Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.1213
Peer-review started: June 11, 2015
First decision: July 20, 2015
Revised: August 17, 2015
Accepted: November 9, 2015
Article in press: November 9, 2015
Published online: January 21, 2016
Processing time: 220 Days and 0.7 Hours
Gastric cancer is a leading cause of cancer-related deaths. However, the mechanisms underlying gastric carcinogenesis remain largely unclear. The association of non-coding RNAs (ncRNAs) with cancer has been widely studied during the past decade. In general, ncRNAs have been classified as small ncRNAs, including microRNAs (miRNAs), and long non-coding RNAs (lncRNAs). Emerging evidence shows that miRNAs and lncRNAs play key roles in the formation and progression of many cancers. In this review, we focus on the regulation of miRNAs and lncRNAs in gastric cancer. miRNAs and lncRNAs appear to be involved in gastric tumor growth, invasion, and metastasis and in establishment of the gastric tumor microenvironment through various mechanisms. Furthermore, we also discuss the possibilities of establishing miRNAs and lncRNAs as potential biomarkers and therapeutic targets for gastric cancer. Taken together, we summarize the emerging roles of ncRNAs in gastric cancer development and their possible clinical significance.
Core tip: Non-coding RNAs (ncRNAs) are recognized as an important player in multiple physiological and pathological processes through diverse mechanisms. This review summarizes the current knowledge on dysregulation of microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) in gastric tumor growth, invasion and metastasis. Moreover, the possibilities of targeting miRNAs and lncRNAs in gastric cancer diagnosis, prognosis and treatment are also discussed.
- Citation: Xie SS, Jin J, Xu X, Zhuo W, Zhou TH. Emerging roles of non-coding RNAs in gastric cancer: Pathogenesis and clinical implications. World J Gastroenterol 2016; 22(3): 1213-1223
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/1213.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.1213
Gastric cancer is one of the leading causes of cancer-related deaths worldwide, with an estimated 951600 new cases and 723100 deaths in 2012[1]. The development of medical and surgical therapy has improved the survival rate of gastric cancer patients. However, survival remains unsatisfactory with < 25% overall 5-year survival rates[2]. The high mortality of gastric cancer is mainly attributed to delayed diagnosis due to the lack of appropriate biomarkers and specific early symptoms. Therefore, it is important to elucidate the mechanisms of gastric carcinogenesis and explore new biomarkers and therapeutic targets for gastric cancer.
The human genome sequencing project revealed that < 2% of the human genome expresses protein-coding RNAs[3,4]. However, several studies have shown that ≥ 90% of the genome is actively transcribed to a diversity of RNAs[5,6]. These RNAs without protein-coding capacity are defined as non-coding RNAs (ncRNAs). Generally, ncRNAs are classified as long ncRNAs (lncRNAs) (> 200 nt) and small ncRNAs (≤ 200 nt). Recent studies showed there are many types of small ncRNAs, including microRNAs (miRNAs), small interfering RNAs, piwi-interacting RNAs, small nuclear RNAs and small nucleolar RNAs[7].
ncRNAs contribute to many biological processes, such as cell proliferation, migration, signaling, development and differentiation. Therefore, they are implicated in the pathogenesis of various diseases, including cancers[8,9]. Increasing data demonstrate that dysregulation of miRNAs and lncRNAs is involved in the development of many human cancers, such as breast, colorectal, lung, liver and gastric cancer[10-12]. Here, we outline our current understanding of the role of miRNAs and lncRNAs in gastric carcinogenesis and highlight their potential clinical value.
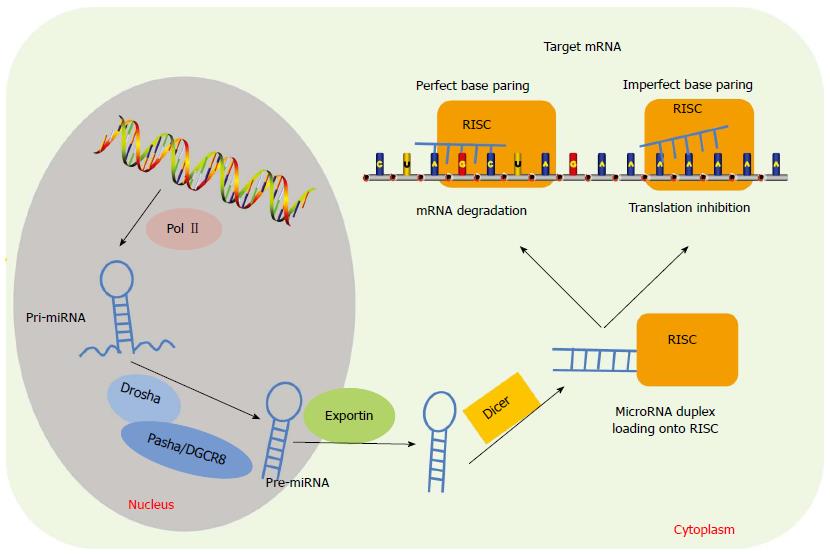
miRNAs are 20-22 nt members of the ncRNA family that regulate genes by triggering mRNA degradation or by translational repression via perfect or imperfect base matching between miRNAs and their target mRNAs (Figure 1). Each miRNA has been shown to target up to 200 mRNAs and therefore they influence many cellular processes, such as cell proliferation, apoptosis, migration, invasion and metabolism[13]. Many studies have demonstrated that dysregulation of miRNAs is associated with the pathogeneses of various cancers, including gastric cancer, through promoting tumor growth, invasion and metastasis (Supplemental Table 1).
| lncRNAs | Expression | Biological processes | Targets | Ref. |
| ABDH11-AS1 | Up | Unknown | Unknown | [41] |
| AC138128.1 | Up | Unknown | Unknown | [42] |
| AK058003 | Up | Promote migration and invasion | γ-Synuclein | [3] |
| ANRIL | Up | Promote proliferation and tumorigenesis | miR-99a/miR-449a | [44] |
| CARLo-5 | Up | Promote proliferation | Unknown | [45] |
| CCAT1 | Up | Promote proliferation and migration | Unknown | [46,47] |
| CCAT2 | Up | Unknown | Unknown | [48] |
| GACAT3 | Up | Unknown | Unknown | [49,50] |
| GAPLINC | Up | Promote proliferation, invasion and tumorigenesis | CD44 | [51] |
| GHET1 | Up | Promote proliferation and tumorigenesis | c-myc | [52] |
| H19 | Up | Promote proliferation and suppress apoptosis; enhance metastasis | p53, miR-675/RUX1, CALN1 | [53-55] |
| HIF1A-AS2 | Up | Unknown | unknown | [56] |
| HOTAIR | Up | Promote migration, invasion, EMT and metastasis | Snail, MMP1, MMP3, HER2, SUZ12 | [57-62] |
| HULC | Up | Promote proliferation, invasion and EMT; suppress apoptosis | Unknown | [63] |
| linc00152 | Up | Unknown | Unknown | [64] |
| linc-UBC1 | Up | Promote proliferation and invasion | Unknown | [65] |
| LSINCT5 | Up | Promote proliferation | Unknown | [66] |
| MALAT1 | Up | Promote proliferation | SF2/ASF | [67] |
| MRUL | Up | Promote MDR | ABCB1 | [68] |
| PVT1 | Up | Promote cell proliferation | p15, p16 | [69] |
| SPRY4-IT1 | Up | Promote tumorigenesis | Unknown | [70] |
| TINCR | Up | Regulate cell proliferation and apoptosis | KLF2 | [71] |
| UCA1 | Up | Unknown | Unknown | [72] |
| LEIGC | Down | Inhibit migration and EMT | Unknown | [73] |
| FENDRR | Down | Suppress migration, invasion and metastasis | FN1 | [74] |
| AA174084 | Down | Unknown | Unknown | [75] |
| BM742401 | Down | Suppress migration, invasion and metastasis | Unknown | [76] |
| FER1L4 | Down | Unknown | miR-106a-5p | [77,78] |
| GACAT1 | Down | Unknown | Unknown | [79,80] |
| GACAT2 | Down | Unknown | Unknown | [50,81] |
| GAS5 | Down | Inhibit proliferation and tumorigenesis | E2F1, p21 | [82] |
| LET | Down | Unknown | Unknown | [83] |
| MEG3 | Down | Inhibit proliferation and promote apoptosis | p53 | [54,85] |
| ncRuPAR | Down | Unknown | PAR1 | [86] |
In 2011, Hanahan and Weinberg summarized the hallmarks of cancer[14]. Some hallmarks, including sustaining proliferative signaling, evasion of growth suppressors, resistance of cell death, and enabling replicative immortality, appear to promote tumor growth[13]. In gastric cancer, the activity of numerous miRNAs has been shown to enhance tumor growth through stimulation of these hallmark processes.
The first studies on the role of miRNAs in gastric cancer were on Let-7a, which is downregulated in gastric cancer tissues. Let-7a directly targets RAB40C, which is a member of the small GTPase RAS family, and downregulation of Let-7a results in the suppression of cell proliferation in vitro and tumor growth in vivo through regulation of RAB40C[15,16]. Our group and others found that expression of another miRNA, miR-375, is frequently decreased in gastric cancer tissues[17-19]. miR-375 plays a crucial role in gastric cancer growth by inhibiting Janus kinase (JAK)2; a key molecule in the cytokine signaling pathway[17]. JAK2 levels are inversely correlated with the levels of miR-375 in gastric cancer tissues. Data from Moriyama’s group also indicate that ectopic expression of miR-375 results in the suppression of cell viability via the caspase-mediated apoptosis pathway by directly targeting phosphoinositide-dependent kinase 1 and 14-3-3ζ in gastric cancer cells[18]. In addition, other groups showed that receptor tyrosine kinase ERBB2 is also targeted by miR-375 in gastric cancer[19]. Thus, these data suggest that miR-375 is involved in gastric carcinogenesis by sustaining proliferative signaling.
Recently, we and another group discovered that miR-215 is upregulated in gastric cancer tissues and induces cell proliferation by binding tumor suppressor gene retinoblastoma 1; a key cell cycle regulator[20,21]. Expression of another miRNA, miR-106a, is also elevated in gastric cancer tissues. miR-106a significantly enhances gastric cancer cell proliferation and prevents apoptosis through interference with the FAS-mediated apoptotic pathway[22,23]. Finally, it has been shown that miR-1182 is downregulated in gastric cancer tissues[24]. miR-1182 targets telomerase reverse transcriptase (hTERT). Telomeres are able to promote replicative immortality, which is controlled by hTERT. In turn, overexpression of hTERT facilitates cell immortality, which increases cell proliferation. Taken together, these studies indicate that, in gastric cancer, aberrant expression of miRNAs results in the promotion of tumor growth through evasion of growth suppressors, resistance of cell death and enabling of replicative immortality.
Recent studies indicated that miRNAs are involved in activating tumor invasion and metastasis. These studies showed that miR-21 expression is frequently elevated in gastric cancer tissues compared with corresponding non-cancerous gastric tissues[24-27]. Furthermore, miRNA-21 is significantly associated with tumor invasion and metastasis. miR-21 apparently promotes gastric tumor invasion by targeting phosphatase and tensin homolog (PTEN)[27].
Several studies showed that miR-148a is downregulated in gastric cancer tissues and that the expression of miR-148a is significantly correlated with TNM stages, lymph node metastasis, and poor prognosis of gastric cancer patients[28-31]. Furthermore, ectopic expression of miR-148a suppresses gastric cancer cell migration and invasion in vitro and lung metastasis in vivo by targeting ROCK1 (rho-associated, coiled-coil-containing protein kinase 1)[28]. miR-148a represses the expression of DNA methyltransferase (DNMT)1, whereas ectopic expression of DNMT1 results in the silencing of miR-148a through hypermethylation of its promoter region[29,30]. These results suggest the existence of a miR-148a/DNMT1 circuit in gastric cancer. In addition, matrix metalloproteinase (MMP)7 and p27, which may contribute to gastric cancer invasion, are also targeted by miR-148a[32,33].
Some miRNAs stimulate the development of gastric cancer through multiple pathways. Our group previously demonstrated that miR-375 is not only involved in tumor growth, but also influences gastric cancer invasion[34]. Moreover, miR-375 expression is negatively regulated by Snail, which binds directly to the putative promoter of miR-375. Snail is a key transcription factor for metastasis.
The crosstalk between cancer cells and their neighboring stroma is required for invasive tumor growth, metastasis, modulation of inflammation and angiogenesis[14]. miRNAs have been shown to play important roles in gastric carcinogenesis induced by Helicobacter pylori (H. pylori) infections. H. pylori infections are a critical risk factor for gastric cancer development. It has previously been shown that downregulation of miR-375 results in the activation of JAK2-signal transducer and activator of transcription (STAT)3 signaling, which promotes H. pylori-mediated inflammation. This in turn facilitates gastric cancer progression[35]. Another miRNA, miR-874, is also downregulated in gastric cancer tissues. Downregulation of miR-874 contributes to tumor angiogenesis through the STAT3/vascular endothelial growth factor-A pathway[36,37]. These results indicate that miRNAs are versatile and involved in the regulation of the tumor microenvironment.
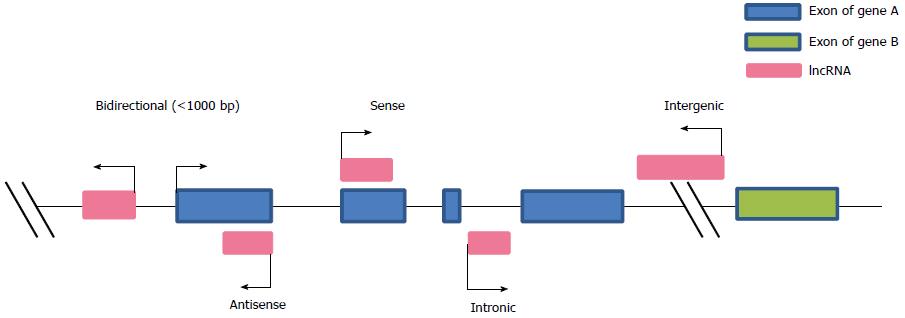
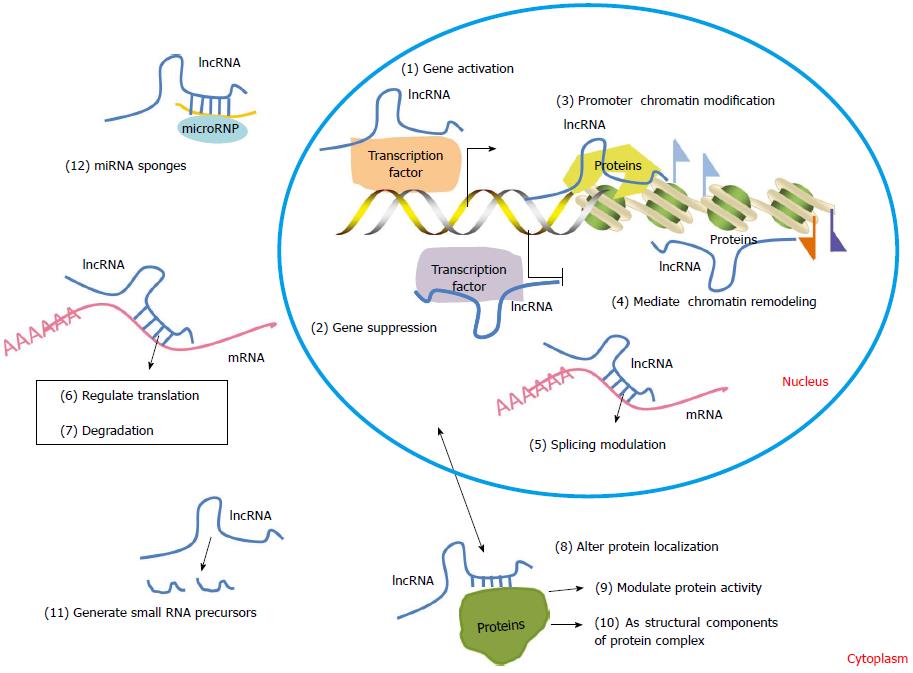
The first lncRNA, H19, was reported by Brannan and colleagues in 1990[38]. To date, the ENCODE project has identified tens of thousands of lncRNAs[5]. According to their locations and characteristics, lncRNAs can be grouped into five categories: sense, antisense, bidirectional, intronic or intergenic (Figure 2)[39]. Increasing data show that lncRNAs may regulate gene expression through diverse mechanisms, including gene activation and suppression, chromatin modification and remodeling, splicing modulation, miRNA sponges and translation (Figure 3).
Research over the last 10 years has accumulated evidence that lncRNAs are important regulators in cell proliferation, apoptosis, migration and differentiation[40]. Similar to miRNAs, lncRNAs are associated with many processes in gene regulation, therefore it may not be surprising that dysregulation of lncRNAs results in tumor growth, invasion and metastasis (Table 1)[41-86].
H19 is a paternally imprinted gene that is highly expressed during embryogenesis but almost completely downregulated shortly after birth[87,88]. Previous studies have shown that H19 is upregulated in gastric cancer and that it is significantly correlated with poor prognosis of gastric cancer patients[53-55]. Furthermore, H19 has been shown to promote gastric cancer cell proliferation, invasion and metastasis through various mechanisms, including processing into miR-675. miR-675 has many targets, such as calneuron 1 and tumor suppressor runt domain transcription factor 1[54,55].
Accumulating evidence indicates that a number of lncRNAs, including antisense ncRNA in the INK4 locus (ANRIL), gastric carcinoma high expressed transcript 1 (GHET1), metastasis associated lung adenocarcinoma transcript 1 (MALAT1), PVT1 oncogene (PVT1) and SPRY4 intronic transcript 1 (SPRY4-IT1), are significantly upregulated in gastric cancer tissues compared with paired non-cancerous tissues, and they are therefore associated with the prognosis of gastric cancer patients[44,52,67,69,70]. ANRIL enhances gastric cancer cell proliferation by silencing miR-99a/miR-449a via binding to polycomb repressive complex 2[44]. Ectopic expression of ANRIL increases the expression of transcription factor E2F1 through repression of miR-449a. Simultaneously, E2F1 promotes ANRIL expression, thus forming a positive feedback loop. GHET1 has been demonstrated to increase the stability of c-myc mRNA by enhancing the physical interaction between c-myc mRNA and insulin-like growth factor 2 mRNA binding protein 1. Stabilization of c-myc mRNA was shown to promote gastric cancer cell growth[52]. MALAT1, a lncRNA associated with metastasis of many cancers, facilitates gastric cancer cell proliferation by recruiting SF2/ASF (serine/arginine-rich splicing factor 1)[67]. PVT1 represses the expression of tumor suppressor genes p15 and p16, which promotes gastric cancer cell proliferation via binding to the zeste homolog 2 enhancer[69]. Finally, SPRY4-IT1 has been found to increase the proliferation, colony formation, and invasion of gastric cancer cells, partially by increasing the expression of MMP-related genes and cyclin D[70].
Other lncRNAs, such as growth arrest-specific transcript (GAS)5 and maternally expressed gene (MEG)3, are frequently downregulated in gastric cancer tissues. They are correlated with poor prognosis of gastric cancer patients[82,84,85]. Ectopic expression of GAS5 decreases gastric cancer cell proliferation and induces apoptosis, partially via regulating E2F1 and p21 expression[82]. Expression of MEG3 is regulated by miR-148a via DNMT1, which inhibits gastric cancer cell proliferation[84,85].
Several studies have shown that lncRNAs are involved in the regulation of tumor invasion and metastasis. The lncRNA Hox transcript antisense intergenic RNA (HOTAIR) is elevated in human cancers, including gastric cancer, and enhances tumor invasion and metastasis[57-62,89]. Knockdown of HOTAIR reverses the epithelial-mesenchymal transition (EMT) process and inhibits invasion by suppressing the expression of MMP1 and MMP3[57]. Furthermore, HOTAIR functions as a competing endogenous RNA and effectively represses HER2 expression through competition for miR-331-3p binding in gastric cancer[59].
Another lncRNA, fetal-lethal noncoding developmental regulatory RNA (FENDRR), is downregulated in gastric cancer tissues. FENDRR inhibits gastric cancer cell migration and invasion via repressing the expression of fibronectin 1 and MMP2/MMP9. Reduced FENDRR expression is significantly correlated with metastasis, TNM stages and poor prognosis of gastric cancer patients[74].
The high mortality of gastric cancer is mainly attributed to failure of early detection and the lack of an effective therapy. Early gastric cancer is either asymptomatic or presented with non-specific symptoms. Also, endoscopic screening is not a common practice in less-developed countries[90]. The current diagnostic biomarkers, including the serological markers carbohydrate antigen (CA)19-9 and carcinoembryonic antigen (CEA), have a low specificity and sensitivity for gastric cancer diagnosis. Thus, there is an urgent need for the discovery of new biomarkers for non-invasive early detection.
Emerging data indicate that gastric cancer patients have different ncRNA serum profiles compared with the healthy controls[91-94]. These profiles appear to be specific in cancer patients and show a higher sensitivity than conventional tumor biomarkers such as CEA and CA19-9. A signature of five specific serum miRNAs (miR-1, miR-20a, miR-27a, miR-34 and miR-423-5p) was able to detect gastric cancer with a sensitivity of 80% and a specificity of 81%. Furthermore, a profile of the three serum lncRNAs CUDR (cancer up-regulated drug resistant), LSINCT-5 (long stress-induced non-coding transcript 5) and PTENP1 (phosphatase and tensin homolog pseudogene 1) showed a better diagnostic accuracy [area under the curve (AUC): 0.92, 95%CI: 0.807-0.974] compared with CEA (AUC: 0.574, 95%CI: 0.432-0.708) and CA19-9 (AUC: 0.580, 95%CI: 0.438-0.713)[94]. These data indicate that ncRNAs may be promising new targets for the development of gastric cancer diagnostic tools.
The expression levels of ncRNAs have been significantly associated with gastric cancer clinical features such as tumor size, invasion and metastasis. For instance, elevated expression of miRNAs such as miR-27a, miR-335, miR-196a and miR-142-5p is associated with a high frequency of recurrence and poor survival of gastric cancer patients[95-98]. Similarly, expression levels of the lncRNAs H19, ANRIL, GHET1, HOTAIR, GAS5, LET, GAPLINC and FENDRR are significantly correlated with the 5-year survival rate of gastric cancer patients[54,44,52,58,82,83,51,74]. Therefore, ncRNAs may be good indicators in gastric cancer prognosis.
Several studies have reported that ncRNAs could affect the resistance of gastric cancer to chemotherapy. For instance, inhibition of miR-21 and miR-223 markedly suppresses gastric cancer cell proliferation by increasing cisplatin sensitivity[99,100]. Furthermore, knockdown of multidrug-resistance-related and upregulated lncRNA increases chemosensitivity of multidrug-resistant gastric cancer cell sublines by facilitating the expression of ABCB1 (ATP-binding cassette, subfamily B, member 1)[68]. Therefore, ncRNAs may be valuable new targets to include in future gastric cancer treatments.
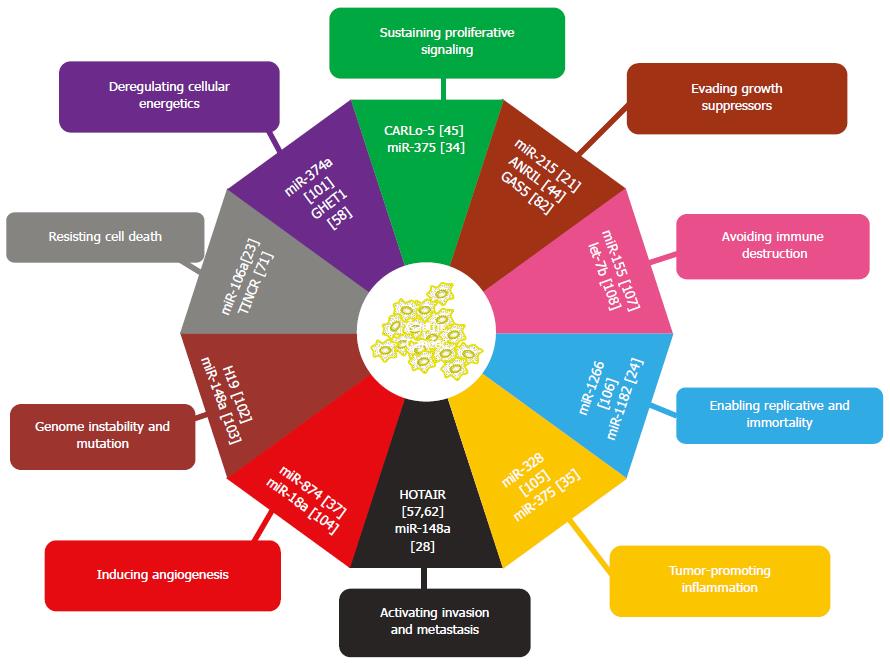
In the last decade, increasing numbers of ncRNAs, including miRNAs and lncRNAs, have been documented to affect gastric cancer. These ncRNAs are aberrantly expressed in gastric cancer tissues, play critical roles in the gastric carcinogenesis, and have potential applications in the diagnosis, prognosis or treatment of gastric cancer. Since tumor progression is a complex and multistep process, several hallmarks have been described that enable normal cells to become tumorigenic and malignant during tumor pathogenesis[14]. A large number of miRNAs and lncRNAs are involved in the regulation of these hallmarks (Figure 4). Aberrant expression of some ncRNAs, including miR-375, ANRIL, miR-106a, miR-1182 and miR-374a, results in gastric cancer growth by promoting hallmark processes such as sustaining proliferative signaling, evasion of growth suppressors, resistance of cell death, enabling replicative immortality and deregulation of cellular energetics. Other ncRNAs, such as miR-328, miR-874 and let-7b, are involved in the interaction between gastric cancer cells and their neighboring stroma and activate invasion and metastasis by facilitating tumor-promoting inflammation, inducing angiogenesis and avoiding immune destruction. Furthermore, genome instability and mutations appear to drive or exacerbate these hallmarks in gastric cancer.
Even though aberrant expression of a number of ncRNAs has been described to stimulate gastric cancer, the underlying molecular mechanisms on the function of these ncRNAs in gastric carcinogenesis are not well understood. Most of the current studies on ncRNAs in gastric cancer focus on their expression profiles. The role of mutations in ncRNAs involved in gastric carcinogenesis should be determined in future studies. New technologies, such as next-generation DNA sequencing and CRISPR-Cas9 genome editing, will further help us to find and characterize the exact role of ncRNAs in gastric cancer.
Clinical applications for ncRNAs in gastric cancer are also in their infancy. Although some ncRNAs may show potential as therapeutic targets, many obstacles, including stability, reliable delivery systems and off-target effects, have to be overcome before clinical trials could commence.
We are grateful to Stijn van der Veen for help with editing the language of our manuscript.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21466] [Article Influence: 1951.5] [Reference Citation Analysis (6)] |
| 2. | Saka M, Morita S, Fukagawa T, Katai H. Present and future status of gastric cancer surgery. Jpn J Clin Oncol. 2011;41:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14554] [Cited by in RCA: 13491] [Article Influence: 963.6] [Reference Citation Analysis (0)] |
| 5. | Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799-816. [PubMed] |
| 6. | Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J, Hofacker IL. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484-1488. [PubMed] |
| 7. | Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15:17-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1635] [Cited by in RCA: 1826] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 8. | He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522-531. [PubMed] |
| 9. | Di Gesualdo F, Capaccioli S, Lulli M. A pathophysiological view of the long non-coding RNA world. Oncotarget. 2014;5:10976-10996. [PubMed] |
| 10. | Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3151] [Cited by in RCA: 3769] [Article Influence: 251.3] [Reference Citation Analysis (0)] |
| 11. | Wang J, Song YX, Wang ZN. Non-coding RNAs in gastric cancer. Gene. 2015;560:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Jiang C, Chen X, Alattar M, Wei J, Liu H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric cancer. Cancer Gene Ther. 2015;22:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [PubMed] |
| 14. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48813] [Article Influence: 3254.2] [Reference Citation Analysis (12)] |
| 15. | Zhang HH, Wang XJ, Li GX, Yang E, Yang NM. Detection of let-7a microRNA by real-time PCR in gastric carcinoma. World J Gastroenterol. 2007;13:2883-2888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Yang Q, Jie Z, Cao H, Greenlee AR, Yang C, Zou F, Jiang Y. Low-level expression of let-7a in gastric cancer and its involvement in tumorigenesis by targeting RAB40C. Carcinogenesis. 2011;32:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, Yao H, Liu X, Ke Y, Si J. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 18. | Tsukamoto Y, Nakada C, Noguchi T, Tanigawa M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M. MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res. 2010;70:2339-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 19. | Shen ZY, Zhang ZZ, Liu H, Zhao EH, Cao H. miR-375 inhibits the proliferation of gastric cancer cells by repressing ERBB2 expression. Exp Ther Med. 2014;7:1757-1761. [PubMed] |
| 20. | Xu YJ, Fan Y. MiR-215/192 participates in gastric cancer progression. Clin Transl Oncol. 2015;17:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Deng Y, Huang Z, Xu Y, Jin J, Zhuo W, Zhang C, Zhang X, Shen M, Yan X, Wang L. MiR-215 modulates gastric cancer cell proliferation by targeting RB1. Cancer Lett. 2014;342:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Xiao B, Guo J, Miao Y, Jiang Z, Huan R, Zhang Y, Li D, Zhong J. Detection of miR-106a in gastric carcinoma and its clinical significance. Clin Chim Acta. 2009;400:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Wang Z, Liu M, Zhu H, Zhang W, He S, Hu C, Quan L, Bai J, Xu N. miR-106a is frequently upregulated in gastric cancer and inhibits the extrinsic apoptotic pathway by targeting FAS. Mol Carcinog. 2013;52:634-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Zhang D, Xiao YF, Zhang JW, Xie R, Hu CJ, Tang B, Wang SM, Wu YY, Hao NB, Yang SM. miR-1182 attenuates gastric cancer proliferation and metastasis by targeting the open reading frame of hTERT. Cancer Lett. 2015;360:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Chan SH, Wu CW, Li AF, Chi CW, Lin WC. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res. 2008;28:907-911. [PubMed] |
| 26. | Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W, Xiao S, Lu H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest. 2008;88:1358-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 380] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 27. | Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY, Yan M. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep. 2012;27:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 28. | Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, Liu L, Jia D, Tian Q, Wu J. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574-7583. [PubMed] |
| 29. | Zhu A, Xia J, Zuo J, Jin S, Zhou H, Yao L, Huang H, Han Z. MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in gastric cancer. Med Oncol. 2012;29:2701-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Zuo J, Xia J, Ju F, Yan J, Zhu A, Jin S, Shan T, Zhou H. MicroRNA-148a can regulate runt-related transcription factor 3 gene expression via modulation of DNA methyltransferase 1 in gastric cancer. Mol Cells. 2013;35:313-319. [PubMed] |
| 31. | Chen Y, Song Y, Wang Z, Yue Z, Xu H, Xing C, Liu Z. Altered expression of MiR-148a and MiR-152 in gastrointestinal cancers and its clinical significance. J Gastrointest Surg. 2010;14:1170-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 32. | Sakamoto N, Naito Y, Oue N, Sentani K, Uraoka N, Zarni Oo H, Yanagihara K, Aoyagi K, Sasaki H, Yasui W. MicroRNA-148a is downregulated in gastric cancer, targets MMP7, and indicates tumor invasiveness and poor prognosis. Cancer Sci. 2014;105:236-243. [PubMed] |
| 33. | Guo SL, Peng Z, Yang X, Fan KJ, Ye H, Li ZH, Wang Y, Xu XL, Li J, Wang YL. MiR-148a promoted cell proliferation by targeting p27 in gastric cancer cells. Int J Biol Sci. 2011;7:567-574. [PubMed] |
| 34. | Xu Y, Jin J, Liu Y, Huang Z, Deng Y, You T, Zhou T, Si J, Zhuo W. Snail-regulated MiR-375 inhibits migration and invasion of gastric cancer cells by targeting JAK2. PLoS One. 2014;9:e99516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Miao L, Liu K, Xie M, Xing Y, Xi T. miR-375 inhibits Helicobacter pylori-induced gastric carcinogenesis by blocking JAK2-STAT3 signaling. Cancer Immunol Immunother. 2014;63:699-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Jiang B, Li Z, Zhang W, Wang H, Zhi X, Feng J, Chen Z, Zhu Y, Yang L, Xu H. miR-874 Inhibits cell proliferation, migration and invasion through targeting aquaporin-3 in gastric cancer. J Gastroenterol. 2014;49:1011-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Zhang X, Tang J, Zhi X, Xie K, Wang W, Li Z, Zhu Y, Yang L, Xu H, Xu Z. miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget. 2015;6:1605-1617. [PubMed] |
| 38. | Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10:28-36. [PubMed] |
| 39. | Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3516] [Cited by in RCA: 4059] [Article Influence: 312.2] [Reference Citation Analysis (0)] |
| 40. | Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4004] [Cited by in RCA: 3983] [Article Influence: 234.3] [Reference Citation Analysis (0)] |
| 41. | Lin X, Yang M, Xia T, Guo J. Increased expression of long noncoding RNA ABHD11-AS1 in gastric cancer and its clinical significance. Med Oncol. 2014;31:42. [PubMed] |
| 42. | Chen X, Sun J, Song Y, Gao P, Zhao J, Huang X, Liu B, Xu H, Wang Z. The novel long noncoding RNA AC138128.1 may be a predictive biomarker in gastric cancer. Med Oncol. 2014;31:262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Wang Y, Liu X, Zhang H, Sun L, Zhou Y, Jin H, Zhang H, Zhang H, Liu J, Guo H. Hypoxia-inducible lncRNA-AK058003 promotes gastric cancer metastasis by targeting γ-synuclein. Neoplasia. 2014;16:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Zhang EB, Kong R, Yin DD, You LH, Sun M, Han L, Xu TP, Xia R, Yang JS, De W. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5:2276-2292. [PubMed] |
| 45. | Zhang Y, Ma M, Liu W, Ding W, Yu H. Enhanced expression of long noncoding RNA CARLo-5 is associated with the development of gastric cancer. Int J Clin Exp Pathol. 2014;7:8471-8479. [PubMed] |
| 46. | Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139:437-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 47. | Mizrahi I, Mazeh H, Grinbaum R, Beglaibter N, Wilschanski M, Pavlov V, Adileh M, Stojadinovic A, Avital I, Gure AO. Colon Cancer Associated Transcript-1 (CCAT1) Expression in Adenocarcinoma of the Stomach. J Cancer. 2015;6:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 48. | Wang CY, Hua L, Yao KH, Chen JT, Zhang JJ, Hu JH. Long non-coding RNA CCAT2 is up-regulated in gastric cancer and associated with poor prognosis. Int J Clin Exp Pathol. 2015;8:779-785. [PubMed] |
| 49. | Xu C, Shao Y, Xia T, Yang Y, Dai J, Luo L, Zhang X, Sun W, Song H, Xiao B. lncRNA-AC130710 targeting by miR-129-5p is upregulated in gastric cancer and associates with poor prognosis. Tumour Biol. 2014;35:9701-9706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Chen S, Li P, Xiao B, Guo J. Long noncoding RNA HMlincRNA717 and AC130710 have been officially named as gastric cancer associated transcript 2 (GACAT2) and GACAT3, respectively. Tumour Biol. 2014;35:8351-8352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Hu Y, Wang J, Qian J, Kong X, Tang J, Wang Y, Chen H, Hong J, Zou W, Chen Y. Long noncoding RNA GAPLINC regulates CD44-dependent cell invasiveness and associates with poor prognosis of gastric cancer. Cancer Res. 2014;74:6890-6902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 794] [Reference Citation Analysis (0)] |
| 52. | Yang F, Xue X, Zheng L, Bi J, Zhou Y, Zhi K, Gu Y, Fang G. Long non-coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c-Myc mRNA stability. FEBS J. 2014;281:802-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 53. | Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J, Fang G. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159-3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 54. | Li H, Yu B, Li J, Su L, Yan M, Zhu Z, Liu B. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318-2329. [PubMed] |
| 55. | Zhuang M, Gao W, Xu J, Wang P, Shu Y. The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun. 2014;448:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 56. | Chen WM, Huang MD, Kong R, Xu TP, Zhang EB, Xia R, Sun M, De W, Shu YQ. Antisense Long Noncoding RNA HIF1A-AS2 Is Upregulated in Gastric Cancer and Associated with Poor Prognosis. Dig Dis Sci. 2015;60:1655-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, Yu PF, Cheng XD. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 58. | Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, Sato I, Yamaguchi K, Tanaka N. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8:e77070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 59. | Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, Kong R, Xia R, Lu KH, Li JH. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 659] [Cited by in RCA: 799] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 60. | Emadi-Andani E, Nikpour P, Emadi-Baygi M, Bidmeshkipour A. Association of HOTAIR expression in gastric carcinoma with invasion and distant metastasis. Adv Biomed Res. 2014;3:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Lee NK, Lee JH, Park CH, Yu D, Lee YC, Cheong JH, Noh SH, Lee SK. Long non-coding RNA HOTAIR promotes carcinogenesis and invasion of gastric adenocarcinoma. Biochem Biophys Res Commun. 2014;451:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Zhang ZZ, Shen ZY, Shen YY, Zhao EH, Wang M, Wang CJ, Cao H, Xu J. HOTAIR Long Noncoding RNA Promotes Gastric Cancer Metastasis through Suppression of Poly r(C)-Binding Protein (PCBP) 1. Mol Cancer Ther. 2015;14:1162-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 63. | Zhao Y, Guo Q, Chen J, Hu J, Wang S, Sun Y. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigation. Oncol Rep. 2014;31:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 64. | Pang Q, Ge J, Shao Y, Sun W, Song H, Xia T, Xiao B, Guo J. Increased expression of long intergenic non-coding RNA LINC00152 in gastric cancer and its clinical significance. Tumour Biol. 2014;35:5441-5447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 65. | Hu Y, Pan J, Wang Y, Li L, Huang Y. Long noncoding RNA linc-UBC1 is negative prognostic factor and exhibits tumor pro-oncogenic activity in gastric cancer. Int J Clin Exp Pathol. 2015;8:594-600. [PubMed] |
| 66. | Xu MD, Qi P, Weng WW, Shen XH, Ni SJ, Dong L, Huang D, Tan C, Sheng WQ, Zhou XY. Long non-coding RNA LSINCT5 predicts negative prognosis and exhibits oncogenic activity in gastric cancer. Medicine (Baltimore). 2014;93:e303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Wang J, Su L, Chen X, Li P, Cai Q, Yu B, Liu B, Wu W, Zhu Z. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF. Biomed Pharmacother. 2014;68:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 68. | Wang Y, Zhang D, Wu K, Zhao Q, Nie Y, Fan D. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. 2014;34:3182-3193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 69. | Kong R, Zhang EB, Yin DD, You LH, Xu TP, Chen WM, Xia R, Wan L, Sun M, Wang ZX. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol Cancer. 2015;14:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 70. | Peng W, Wu G, Fan H, Wu J, Feng J. Long noncoding RNA SPRY4-IT1 predicts poor patient prognosis and promotes tumorigenesis in gastric cancer. Tumour Biol. 2015;36:6751-6758. [PubMed] |
| 71. | Xu TP, Liu XX, Xia R, Yin L, Kong R, Chen WM, Huang MD, Shu YQ. SP1-induced upregulation of the long noncoding RNA TINCR regulates cell proliferation and apoptosis by affecting KLF2 mRNA stability in gastric cancer. Oncogene. 2015;34:5648-5661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 72. | Zheng Q, Wu F, Dai WY, Zheng DC, Zheng C, Ye H, Zhou B, Chen JJ, Chen P. Aberrant expression of UCA1 in gastric cancer and its clinical significance. Clin Transl Oncol. 2015;17:640-646. [PubMed] |
| 73. | Han Y, Ye J, Wu D, Wu P, Chen Z, Chen J, Gao S, Huang J. LEIGC long non-coding RNA acts as a tumor suppressor in gastric carcinoma by inhibiting the epithelial-to-mesenchymal transition. BMC Cancer. 2014;14:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Xu TP, Huang MD, Xia R, Liu XX, Sun M, Yin L, Chen WM, Han L, Zhang EB, Kong R. Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression. J Hematol Oncol. 2014;7:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 75. | Shao Y, Ye M, Jiang X, Sun W, Ding X, Liu Z, Ye G, Zhang X, Xiao B, Guo J. Gastric juice long noncoding RNA used as a tumor marker for screening gastric cancer. Cancer. 2014;120:3320-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 76. | Park SM, Park SJ, Kim HJ, Kwon OH, Kang TW, Sohn HA, Kim SK, Moo Noh S, Song KS, Jang SJ. A known expressed sequence tag, BM742401, is a potent lincRNA inhibiting cancer metastasis. Exp Mol Med. 2013;45:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 77. | Liu Z, Shao Y, Tan L, Shi H, Chen S, Guo J. Clinical significance of the low expression of FER1L4 in gastric cancer patients. Tumour Biol. 2014;35:9613-9617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 78. | Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi Y, Guo J. Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci Rep. 2014;4:6088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 79. | Sun W, Wu Y, Yu X, Liu Y, Song H, Xia T, Xiao B, Guo J. Decreased expression of long noncoding RNA AC096655.1-002 in gastric cancer and its clinical significance. Tumour Biol. 2013;34:2697-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Xiao B, Guo J. Long noncoding RNA AC096655.1-002 has been officially named as gastric cancer-associated transcript 1, GACAT1. Tumour Biol. 2013;34:3271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Shao Y, Chen H, Jiang X, Chen S, Li P, Ye M, Li Q, Sun W, Guo J. Low expression of lncRNA-HMlincRNA717 in human gastric cancer and its clinical significances. Tumour Biol. 2014;35:9591-9595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 82. | Sun M, Jin FY, Xia R, Kong R, Li JH, Xu TP, Liu YW, Zhang EB, Liu XH, De W. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 268] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 83. | Zhou B, Jing XY, Wu JQ, Xi HF, Lu GJ. Down-regulation of long non-coding RNA LET is associated with poor prognosis in gastric cancer. Int J Clin Exp Pathol. 2014;7:8893-8898. [PubMed] |
| 84. | Sun M, Xia R, Jin F, Xu T, Liu Z, De W, Liu X. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol. 2014;35:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 85. | Yan J, Guo X, Xia J, Shan T, Gu C, Liang Z, Zhao W, Jin S. MiR-148a regulates MEG3 in gastric cancer by targeting DNA methyltransferase 1. Med Oncol. 2014;31:879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 86. | Liu L, Yan B, Yang Z, Zhang X, Gu Q, Yue X. ncRuPAR inhibits gastric cancer progression by down-regulating protease-activated receptor-1. Tumour Biol. 2014;35:7821-7829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Poirier F, Chan CT, Timmons PM, Robertson EJ, Evans MJ, Rigby PW. The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development. 1991;113:1105-1114. [PubMed] |
| 88. | Tabano S, Colapietro P, Cetin I, Grati FR, Zanutto S, Mandò C, Antonazzo P, Pileri P, Rossella F, Larizza L. Epigenetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction. Epigenetics. 2010;5:313-324. [PubMed] |
| 89. | Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320-6326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 1048] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 90. | di Mario F, Cavallaro LG. Non-invasive tests in gastric diseases. Dig Liver Dis. 2008;40:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Shiotani A, Murao T, Kimura Y, Matsumoto H, Kamada T, Kusunoki H, Inoue K, Uedo N, Iishi H, Haruma K. Identification of serum miRNAs as novel non-invasive biomarkers for detection of high risk for early gastric cancer. Br J Cancer. 2013;109:2323-2330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 92. | Song MY, Pan KF, Su HJ, Zhang L, Ma JL, Li JY, Yuasa Y, Kang D, Kim YS, You WC. Identification of serum microRNAs as novel non-invasive biomarkers for early detection of gastric cancer. PLoS One. 2012;7:e33608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 93. | Liu R, Zhang C, Hu Z, Li G, Wang C, Yang C, Huang D, Chen X, Zhang H, Zhuang R. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 329] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 94. | Dong L, Qi P, Xu MD, Ni SJ, Huang D, Xu QH, Weng WW, Tan C, Sheng WQ, Zhou XY. Circulating CUDR, LSINCT-5 and PTENP1 long noncoding RNAs in sera distinguish patients with gastric cancer from healthy controls. Int J Cancer. 2015;137:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 95. | Huang D, Wang H, Liu R, Li H, Ge S, Bai M, Deng T, Yao G, Ba Y. miRNA27a is a biomarker for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer. J Cell Biochem. 2014;115:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 96. | Yan Z, Xiong Y, Xu W, Gao J, Cheng Y, Wang Z, Chen F, Zheng G. Identification of hsa-miR-335 as a prognostic signature in gastric cancer. PLoS One. 2012;7:e40037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 97. | Tsai KW, Liao YL, Wu CW, Hu LY, Li SC, Chan WC, Ho MR, Lai CH, Kao HW, Fang WL. Aberrant expression of miR-196a in gastric cancers and correlation with recurrence. Genes Chromosomes Cancer. 2012;51:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 98. | Zhang X, Yan Z, Zhang J, Gong L, Li W, Cui J, Liu Y, Gao Z, Li J, Shen L. Combination of hsa-miR-375 and hsa-miR-142-5p as a predictor for recurrence risk in gastric cancer patients following surgical resection. Ann Oncol. 2011;22:2257-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (3)] |
| 99. | Yang SM, Huang C, Li XF, Yu MZ, He Y, Li J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 100. | Zhou X, Jin W, Jia H, Yan J, Zhang G. MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J Exp Clin Cancer Res. 2015;34:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 101. | Xu X, Wang W, Su N, Zhu X, Yao J, Gao W, Hu Z, Sun Y. miR-374a promotes cell proliferation, migration and invasion by targeting SRCIN1 in gastric cancer. FEBS Lett. 2015;589:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 102. | Lu Y, Lu P, Zhu Z, Xu H, Zhu X. Loss of imprinting of insulin-like growth factor 2 is associated with increased risk of lymph node metastasis and gastric corpus cancer. J Exp Clin Cancer Res. 2009;28:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 103. | Song P, Zhu H, Zhang D, Chu H, Wu D, Kang M, Wang M, Gong W, Zhou J, Zhang Z. A genetic variant of miR-148a binding site in the SCRN1 3’-UTR is associated with susceptibility and prognosis of gastric cancer. Sci Rep. 2014;4:7080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Zheng Y, Li S, Ding Y, Wang Q, Luo H, Shi Q, Hao Z, Xiao G, Tong S. The role of miR-18a in gastric cancer angiogenesis. Hepatogastroenterology. 2013;60:1809-1813. [PubMed] |
| 105. | Ishimoto T, Izumi D, Watanabe M, Yoshida N, Hidaka K, Miyake K, Sugihara H, Sawayama H, Imamura Y, Iwatsuki M. Chronic inflammation with Helicobacter pylori infection is implicated in CD44 overexpression through miR-328 suppression in the gastric mucosa. J Gastroenterol. 2015;50:751-757. [PubMed] |
| 106. | Chen L, Lü MH, Zhang D, Hao NB, Fan YH, Wu YY, Wang SM, Xie R, Fang DC, Zhang H. miR-1207-5p and miR-1266 suppress gastric cancer growth and invasion by targeting telomerase reverse transcriptase. Cell Death Dis. 2014;5:e1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 107. | Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Müller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic Gastritis and Colitis. J Immunol. 2011;187:3578-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 108. | Teng GG, Wang WH, Dai Y, Wang SJ, Chu YX, Li J. Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS One. 2013;8:e56709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: De Re V, Kleeff J, Wei QZ S- Editor: Yu J L- Editor: Kerr C E- Editor: Liu XM