suggest that ZA can enhance the activity of sorafenib or rescue sorafenib-resistant HCC.
The human EGFR pathway has been implicated in the progression of liver fibrosis to cirrhosis and hepatocarcinogenesis[86]. Increased EGF expression is part of a 186-gene signature associated with an increased risk of recurrence and poor survival following resection[87]. In mouse and rat models with chemically or surgically induced liver injury, erlotinib decreased and even reversed fibrosis in some animals, inhibited hepatic stellate cell activation, and prevented hepatocarcinogenesis[86]. These physiological changes were associated with upregulation and downregulation of good and poor-prognosis genes, respectively, thus reversing the poor risk gene signature. A study of erlotinib for the chemoprevention of HCC is currently underway (NCT02273362). Given the inhibitory effects of ZA on HER, it would be interesting to assess combination therapy with erlotinib and ZA in primary as well as secondary prevention of HCC. A phase II clinical trial of sorafenib and ZA for advanced HCC was initiated in 2010 (NCT01259193), but results have not been reported yet.
There are no data, preclinical or clinical, on the activity of BPs in small bowel cancers, likely owing to the rarity of this disease. Next-generation sequencing has identified alterations in ERBB2/HER2 in 15%-30% of duodenal adenocarcinomas[88,89], providing a basis for assessing HER-2-targeted therapies with or without BPs.
In vitro studies in cholangiocarcinomas have shown that ZA causes cell cycle arrest and decreases tumor colony formation, but does not cause apoptosis[90]. The combination of ZA with ABT-737, a BH3 mimetic that sequesters pro-survival BCL-2 proteins, is synergistic in causing apoptosis in cholangiocarcinoma cell lines[91]. The microenvironment of cholangiocarcinomas contains an active immune cell infiltrate that includes TAMs[92], which may be targeted by NBPs to induce tumor cell death and prevent disease dissemination.
Bisphosphonates have pleiotropic biologic effects on cancer cells and their microenvironment, providing a rationale for evaluating their use as therapeutic adjuncts in the management, and possibly prevention, of cancer. Mechanistically, BPs target key processes that are universally operational in oncogenesis, maintenance and progression, suggesting their utility across a broad array of malignancies. Though the reported experience on the clinical use of BPs in digestive cancers is limited, preclinical studies across this diverse disease group consistently show that BPs exert antitumor effects as monotherapies, and may increase the efficacy of other systemic agents when given in combination. The combination of anti-HER agents and NBPs is of particular interest given recent mechanistic insights into the interactions of BPs with the HER family as well as the prevalence of HER aberrations in digestive malignancies. Studies to assess the clinical relevance of BPs as antineoplastic adjuncts in digestive cancers represent a largely untapped research opportunity.
| 1. | Cancer Facts and Figures 2014. American Cancer Society. Accessed January 18. 2015; Available from: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. |
| 2. | Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, von Minckwitz G, Miller J, Schenk N, Coleman R. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol. 2010;21:2188-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 792] [Article Influence: 46.6] [Reference Citation Analysis (1)] |
| 4. | Coleman R, Cameron D, Dodwell D, Bell R, Wilson C, Rathbone E, Keane M, Gil M, Burkinshaw R, Grieve R. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Gnant M, Clézardin P. Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev. 2012;38:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Mystakidou K, Katsouda E, Parpa E, Kelekis A, Galanos A, Vlahos L. Randomized, open label, prospective study on the effect of zoledronic acid on the prevention of bone metastases in patients with recurrent solid tumors that did not present with bone metastases at baseline. Med Oncol. 2005;22:195-201. [PubMed] |
| 7. | Zarogoulidis K, Boutsikou E, Zarogoulidis P, Eleftheriadou E, Kontakiotis T, Lithoxopoulou H, Tzanakakis G, Kanakis I, Karamanos NK. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer. 2009;125:1705-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989-1999. [PubMed] |
| 10. | Avilés A, Nambo MJ, Neri N, Castañeda C, Cleto S, Huerta-Guzmán J. Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med Oncol. 2007;24:227-230. [PubMed] |
| 11. | Cardwell CR, Abnet CC, Veal P, Hughes CM, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of cancer. Int J Cancer. 2012;131:E717-E725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Chlebowski RT, Anderson G, Manson JE, Pettinger M, Yasmeen S, Lane D, Langer RD, Hubbell FA, McTiernan A, Hendrix S. Estrogen alone in postmenopausal women and breast cancer detection by means of mammography and breast biopsy. J Clin Oncol. 2010;28:2690-2697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Rennert G, Pinchev M, Rennert HS. Use of bisphosphonates and risk of postmenopausal breast cancer. J Clin Oncol. 2010;28:3577-3581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Newcomb PA, Trentham-Dietz A, Hampton JM. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br J Cancer. 2010;102:799-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Pazianas M, Abrahamsen B, Eiken PA, Eastell R, Russell RG. Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate--Danish National Register Based Cohort Study. Osteoporos Int. 2012;23:2693-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Singh S, Singh AG, Murad MH, Limburg PJ. Bisphosphonates are associated with reduced risk of colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:232-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Rennert G, Pinchev M, Rennert HS, Gruber SB. Use of bisphosphonates and reduced risk of colorectal cancer. J Clin Oncol. 2011;29:1146-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Clézardin P. Bisphosphonates’ antitumor activity: an unravelled side of a multifaceted drug class. Bone. 2011;48:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Sun M, Iqbal J, Singh S, Sun L, Zaidi M. The crossover of bisphosphonates to cancer therapy. Ann N Y Acad Sci. 2010;1211:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235-242. [PubMed] |
| 21. | Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1179] [Cited by in RCA: 1123] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 22. | Yuen T, Stachnik A, Iqbal J, Sgobba M, Gupta Y, Lu P, Colaianni G, Ji Y, Zhu LL, Kim SM. Bisphosphonates inactivate human EGFRs to exert antitumor actions. Proc Natl Acad Sci USA. 2014;111:17989-17994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Stachnik A, Yuen T, Iqbal J, Sgobba M, Gupta Y, Lu P, Colaianni G, Ji Y, Zhu LL, Kim SM. Repurposing of bisphosphonates for the prevention and therapy of nonsmall cell lung and breast cancer. Proc Natl Acad Sci USA. 2014;111:17995-18000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Russell RG. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci. 2006;1068:367-401. [PubMed] |
| 25. | Lehenkari PP, Kellinsalmi M, Näpänkangas JP, Ylitalo KV, Mönkkönen J, Rogers MJ, Azhayev A, Väänänen HK, Hassinen IE. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002;61:1255-1262. [PubMed] |
| 26. | Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15:1467-1476. [PubMed] |
| 27. | Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581-589. [PubMed] |
| 28. | Brown HK, Ottewell PD, Coleman RE, Holen I. The kinetochore protein Cenp-F is a potential novel target for zoledronic acid in breast cancer cells. J Cell Mol Med. 2011;15:501-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Cabillic F, Toutirais O, Lavoué V, de La Pintière CT, Daniel P, Rioux-Leclerc N, Turlin B, Mönkkönen H, Mönkkönen J, Boudjema K. Aminobisphosphonate-pretreated dendritic cells trigger successful Vgamma9Vdelta2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol Immunother. 2010;59:1611-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Yamasaki A, Onishi H, Morisaki T, Katano M. Induction of cytotoxic T lymphocytes by CEA peptide-pulsed T-cells isolated from patients with advanced cancer. Anticancer Res. 2011;31:2419-2424. [PubMed] |
| 31. | Mönkkönen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsäläinen J, Mönkkönen J. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006;147:437-445. [PubMed] |
| 32. | Tanaka Y, Kobayashi H, Terasaki T, Toma H, Aruga A, Uchiyama T, Mizutani K, Mikami B, Morita CT, Minato N. Synthesis of pyrophosphate-containing compounds that stimulate Vgamma2Vdelta2 T cells: application to cancer immunotherapy. Med Chem. 2007;3:85-99. [PubMed] |
| 33. | Tang X, Zhang Q, Shi S, Yen Y, Li X, Zhang Y, Zhou K, Le AD. Bisphosphonates suppress insulin-like growth factor 1-induced angiogenesis via the HIF-1alpha/VEGF signaling pathways in human breast cancer cells. Int J Cancer. 2010;126:90-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Heikkilä P, Teronen O, Moilanen M, Konttinen YT, Hanemaaijer R, Laitinen M, Maisi P, van der Pluijm G, Bartlett JD, Salo T. Bisphosphonates inhibit stromelysin-1 (MMP-3), matrix metalloelastase (MMP-12), collagenase-3 (MMP-13) and enamelysin (MMP-20), but not urokinase-type plasminogen activator, and diminish invasion and migration of human malignant and endothelial cell lines. Anticancer Drugs. 2002;13:245-254. [PubMed] |
| 35. | Sabatino R, Antonelli A, Battistelli S, Schwendener R, Magnani M, Rossi L. Macrophage depletion by free bisphosphonates and zoledronate-loaded red blood cells. PLoS One. 2014;9:e101260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Hamadeh IS, Ngwa BA, Gong Y. Drug induced osteonecrosis of the jaw. Cancer Treat Rev. 2015;41:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Saita Y, Ishijima M, Kaneko K. Atypical femoral fractures and bisphosphonate use: current evidence and clinical implications. Ther Adv Chronic Dis. 2015;6:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Dalle Carbonare L, Zanatta M, Gasparetto A, Valenti MT. Safety and tolerability of zoledronic acid and other bisphosphonates in osteoporosis management. Drug Healthc Patient Saf. 2010;2:121-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Trojan J, Kim SZ, Engels K, Kriener S, Mitrou PS, Chow KU. In vitro chemosensitivity to gemcitabine, oxaliplatin and zoledronic acid predicts treatment response in metastatic gastric cancer. Anticancer Drugs. 2005;16:87-91. [PubMed] |
| 40. | Weinreich J, Schott TC, Königsrainer I, Küper M, Königsrainer A, Schott H. Cytostatic activity of a 5-fluoro-2’-deoxyuridine-alendronate conjugate against gastric adenocarcinoma and non-malignant intestinal and fibroblast cell lines. Anticancer Res. 2012;32:4299-4305. [PubMed] |
| 41. | Mi YJ, Gao J, Xie JD, Cao JY, Cui SX, Gao HJ, Yao SP, Liu T, Zhang YY, Guo CH. Prognostic relevance and therapeutic implications of centromere protein F expression in patients with esophageal squamous cell carcinoma. Dis Esophagus. 2013;26:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Wang HJ, Liu Y, Fan LQ, Han CL, Jiang Y, Cheng SJ, Li Y. A new bisphosphonate derivative, CP, induces gastric cancer cell apoptosis via activation of the ERK1/2 signaling pathway. Acta Pharmacol Sin. 2013;34:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Full Text] |
| 44. | de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, Pryor-Tillotson S, Seleznick MJ, Pinkas H, Wang KK. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335:1016-1021. [PubMed] |
| 45. | Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Wright E, Schofield PT, Seed P, Molokhia M. Bisphosphonates and risk of upper gastrointestinal cancer--a case control study using the General Practice Research Database (GPRD). PLoS One. 2012;7:e47616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 48. | Abrahamsen B, Eiken P, Eastell R. More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360:1789; author reply 1791-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 49. | Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA. 2010;304:657-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 50. | Nguyen DM, Schwartz J, Richardson P, El-Serag HB. Oral bisphosphonate prescriptions and the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci. 2010;55:3404-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to bisphosphonates and risk of gastrointestinal cancers: series of nested case-control studies with QResearch and CPRD data. BMJ. 2013;346:f114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Ho YF, Lin JT, Wu CY. Oral bisphosphonates and risk of esophageal cancer: a dose-intensity analysis in a nationwide population. Cancer Epidemiol Biomarkers Prev. 2012;21:993-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Sun K, Liu JM, Sun HX, Lu N, Ning G. Bisphosphonate treatment and risk of esophageal cancer: a meta-analysis of observational studies. Osteoporos Int. 2013;24:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | FDA Drug Safety Podcast for Healthcare Professionals: Ongoing safety review of oral osteoporosis drugs (bisphosphonates) and potential increased risk of esophageal cancer. Accessed October 27, 2015. Available from: http://www.fda.gov/Drugs/DrugSafety/DrugSafetyPodcasts/ucm264096.htm. |
| 55. | Morden NE, Munson JC, Smith J, Mackenzie TA, Liu SK, Tosteson AN. Oral bisphosphonates and upper gastrointestinal toxicity: a study of cancer and early signals of esophageal injury. Osteoporos Int. 2015;26:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Oh YH, Yoon C, Park SM. Bisphosphonate use and gastrointestinal tract cancer risk: meta-analysis of observational studies. World J Gastroenterol. 2012;18:5779-5788. [PubMed] [DOI] [Full Text] |
| 57. | Pazianas M, Russell RG. Potential therapeutic effects of oral bisphosphonates on the intestine. Ann N Y Acad Sci. 2011;1240:E19-E25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Yang Y, Wang X, Huycke T, Moore DR, Lightfoot SA, Huycke MM. Colon Macrophages Polarized by Commensal Bacteria Cause Colitis and Cancer through the Bystander Effect. Transl Oncol. 2013;6:596-606. [PubMed] |
| 59. | Sewing L, Steinberg F, Schmidt H, Göke R. The bisphosphonate zoledronic acid inhibits the growth of HCT-116 colon carcinoma cells and induces tumor cell apoptosis. Apoptosis. 2008;13:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287-7296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 61. | Eiken P, Vestergaard P. Oral bisphosphonates and colon cancer: an update. Ther Adv Musculoskelet Dis. 2015;7:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Tassone P, Tagliaferri P, Viscomi C, Palmieri C, Caraglia M, D’Alessandro A, Galea E, Goel A, Abbruzzese A, Boland CR. Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro. Br J Cancer. 2003;88:1971-1978. [PubMed] |
| 63. | Märten A, Lilienfeld-Toal Mv, Büchler MW, Schmidt J. Zoledronic acid has direct antiproliferative and antimetastatic effect on pancreatic carcinoma cells and acts as an antigen for delta2 gamma/delta T cells. J Immunother. 2007;30:370-377. [PubMed] |
| 64. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [PubMed] |
| 65. | Zhao M, Tominaga Y, Ohuchida K, Mizumoto K, Cui L, Kozono S, Fujita H, Maeyama R, Toma H, Tanaka M. Significance of combination therapy of zoledronic acid and gemcitabine on pancreatic cancer. Cancer Sci. 2012;103:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Souchek JJ, Baine MJ, Lin C, Rachagani S, Gupta S, Kaur S, Lester K, Zheng D, Chen S, Smith L. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. Br J Cancer. 2014;111:1139-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 67. | Partecke LI, Günther C, Hagemann S, Jacobi C, Merkel M, Sendler M, van Rooijen N, Käding A, Nguyen Trung D, Lorenz E. Induction of M2-macrophages by tumour cells and tumour growth promotion by M2-macrophages: a quid pro quo in pancreatic cancer. Pancreatology. 2013;13:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61:1373-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 69. | Sanford DE, Porembka MR, Panni RZ, Mitchem JB, Belt BA, Plambeck-Suess SM, Lin G, Denardo DG, Fields RC, Hawkins WG. A Study of Zoledronic Acid as Neo-Adjuvant, Perioperative Therapy in Patients with Resectable Pancreatic Ductal Adenocarcinoma. J Cancer Ther. 2013;4:797-803. [PubMed] |
| 70. | Montella L, Addeo R, Palmieri G, Caraglia M, Cennamo G, Vincenzi B, Guarrasi R, Mamone R, Faiola V, Frega N. Zoledronic acid in the treatment of bone metastases by hepatocellular carcinoma: a case series. Cancer Chemother Pharmacol. 2010;65:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Natori T, Yamaguchi M. [Complete response after sorafenib therapy plus zoledronic acid for advanced hepatocellular carcinoma with bone metastasis - a case report]. Gan To Kagaku Ryoho. 2013;40:635-637. [PubMed] |
| 72. | Ohnishi T, Takeda E, Yogita S, Miyake H, Kinoshita T, Terashima Y, Matsumoto T, Tashiro S. Effects of alendronate on bone metastases and hypercalcemia after surgery for hepatocellular carcinoma. Jpn J Clin Oncol. 2000;30:410-413. [PubMed] |
| 73. | Katamura Y, Aikata H, Hashimoto Y, Kimura Y, Kawaoka T, Takaki S, Waki K, Hiramatsu A, Kawakami Y, Takahashi S. Zoledronic acid delays disease progression of bone metastases from hepatocellular carcinoma. Hepatol Res. 2010;40:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Attili VS, Babu KG, Lokanatha D, Bapsy PP, Ramachandra C, Rajshekar H. Bone metastasis in hepatocellular carcinoma: need for reappraisal of treatment. J Cancer Res Ther. 2008;4:93-94. [PubMed] |
| 75. | Wada A, Fukui K, Sawai Y, Imanaka K, Kiso S, Tamura S, Shimomura I, Hayashi N. Pamidronate induced anti-proliferative, apoptotic, and anti-migratory effects in hepatocellular carcinoma. J Hepatol. 2006;44:142-150. [PubMed] |
| 76. | Kogure T, Ueno Y, Kimura O, Kondo Y, Inoue J, Fukushima K, Iwasaki T, Shimosegawa T. A novel third generation bisphosphonate, minodronate (YM529), prevented proliferation and migration of hepatocellular carcinoma cells through inhibition of mevalonate pathway. Hepatol Res. 2009;39:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Ilyas A, Hashim Z, Naeem N, Haneef K, Zarina S. The effect of alendronate on proteome of hepatocellular carcinoma cell lines. Int J Proteomics. 2014;2014:532953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Liu Q, Tao YH, Bai RZ, Chang SJ, Hua D. Zoledronic acid inhibits growth of hepatocellular carcinoma cells in vitro and in vivo. Chin Med J (Engl). 2013;126:1486-1490. [PubMed] |
| 79. | Honda Y, Takahashi S, Zhang Y, Ono A, Murakami E, Shi N, Kawaoka T, Miki D, Tsuge M, Hiraga N. Effects of bisphosphonate zoledronic acid in hepatocellular carcinoma, depending on mevalonate pathway. J Gastroenterol Hepatol. 2015;30:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Matsubara T, Kanto T, Kuroda S, Yoshio S, Higashitani K, Kakita N, Miyazaki M, Sakakibara M, Hiramatsu N, Kasahara A. TIE2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlates with angiogenesis. Hepatology. 2013;57:1416-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 81. | Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263-266. [PubMed] |
| 82. | Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420-3430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 317] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 83. | Zhu XD, Sun HC, Xu HX, Kong LQ, Chai ZT, Lu L, Zhang JB, Gao DM, Wang WQ, Zhang W. Antiangiogenic therapy promoted metastasis of hepatocellular carcinoma by suppressing host-derived interleukin-12b in mouse models. Angiogenesis. 2013;16:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Hoh A, Dewerth A, Vogt F, Wenz J, Baeuerle PA, Warmann SW, Fuchs J, Armeanu-Ebinger S. The activity of γδ T cells against paediatric liver tumour cells and spheroids in cell culture. Liver Int. 2013;33:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 85. | Bouet-Toussaint F, Cabillic F, Toutirais O, Le Gallo M, Thomas de la Pintière C, Daniel P, Genetet N, Meunier B, Dupont-Bierre E, Boudjema K. Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother. 2008;57:531-539. [PubMed] |
| 86. | Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, DePeralta DK, Chen X, Kuroda T. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 294] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 87. | Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1017] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 88. | Laforest A, Aparicio T, Zaanan A, Silva FP, Didelot A, Desbeaux A, Le Corre D, Benhaim L, Pallier K, Aust D. ERBB2 gene as a potential therapeutic target in small bowel adenocarcinoma. Eur J Cancer. 2014;50:1740-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 89. | Chmielecki J, Ross JS, Wang K, Frampton GM, Palmer GA, Ali SM, Palma N, Morosini D, Miller VA, Yelensky R. Oncogenic alterations in ERBB2/HER2 represent potential therapeutic targets across tumors from diverse anatomic sites of origin. Oncologist. 2015;20:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 90. | Romani AA, Desenzani S, Morganti MM, La Monica S, Borghetti AF, Soliani P. Zoledronic acid determines S-phase arrest but fails to induce apoptosis in cholangiocarcinoma cells. Biochem Pharmacol. 2009;78:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 91. | Romani AA, Desenzani S, Morganti MM, Baroni MC, Borghetti AF, Soliani P. The BH3-mimetic ABT-737 targets the apoptotic machinery in cholangiocarcinoma cell lines resulting in synergistic interactions with zoledronic acid. Cancer Chemother Pharmacol. 2011;67:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Cadamuro M, Morton SD, Strazzabosco M, Fabris L. Unveiling the role of tumor reactive stroma in cholangiocarcinoma: an opportunity for new therapeutic strategies. Available from: http://www.amepc.org/tgc/article/view/1856/2835. |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Garfield D, Kucherlapati MH, Massironi S, Picardi A, Yamagata M S- Editor: Qi Y L- Editor: A E- Editor: Liu XM