Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.1160
Peer-review started: May 7, 2015
First decision: August 25, 2015
Revised: September 22, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: January 21, 2016
Processing time: 262 Days and 2.7 Hours
Despite improvements in adjuvant therapies for gastric cancer in recent years, the disease is characterized by high recurrence rates and a dismal prognosis. The major improvement in the treatment of recurrent or metastatic gastric cancer in recent years has been the incorporation of trastuzumab, a monoclonal antibody that inhibits human epidermal growth factor receptor 2 (HER2) heterodimerization, after the demonstrated predictive value of the overexpression and/or amplification of this receptor. Beyond HER2, other genetic abnormalities have been identified, and these mutations may be targetable by tyrosine kinase inhibitors or monoclonal antibodies. The demonstration of four distinct molecular subtypes of gastric cancer by the Cancer Genome Atlas study highlight the enormous heterogeneity of the disease and its complex interplay between genetic and epigenetic alterations and provide a roadmap to implement genome-guided personalized therapy in gastric cancer. In the present review, we aim to discuss, from a clinical point of view, the genomic landscape of gastric cancer described in recent studies, the therapeutic insights derived from these findings, and the clinical trials that have been conducted and those in progress that take into account tailored therapies for gastric cancer.
Core tip: Gastric cancer is a highly heterogeneous disease. Recently, significant improvements have been made in the description of the genomic landscape of the disease, and these improvements provide a roadmap to implement genome-guided personalized therapy for gastric cancer. The present review aims to discuss the therapeutic insights derived from these recent findings and present the clinical trials that have been conducted and those in progress that take into account tailored therapies for gastric cancer from a clinical point of view.
- Citation: Jácome AA, Coutinho AK, Lima EM, Andrade AC, Santos JSD. Personalized medicine in gastric cancer: Where are we and where are we going? World J Gastroenterol 2016; 22(3): 1160-1171
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/1160.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.1160
Gastric cancer (GC) is the third cause of cancer-related mortality worldwide[1]. Its incidence is closely related to environmental factors, reflecting a characteristic geographical distribution. Eastern Asia, Central and Eastern Europe and South America represent areas of higher risk of developing the disease, whereas Northern America and most parts of Africa are low-risk areas[1].
Surgical treatment is the therapeutic modality that offers the greatest possibility of cure. The improvement in adjuvant therapies has increased cure rates, but approximately 20%-40% of patients continue to experience recurrence of the disease[2,3]. The prognosis of patients with recurrent or metastatic disease is dismal, with an estimated median survival of 8 mo[4].
Discoveries in the molecular biology of malignant neoplasms, especially the identification of driver mutations, have allowed the identification of biomarkers with prognostic and predictive values, which has improved the individualization of therapy. The first step in this direction in the treatment of GC was provided in a previous study [Trastuzumab in combination with chemotherapy vs chemotherapy alone for treatment of human epidermal growth factor receptor 2 (HER2)-positive advanced gastric and gastro-oesophageal junction cancer (ToGA)], which demonstrated that the addition of the monoclonal antibody trastuzumab to chemotherapy for the treatment of patients with HER2-positive disease conferred a significant overall survival benefit[5].
Since the ToGA study, important advances have been achieved in the molecular characterization of gastric cancer, mainly via gene expression profile studies, which identified four distinct molecular subtypes of the disease[6]. Progress has also been made in identifying new driver mutations and potential therapeutic targets.
The present comprehensive review aims to discuss the current state of personalized treatment of GC, highlighting the complex interplay between genetic and epigenetic alterations from a clinical point of view. Completed and ongoing clinical trials involving targeted therapies and the therapeutic perspectives based on recent discoveries in the molecular biology of the disease are discussed.
The standard treatment of localized or resectable locally advanced GC is surgical resection plus D2 lymphadenectomy associated with adjuvant therapies, with the exception of stage IA disease patients, who are treated with surgery alone[7]. The options for adjuvant treatments currently include adjuvant chemoradiotherapy[8,9], perioperative chemotherapy[10] and adjuvant chemotherapy[11,12], and all of these options have been demonstrated to confer a survival benefit. However, targeted therapies have not been shown to confer a survival benefit as an adjuvant treatment for GC. Ongoing phase II trials are evaluating the combination of capecitabine, oxaliplatin and trastuzumab in the neoadjuvant and adjuvant setting of HER2-positive GC patients (clinicaltrials.gov NCT 01748773, NCT01130337).
The treatment of unresectable locally advanced or metastatic GC is based predominantly on systemic therapy. The cornerstone of the systemic treatment of advanced disease has not significantly changed since the 2000s and remains based on a platinum-fluoropyrimidine combination[13], with the optional addition of anthracyclines[14] or docetaxel[15] to the doublet-therapy. The major advance in the first-line therapy of GC was the addition of trastuzumab for the treatment of HER2-positive patients, which yielded a 35% reduction in the risk of death in patients harboring immunohistochemistry-confirmed HER2 3+ tumors or tumors that exhibited ERBB2 amplification by fluorescence in situ hybridization (FISH)[5].
Recently, the benefit of second-line therapy associated with best supportive care (BSC) was demonstrated. Paclitaxel[16], docetaxel[17], or irinotecan[16] are options after platin and fluoropyrimidine failure. The second monoclonal antibody incorporated into the therapeutic arsenal of advanced GC was ramucirumab, a monoclonal antibody that inhibits vascular endothelial growth factor receptor-2 (VEGFR-2). This monoclonal antibody improved overall survival compared with BSC[18], and it also conferred a benefit when added to paclitaxel as a second-line therapy[19].
GC is a highly heterogeneous disease. The vast majority of cases histologically present as adenocarcinomas and are classified into different histological subtypes based on Lauren’s classification: intestinal-type, with a well-defined glandular pattern; diffuse-type, infiltrative, characterized by minor cell cohesion; and mixed-type, which exhibits intermediate characteristics between the two aforementioned groups[20]. The intestinal-type features better-recognized risk factors and is closely related to Helicobacter pylori infection, especially CagA+ subtype[21], obesity[22] and gastroesophageal reflux disease[23]. The diffuse-type is not associated with clearly defined environmental risk factors but is associated with germline mutations of CDH1, which are responsible for the expression of E-cadherin, or mismatch repair genes (Lynch Syndrome)[6]. However, hereditary GC is responsible for the minority of GC cases. Unlike cases associated with germline mutations, sporadic mismatch repair-deficient GC are characterized by the epigenetic silencing of MLH1 in the context of a CpG island methylator phenotype[6].
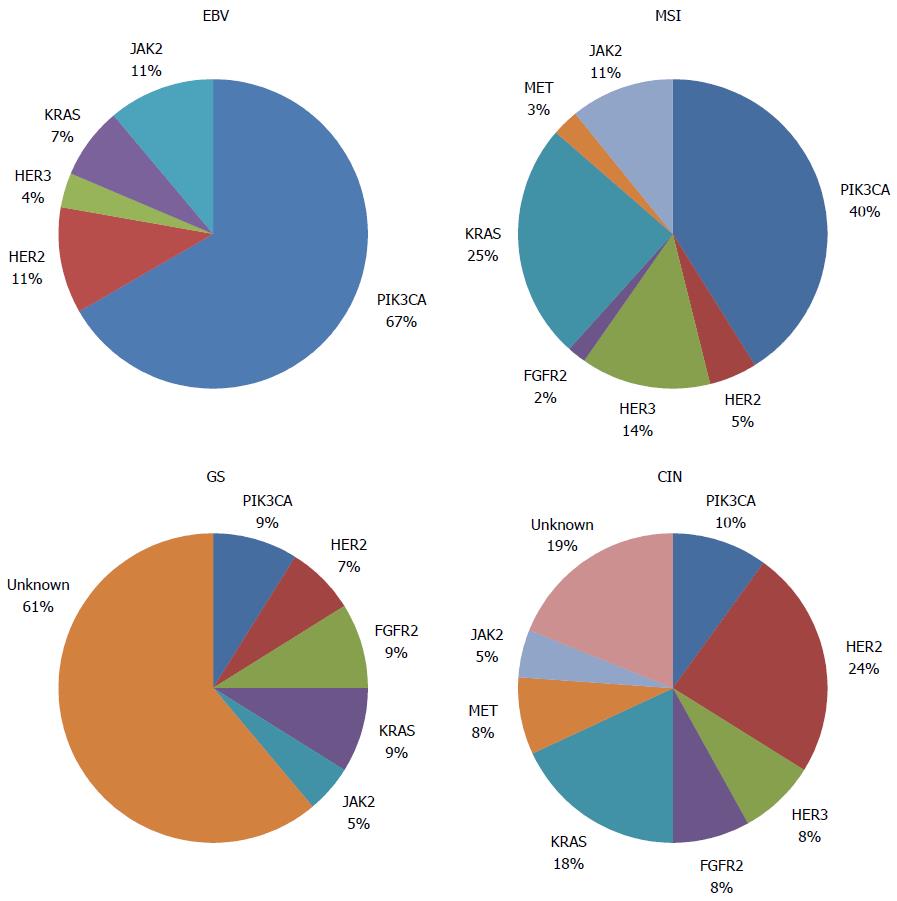
This marked phenotypic diversity encompasses various molecular subtypes. The recently presented Cancer Genome Atlas (TCGA) data, which was obtained using six molecular platforms (array-based somatic copy number analysis, whole-exome sequencing, array-based DNA methylation profiling, messenger RNA sequencing, microRNA (miRNA) sequencing and reverse-phase protein array), proposed a molecular classification that divides GC into four subtypes: EBV-positive, microsatellite unstable (MSI), genomically stable (GS), and chromosomal instability (CIN)[6].
Only 9% of tumors are EBV-positive and exhibit extensive DNA hypermethylation, the highest prevalence in solid tumors reported by TCGA. These tumors are predominantly located in the gastric fundus or body and preferentially occur in males. ARID1A and BCOR mutations are prevalent, whereas TP53 mutations are rare. Specifically, this type is associated with phosphatidylinositol 3-kinase CA (PIK3CA) mutations (80% of this subgroup) as well as the amplification of JAK2, CD274 and PDCD1LG2, which encode a receptor tyrosine kinase, PD-L1 and PD-L2, respectively. Based on these findings, JAK2 inhibitors and PD-L1/2 antagonists might be explored for the treatment of EBV-positive tumors.
MSI tumors are more frequent in females and older patients. These tumors constitute 22% of GC cases, and, as expected, strongly correlate with MLH1 hypermethylation. Unlike MSI colorectal cancer, BRAF V600E mutations have not been identified in MSI GC. Targetable amplifications are generally absent, but mutations in oncogenic signaling proteins, such as PIK3CA, EGFR, ERBB2 and ERBB3, have been noted.
GS tumors are associated with diffuse-type histology and tend to be diagnosed in younger patients. CDH1 mutations are enriched in this subgroup, which represents 20% of GC patients. As in the EBV-subtype, ARID1A mutations are prevalent, and the RHOA gene is almost exclusively found in GS tumors. RHOA controls actin-myosin-dependent cell contractility and cellular motility and activates STAT3 to promote tumorigenesis. Fusions involving RHO-family GTPase-activating proteins (CLDN18 and ARHGAP26, which are involved in junction adhesion structures and cellular motility, respectively) were enriched in the GS subtype and are mutually exclusive to RHOA mutations. The chimeric protein resulting from this fusion impacts cellular adhesion and may contribute to the invasive phenotype of the diffuse-type histology of GC.
The CIN subtype represents the largest group and accounts for 50% of GC cases. It has a predilection for the gastroesophageal junction, is associated with intestinal-type histology, and exhibits the highest rates of ERBB2 amplification among the molecular subtypes. Elevated rates of EGFR amplification and TP53 mutation have also been demonstrated, which is consistent with the marked aneuploidy found in this subtype.
Among the patients evaluated in the TCGA study, survival did not differ among the four molecular subtypes[6]. When comparing the distribution of subtypes between Western and Eastern patients, we observed a similar distribution in both populations. Notably, this comparison was made considering only Vietnam and South Korea as Eastern countries. Additional studies of larger samples of patients are needed to clarify the relationships of geographic regions and ethnic characteristics with the biological profile of GC.
The TCGA study data proposed the classification of gastric cancer into four distinct subtypes, but this classification would benefit from validation by additional studies, especially the assessment of prognosis and predictive value. From a clinical point of view, as well as the molecular classification of breast cancer, more accessible surrogate markers than DNA sequencing, such as immunohistochemistry, need to be identified. Furthermore, the predictive value of these molecular subtypes needs to be evaluated to guide therapeutic decisions.
The identification of driver mutations and the recognition of the complex relationship between genetic and epigenetic alterations are the first steps towards the realization of genome-guided personalized therapy. In recent years, several genetic abnormalities have been demonstrated in GC, which consequently identified potential therapeutic targets. These discoveries were facilitated by exome sequencing studies but relied on small patient samples. Thus, the global molecular portrait of GC remains incomplete.
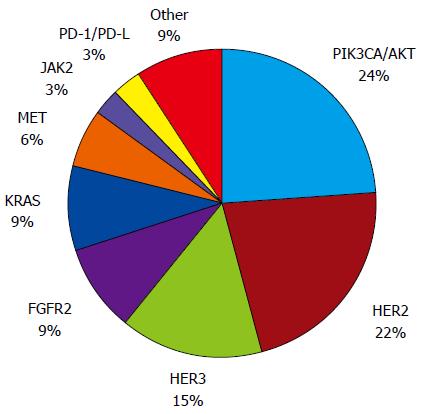
Several genetic abnormalities and driver mutations have been described, and some of these abnormalities have been recognized, validated, and demonstrated to be targetable, including in completed and promising ongoing clinical trials (Figure 1 and Table 1). Furthermore, the rates of genetic and epigenetic abnormalities and driver mutations appear to differ by GC molecular subtype[6] (Figure 2).
| Target | Drug | Study phase | Status |
| HER2 | Trastuzumab | Phase III | Available results[5] |
| T-DM1 | Phase II | Ongoing (NCT01702558) | |
| Phase III | Ongoing (NCT01641939) | ||
| Lapatinib | Phase III | Available results[32] | |
| Phase III | Available results[33] | ||
| HER2/HER3 | Pertuzumab | Phase II | Available results[31] |
| Phase III | Ongoing (NCT01774786) | ||
| EGFR | Cetuximab | Phase III | Available results[37] |
| Panitumumab | Phase III | Available results[38] | |
| Nimotuzumab | Phase II1 | Available results[39] | |
| Erlotinib | Phase II | Available results[40] | |
| VEGF | Bevacizumab | Phase III | Available results[52] |
| VEGFR-2 | Ramucirumab | Phase III | Available results[18] |
| Phase III | Available results[19] | ||
| Phase II1 | Available results[53] | ||
| Phase III | Ongoing (NCT02314117) | ||
| Apatinib | Phase III | Available results[54] | |
| PIK3/Akt/mTOR | Everolimus | Phase III | Available results[61] |
| Phase III | Ongoing (NCT01248403) | ||
| Phase I | Ongoing (NCT01049620) | ||
| MK-2206 | Phase II | Ongoing (NCT01260701) | |
| BYL719 | Phase I | Ongoing (NCT01613950) | |
| FGFR2 | Dovitinib | Phase I/II | Ongoing (NCT01921673) |
| Phase II | Ongoing (NCT01719549) | ||
| AZD4547 | Phase II1 | Available results[68] | |
| MET | Rilotumumab | Phase II | Available results[73] |
| Phase III | Available results[74] | ||
| Phase III | Available results[75] | ||
| Onartuzumab | Phase III | Available results[72] | |
| Foretinib | Phase II | Available results[71] | |
| Tivantinib | Phase II | Available results[76] | |
| PD-L1 | Pembrolizumab | Phase Ib | Available results[84] |
| Phase II | Ongoing (NCT02335411) | ||
| CTLA-4 | Tremelimumab | Phase I/II | Ongoing (NCT02340975) |
| JAK2 | AZD1480 | Phase I | Ongoing (NCT01219543) |
| PARP | Olaparib | Phase III | Ongoing (NCT01924533) |
The epidermal growth factor receptors HER1 (also denoted EGFR), HER2, HER3 and HER4 comprise the EGFR family[24,25]. Except for HER3, these receptors all share the same molecular structure: an extracellular domain that binds to the ligand, a transmembrane portion, and an intracellular domain with tyrosine kinase activity[26]. The binding of different ligands to the extracellular domains triggers intracellular signaling reactions involved in cell differentiation, proliferation and survival. The binding of the ligand to the extracellular domain induces the HER1 homodimerization and heterodimerization of the remaining receptors, especially HER2.
The first biomarker with demonstrated predictive value in the treatment of GC was HER2. Approximately 22% of patients are estimated to overexpress HER2 or amplify ERBB2[5]. Specifically, ERBB2 amplification (24%) appears to be prevalent in the CIN subtype[6]. The prognostic value of HER2 remains controversial[27], but meta-analysis data suggest that patients harboring this gene amplification have a worse prognosis[28,29]. The ToGA study evaluated the impact of the addition of the monoclonal antibody trastuzumab to standard chemotherapy on the overall survival of patients with advanced GC. The addition of trastuzumab to cisplatin plus fluoropyrimidines for the treatment of HER2-positive patients reduced the relative risk of death by 26% (HR = 0.74, 95%CI: 0.60-0.91), permitting an increase in overall survival from 11.1 mo to 13.8 mo[5]. In an exploratory analysis, the risk reduction was more pronounced in the HER2-enriched population, which exhibited 3+ or 2+ immunohistochemistry and FISH-positive status. In this population, the addition of trastuzumab improved survival from 11.8 mo to 16.0 mo (HR = 0.65, 95%CI: 0.51-0.83). The ToGA study ushered in the era of targeted therapy for advanced GC, allowing the inclusion of the first monoclonal antibody in the therapeutic arsenal, which led to the approval of the drug in several countries.
The lack of tyrosine kinase activity in HER3 initially suggested that this receptor is of minor importance in cell proliferation and differentiation, but increasing evidence has demonstrated its role as an important regulator of HER2 activity[26]. The benefit demonstrated by the addition of pertuzumab - a drug that inhibits HER2-HER3 heterodimerization - to trastuzumab in the treatment of HER2-positive breast cancer supports the importance of this heterodimer in the proliferation of tumor cells with HER2 overexpression and/or amplification. These findings identify this new monoclonal antibody as a potentially effective agent in the treatment of GC[30]. ERBB3 amplification appears to be absent in GS-molecular subtype[6]. A phase II trial has shown that the combination of pertuzumab with trastuzumab, capecitabine and cisplatin yielded promising in patients with advanced GC[31], and a phase III clinical trial is ongoing (clinicaltrials.gov NCT01774786). Studies evaluating T-DM1 in HER2-positive advanced GC are currently underway, both in a phase II trial in association with capecitabine (clinicaltrials.gov NCT01702558) and in a phase III trial comparing T-DM1 with taxane after the failure of first-line chemotherapy and trastuzumab (clinicaltrials.gov NCT01641939). Strategies to inhibit the HER2 pathway using lapatinib, a tyrosine kinase inhibitor of HER1 and HER2 receptors, have been unsuccessful. When added to paclitaxel as a second-line therapy for HER2-positive Asian patients, lapatinib did not improve overall survival (11.0 mo vs 8.9 mo, HR = 0.84, 95%CI: 0.64-1.11) in a phase III trial (TyTAN study), despite increasing the response rates (27% vs 9%, OR = 3.85, 95%CI: 1.80-8.87)[32]. In a first-line setting, lapatinib also did not improve the overall survival of HER2-positive advanced GC patients when combined with capecitabine and oxaliplatin in a placebo-controlled, multicenter phase III study (LOGiC study) (clinicaltrials.gov NCT00680901)[33].
EGFR (HER1) is overexpressed in a variable proportion of patients with GC, but gene amplification is found in only a small portion of patients (2%)[34-36]. Strategies to inhibit the EGFR pathway using both monoclonal antibodies and tyrosine kinase inhibitors for the treatment of GC have yielded disappointing results. Specifically, randomized trials of cetuximab[37], panitumumab[38], nimotuzumab[39] and erlotinib[40] in an unselected population of patients showed no clinical benefit. However, evaluation in an enriched population may reveal new findings.
VEGF is a key mediator of physiologic and pathologic angiogenesis[41]. Its activities are mediated by two receptor tyrosine kinases, VEGFR-1 and VEGFR-2. VEGF- and VEGFR-2-mediated signaling seems to play an important role in the pathogenesis of GC[42,43].
Bevacizumab, a monoclonal antibody that targets VEGF-A, exhibits clinical activity in many solid tumors, such as colon cancer[44], lung cancer[45], breast cancer[46], glioblastoma[47], ovarian cancer[48,49] and cervical cancer[50]. Furthermore, based on compelling results in experimental models and phase II trial[51], it was evaluated in a first-line setting for the treatment of advanced GC. Specifically, it was added to standard therapy consisting of cisplatin and fluoropyrimidines in a phase III trial (AVAGAST study)[52]. This trial failed to show significant changes in overall survival in the overall population in response to the addition of bevacizumab (12.1 mo with bevacizumab vs 10.1 mo with placebo, HR = 0.87, 95%CI: 0.73-1.03), but populations with diverse geographical and ethnic backgrounds derived different benefits from the use of this anti-angiogenic agent. Specifically, subgroup analysis showed that bevacizumab improved overall survival in Pan-American patients (11.5 mo vs 6.8 mo, HR = 0.63, 95%CI: 0.43-0.94), whereas Asian (HR = 0.97) and European patients (HR = 0.85) derived no benefit. Notably, bevacizumab improved progression-free survival (6.7 mo vs 5.3 mo, HR = 0.80, 95%CI: 0.68-0.93) and the overall response rate (46% vs 37.4%, P = 0.0315) in the overall population, maintaining the hypothesis that angiogenesis pathway inhibition may be an important therapeutic target and should be further explored.
Ramucirumab, a fully human IgG1 monoclonal antibody VEGFR-2 antagonist that prevents ligand-binding and receptor-mediated pathway activation in endothelial cells, was evaluated in a phase III, placebo-controlled trial as a second-line therapy for advanced GC patients who had progressed after standard therapy consisting of platinum-fluoropyrimidines (REGARD study)[18]. This monoclonal antibody improved overall survival (5.2 mo vs 3.8 mo, HR = 0.77, 95%CI: 0.60-0.99), progression-free survival (2.1 mo vs 1.3 mo, HR = 0.48) and duration of disease control (4.2 mo vs 2.9 mo, P = 0.036). Based on the positive results of the REGARD study, ramucirumab was also evaluated in a second-line setting in association with paclitaxel in a phase III trial compared with paclitaxel plus placebo (RAINBOW study)[19]. Ramucirumab plus paclitaxel improved overall survival (9.6 mo vs 7.4 mo, HR = 0.80, 95%CI: 0.67-0.96), progression-free survival (4.4 mo vs 2.9 mo, HR = 0.63, 95%CI: 0.53-0.75) and disease control (80% vs 64%, P < 0.001). Both of these studies validate the role of VEGFR-2 as an important therapeutic target in GC. Similar to the AVAGAST study, the RAINBOW study showed that anti-angiogenic agents did not benefit Asian patients. This finding may be due to the post-study therapies that are commonly used in Eastern countries[19,52]. However, genetic and epigenetic alterations associated with geographical and ethnic backgrounds may also be responsible for these differences. Thus, a biomarker that would permit the use of both bevacizumab and ramucirumab in enriched populations needs to be identified; this biomarker may allow angiogenesis inhibitors to be used to a maximum benefit. Motivated by these results involving ramucirumab, the RAINFALL study, a phase III trial evaluating the association of ramucirumab with cisplatin plus capecitabine in a first-line setting of patients with advanced disease (clinicaltrials.gov NCT02314117), is ongoing, despite the unsuccessful results of a placebo-controlled, randomized phase II trial that evaluated ramucirumab associated with FOLFOX as a first-line therapy[53].
Apatinib, a tyrosine kinase inhibitor that selectively inhibits VEGFR-2, was also evaluated in a placebo-controlled, phase III trial involving previously treated advanced GC patients. Improvements in overall survival (195 d vs 140 d, HR = 0.71, 95%CI: 0.54-0.94) and progression-free survival (78 d vs 53 d, HR = 0.44, 95%CI: 0.33-0.61) were demonstrated[54]. These findings, together with the aforementioned results obtained using ramucirumab, reinforce the role of VEGFR-2 as a therapeutic target in advanced GC.
Phosphatidylinositol 3-kinases (PIK3s) are heterodimeric lipid kinases that consist of several regulatory subunits. In response to stimulation by growth factors, PIK3CA, which encodes the p110alpha catalytic subunit of PIK3, activates downstream effectors, including pAkt and mTOR[55]. PIK3CA amplification contributes to cell proliferation and survival in gastric tumorigenesis by activating the PIK3/pAkt pathway[56]. PIK3CA mutations have been detected at various frequencies in the overall population of GC patients (4%-15%)[57-59], and these mutations appear to be widely distributed among molecular subtypes, with the highest incidence in the EBV subtype (80%) and the lowest incidence in the CIN subtype (3%)[6].
Clinical trial results evaluating PIK3/pAkt pathway inhibitors in GC are not available. Ongoing clinical trials of Akt inhibitors, such as MK-2206, as a second-line therapy are ongoing in a phase II study of advanced GC (clinicaltrials.gov NCT01260701). Because PIK3 mutations can induce resistance to HER2 inhibition, MK-2206 is also being tested in association with lapatinib and trastuzumab in HER2-positive GC patients (clinicaltrials.gov NCT01705340). BYL719, another PIK3 inhibitor, is under evaluation in association with AUY922, a HSP90 inhibitor, in a phase I trial of patients who harbor molecularly altered PIK3 or exhibit ERBB2 amplification (clinicaltrials.gov NCT01613950).
The PI3K/Akt/mTOR pathway is activated in several solid tumors and is estimated to be activated in up to 60% of GC patients[60] via PTEN loss-of-function or PIK3CA-activating mutations. Everolimus, a well-known mTOR inhibitor, has been studied in a placebo-controlled, phase III trial in advanced GC patients who failed standard therapies (GRANITE-1 study)[61]. However, everolimus did not confer an overall survival benefit over BSC in this study (5.4 mo vs 4.3 mo, HR = 0.90, 95%CI: 0.75-1.08). Biomarkers that can identify patients for whom everolimus is beneficial have been elusive, and the results of the biomarker analysis of the GRANITE-1 study are awaited. Despite the initial disappointing results, strategies for PI3K/Akt/mTOR pathway inhibition are currently under investigation, such as everolimus in combination with capecitabine and oxaliplatin in a phase I study (clinicaltrials.gov NCT01049620) and everolimus in combination with paclitaxel in a phase III trial in a second-line setting (clinicaltrials.gov NCT01248403).
Fibroblast growth factor receptor (FGFR) is a member of the transmembrane receptor tyrosine kinase family[62,63], which is represented by four members (FGFR1-4) that are involved in cell signaling by interacting with fibroblast growth factors (FGFs). The activation of FGFRs by FGFs leads to the autophosphorylation and activation of several downstream signaling pathways, including mitogen-activated protein kinase and PIK3/Akt/mTOR/p70S6kinase, which are crucial effectors in oncogenic signaling[62]. Studies have demonstrated FGFR2 amplification in 4% to 6% of GC patients, and this amplification seems to be a prognostic factor because patients harboring this genetic alteration exhibit a poor survival rate[64,65]. FGFR2 amplification seems to be absent in EBV-positive molecular subtype tumors[6]. FGFR2 inhibitors, such as ponatinib[66], dovitinib[67] and AZD4547[63], have demonstrated activity against FGFR2-amplified cell lines in vitro. AZD4547 was compared with paclitaxel in a randomized phase II trial of 71 advanced GC patients with FGFR2 amplification who had failed first-line therapy. Improvement in progression-free survival with the use of this FGFR2 inhibitor were not observed (1.8 mo on AZD4547 vs 3.5 mo on paclitaxel arms)[68]. Dovitinib is being evaluated as a monotherapy in a phase II trial of patients with previously treated advanced disease who harbor FGFR2 polysomy or amplification (clinicaltrials.gov NCT01719549). It is also being evaluated in association with docetaxel as a second-line therapy in a phase I/II trial, irrespective of FGFR2 status (clinicaltrials.gov NCT01921673).
Mesenchymal-epithelial transition (MET) receptor is also a transmembrane receptor tyrosine kinase and belongs to the hepatocyte growth factor (HGF) receptor family[62]. Approximately 2% to 4% of GC patients are estimated to present MET-amplification[69-71], which seems to confer poor prognosis[70]. Its amplification seems to be absent in EBV-positive and GS molecular subtypes[6]. In a report of four GC patients with advanced disease and MET-amplification, the tumors of two patients responded to crizotinib for a limited time[70]. The use of foretinib in MET-amplified GC patients was also disappointing because none of the 69 patients responded to this tyrosine kinase inhibitor[71]. The strategy to target MET using monoclonal antibodies that bind to the MET receptor or the circulating ligands for MET, such as hepatocyte growth factor, have also been disappointing. Onartuzumab, a MET antibody, was tested in association with mFOLFOX6 in a phase III trial of HER2-negative, MET-positive patients based on immunohistochemistry. The enrollment of this trial was stopped early due to negative final results from a phase II trial assessing mFOLFOX6 plus onartuzumab. This monoclonal antibody did not improve overall survival (11.0 mo vs 11.3 mo, HR = 0.82) or progression-free survival (6.7 mo vs 6.8 mo, HR = 0.90)[72]. Rilotumumab, a fully monoclonal antibody that targets HGF, showed promising results in association with ECX (epirubicin, cisplatin and capecitabine) in a randomized, placebo-controlled phase II trial[73], which motivated the initiation of phase III trials. The RILOMET-1 trial was a phase III study of MET-positive GC patients with advanced disease that compared ECX plus rilotumumab or placebo as a first-line therapy. This trial was closed prematurely due to the higher number of deaths in the rilotumumab arm[74]. Overall survival (9.6 mo vs 11.5 mo, HR = 1.37, 95%CI: 1.06-1.78), progression-free survival (5.7 mo vs 5.7 mo, HR = 1.30, 95%CI: 1.05-1.62) and response rate (30% vs 39%, OR = 0.67, 95%CI: 0.46-0.96) were worse in the rilotumumab arm. Rilotumumab was also evaluated in association with mFOLFOX6 in a randomized phase II trial (MEGA trial) of 162 unselected patients with advanced GC. Adding this monoclonal antibody was more toxic and not more effective than mFOLFOX6 alone[75].
Tivantinib, a selective small-molecule inhibitor of MET, was also evaluated in a single-arm phase II study of Asian patients with previously treated advanced GC[76]. None of the 30 patients presented objective responses and no correlation was identified between efficacy and biomarkers, including gene amplification of MET, expression of MET, expression of p-Met and expression of HGF.
The blockade of immune checkpoints is one of the most promising approaches to activating therapeutic antitumor immunity. The most extensively described immune checkpoints are the Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and Programmed cell death 1 (PD-1) receptors, which are linked to inhibitory pathways in the immune system and are crucial for maintaining self-tolerance and modulating the physiological immune responses to minimize collateral tissue damage[77]. PD-1 binds two ligands: PD-L1 and PD-L2. Therapeutic strategies to block the CTLA-4 and PD-1 pathway, both alone and in combination, have been successful in metastatic melanoma[78-80] and are promising for the treatment of other malignant neoplasms.
Elevated PD-L1 and PD-L2 expression has been demonstrated in EBV-positive GC[6]. In an unselected GC population, PD-L1 was overexpressed in 42%-50% of patients[81,82], and this overexpression correlated with poor survival rates[82]. The inhibition of PD-L1 in GC using monoclonal antibodies was explored in experimental models[83] and evaluated in a phase Ib trial, which demonstrated an antitumor activity of pembrolizumab in an enriched PD-L1-positive population consisting of 39 GC patients[84]. Pembrolizumab is also currently being tested in a phase II study evaluating its efficacy in previously treated HER2-negative advanced GC (clinicaltrials.gov NCT02335411). The inhibition of CTLA-4 by tremelimumab is also being investigated in in a phase Ib/II trial of GC patients with refractory disease (clinicaltrials.gov NCT02340975).
The Janus kinase (JAK) family of proteins consists of non-receptor tyrosine kinases that phosphorylate cytoplasmic targets, including the signal transducers and activators of transcription (STATs)[85]. The JAK/STAT pathway mediates signaling via cytokines, which is required for cell proliferation, survival and differentiation. JAK2 is a member of this family, and its mutation is a well-known genetic alteration in myeloproliferative disorders[86]. In GC, JAK2 overexpression seems to be enriched in the EBV-molecular subtype[6]. The inhibition of the JAK2/STAT3 pathway with WP1066 reduced GC growth in experimental models and may form the basis for further clinical studies[87]. The clinical use of a JAK2 inhibitor, AZD1480, has been evaluated in a phase I, dose-escalation study of Asian patients with solid malignancies, including GC (clinicaltrials.gov NCT01219543).
Poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) plays an essential part in the repair of single-stranded DNA breaks via the base excision-repair pathway, and it has been proposed to maintain the low-fidelity nonhomologous end joining DNA repair machinery[88]. Thus, PARP inhibition leads to the formation of double-stranded DNA breaks that cannot be accurately repaired in tumors with a homologous recombination deficiency, a concept known as synthetic lethality[88]. PARP inhibitors have been evaluated in ovarian[88,89] and breast cancer[90] BRCA 1/2-deficient patients. Olaparib is a potent oral PARP inhibitor that induces synthetic lethality in BRCA1/2-deficient tumor cells, and it is currently being evaluated in a phase III trial of advanced gastric cancer patients who have failed first-line therapy (clinicaltrials.gov NCT01924533).
In recent years, the molecular biology of GC has been elucidated, highlighting the enormous heterogeneity of the disease and its complex interplay between genetic and epigenetic alterations.
The predictive value of the overexpression and/or amplification of HER2 demonstrated by the ToGA study[5], which allowed the incorporation of trastuzumab in the therapeutic arsenal of the disease, was the first step toward greater therapy individualization. Likewise, several trials concluded and ongoing trials demonstrate or suggest the predictive value of other biomarkers before new drugs are incorporated into clinical practice.
The demonstration of four distinct molecular GC subtypes by the TCGA study constitutes a great leap forward in patient stratification and the development of targeted therapies[6]. The reproducibility of these findings in other populations would further improve these data. Specifically, new targetable driver mutations and genetic abnormalities beyond HER2 need to be identified. The recognition of subtypes of the disease with unique gene expression profiles that are enriched for certain biomarkers provides a roadmap to implement genome-guided personalized therapy for GC.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21466] [Article Influence: 1951.5] [Reference Citation Analysis (6)] |
| 2. | D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808-816. [PubMed] |
| 3. | Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236-242. [PubMed] |
| 4. | Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 436] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 5. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5533] [Article Influence: 345.8] [Reference Citation Analysis (3)] |
| 6. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 5095] [Article Influence: 424.6] [Reference Citation Analysis (4)] |
| 7. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1338] [Article Influence: 83.6] [Reference Citation Analysis (1)] |
| 8. | Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 636] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 9. | Park SH, Sohn TS, Lee J, Lim do H, Hong ME, Kim KM, Sohn I, Jung SH, Choi MG, Lee JH. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol. 2015;33:3130-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 10. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4739] [Article Influence: 237.0] [Reference Citation Analysis (7)] |
| 11. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1119] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 12. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1334] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 13. | Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648-2657. [PubMed] |
| 14. | Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362:858-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1472] [Article Influence: 73.6] [Reference Citation Analysis (1)] |
| 16. | Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438-4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (5)] |
| 17. | Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S, Middleton GW. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 457] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 18. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1625] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 19. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1842] [Article Influence: 153.5] [Reference Citation Analysis (7)] |
| 20. | Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 446] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 21. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. [PubMed] |
| 22. | Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, Stanford JL, Dubrow R, Schoenberg JB, Mayne ST, Farrow DC. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150-155. [PubMed] |
| 23. | Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2042] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 24. | Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37 Suppl 4:S9-S15. [PubMed] |
| 25. | Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1954] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 26. | Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 2008;15:413-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Gu J, Zheng L, Wang Y, Zhu M, Wang Q, Li X. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol. 2014;35:5315-5321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Chen C, Yang JM, Hu TT, Xu TJ, Yan G, Hu SL, Wei W, Xu WP. Prognostic role of human epidermal growth factor receptor in gastric cancer: a systematic review and meta-analysis. Arch Med Res. 2013;44:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Liang JW, Zhang JJ, Zhang T, Zheng ZC. Clinicopathological and prognostic significance of HER2 overexpression in gastric cancer: a meta-analysis of the literature. Tumour Biol. 2014;35:4849-4858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 1892] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 31. | Kang YK, Rha SY, Tassone P, Barriuso J, Yu R, Szado T, Garg A, Bang YJ. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer. 2014;111:660-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 510] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 33. | Hecht J, Bang Y, Qin S, Chung H, Xu J, Park J, Jeziorski K, Shparyk Y, Hoff P, Sobrero A. Lapatinib in combination with capecitabine plus oxaliplatin (CapeOx) in HER2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma (AC): The TRIO-013/LOGiC Trial. J Clin Oncol. 2013;31:4001. |
| 34. | Jácome AA, Wohnrath DR, Scapulatempo Neto C, Carneseca EC, Serrano SV, Viana LS, Nunes JS, Martinez EZ, Santos JS. Prognostic value of epidermal growth factor receptors in gastric cancer: a survival analysis by Weibull model incorporating long-term survivors. Gastric Cancer. 2014;17:76-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL, Soares FA. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29:3030-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 36. | Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 37. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 695] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 38. | Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 594] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 39. | Kim Y, Sasaki Y, Lee K, Rha S, Park S, Boku N, Komatsu Y, Kim T, Kim S, Sakata Y. Randomized phase II study of nimotuzumab, an anti-EGFR antibody, plus irinotecan in patients with 5-fluorouracil-based regimen-refractory advanced or recurrent gastric cancer in Korea and Japan: Preliminary results. J Clin Oncol. 2011;29 (suppl 4); 87. |
| 40. | Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, Hackett CB, Urba SG, Zaner KS, Blanke CD. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922-4927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 41. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 7054] [Article Influence: 306.7] [Reference Citation Analysis (0)] |
| 42. | Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F, Galizia G. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008;15:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 43. | Cabuk D, Basaran G, Celikel C, Dane F, Yumuk PF, Iyikesici MS, Ekenel M, Turhal NS. Vascular endothelial growth factor, hypoxia-inducible factor 1 alpha and CD34 expressions in early-stage gastric tumors: relationship with pathological factors and prognostic impact on survival. Oncology. 2007;72:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7794] [Article Influence: 354.3] [Reference Citation Analysis (8)] |
| 45. | Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4457] [Cited by in RCA: 4476] [Article Influence: 223.8] [Reference Citation Analysis (7)] |
| 46. | Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2393] [Cited by in RCA: 2323] [Article Influence: 122.3] [Reference Citation Analysis (8)] |
| 47. | Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733-4740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1951] [Cited by in RCA: 1895] [Article Influence: 111.5] [Reference Citation Analysis (4)] |
| 48. | Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1658] [Cited by in RCA: 1856] [Article Influence: 123.7] [Reference Citation Analysis (0)] |
| 49. | Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1501] [Cited by in RCA: 1678] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 50. | Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 1074] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 51. | Shah MA, Ramanathan RK, Ilson DH, Levnor A, D’Adamo D, O’Reilly E, Tse A, Trocola R, Schwartz L, Capanu M. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 302] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 52. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 907] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 53. | Yoon H, Johanna C, Braiteh F, Firdaus I, Philip P, Cohn A, Lewis N, Anderson D, Arrowsmith E, Schwartz J. Ramucirumab (RAM) plus FOLFOX as front-line therapy (Rx) for advanced gastric or esophageal adenocarcinoma (GE-AC): Randomized, double-blind, multicenter phase 2 trial. J Clin Oncol. 2014;32:4004. |
| 54. | Qin S. Phase III study of apatinib in advanced gastric cancer: A randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2014;32:4003. |
| 55. | Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 612] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 56. | Byun DS, Cho K, Ryu BK, Lee MG, Park JI, Chae KS, Kim HJ, Chi SG. Frequent monoallelic deletion of PTEN and its reciprocal associatioin with PIK3CA amplification in gastric carcinoma. Int J Cancer. 2003;104:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 57. | Li VS, Wong CW, Chan TL, Chan AS, Zhao W, Chu KM, So S, Chen X, Yuen ST, Leung SY. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Velho S, Oliveira C, Ferreira A, Ferreira AC, Suriano G, Schwartz S, Duval A, Carneiro F, Machado JC, Hamelin R. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur J Cancer. 2005;41:1649-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 275] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 59. | Zhou J, Chen GB, Tang YC, Sinha RA, Wu Y, Yap CS, Wang G, Hu J, Xia X, Tan P. Genetic and bioinformatic analyses of the expression and function of PI3K regulatory subunit PIK3R3 in an Asian patient gastric cancer library. BMC Med Genomics. 2012;5:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Lang SA, Gaumann A, Koehl GE, Seidel U, Bataille F, Klein D, Ellis LM, Bolder U, Hofstaedter F, Schlitt HJ. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer. 2007;120:1803-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 62. | Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2716] [Cited by in RCA: 2726] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 63. | Xie L, Su X, Zhang L, Yin X, Tang L, Zhang X, Xu Y, Gao Z, Liu K, Zhou M. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res. 2013;19:2572-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 64. | Su X, Zhan P, Gavine PR, Morgan S, Womack C, Ni X, Shen D, Bang YJ, Im SA, Ho Kim W. FGFR2 amplification has prognostic significance in gastric cancer: results from a large international multicentre study. Br J Cancer. 2014;110:967-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 65. | Jung EJ, Jung EJ, Min SY, Kim MA, Kim WH. Fibroblast growth factor receptor 2 gene amplification status and its clinicopathologic significance in gastric carcinoma. Hum Pathol. 2012;43:1559-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Gozgit JM, Wong MJ, Moran L, Wardwell S, Mohemmad QK, Narasimhan NI, Shakespeare WC, Wang F, Clackson T, Rivera VM. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther. 2012;11:690-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 67. | Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, Zhang S, Lee M, Wu J, Lim KH. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 537] [Article Influence: 38.4] [Reference Citation Analysis (1)] |
| 68. | Bang YJ, Van Cutsem E, Mansoor W, Petty R, Chao Y, Cunningham D, Ferry D, Landers D, Stockman P, Smith N. A randomized, open-label phase II study of AZD4547 (AZD) versus Paclitaxel (P) in previously treated patients with advanced gastric cancer (AGC) with Fibroblast Growth Factor Receptor 2 (FGFR2) polysomy or gene amplification (amp): SHINE study. J Clin Oncol. 2015;33:4014. |
| 69. | Kiyose S, Nagura K, Tao H, Igarashi H, Yamada H, Goto M, Maeda M, Kurabe N, Suzuki M, Tsuboi M. Detection of kinase amplifications in gastric cancer archives using fluorescence in situ hybridization. Pathol Int. 2012;62:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Lennerz JK, Kwak EL, Ackerman A, Michael M, Fox SB, Bergethon K, Lauwers GY, Christensen JG, Wilner KD, Haber DA. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29:4803-4810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 71. | Shah MA, Wainberg ZA, Catenacci DV, Hochster HS, Ford J, Kunz P, Lee FC, Kallender H, Cecchi F, Rabe DC. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One. 2013;8:e54014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 72. | Shah M, Bang Y, Lordick F, Tabernero J, Chen M, Hack S, Phan S, Shames D, Cunningham D. METGastric: A phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2-) and MET-positive (MET ) adenocarcinoma of the stomach or gastroesophageal junction (GEC). J Clin Oncol. 2015;33:4012. |
| 73. | Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas A, Jiang Y. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (3)] |
| 74. | Cunningham D, Tebbutt N, Davidenko I, Murad M, Al-Batran S, Ilson D, Tjulandin S, Gotovkin E, Karaszewska B, Bondarenko I. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol. 2015;33:4000. |
| 75. | Malka D, Castan F, Francois E, Bouche O, Bennouna J, Ghiringhelli F, De La Fouchardiere C, Borg C, Samalin E, Bachet J. FOLFOX alone or combined to rilotumumab or panitumumab as first-line treatment in patients (pts) with advanced gastroesophageal adenocarcinoma (AGEA): An open-label, randomized phase II trial (PRODIGE 17 ACCORD 20 MEGA). J Clin Oncol. 2015;33:4016. |
| 76. | Kang YK, Muro K, Ryu MH, Yasui H, Nishina T, Ryoo BY, Kamiya Y, Akinaga S, Boku N. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 77. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10728] [Article Influence: 766.3] [Reference Citation Analysis (34)] |
| 78. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 12039] [Article Influence: 752.4] [Reference Citation Analysis (0)] |
| 79. | Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2712] [Cited by in RCA: 2756] [Article Influence: 212.0] [Reference Citation Analysis (3)] |
| 80. | Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3964] [Cited by in RCA: 4440] [Article Influence: 403.6] [Reference Citation Analysis (0)] |
| 81. | Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu GL, Luo H, Yang YX, Dai XY, Zhou SF. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther. 2015;9:901-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 82. | Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 406] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 83. | Sun J, Xu K, Wu C, Wang Y, Hu Y, Zhu Y, Chen Y, Shi Q, Yu G, Zhang X. PD-L1 expression analysis in gastric carcinoma tissue and blocking of tumor-associated PD-L1 signaling by two functional monoclonal antibodies. Tissue Antigens. 2007;69:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Muro K, Bang Y, Shankaran V, Geva R, Catenacci D, Gupta S, Eder J, Berger R, Gonzalez E, Pulini J. A phase Ib study of pembrolizumab (Pembro; MK-3475) in patients (Pts) with advanced gastric cancer. Ann Oncol. 2014;25:1-41. [DOI] [Full Text] |
| 85. | Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2647] [Cited by in RCA: 2781] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 86. | Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 441] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 87. | Judd LM, Menheniott TR, Ling H, Jackson CB, Howlett M, Kalantzis A, Priebe W, Giraud AS. Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo. PLoS One. 2014;9:e95993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 88. | Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1373] [Cited by in RCA: 1467] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 89. | Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, Colombo N, Špaček J, Vuylsteke P, Hirte H. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 453] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 90. | O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 609] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Carter WG, Huang KH, Hironaka S, Kleeff J S- Editor: Qi Y L- Editor: A E- Editor: Ma S