Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.1045
Peer-review started: April 26, 2015
First decision: June 2, 2015
Revised: July 22, 2015
Accepted: September 13, 2015
Article in press: September 22, 2015
Published online: January 21, 2016
Processing time: 265 Days and 17.4 Hours
Inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn’s disease are chronic, life-long, and relapsing diseases of the gastrointestinal tract. Currently, there are no complete cure possibilities, but combined pharmacological and nutritional therapy may induce remission of the disease. Malnutrition and specific nutritional deficiencies are frequent among IBD patients, so the majority of them need nutritional treatment, which not only improves the state of nutrition of the patients but has strong anti-inflammatory activity as well. Moreover, some nutrients, from early stages of life are suspected as triggering factors in the etiopathogenesis of IBD. Both parenteral and enteral nutrition is used in IBD therapy, but their practical utility in different populations and in different countries is not clearly established, and there are sometimes conflicting theories concerning the role of nutrition in IBD. This review presents the actual data from research studies on the influence of nutrition on the etiopathogenesis of IBD and the latest findings regarding its mechanisms of action. The use of both parenteral and enteral nutrition as therapeutic methods in induction and maintenance therapy in IBD treatment is also extensively discussed. Comparison of the latest research data, scientific theories concerning the role of nutrition in IBD, and different opinions about them are also presented and discussed. Additionally, some potential future perspectives for nutritional therapy are highlighted.
Core tip: Inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn’s disease are chronic, life-long, and relapsing diseases of the gastrointestinal tract. Currently, there are no complete cure possibilities, but combined pharmacological and nutritional therapy may induce remission of the disease. Both parenteral and enteral nutrition is used in IBD therapy, but their practical utility is not clearly established, and the evidence is sometimes conflicting. This review presents the latest findings of research studies regarding the influence of nutrition on the etiopathogenesis of IBD, its mechanisms of action, and its use as a therapeutic method. Additionally, some potential future perspectives for nutritional therapy are highlighted.
- Citation: Wędrychowicz A, Zając A, Tomasik P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J Gastroenterol 2016; 22(3): 1045-1066
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/1045.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.1045
Inflammatory bowel disease (IBD) is a collection of chronic inflammatory disorders of the gastrointestinal tract, including ulcerative colitis (UC) and Crohn’s disease (CD), which is characterized by periods of remission and flare-up of the disease[1]. The inflammatory process observed in UC is continuous and limited to the mucosa of the colon, while in contrast; CD is characterized by transmural inflammation and skip lesions, located in any region of the gastrointestinal tract[2]. Both CD and UC, despite multiple differences, share similar symptoms, including abdominal pain, diarrhea, extraintestinal manifestations, and malnutrition[1,2].
The pathogenesis of malnutrition in IBD is multifactorial. Chronic nutrition deficiencies are probably secondary to inadequate calorie intake and increased energy expenditure during chronic and relapsing inflammatory lesions[3]. In addition, pharmacological and surgical treatment may impair digestion and absorption of nutrients because of different drug-nutrient interactions and reduced absorptive area of the intestine secondary to surgical resections[4]. Nevertheless, direct inhibitory effects of pro-inflammatory mediators secreted from the inflamed tissue are also very important for the state of malnutrition[2].
The management of IBD includes pharmacological, nutritional, and surgical therapy. The main goal of the treatment is induction and maintenance of remission, correction of nutritional deficiencies, and prevention of complications[1].
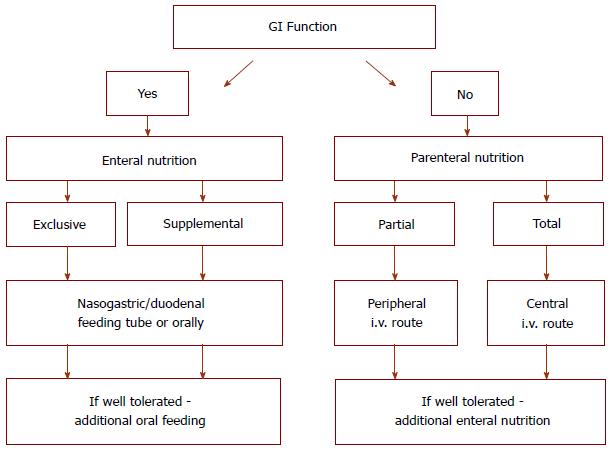
Nutritional therapy may be carried out through enteral (EN) or parenteral nutrition (PN). Moreover, nutritional treatment can be divided into primary and support therapy. The aim of the first is to induce and maintain remission and the second to support the long-lasting effects of drug therapy[4]. PN may be an alternative method of nutrition to enteral intake of nutrients in IBD patients, mainly used for those with contraindications or EN intolerance, especially when there are symptoms of severe malnutrition. Total parenteral nutrition (TPN) may be also used during the acute inflammatory phase (obstruction, toxic megacolon, and active fistulas) in the preoperative period and in patients with short bowel syndrome (SBS) due to previous extensive bowel resections[5,6].
Indications for EN in the treatment of IBD include exclusive enteral nutrition (EEN) for active disease, supplemental EN to maintain disease remission, and nutritional support to achieve adequate weight gain and growth[4]. Treatment by EN is more effective in achieving clinical remission in children with IBD than in adults[7,8]. Recent meta-analysis of clinical trials indicated that the efficacy of EEN might be comparable to that of corticosteroid therapy[9]. EEN decreases disease activity and promotes mucosal healing in patients with IBD, but the mechanism of action of this therapy is still poorly understood.
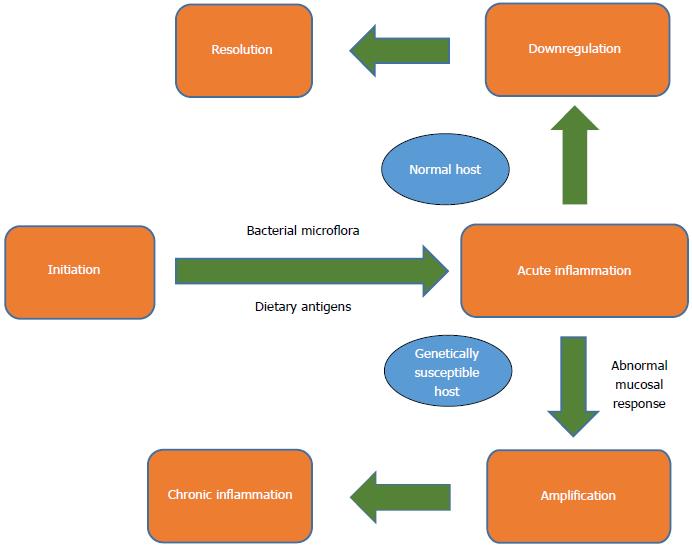
It is well known that the molecular basis of IBD is linked with the immunology of T-cells. Most popular theories suggest that T cells are activated due to a confluence of genetic and environmental factors, which generate an immune imbalance, leading to the inflammation characteristic of these diseases (Figure 1)[10]. The connection of improper activation of T-cells with diet or diet-related factors is still not a well-established theory. Several dietary risk factors have been suggested from the early beginning of life.
Several recent studies showed a protective role of breastfeeding against the risk of IBD. A New Zealand study with a relatively large group of participants indicated that breastfeeding has a protective effect against IBD [CD odds ratio (OR) = 0.55 (0.41-0.74), UC OR = 0.71 (0.52-0.96)] with a duration-response effect[11]. Many studies limit those beneficial effects to subjects with at least 6 mo of nursing. Slovak studies established that breastfeeding for less than 6 mo was a risk factor for both CD (OR = 2.7) and UC (OR = 1.7)[12]. In a Danish study, breastfeeding for more than 6 mo decreased the odds for IBD (OR = 0.50; 95%CI: 0.23-1.11)[13]. However, some giant multicenter studies did not support these findings - in an extremely large study (but in women only), no significant association was found between breastfeeding and IBD in adulthood[14,15]. Conversely, a meta-analysis found a significant protective effect of breastfeeding (OR = 0.69, 95%CI: 0.51-0.94) against the development of IBD in pediatric patients[16]. An older meta-analysis, based mainly on studies from the previous century, found that breastfeeding had a protective effect on the development of UC (OR = 0.61, 95%CI: 0.44-0.84) but not CD[17]. The mechanism by which breastfeeding protects against IBD is not precisely known. Feeding in the first months of life is crucial for the development of gut microflora. The gut microbiome stimulates innate and acquired immunity, promotes the maturation of the mucosal immune system and the integrity of the mucosa, and develops tolerance to food antigens as well[18,19]. Some authors assign a special role in this process to lactoferrin[20]. This peptide, present in human milk only (absent in formula), possesses anti-inflammatory, antibacterial, and antiviral properties[21].
Several Danish studies showed that a high intake of digestible sugar increased the risk of total IBD [OR = 2.5 (1.0-6.2)] and CD [OR = 2.9 (1.0-8.5)][13,22]. In an older study, only high sucrose consumption was associated with an increased risk for IBD (OR = 2.85, P = 0.03). The EpiCom cohort (1560 IBD patients from 31 European countries) study revealed that increased sugar consumption and daily fast food were associated with earlier onset of IBD and an increased risk of disease severity and surgery in UC[23]. A more detailed study showed that lactose consumption has no effect, while fructose intake was negatively associated with risk for IBD[24]. Some foodstuffs, such as bread, are difficult to categorize correctly, thus the results of such studies can be contradictory. In one study, high bread intake was protective against IBD - [OR = 0.42 (95%CI: 0.26-0.70)][13], while another study delivered quite the opposite result - high bread intake increased risk of IBD onset (RR = 6.38)[25]. A detailed study showed that frequent intake of white bread [OR = 4.9 (95%CI: 1.0-23.4, P = 0.05)] was associated with an increased risk of CD onset and that a diet rich in non-digestible fibers, like vegetable and whole meal bread, is protective against IBD [CD: 0.3 (0.1-1.0)], UC [0.3 (0.1-0.8)]; OR: 0.5 (0.3-0.9), CD [0.4 (0.2-0.9) respectively][22]. However, another study found that high fiber diet was protective against the risk of CD but not UC[26]. Fiber has anti-inflammatory properties as well and positively modulates the internal microbiome[18]. Fibers are fermented by colonizing bacteria into short chain fatty acids. These fatty acids activate peroxisome proliferator-activated receptor γ, a nuclear transcription factor with anti-inflammatory effects[27]. Digestible sugars in the diet modify the intestinal microbiome, increasing the prevalence of Prevotella, whereas protein and animal fat in the diet cause an increase in Bacteroides[28].
Starch, one of the most important carbohydrates present in the human diet, strongly influences the microbiome as well. It is a necessary substrate for intestinal bacteria, especially to promote the development of Klebsiella microbes[29]. Because the levels of anti-Klebsiella antibodies were high in CD patients, it has been theorized that Klebsiella species are an important factor in the induction of autoimmunological reactions through the mechanism of molecular mimicry. Furthermore, strong positive correlation and cross-reactive antibodies were observed between Klebsiella and collagen in CD and ankylosing spondylitis patients[30]. Additionally, the “low starch diet” used in ankylosing spondylitis patients decreased the level of inflammatory mediators and reduced the clinical symptoms of disease[31].
Recently, interesting studies regarding the use of the “specific carbohydrate diet” (SCD) in CD children were published. SCD, initially used in celiac disease treatment almost 70 years ago, contains almond, nut, and coconut flours and excludes grains (wheat, rice, corn). Additionally, the majority of dairy products are restricted, except fermented yogurt, and the only sugar allowed is fructose (honey). Two small, independent United States retrospective studies that assessed SCD as the sole method of treatment in CD children showed that the use of SCD for 5 to 30 (or 12 to 52, respectively) months had positive effects on laboratory inflammatory markers and clinical presentation of the disease, including mucosal healing[32,33]. The direct reason for the improvement was not known, but modification of the intestinal microbiome was the most probable cause. Nevertheless, prospective studies are necessary to confirm these preliminary results.
It has been known for years that high animal fat intake is associated with an increased risk for UC (OR = 4.09, P = 0.02) as is cholesterol intake (OR = 4.57, P = 0.02)[24]. In another study, high consumption of mono- and disaccharides and total fats consistently increased the risk for CD and UC[34]. Several studies showed that prolonged, habitual intake of fast food, commonly rich in fats and digestible sugars, is a risk factor for CD[23].
Significant attention has been paid to the role of unsaturated fatty acids in the pathogenesis of IBD. A diet rich in olive oil and fish, but also in vegetables, fruits, grains, and nuts, was inversely associated with CD in both genders (girls: OR = 0.3, 95%CI: 0.1-0.9; boys: OR = 0.2, 95%CI: 0.1-0.5)[35]. Increased intake of ω-6 polyunsaturated fatty acids (PUFA, beef, pork, corn, sunflower oils, and polyunsaturated margarines) in place of ω-3 PUFA (fish) was associated with an increased incidence of IBD[36,37]. High intakes of ω-6 PUFA linoleic acid were associated with a greater risk of developing UC (P = 0.01; OR = 2.49, 95%CI: 1.23-5.07 for the highest quartile) in a dose-dependent manner (P = 0.02). This association was observed in both genders, but it was significant only in women (P = 0.03). On the other hand, high intakes of the ω-3 PUFA docosahexaenoic acid were associated with lower risks of developing UC (P = 0.03)[38]. The action of saturated and unsaturated fats could be realized through modulation of Toll-like receptors in macrophages[39].
Some evidence linked fat intake with dysbiosis. The incidence of colitis was markedly increased in milk fat-fed interleukin (IL)-10-deficient mice but not in normal mice or polyunsaturated fat-fed IL-10-deficient mice. Bilophila wadsworthi was observed in the feces of milk fat-fed mice, indicating dysbiosis[40]. This finding was clearly linked with taurine conjugation of hepatic bile acids by Bilophila wadsworthi, with direct evidence showing that IL-10-deficient mice fed a low-fat diet containing taurocholic acid, but not glycocholic acid, developed colitis[41].
The role of protein consumption in IBD etiology is controversial. In a French study, high total protein intake, specifically animal protein, was associated with a significantly increased risk of IBD in future life (hazards ratio for the third vs first tertile and 95% confidence interval being 3.31 and 1.41-7.77 (P trend = 0.007) and 3.03 and 1.45-6.34 (P trend = 0.005) for total and animal protein, respectively). High consumption of meat or fish was suspected as a risk factor, but high consumption of eggs or dairy products was not[42]. A German twin study indicated that high consumption of processed meat, including sausage, was associated with an increased risk of CD (OR = 7.9, 95%CI: 2.15-38.12 - monozygotic twins OR = 10.75, 95%CI: 4.82-25.55 - dizygotic twins) as well as UC (OR = 5.69, 95%CI: 1.89-19.48; OR = 18.11, 95%CI: 7.34-50.85; mono and di-zygotic twins respectively)[43]. Some papers have documented similar relationships in Japan, where increased intake of dairy products and meat accompanied the rising trend of UC[44]. One of the promising explanations of this phenomenon is allergy to cow’s milk proteins. In recent studies it was shown that allergy to cow’s milk protein at infancy was associated with CD (OR = 1.92, 95%CI: 1.09-3.36, P < 0.05) and UC (OR = 1.71, 95%CI: 1.04-2.83, P < 0.05) in later childhood[45].
An alternative explanation may be connected with Mycobacterium avium subspecies paratuberculosis (MAP) infection, which is strongly suspected in the pathogenesis of multiple diseases, including IBD[46,47]. MAP is broadly distributed in the environment, water, and food. Some nutrients, such as milk, meat and sugar, may fortify its invasiveness[48,49].
Magnesium (P = 0.04), vitamin C, and fruits were negatively associated with risk for IBD, while a positive association was found for retinol (P = 0.01). Potassium intake and vegetable consumption showed a negative association only with risk for CD[24]. Experiments in animal models indicated a significant role for selenium shortage in the pathogenesis of IBD, as it is a cofactor of anti-inflammatory proteins[50], but no correlation was found in the case of folate[51]. Vitamin D is also significant in IBD development. Data obtained in a mouse model of colitis demonstrated that 1,25(OH)2D3 or vitamin D receptor deficiency caused dysbiosis, leading to increased susceptibility to gastrointestinal injury[52]. On the other hand, increased intake of vitamin D protected against IBD in an animal model and in humans, but only against CD[53,54].
Increased dietary sulfur is a result of its use as a preservative food additive[55]. This additive is linked with an increased number of UC cases[56], and it is suggested that sulfur may be toxic to human colonocytes following its metabolism by colonic bacteria to hydrogen sulfide (H2S)[57]. The polysaccharide dietary additive maltodextrin was shown to impair cellular anti-bacterial responses and suppress intestinal anti-microbial defense mechanisms, which could result in IBD onset[58].
Microparticles, such as titanium dioxide, aluminum silicates, and talc, can act as antigen transporters from the intestinal lumen to the mucosa and have been linked with the development of CD[59]. Aluminum silicates are used as a co-compound of anti-acids, and sodium aluminosilicate and talc (E-553b) are added to powdered foods to prevent lump formation. Titanium dioxide is a food and toothpaste colorant (E171). These microparticles are absorbed by the specialized M cells but are undegradable and accumulate in macrophages and lymphoid tissue and induce the secretion of proinflammatory IL-1β and IL-18[60]. As adjuvants facilitate the interactions of mucosa with antigens, they impair the local immune system[61]. Many others substances from toothpaste are suspected to be linked to IBD etiology. Tricalcium phosphate and quartz (abrasives) in animal models penetrated the epithelium and created enteric lesions similar to those in Crohn’s disease. Also carrageenans, widely used in foodstuffs and toothpastes for their gelling, thickening, and stabilizing properties, injured enterocytes in animal models, in a manner typical of IBD in humans[62].
A high intake of fluids (P = 0.04) was negatively associated with the risk of IBD[24]. Furthermore, green tea and coffee reduce the risk of IBD in Asian and Australian populations. Daily tea consumption was associated with a reduced risk of CD (aOR = 0.62; 95%CI: 0.43-0.91), whereas both daily tea (aOR = 0.63; 95%CI: 0.46-0.86) and coffee consumption (aOR = 0.51; 95%CI: 0.36-0.72) were associated with a reduced risk of UC. Caffeine ameliorated acute colitis in intestinal epithelial cells in an in vitro study[63]. Green tea is rich in polyphenols - antioxidants that attenuate the severity of colitis in an animal model[64]. A similar protective mechanism is postulated in the case of wine consumption[65]. However, wine also contains sulfur dioxide, which acts as a potential negative factor[66]. Diet and beverages are a major modifiable influence of the microbiome but are also a source of microorganisms. Water was found to be the route of transmission of several pathogens linked with IBD, such as in the potentially protective role of Helicobacter pylori infection against IBD[67,68].
In summary, conflicting data likely arise from different study designs. Some studies were retrospective, others prospective. Retrospective studies are burdened with errors associated with memory, and prospective studies have biases based on some social issues, such as modification of answers to fit with promoted healthy lifestyle standards[69]. Another possible source of bias is connected with different groups of patients (children vs adults and different genders, different races), small sample sizes, and heterogeneity of disease subtypes. In addition, type of food vs nutrients results in severe inconsistencies between studies[70]. For instance, the influence of fruits and vegetables on IBD onset is multifactorial, as they are a source of digestible sugars, fiber, microelements, vitamins, and proteins but also potential allergens. They may also carry some preservatives, depending on the country of origin. Therefore, it is impossible to consistently interpret the results of all of these different studies.
Malnutrition is common among IBD patients and can be recognized by protein-energy malnutrition, altered body composition, and micronutrient deficiencies. Malnutrition is one of the most important factors linked with poor outcome in patients with IBD[71]. The most common type in adult IBD patients is protein-energy malnutrition, predominantly seen as weight loss[72]. In a recent Romanian study, the prevalence of malnutrition based on body mass index (BMI) in IBD patients was established as 30.6%[73]. The rate of patients suffering from protein-energy malnutrition was significantly higher among IBD patients (OR = 5.57, 95%CI: 5.29-5.86) than it was among non-IBD cases, for both CD (P < 0.0001) and UC patients (P < 0.0001)[74]. In a Serbian study, of 76 IBD patients (23 CD and 53 UC), 52 (68.4%) met the criteria for malnourishment, and 24 (31.6% of the entire cohort) were severely malnourished[75]. A similar situation was observed in children - up to 60% of newly diagnosed CD children - but only 35% of patients with UC present symptoms of malnutrition[76].
An unexpected high proportion of patients with IBD (mostly UC patients) are overweight[77]. A recent Untied States study showed that obesity prevalence in IBD patients reflects the obesity index in the general population[78]. A very promising observation is that clinical outcomes in obese patients are better than in non-obese patients with IBD, and obesity (defined using BMI index) is a marker of a less severe disease course in IBD[78]. A relatively high percentage of obese patients during diagnosis could be related to an increase in obesity in the general population, accompanied by earlier IBD recognition.
IBD sometimes develops in patients treated previously for obesity. Some surgical interventions, such as gastric bypass, may result in severe malnutrition in the case of IBD in later life[79].
There are several reasons for malnutrition and disturbed body composition in IBD patients (Table 1). These factors depend on pathophysiology but could be superimposed with iatrogenic factors. During active phase of the disease, energy intake in adults and children is lower than in healthy controls[80]. Healthy siblings consume on average 1757 kJ (420 kcal) more than their siblings with IBD (matched for height, sex, and weight). Imbalance is deepened through a higher basic metabolic rate in CD compared to healthy controls[81]. What is more, children with CD fail to adapt their resting energy expenditure to their diminished body mass[82]. The explanation for this phenomenon is based on pro-inflammatory cytokine action accompanying an increased lipid oxidation rate in CD patients[83,84]. However, diet-induced thermogenesis was lower in CD patients in an active compared to an inactive stage of disease[85]. Lack of appetite could be enhanced by side effects of some drugs, such as nausea, during methotrexate intake[86]. On the other hand, treatment with corticosteroids increases appetite but is also associated with lean mass reduction. A similar effect was found for mesalamine, which is predictive of lean mass for height Z-score less than -1.00[87].
| Reason | Contributing factor |
| Increased nutritional requirements | Active inflammation |
| Increased nutrient losses | Diarrhea, intestinal and fistulae protein losses |
| Impaired dietary intake | Nausea, vomiting, loss of appetite |
| Nutrient malabsorption | Bacterial overgrowth, secondary lactose malabsorption |
| Iatrogenic | Drug - nutrient interactions, |
| (drug-related, surgery-related) | reduced intestinal absorptive area |
Malabsorption seems to be major contributor to low weight in adult CD patients. Increased gastrointestinal nutrient losses are observed in patients after ileal resection or with bile acid malabsorption[88]. Bile acid malabsorption is common in IBD patients, whether the disease is localized in the ileum or not. It leads to malfunction of lipid digestion with steatorrhea, impaired intestinal motility, and/or significant changes in the intestinal microflora environment. Increase of fat in stool could also be a result of a deficit in pancreatic enzyme secretion. Gastric acid and pancreatic enzyme impaired secretion were observed in 80% of CD patients[89]. Loss of nutrients can also occur as a result of protein enteropathy from a ruptured, permeable gut. Studies using whole gut lavage have shown that disease activity closely paralleled gastrointestinal protein loss[90].
Several studies have documented the beneficial influence of therapy on total weight. In a United Kingdom study, 29% of children were severely malnourished (BMI Z-scores < -2 SD) during diagnosis of the CD and only 4% in UC patients. At 6 and 12 mo post-diagnosis, 5% and 1% of the CD children and 0% and 5% of UC patients had low BMI z-score values, respectively[84]. A similar beneficial effect of therapy was observed in a French study[91]. In these children, weight and BMI z-scores improved post-diagnosis, and the prevalence of CD children with low BMI (< -2 SD) was half of that at diagnosis.
Lean body mass: Weight and BMI are very superficial factors in the analysis of nutritional status. Therefore, profound measurements of compartments of the body are frequently performed. Typically, lean body mass in patients with IBD is lower than in the healthy population. A recent study showed that a decrease in lean mass was observed in 21% of adult patients with IBD[92]. In addition, a systematic review analyzing adult patients with IBD up to the year 2013 reported similar data. Independent of the methodology used, in adult patients with IBD, reduced fat-free mass was observed in 28% of CD and 13% of UC cases as compared to healthy controls[93]. No consistent association between body composition and disease activity, duration, extent, or therapies was found. Several studies showed that lean mass was also reduced in children with IBD compared to controls[94]. In a German study, significantly reduced lean mass was found in children with well-controlled IBD compared to controls [difference -0.72; 95%CI: -1.10-(-0.34)][95]. In a British study, fat free mass correlated negatively with disease activity in children with CD regardless of changes in weight[96]. Lean mass in CD adjusted for age and height was significantly lower than in controls. In a regression model including height, age, Tanner stage, and race, CD was associated with a 6% reduction in lean mass[97]. Only in one study, reduction of lean mass was predominantly observed in boys[98].
Fat mass: In a recent systematic review, fat mass was found not to be statistically different in 66% of CD patients (n = 419) and in 75% of UC patients (n = 220) compared to controls[93]. Thirty one percent of patients (n = 192) with CD and 13% of patients (n = 39) with CU showed a statistically significant reduction of fat mass. Twelve percent of patients with CU (n = 36) and only 3% with CD (n = 20) developed fat tissue better than controls - increased in 12% of patients compared to controls[99,100]. Another study concluded that physical activity of adult IBD patients is low and correlated inversely with fat mass[101]. In children, some studies found no differences in fat mass between CD patients and control groups[102]. However, there are several papers describing a gender difference in fat mass in children with IBD. While one study showed that deficit of fat is predominant in girls[98], others reported a significantly higher percentage body fat in girls with CD compared to boys or to the control group[102,103].
Fat distribution, especially fat localized around the intestine lumen, is suspected to be pathognomonic as well as a pathogenic factor for IBD[104]. Fat deposited around the small or large intestine is called “fat wrapping”[105]. In CD, fat surrounding over 50% of the bowel circumference is typical[106]. Moreover, it is suggested that proinflammatory and modulating immune system adipokines secreted by this fatty tissue play a significant role in the disease etiopathology[107].
Bone mass: Osteopenia and osteoporosis are common complications of IBD and affect almost every second patient[108]. In a Romanian study, osteopenia was found in 48.07% of the patients with UC and in 56.41% of the patients with CD; and osteoporosis was present in 18.26% of the patients with UC and 15.38% of those with CD[109]. When examined using dual-energy X-ray absorptiometry (DEXA), patients with CD had significantly lower femoral Z scores than patients with UC. Femoral Z score was strongly associated with disease duration[110].
There are several discrepancies concerning the influence of age, gender, location of lesions, and BMI on bone mass. Early onset of IBD (before 30 years of age) is a risk factor for profound decrease in bone mineral density compared with patients diagnosed in old age[111]. However, age over 50 is also a risk factor for osteopenia and osteoporosis[112]. A recent Italian study showed that in newly diagnosed patients with IBD, osteoporosis and osteopenia were seen mostly in male patients over 30 years of age (63%) and in young women (62%)[113]. However, a similar United States study performed between 2008-2012 showed no influence of age, BMI, or disease location on bone mineral density[114]. Another American study noticed a positive correlation between bone mineral density and BMI[115]. Additionally, a recent Slovak study showed that CD patients with ileic/ileocolic location and history of proctocolectomy/total colectomy were at a higher risk of developing osteoporosis than other IBD patients[110].
A Canadian study using the World Health Organization Fracture Risk Assessment tool (FRAX) showed that IBD patients aged over 50 are at increased risk of hip fracture only (HR = 2.14; 95%CI: 1.26-3.65)[116]. Interestingly, the same authors in a similar study concluded that CD was associated with an increased risk of osteoporosis of the lumbar spine and trochanter only[117]. Some studies have shown an increased risk of vertebral fractures in children with IBD[118]. These observations are supported by a Swedish study that showed a permanent decrease in children’s BMD Z scores for the lumbar spine using DEXA[119]. Patients with a severe disease course complicated with ostomy and low BMD also had a five times higher frequency of fragility fractures than IBD patients with normal BMD[115].
The most popular explanation for loss of mineral bone matrix is malabsorption of calcium and vitamin D as well as treatment side effects, mainly from long-term, systematic steroids[86,120]. Biological therapy is linked with better outcomes in bone mineral density[121]. Recent studies have linked bone mineral density in IBD with genetic factors[122,123]. It was also suggested that adipokines, as well as inflammatory mediators, interfere in the balance between osteoblasts and osteoclasts[124].
Vitamin and micronutrient deficiencies: Patients with IBD usually do not present overt symptoms of vitamin or micronutrient deficiency. The most detailed studies were performed on vitamin D as a risk factor for decreased bone mineral density. The conflicting results of those studies seem to depend on the different ethnic groups studied, living at different latitudes with different exposure to sunlight, different cut-offs for deficiency of vitamin D, and different methods used for vitamin measurement.
In children and adolescents from Massachusetts, United States, no difference was found in mean serum 25(OH)D concentration between IBD patients and healthy controls. However, patients with active inflammation processes confirmed by elevated erythrocyte sedimentation rate had significantly lower 25(OH)D levels than controls[125]. A New Zealand study showed that during winter, 76% of tested IBD patients were vitamin D deficient (< 50 nmol/L), and all of them had insufficient 25(OH)D levels (< 75 nmol/L). During summer, only 10% of patients were deficient, but an insufficient level of vitamin D was still discovered in 55% of patients. In the latter study, no relation was found between vitamin D deficiency and activity of CD[126]. Conversely, a Romanian study showed that vitamin D levels were significantly lower in CD patients with moderate to severe disease activity compared to CD patients in remission or with mild disease activity (16 ± 6 ng/mL; 26 ± 7 ng/mL; 31 ± 9 ng/mL, respectively)[127]. Another American study from the southern part of the United States (Houston) reported slightly lower rates of deficiency and insufficiency of vitamin D in a population of IBD patients, but the cut-off was different. Insufficiency of 25(OH)D (between 20 and 30 ng/mL) was found in 37% of IBD patients and deficiency (levels < 20 ng/mL) was found in 23% of patients. A positive correlation between low vitamin D status and diminished bone mineral density was also shown[114].
Vitamin D deficiency occurs likewise in Chinese patients with UC (10.32 ± 4.46 ng/mL vs controls, P < 0.001) and CD patients (11.57 ± 5.02 ng/mL vs controls, P = 0.029) and is closely associated with the severity of the disease[128]. Furthermore, plasma 25(OH)D level < 20 ng/mL was associated with an increased risk of surgery (OR = 1.76, 95%CI: 1.24-2.51) and hospitalization (OR = 2.07, 95%CI: 1.59-2.68) of IBD patients compared to those patients with sufficient levels of vitamin D[129].
The beneficial effect of vitamin D supplementation is connected with its immunomodulatory role in IBD. In IBD patients, vitamin D levels are strongly inversely correlated with intestinal inflammation[130]. Animal models have suggested that increased vitamin D intake prevents inflammation-associated colon cancer[53]. Additionally, supplementation with vitamin D and vitamin D plasma levels correlated with quality of life in UC and CD patients during the winter/spring period in central European countries[131].
A further typical complication of IBD is anemia. Some discrepancies in different reports are a result of two different anemia mechanisms (iron deficiency anemia and anemia of chronic diseases) and various cut-offs for diagnostic hemoglobin levels and other markers for anemia like ferritin, transferrin, or hepcidin[132]. Anemia is connected with deficits in iron, zinc, copper, folate, and vitamin B12. According to a recent review and meta-analysis of over 2000 patients data from European countries, the overall prevalence of anemia in IBD patients was 24% (95%CI: 18-31), and in 57% of cases, it was iron-deficiency anemia[133]. In the Canadian Manitoba IBD Cohort Study, iron deficiency was identified in 20% of adult CD patients and 27% of UC patients, but anemia was diagnosed only in one third of these patients[134]. Iron and zinc plasma concentrations were also significantly lower in children with newly diagnosed IBD[135]. A newer study confirmed these data. Zinc deficiency in children with IBD is relatively common - affecting 40% of patients - especially when compared to deficiency in a healthy, age matched population (19%)[136]. In adults, higher zinc as well as lower serum levels were observed in CU patients as compared to controls[137,138]. Selenium is another micronutrient with antioxidant properties, but it did not differ in IBD children compared to healthy controls[135]. In adults, the concentration of selenium was significantly lower in UC patients than controls[138]. Surprisingly, the mean serum concentration of copper was higher in CD children compared with age-matched patients suffering from UC or controls[139]. In adults, both CD and UC patients had higher levels of copper than controls[137]. No gender differences were observed, but men with pancolitis had significantly higher copper levels than men with proctitis.
A Brazilian study established vitamin B12 deficiency in 6% of patients in an IBD population (equally in CD and UC patients)[140]. Another study found vitamin B12 deficiency limited to CD patients with significant (over 20 cm) ileal resection[141]. In addition, folate concentration was not different between IBD children and adults when compared to age matched control groups[135,142]. Vitamin A deficiency in an American study was found in 16% of pediatric patients with IBD, while vitamin E deficiency in the same study was rare (5%), similar to the index in the healthy population (8%)[136]. Another study found no differences in mean vitamin A and E concentration between IBD children and healthy controls[135]. Modulation of gastrointestinal microflora in IBD influenced the bioavailability of vitamin K. In a Polish pediatric population, the prevalence of vitamin K deficiency was 54.0% in CD and 43.7% in UC. Vitamin K status in this study was assessed indirectly using determination of a protein induced by lack of vitamin K (PIVKA-II)[143]. Single studies have analyzed other vitamins: 30% of adult IBD patients have vitamin B6 deficiency, and a decrease in vitamin C levels was observed in adult IBD patients in remission[72,144].
In summary, nutrition is a key factor in IBD - it is involved in the pathogenesis and in the treatment of these diseases. Nutrition in IBD is still a challenge for patients and for medical care. Recent papers have shown that patients’ knowledge in this matter is relatively weak and needs improvement[145]. Furthermore, the increasing number of children with IBD opens a new chapter in nutritional science. In these cases, nutrition must ensure proper development as well as promoting remission of the diseases.
Estimates suggest that 20%-85% of patients suffering from IBD are malnourished or underweight[4,138,146]. Malnutrition occurs more frequently in patients with CD than those with UC. There are several reasons why patients with IBD are malnourished, and they are mostly related to the significant importance of the small bowel condition in patient body weight[147].
The relationship between metabolic condition and IBD is complex. Nutritional therapy is carried out through the combination of EN and PN (Figure 2). Moreover, nutritional treatment can be divided into primary and support therapy. The aim of primary therapy is to induce and maintain remission, and support therapy aims to enable the long-lasting effects of pharmacological treatment.
PN can benefit patients with IBD as an alternative for enteral intake of proteins, fats, vitamins, and minerals. PN is used in patients with contraindications or EN intolerance, especially those with symptoms of severe malnutrition. TPN is also used during the acute inflammatory phase (obstruction, toxic megacolon, active fistulas), in the preoperative period, and in groups of patients with SBS due to previous extensive bowel resections[5,6].
PN is recommended for severely malnourished patients unable to take food spontaneously or by tube (nasogastric or nasoduodenal tube, gastrostomy). Severe malnutrition is defined as a loss of more than 5% of body mass during 1 mo or 10% in 6 mo and body mass index (BMI) less than 19 kg/m2[146]. An accompanying marker of malnutrition is low serum albumin level, which often correlates with the acute phase and poor prognosis.
Proper restitution of nutritional state ought to be considered, especially in pediatric patients. IBD by itself or in combination with the effects of medication (steroids) can severely impair normal growth and development, sometimes with definitive deterioration of children’s condition. A combination of under-nutrition and growth failure is typical for pediatric patients with IBD, although nutritional disturbances are more common in children with CD compared with those with UC. IBD therapy of adult patients mostly concentrates on inducing and maintaining remission through EN. In children, improvement of nutritional status and thereby restoration of somatic and psychological condition needs to be one of the main goals of therapy, achieved in severe cases only with the introduction of PN[148].
Thus, PN as TPN or supportive therapy is particularly indicated in the preoperative period in severely malnourished patients for the restitution or improvement of their metabolic condition. Expected benefits are improved wound healing, prevention of postoperative complications (anastomotic leaks), and limited perioperative weight loss. There are studies strongly indicating a correlation between low preoperative serum albumin level and higher risk of poor mucosal healing or anastomotic leak[149,150]. In the early postoperative period, TPN provides full calorific, electrolyte, microelement, vitamin, and fluid requirements. In addition, when there are complications in the course of IBD or during the postoperative period, such as bowel obstruction, fistula, or toxic megacolon, PN is the main route for metabolic support. Combined feeding is indicated as early as possible postoperatively, and EN combined with PN diminishes inflammation and induces remission. It also can improve mucosal healing by its local immune-stimulating effects[151]. The duration of PN during perioperative periods depends on the type of surgery and the patient’s condition. In patients with CD and multiple small bowel resections resulting in SBS, PN is indicated as a prolonged supportive therapy[146].
In fact, the available data show that while PN seems to play a primary role in the management of patients with active CD, it does not have a primary therapeutic effect in active UC and does not induce clinical remission of this type of IBD. The use of artificial nutrition in the treatment of active CD is mostly based on the theoretical background of temporary bowel rest, decreasing motoric function, and thus antigenic stimulation by food components, leading to increased bowel permeability. Coincident metabolic support allows patients to restitute their metabolic status with better and faster intestinal cell renewal and better mucosal healing[152].
To date, few controlled clinical trials have been conducted to show the effect of TPN on inducing remission in active CD. Three months after starting PN, the remission rate varied between 20% and 80%[153]. In other reports, authors found the effectiveness of fistula healing during TPN improved from 43% to 63% in patients linked with weight gain and metabolic restitution[154,155].
Moreover, the latest studies in adults have not shown a statistically significant decline in mortality in surgical patients with PN support in IBD, except for the severely malnourished[146,156]. There are no large randomized studies concentrating exclusively on the effects of PN in patients with IBD[146,157,158]. Thus, there are no data on PN support during the preoperative and perioperative period and disease relapse[159]. However, theory and clinical experience has strongly established PN as a supportive therapy, especially in children in the preoperative period or during perioperative complications.
PN seems to have a limited role as a maintenance therapy in remission. It may be indicated in some restricted cases, such as SBS after extensive bowel resection, bowel obstruction, high-output fistula, patients with severe dysmotility, and patients intolerant to EN[147]. For patients with SBS and limited absorption, PN is advocated for years until they reach maximal bowel adaptation (hyperplasia and bowel elongation and dilatation). Nevertheless, in some cases, PN must be considered as life-long therapy.
Bowel obstruction in the first 2 years after surgery has a frequency of around 15%, reaching 18% after 10 years and 29% after 30 years[159]. Because each subsequent laparotomy/laparoscopy increases the risk of adhesions, conservative treatment is favored and recommended for as long as possible with the support of PN in cases of subileus, repeated incomplete bowel obstruction, and severe dysmotility. Similarly, in patients in remission with high-output jejuno/ileostomy, PN plays a crucial role in metabolite and fluid supply.
Long lasting PN increases the risk of complications related to metabolic disturbances, liver failure, cholestasis, central line infection, and catheter-related venous thrombosis, thus significantly impacting patient quality of life. If the gut can be used safely, EN is actually the preferred feeding method for IBD patients and must be the first choice in the induction and remission phase of treatment[147,160,161].
Generally, PN is accomplished through central venous catheters (CVC) placed in central veins, mostly in the superior vena cava. There are special types of catheters for long-lasting intravenous therapy, and the access, especially in pediatric patients, is performed under general anesthesia. Because of the limited number of central veins, it is very important in IBD patients with a possibility of long-lasting PN to reserve central veins for nutritional access.
About 5%-15% of patients have complications related to central venous access. There are four main risks of PN: line infections, liver failure, metabolic disturbances, and vein thrombosis. Most PN are carried out at home as home parenteral nutrition (HPN) according to the “all-in-one” method, administered by the patient or by family members. Deviation from the correct method of PN administration may result in temporary serum glucose level disturbances, fluid and electrolyte imbalances, and hunger strikes. Patients on long-lasting PN are at a significantly increased risk for developing metabolic bone disease, and vitamin, microelement, and serum leptin disturbances[162-164].
Liver failure seems to be the most important complication, apart from septicemia, correlated with PN. Acute hepatic complications after the introduction of PN include liver dysfunction and cholestasis, painful hepatomegaly, and hyperammonemia. They can develop at any age but are most common among pediatric patients. PN-associated cholestasis is linked with the administration of soybean-based intravenous fat emulsion[165]. In adults, the most common complication is liver steatosis. Liver disease incidence is strictly related to HPN duration. Moreover, liver dysfunction may be transient, as evidenced by elevated transaminases, bilirubin, and alkaline phosphatase; and it commonly occurs when HPN is started. Delayed or persistent elevations may result from an excess of amino acids in the liquid nutritional mixture. In some patients progressive fibrosis of the liver may occasionally develop. Long-term TPN is also a well-documented factor for cholelithiasis, with frequency of about 40%[166,167].
CVC related septicemia and vein thrombosis are the most common complications leading to catheter replacement. The septicemia rate in the first 1000 d of HPN based on large group analysis ranged between 12% to 0.5-0.6/1000 HPN days, with Staphylococcal septicemia being dominant[168-170]. A higher frequency was observed in the first 1000 HPN days, especially in pediatric patients. Pediatric patients were also more likely to need catheter replacement because of injury (disruption) and spontaneous removal more often.
TPN is realized through continuous intravenous flow of fluids, which obviously influences quality of life. Compared to EN, PN generates much higher costs and very often requires professional nursing. New types of central line catheters inserted peripherally or without general anesthesia may partially improve patients’ quality of life[171].
In summary, PN is a significant element of treatment in the group of IBD patients, is often life-saving and should be implemented when required. However, there are no evidence-based guidelines relating to the effectiveness of PN in the therapy of IBD. Thus, EN should be re-introduced as soon as possible, being more suitable physiologically, safer, and less expensive.
For more than 40 years, enteral feeding has been used in the nutritional therapy of IBD. From the early 1970s, when the first primary therapeutic effects of an elemental diet in CD patients were shown, multiple studies regarding EN were carried out. The observed therapeutic effect was different depending on the type of IBD (CD vs UC), the age of the patients, their clinical presentations, and their concomitant treatment[172].
Generally, EEN is the provision of 100% of a person’s nutritional requirements from a liquid nutritional formula either orally or via a feeding tube. EEN is usually provided for 6-8 wk and then a normal diet is gradually reintroduced[173].
Protocols of EEN may be different regarding composition of the enteral formula and route of administration. Three main types of enteral formulas are used in EEN depending on the degree of protein hydrolysis. Polymeric formulas are designed to mimic the general diet with non-hydrolyzed proteins, carbohydrates, and fat. The source of protein is usually casein, carbohydrates are provided as corn maltodextrin, while the source of fat is canola or soybean. Semi-elemental and elemental formulas are planned for use in patients with malabsorption, so the nutrients are partially (semi elemental formulas) or fully hydrolyzed (elemental formulas). Protein is usually in the form of dipeptides or tripeptides in semi elemental formulas and free amino acids in elemental ones. Carbohydrates are supplied as hydrolyzed cornstarch, maltodextrin, or fructose, and lipids as fatty acids esters or medium-chain triglycerides[174]. Additionally, diet may be of standard concentration (1 kcal/mL) or high concentration (2 kcal/mL). Depending on the fat concentration, very low fat diets (< 3 g/1000 kcal), low fat diets (< 20 g/1000 kcal), and high fat diets (> 20 g/1000 kcal) may be distinguished[3].
In the majority of early clinical studies with EEN, elemental formulas were used[175]. Nevertheless, the latest clinical studies showed that polymeric formulas were as effective as elemental formulas at inducing disease remission both in children and adults[172,176].
The mechanism underlying the therapeutic response to EEN still remains unclear. There are a few theories that try to explain its anti-inflammatory properties. Initially, low antigenic load was thought to be responsible for curative effects, but later studies showed that a polymeric diet was as effective as an elemental one in inducing remission in active CD[7]. Another theory indicated change of the profile of fatty acids in the enteral diet and its possible influence on bacterial microflora of the gastro-intestinal tract, resulting in the therapeutic effect on CD[8].
The latest studies have indicated downregulation of mucosal pro-inflammatory cytokines as a possible factor responsible for inducing remission in IBD[177,178]. One of the latest theories indicated a strong anti-inflammatory effect of EN formulas on intestinal mucosa accompanied by histopathological healing. EN formulas enriched with tumor growth factor beta (TGF-β) induced clinical remission of CD. This was associated with mucosal healing and a decrease in pro-inflammatory cytokines, such as IL-1, IL-8 and interferon gamma, in mucosal specimens of the terminal ileum and colon; and an increase of TGF-β in the terminal ileum. This suggested that clinical remission of CD during EEN is rather a result of a reduction of the inflammatory process than an improvement in the nutritional status of the patient[179]. Another clinical study showed that the anti-inflammatory effect of EEN preceded the nutritional effect and was maintained until the end of the study[180]. The specific diet used in this study contained TGF-β, which was postulated as the therapeutic activity of the formula used[177]. This observation was confirmed in a retrospective pediatric study, where the authors reported more effective results in decreasing clinical activity of CD and improving nutritional status of patients after EEN with polymeric formula enriched with TGF-β2 when compared with patients fed with non-TGF-β2 enriched formula[181]. Moreover, a recently published prospective study on a pediatric population showed a correlation of daily protein and calorie intake during EEN with both clinical response and the concentration of TGF-β1 in CD children, confirming a potential role of TGF-β in the stimulation of healing processes in IBD[182]. Additionally, the time needed for clinical response in CD children treated with EEN was comparable when using TGF-β enriched formulas and formulas stimulating TGF-β release[181-183].
Overall, no single component of EN has been defined as explaining the curative effect of this therapy. Nevertheless, some components of EN, such as glutamine, arginine, and polyunsaturated fatty acids, were shown to have beneficial properties in IBD patients[184].
Despite the fact that some experimental and clinical studies showed a positive effect of arginine and glutamine on clinical symptoms and inflammatory mediators in different inflammatory diseases, including CD, their role in IBD is poorly documented[185,186]. Although early studies showed an improvement in the clinical course of diseases after arginine-enriched formulas were used in EN, the latest meta-analyses did not confirm these findings[187,188]. Additionally, a glutamine-enriched enteral diet was not confirmed to be superior to a standard polymeric diet in the treatment of active CD patients[189]. Moreover, data from clinical studies on EN in critically ill patients showed conflicting results. In some groups of patients, increased mortality was observed after glutamine-enriched EN[174]. Based on these results, the latest European Society for Parenteral and Enteral Nutrition (ESPEN) guidelines did not recommend the use of immune mediating nutrients in EN[190].
Several recent studies have shown that EEN strongly influenced the physical and biochemical characteristics of stool, especially sulfide and butyric acid concentration[191]. Changes in stool metabolic activities interact with gut microbiome composition, which may be also connected with the curative properties of EEN[192].
The role of invasive bacterial species as triggers and coinfection factors in IBD has not been fully identified. Recent studies reported an increased prevalence of MAP and adherent-invasive Escherichia coli in intestinal tissues, stool cultures, and blood of CD and UC patients, especially in the active stage of disease[49]. EEN may influence invasive bacterial species and decrease their prevalence through modification of bacterial diversity and modulation of predominant intestinal bacterial microflora. This may also be a potential mode of action of nutritional therapy in IBD[193,194].
EEN is an effective treatment option for children and adolescents with IBD, currently recommended as the first line of the therapy in active CD[195]. Nevertheless, latest evidence suggests that this therapy may be more effective in children than in adults, especially in CD compared with UC[196,197]. Additionally, nutritional support is beneficial for the development and prevention of growth retardation, which is a frequent clinical symptom in children with IBD, especially with CD. A Cochrane systematic review confirmed the positive therapeutic role of EEN on development and growth parameters in children with CD. Moreover, children treated with EEN avoided corticosteroid therapy and steroid-dependent adverse effects during the first year after IBD diagnosis[191]. In many pediatric IBD centers in Europe, EEN therapy is frequently chosen as the primary induction therapy in CD. In contrast, in North America, only a few centers prefer EEN in newly diagnosed CD children[198]. The British Society of Gastroenterology guidelines suggest EEN as the first line therapy in children and adolescents, especially in those with small bowel involvement in CD[9]. Additionally, case reports of three children with newly diagnosed perianal CD found that combined EEN with pharmacological and surgical therapy may be effective as an induction treatment resulting in the remission of perianal CD lesions[199].
EN as induction therapy was confirmed as being most effective when it was administered as EEN via nasogastric tube excluding a normal diet[200]. Recently, however, a retrospective study analyzing the efficiency of a fractionated oral diet compared to continuous EEN in children with CD was published. The authors found comparable clinical efficacy of the treatment in both groups of patients, except for weight gain, which was higher in the continuous feeding group. Additionally, they did not observe intolerance of the volume of the nutrient administered orally that had been observed in previous studies[201,202].
The first studies on EEN, both for polymeric and elemental diets, as primary nutrition therapy in adult CD patients showed a therapeutic effect that was as strong as steroid therapy in inducing clinical remission. However, a later Cochrane systematic review showed that steroid therapy is more effective than EEN in adults[176]. When taking into consideration the potential adverse effects of both methods of treatment, EN is a much safer method of treatment and is still used as a therapeutic method.
Nevertheless, in different parts of the world there are different, sometimes contradictory, rules regarding the place of EEN in management of IBD. Currently, EEN is recommended as the first line of the therapy in active CD in adults, when treatment with corticosteroids is not feasible[195]. Recent European and North American clinical guidelines recommend EEN as a therapeutic option only if a patient declines pharmacological therapy or as an adjunctive treatment to support nutritional status rather than as primary treatment[203,204].
The British Society of Gastroenterology guidelines suggest EEN in adult patients in preference to steroids, immunosuppressants, or surgery, especially in patients non-responsive to 5-aminosalicylic acid (5-ASA) or with contraindications to steroid therapy[9]. In contrast, in Japan, EEN is still used in adult patients with IBD as the preferred first line therapy[205].
It is not currently known why the benefits of EEN therapy achieved in children are not demonstrated in adults. One of the reasons may be that EEN therapy induces remission of the disease by reducing mucosal inflammation. In adult IBD patients, who have a longer disease course and more frequent complications, which are often not connected with inflammatory status, EEN may be less effective.
There is limited evidence suggesting that EEN therapy is more effective in newly diagnosed CD patients compared to patients with long-standing CD. In an Australian study in CD children, induction treatment with EEN was successful in 80% of newly diagnosed and 58% of long-standing CD patients[206]. Another pediatric study confirmed these results, showing a higher relapse rate after the second course of EEN (70%) when compared to the first one (67%) during a 1 year period of follow-up[207].
However, a different treatment response dependent on the time since diagnosis is not only demonstrated for EEN therapy. Similarly, response and remission rates achieved after anti-TNF therapy were higher in CD children than in adults, which could be related to disease duration prior to starting treatment[208]. Nevertheless, a recent small study was published that did not confirm these observations. In a group of 22 patients with CD, the authors found that EEN was as effective in newly diagnosed patients as in those with existing disease[172].
Additionally, a large retrospective Chinese study showed that CD patients who completed EEN therapy before surgical treatment of CD had a lower risk of complications and reoperations and longer immunosuppressant-free intervals when compared to patients without EEN therapy[209].
Different nutritional strategies can be considered in maintenance therapy during remission. Two alternatives are cyclical EEN, including nocturnal infusions of enteral diet during one of every 4 mo or supplementary EN with unrestricted daytime diet[210].
Children who continued supplementary EN through a nasogastric tube after finishing induction therapy remained in remission for a longer period of time. A retrospective Scottish study in pediatric patients who, after induction of CD remission, used EEN therapy with continued maintenance EN with 25% of their earlier EEN volume showed remission rates after 1 year of 60% compared to 65% in patients undergoing azathioprine treatment (P = 0.14) and 15% in patients with no treatment (P = 0.001)[211].
In a Japanese randomized controlled trial comparing immunosuppressive therapy and elemental diet as a maintenance therapy in adult CD patients, the authors found a higher remission rate after 2 years of follow-up in a 6-mercaptopurine treated group (56.7%) and an elemental diet treated group (46.9%) compared to a group without maintenance treatment (21.2%)[212]. However, in this study, the majority of patients drank elemental EN orally for a 2 year period. This may be difficult to achieve in other populations, especially in pediatric ones.
Another Japanese randomized controlled trial in adult CD patients compared maintenance enteral therapy supplying 50% of nutritional requirements with unrestricted diet without any supplementation. The study showed a lower relapse rate after 2 years of follow-up in the elemental diet group (35%) compared to the free unrestricted diet group (64%)[213].
Moreover, continuation of EN maintenance feeding before completion of puberty was associated with improved linear growth of children[210]. EEN was also effective in children with perianal CD disease in maintenance remission of lesions[199].
Two pediatric retrospective studies showed that only 31% of children who achieved remission after an EEN course continue EN as maintenance therapy, with a mean time of 10 mo (range, 4-12 mo and 1-15 mo)[206,211].
Thus, poor compliance in maintenance EN therapy, secondary to taste fatigue, may be a main factor limiting the length of EN supplementation and the efficacy of this method of treatment.
EN is an effective therapy option without severe adverse effects. Some minor side effects may occur during administration of a nasogastric tube, such as nausea and vomiting, but they usually disappear quickly[4]. If the volume of formula is increased gradually, it should be better tolerated by the patient. Nevertheless, some patients can better tolerate the prescribed volume at the beginning of the EEN course, hoping for induction of the remission of the disease, but if the disease symptoms are still present, the compliance rate may be lower than expected[214]. From the patient’s point of view, a very important side effect of the EEN regime is the restriction of the possibility of eating and drinking additional food, which may seriously limit compliance with the diet[4].
Both in pediatric and adult studies, some patients did not accept the EEN regimen at all and refused EEN treatment. In addition, some patients did not comply with the EEN regime during nutritional therapy, due to eating additional food to the prescribed formulas[214,215]. This observation also requires attention, because it reduces compliance of the patients and limits the efficacy of EEN.
Elemental formulas compared to polymeric ones have a distinctive smell and flavor due to a higher level of hydrolysis, mainly because of the presence of amino acids, which causes them to be poorly tolerated by patients when they are administered orally[216].
Bitterness, offensive smell, and belching and hiccup may considerably impair patients’ quality of life and compliance during EN with the elemental formula. Modification of the formula by changing the particle size of branched-chain amino acids reduced these symptoms and increased patient compliance[217]. Moreover, some additive flavors, such as apple, pineapple, and fruit flavors, increased sweetness and sourness and masked the bitterness of the formula[217].
The mode of delivery of the formula is very important for patient quality of life and compliance. Many studies with high compliance rates administered elemental formulas via nasogastric tubes rather than orally. Because this method of administration need patients’ compliance and impairs the quality of life, it is better accepted by hospitalized patients. However, for outpatients taking the formula orally may be more socially acceptable.
The influence of EEN on health-related quality of life was confirmed in clinical trials. In a Japanese randomized, controlled trial, the authors did not show significant differences between semi-elemental diet fed and normal diet fed groups[218]. However, another trial confirmed a significant improvement in health-related quality of life after EEN treatment in children with active CD[219]. Additionally, in this prospective cohort study, a weak correlation between mucosal healing after EEN therapy and quality of life was shown, although the majority of patients had high scores on quality of life questionnaires[219].
Interesting data were reported in a Chinese clinical trial with adult active CD patients treated with EEN for 4 wk. The enteral feeding protocol used in this study included multiple low doses of oral polymeric EN formula in the daytime and a self-intubated nasogastric tube feeding at night for 4 wk. Patients who achieved remission also had significantly improved total IBD quality of life scores, intestinal and systemic symptoms, and emotional and social scores. In addition, 61.5% of patients who achieved remission after the EEN course wanted to repeat the EEN course if the disease would relapse again[220]. These data showed that the positive impact of EEN on disease-related quality of life symptoms exceeded its negative influence and limitations related to the method of feeding.
In a retrospective study concerning the therapeutic efficacy of EEN with three different enteral diets (polymeric, semi-elemental, and elemental) when compared to corticosteroid therapy, the authors observed a higher remission rate (80% vs 30%) after 12 mo of follow-up, comparable for all three formulas. Additionally, a higher number of patients treated with EEN achieved endoscopic and histopathological remission after 8 wk of treatment[221]. Another retrospective study on a population of newly diagnosed CD children showed that more than 90% of patients treated with EEN achieved remission after a median time of 6 wk[222]. Moreover, 47% of patients treated with EEN avoided corticosteroid therapy for the next 1-7 years[223]. According to a recently published meta-analysis, concerning five randomized controlled trials on newly diagnosed pediatric CD patients (total n = 147), EEN efficacy was comparable to corticosteroid therapy, with equal rates of clinical remission[224]. A second meta-analysis of children with CD, based on the results of four randomized controlled trails (total n = 144), also found no significant differences in the remission rates between EEN and corticosteroid therapy[225]. The large retrospective study regarding short-term follow-up of children after 8 wk of EEN confirmed earlier observations. Additionally, during 2 years of follow-up the patients’ weight and BMI Z scores significantly improved when compared to baseline. However, EEN course increased height velocity during 6 mo in responding patients but did not improve height Z score in a 2 year term of follow-up[226]. Overall, 57% of patients relapsed during the next 2 years with a median relapse time of 6.5 mo. Nevertheless, 70% of them responded to a second course of EEN that was used as an induction treatment[227].
In a Canadian pediatric study on newly diagnosed CD children, similar remission rates (89% vs 91.3%, P = NS) after 3 mo and similar relapse rates (40.6% vs 28.6%, P = NS) after 12 mo of follow-up in the EEN and corticosteroid groups were reported[226]. Similar observations were presented in an Australian pediatric study during 2 years of follow-up[227].
In a large Dutch pediatric study, assessing a 6 wk EEN course in active CD children, 71% of patients achieved complete remission and 26% partial remission of disease. The median time observed to relapse of CD was 21 wk (range, 2-169 wk), while after a median follow-up time of 18 mo 62% of patients had a relapse of CD. Additionally, Kaplan-Meier analysis showed that the cumulative risk of relapse within the first year of follow-up after EEN induction therapy was 59%[214].
Based on the latest research data, numerous new questions will be asked regarding the influence of nutrition on the pathogenesis of IBD and its methods of application, especially in EEN.
A role for nutritional factors in “IBD integrome” theory is still not quite clear[228]. Detailed potential interactions among nutrition and the genome, microbiome, and immunome may have important clinical value and need to be investigated in future studies.
The confirmed influence of EEN on bacterial microflora modification provokes the question whether the enrichment of enteral diet with prebiotics or probiotics increase the EN efficacy in IBD.
Taking into consideration the potential side effects of EN, we also should ask the question whether EEN manner is mandatory for therapeutic effect, or whether for better patient quality of life we should propose semi-exclusive nutrition.
Furthermore, if changes in the fatty acid profile in an EN diet may result in anti-inflammatory activity, what other diet supplements or fat modifiers may show similar or even stronger therapeutic effects?
Future studies also should specify the indications for different ways of feeding (parenteral vs enteral, tube vs oral) and the precise details of nutritional protocols (time, type of formula, and dose). Finally, increasing patient compliance during nutritional therapy is essential to establish the optimal formula composition regarding its smell and taste.
Answers to the above questions will take nutrition therapy a step further and increase the efficacy of treatment and its tolerance by both adult and pediatric patients.
In summary, the role of nutrition in IBD is essential, it is involved both in pathogenesis and in treatment of these diseases. Although research studies have confirmed its effectiveness in induction and maintenance therapy, outcomes from actual use in children and adult patients with IBD in different countries is not the same. EEN is the first-line therapy option in active CD in children and to a lesser degree in adult patients, while PN should only be considered as an alternative method of nutrition for those with EN intolerance or during perioperative periods of treatment. Future improvements in nutritional protocols and ameliorating the side effects of currently used nutritional therapy and improving the quality of life of the patients would result in better acceptance and better tolerance of this method of treatment by IBD patients.
| 1. | Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3-S9. [PubMed] |
| 2. | Goyette P, Labbé C, Trinh TT, Xavier RJ, Rioux JD. Molecular pathogenesis of inflammatory bowel disease: genotypes, phenotypes and personalized medicine. Ann Med. 2007;39:177-199. [PubMed] |
| 3. | Hartman C, Eliakim R, Shamir R. Nutritional status and nutritional therapy in inflammatory bowel diseases. World J Gastroenterol. 2009;15:2570-2578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 141] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | O’Sullivan M, O’Morain C. Nutrition in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2006;20:561-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Alastair F, Emma G, Emma P. Nutrition in inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2011;35:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Mihai C, Prelipcean CC, Pintilie I, Nedelciuc O, Jigaranu AO, Dranga M, Mihai B. Nutrition in inflammatory bowel diseases. Rev Med Chir Soc Med Nat Iasi. 2013;117:662-669. [PubMed] |
| 7. | Verma S, Brown S, Kirkwood B, Giaffer MH. Polymeric versus elemental diet as primary treatment in active Crohn’s disease: a randomized, double-blind trial. Am J Gastroenterol. 2000;95:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Gassull MA, Fernández-Bañares F, Cabré E, Papo M, Giaffer MH, Sánchez-Lombraña JL, Richart C, Malchow H, González-Huix F, Esteve M; European Group on Enteral Nutrition in Crohn’s Disease. Fat composition may be a clue to explain the primary therapeutic effect of enteral nutrition in Crohn’s disease: results of a double blind randomised multicentre European trial. Gut. 2002;51:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Carter MJ, Lobo AJ, Travis SP; IBD Section, British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-V16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 777] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 10. | Korzenik JR. Past and current theories of etiology of IBD: toothpaste, worms, and refrigerators. J Clin Gastroenterol. 2005;39:S59-S65. [PubMed] |
| 11. | Gearry RB, Richardson AK, Frampton CM, Dodgshun AJ, Barclay ML. Population-based cases control study of inflammatory bowel disease risk factors. J Gastroenterol Hepatol. 2010;25:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Hlavaty T, Toth J, Koller T, Krajcovicova A, Oravcova S, Zelinkova Z, Huorka M. Smoking, breastfeeding, physical inactivity, contact with animals, and size of the family influence the risk of inflammatory bowel disease: A Slovak case-control study. United European Gastroenterol J. 2013;1:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Hansen TS, Jess T, Vind I, Elkjaer M, Nielsen MF, Gamborg M, Munkholm P. Environmental factors in inflammatory bowel disease: a case-control study based on a Danish inception cohort. J Crohns Colitis. 2011;5:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Khalili H, Ananthakrishnan AN, Higuchi LM, Richter JM, Fuchs CS, Chan AT. Early life factors and risk of inflammatory bowel disease in adulthood. Inflamm Bowel Dis. 2013;19:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 15. | Castiglione F, Diaferia M, Morace F, Labianca O, Meucci C, Cuomo A, Panarese A, Romano M, Sorrentini I, D’Onofrio C. Risk factors for inflammatory bowel diseases according to the “hygiene hypothesis”: a case-control, multi-centre, prospective study in Southern Italy. J Crohns Colitis. 2012;6:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Barclay AR, Russell RK, Wilson ML, Gilmour WH, Satsangi J, Wilson DC. Systematic review: the role of breastfeeding in the development of pediatric inflammatory bowel disease. J Pediatr. 2009;155:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Klement E, Cohen RV, Boxman J, Joseph A, Reif S. Breastfeeding and risk of inflammatory bowel disease: a systematic review with meta-analysis. Am J Clin Nutr. 2004;80:1342-1352. [PubMed] |
| 18. | Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I, Bruno G, Petito V, Laterza L, Cammarota G. Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int. 2013;2013:435268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1304] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 20. | Frolkis A, Dieleman LA, Barkema HW, Panaccione R, Ghosh S, Fedorak RN, Madsen K, Kaplan GG; Alberta IBD Consortium. Environment and the inflammatory bowel diseases. Can J Gastroenterol. 2013;27:e18-e24. [PubMed] |
| 21. | Brock JH. The physiology of lactoferrin. Biochem Cell Biol. 2002; 80: 1-6.birth cohorts. Eur J Gastroenterol Hepatol. 2000;12:25-30. |
| 22. | Jakobsen C, Paerregaard A, Munkholm P, Wewer V. Environmental factors and risk of developing paediatric inflammatory bowel disease -- a population based study 2007-2009. J Crohns Colitis. 2013;7:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Burisch J, Pedersen N, Cukovic-Cavka S, Turk N, Kaimakliotis I, Duricova D, Bortlik M, Shonová O, Vind I, Avnstrøm S. Environmental factors in a population-based inception cohort of inflammatory bowel disease patients in Europe--an ECCO-EpiCom study. J Crohns Colitis. 2014;8:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40:754-760. [PubMed] |
| 25. | Bianchi Porro G, Panza E. Smoking, sugar, and inflammatory bowel disease. Br Med J (Clin Res Ed). 1985;291:971-972. [PubMed] |
| 26. | Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145:970-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 544] [Cited by in RCA: 471] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 27. | Viladomiu M, Hontecillas R, Yuan L, Lu P, Bassaganya-Riera J. Nutritional protective mechanisms against gut inflammation. J Nutr Biochem. 2013;24:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Wu GD, Bushmanc FD, Lewis JD. Diet, the human gut microbiota, and IBD. Anaerobe. 2013;24:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Rashid T, Ebringer A, Tiwana H, Fielder M. Role of Klebsiella and collagens in Crohn’s disease: a new prospect in the use of low-starch diet. Eur J Gastroenterol Hepatol. 2009;21:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Ebringer A, Rashid T, Tiwana H, Wilson C. A possible link between Crohn’s disease and ankylosing spondylitis via Klebsiella infections. Clin Rheumatol. 2007;26:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Ebringer A, Wilson C. The use of a low starch diet in the treatment of patients suffering from ankylosing spondylitis. Clin Rheumatol. 1996;15 Suppl 1:62-66. [PubMed] |
| 32. | Suskind DL, Wahbeh G, Gregory N, Vendettuoli H, Christie D. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. J Pediatr Gastroenterol Nutr. 2014;58:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch B, Eshee L, Mason D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2014;59:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Gentschew L, Ferguson LR. Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases. Mol Nutr Food Res. 2012;56:524-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | D’Souza S, Levy E, Mack D, Israel D, Lambrette P, Ghadirian P, Deslandres C, Morgan K, Seidman EG, Amre DK. Dietary patterns and risk for Crohn’s disease in children. Inflamm Bowel Dis. 2008;14:367-373. [PubMed] |
| 36. | Bernstein CN. New insights into IBD epidemiology: Are there any lessons for treatment? Dig Dis. 2010;28:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Ueda Y, Kawakami Y, Kunii D, Okada H, Azuma M, Le DS, Yamamoto S. Elevated concentrations of linoleic acid in erythrocyte membrane phospholipids in patients with inflammatory bowel disease. Nutr Res. 2008;28:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen J, Hallmans G, Palmqvist R, Sjodin H, Hagglund G, Berglund G. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut. 2009;58:1606-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 39. | Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971-16979. [PubMed] |
| 40. | Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1442] [Cited by in RCA: 1476] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 41. | Kanai T, Matsuoka K, Naganuma M, Hayashi A, Hisamatsu T. Diet, microbiota, and inflammatory bowel disease: lessons from Japanese foods. Korean J Intern Med. 2014;29:409-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am J Gastroenterol. 2010;105:2195-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 310] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 43. | Spehlmann ME, Begun AZ, Saroglou E, Hinrichs F, Tiemann U, Raedler A, Schreiber S. Risk factors in German twins with inflammatory bowel disease: results of a questionnaire-based survey. J Crohns Colitis. 2012;6:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Ng SC. Epidemiology of inflammatory bowel disease: focus on Asia. Best Pract Res Clin Gastroenterol. 2014;28:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 45. | Virta LJ, Ashorn M, Kolho KL. Cow’s milk allergy, asthma, and pediatric IBD. J Pediatr Gastroenterol Nutr. 2013;56:649-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Kuenstner JT, Chamberlin W, Naser SA, Collins MT, Dow CT, Aitken JM, Weg S, Telega G, John K, Haas D. Resolution of Crohn’s disease and complex regional pain syndrome following treatment of paratuberculosis. World J Gastroenterol. 2015;21:4048-4062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 47. | Bach H. What Role Does Mycobacterium avium subsp. paratuberculosis Play in Crohn’s Disease? Curr Infect Dis Rep. 2015;17:463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Patel D, Danelishvili L, Yamazaki Y, Alonso M, Paustian ML, Bannantine JP, Meunier-Goddik L, Bermudez LE. The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect Immun. 2006;74:2849-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Nazareth N, Magro F, Machado E, Ribeiro TG, Martinho A, Rodrigues P, Alves R, Macedo GN, Gracio D, Coelho R. Prevalence of Mycobacterium avium subsp. paratuberculosis and Escherichia coli in blood samples from patients with inflammatory bowel disease. Med Microbiol Immunol. 2015;204:681-692. [PubMed] |
| 50. | Kaushal N, Kudva AK, Patterson AD, Chiaro C, Kennett MJ, Desai D, Amin S, Carlson BA, Cantorna MT, Prabhu KS. Crucial role of macrophage selenoproteins in experimental colitis. J Immunol. 2014;193:3683-3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | MacFarlane AJ, Behan NA, Matias FM, Green J, Caldwell D, Brooks SP. Dietary folate does not significantly affect the intestinal microbiome, inflammation or tumorigenesis in azoxymethane-dextran sodium sulphate-treated mice. Br J Nutr. 2013;109:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143:1679-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 53. | Meeker S, Seamons A, Paik J, Treuting PM, Brabb T, Grady WM, Maggio-Price L. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 2014;74:4398-4408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 54. | Ananthakrishnan AN. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9:367-374. [PubMed] |
| 55. | Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002;7:22-44. [PubMed] |
| 56. | Roediger WE, Duncan A, Kapaniris O, Millard S. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology. 1993;104:802-809. [PubMed] |
| 57. | Martin TD, Chan SS, Hart AR. Environmental factors in the relapse and recurrence of inflammatory bowel disease: a review of the literature. Dig Dis Sci. 2015;60:1396-1405. [PubMed] |
| 58. | Nickerson KP, Chanin R, McDonald C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes. 2015;6:78-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Han DY, Fraser AG, Dryland P, Ferguson LR. Environmental factors in the development of chronic inflammation: a case-control study on risk factors for Crohn’s disease within New Zealand. Mutat Res. 2010;690:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Becker HM, Bertschinger MM, Rogler G. Microparticles and their impact on intestinal immunity. Dig Dis. 2012;30 Suppl 3:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Butler M, Boyle JJ, Powell JJ, Playford RJ, Ghosh S. Dietary microparticles implicated in Crohn’s disease can impair macrophage phagocytic activity and act as adjuvants in the presence of bacterial stimuli. Inflamm Res. 2007;56:353-361. [PubMed] |
| 62. | Choi HJ, Kim HG, Kim J, Park SH, Park J, Oh CG, Do KH, Lee SJ, Park YC, Ahn SC. Pro-apoptotic action of macrophage inhibitory cytokine 1 and counteraction of activating transcription factor 3 in carrageenan-exposed enterocytes. Toxicol Lett. 2014;231:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Lee IA, Low D, Kamba A, Llado V, Mizoguchi E. Oral caffeine administration ameliorates acute colitis by suppressing chitinase 3-like 1 expression in intestinal epithelial cells. J Gastroenterol. 2014;49:1206-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Oz HS, Chen T, de Villiers WJ. Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. Front Immunol. 2013;4:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 65. | Nunes C, Ferreira E, Freitas V, Almeida L, Barbosa RM, Laranjinha J. Intestinal anti-inflammatory activity of red wine extract: unveiling the mechanisms in colonic epithelial cells. Food Funct. 2013;4:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Biasi F, Deiana M, Guina T, Gamba P, Leonarduzzi G, Poli G. Wine consumption and intestinal redox homeostasis. Redox Biol. 2014;2:795-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Boehnke KF, Eaton KA, Valdivieso M, Baker LH, Xi C. Animal Model Reveals Potential Waterborne Transmission of Helicobacter pylori Infection. Helicobacter. 2015;20:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol. 2014;20:6374-6385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 69. | Ananthakrishnan AN. Environmental triggers for inflammatory bowel disease. Curr Gastroenterol Rep. 2013;15:302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 70. | Dotson JL, Kappelman MD, Chisolm DJ, Crandall WV. Racial disparities in readmission, complications, and procedures in children with Crohn’s disease. Inflamm Bowel Dis. 2015;21:801-808. [PubMed] |
| 71. | Sandhu A, Mosli M, Yan B, Wu T, Gregor J, Chande N, Ponich T, Beaton M, Rahman A. Self-Screening for Malnutrition Risk in Outpatient Inflammatory Bowel Disease Patients Using the Malnutrition Universal Screening Tool (MUST). JPEN J Parenter Enteral Nutr. 2015;Epub ahead of print. [PubMed] |
| 72. | Hengstermann S, Valentini L, Schaper L, Buning C, Koernicke T, Maritschnegg M, Buhner S, Tillinger W, Regano N, Guglielmi F. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin Nutr. 2008;27:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Gheorghe C, Pascu O, Iacob R, Vadan R, Iacob S, Goldis A, Tantau M, Dumitru E, Dobru D, Miutescu E. Nutritional risk screening and prevalence of malnutrition on admission to gastroenterology departments: a multicentric study. Chirurgia (Bucur). 2013;108:535-541. [PubMed] |
| 74. | Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis. 2008;14:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (2)] |
| 75. | Mijac DD, Janković GL, Jorga J, Krstić MN. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 76. | Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 77. | Kugathasan S, Nebel J, Skelton JA, Markowitz J, Keljo D, Rosh J, LeLeiko N, Mack D, Griffiths A, Bousvaros A. Body mass index in children with newly diagnosed inflammatory bowel disease: observations from two multicenter North American inception cohorts. J Pediatr. 2007;151:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig Dis Sci. 2015;60:2436-2445. [PubMed] |
| 79. | Dodell GB, Albu JB, Attia L, McGinty J, Pi-Sunyer FX, Laferrère B. The bariatric surgery patient: lost to follow-up; from morbid obesity to severe malnutrition. Endocr Pract. 2012;18:e21-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Pons R, Whitten KE, Woodhead H, Leach ST, Lemberg DA, Day AS. Dietary intakes of children with Crohn’s disease. Br J Nutr. 2009;102:1052-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis. 2006;12:185-191. [PubMed] |
| 82. | Azcue M, Rashid M, Griffiths A, Pencharz PB. Energy expenditure and body composition in children with Crohn’s disease: effect of enteral nutrition and treatment with prednisolone. Gut. 1997;41:203-208. [PubMed] |
| 83. | Toptygina AP, Semikina EL, Bobyleva GV, Miroshkina LV, Petrichuk SV. Cytokine profile in children with inflammatory bowel disease. Biochemistry (Mosc). 2014;79:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammatory bowel disease. J Hum Nutr Diet. 2011;24:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 85. | Al-Jaouni R, Hébuterne X, Pouget I, Rampal P. Energy metabolism and substrate oxidation in patients with Crohn’s disease. Nutrition. 2000;16:173-178. [PubMed] |
| 86. | Spooren CE, Pierik MJ, Zeegers MP, Feskens EJ, Masclee AA, Jonkers DM. Review article: the association of diet with onset and relapse in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:1172-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 87. | Burnham JM, Shults J, Semeao E, Foster B, Zemel BS, Stallings VA, Leonard MB. Whole body BMC in pediatric Crohn disease: independent effects of altered growth, maturation, and body composition. J Bone Miner Res. 2004;19:1961-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Vítek L. Bile acid malabsorption in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 89. | Winter TA, O’keefe SJ, Callanan M, Marks T. Impaired gastric acid and pancreatic enzyme secretion in patients with Crohn’s disease may be a consequenece of a poor nutritional state. Inflamm Bowel Dis. 2004;10:618-625. [PubMed] |
| 90. | Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13:11-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 601] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 91. | Vasseur F, Gower-Rousseau C, Vernier-Massouille G, Dupas JL, Merle V, Merlin B, Lerebours E, Savoye G, Salomez JL, Cortot A. Nutritional status and growth in pediatric Crohn’s disease: a population-based study. Am J Gastroenterol. 2010;105:1893-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 92. | Bryant RV, Ooi S, Schultz CG, Goess C, Grafton R, Hughes J, Lim A, Bartholomeusz FD, Andrews JM. Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:895-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 93. | Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 94. | Hill RJ. Update on nutritional status, body composition and growth in paediatric inflammatory bowel disease. World J Gastroenterol. 2014;20:3191-3197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (3)] |
| 95. | Werkstetter KJ, Ullrich J, Schatz SB, Prell C, Koletzko B, Koletzko S. Lean body mass, physical activity and quality of life in paediatric patients with inflammatory bowel disease and in healthy controls. J Crohns Colitis. 2012;6:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 96. | Wiskin AE, Wootton SA, Hunt TM, Cornelius VR, Afzal NA, Jackson AA, Beattie RM. Body composition in childhood inflammatory bowel disease. Clin Nutr. 2011;30:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Burnham JM, Shults J, Semeao E, Foster BJ, Zemel BS, Stallings VA, Leonard MB. Body-composition alterations consistent with cachexia in children and young adults with Crohn disease. Am J Clin Nutr. 2005;82:413-420. [PubMed] |
| 98. | Thayu M, Shults J, Burnham JM, Zemel BS, Baldassano RN, Leonard MB. Gender differences in body composition deficits at diagnosis in children and adolescents with Crohn’s disease. Inflamm Bowel Dis. 2007;13:1121-1128. [PubMed] |
| 99. | Jahnsen J, Falch JA, Mowinckel P, Aadland E. Body composition in patients with inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2003;98:1556-1562. [PubMed] |
| 100. | Katznelson L, Fairfield WP, Zeizafoun N, Sands BE, Peppercorn MA, Rosenthal DI, Klibanski A. Effects of growth hormone secretion on body composition in patients with Crohn’s disease. J Clin Endocrinol Metab. 2003;88:5468-5472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Zaltman C, Braulio VB, Outeiral R, Nunes T, de Castro CL. Lower extremity mobility limitation and impaired muscle function in women with ulcerative colitis. J Crohns Colitis. 2014;8:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 102. | Sentongo TA, Semeao EJ, Piccoli DA, Stallings VA, Zemel BS. Growth, body composition, and nutritional status in children and adolescents with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2000;31:33-40. [PubMed] |
| 103. | Dung NQ, Fusch G, Armbrust S, Jochum F, Fusch C. Use of bioelectrical impedance analysis and anthropometry to measure fat-free mass in children and adolescents with Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44:130-135. [PubMed] |
| 104. | Fink C, Karagiannides I, Bakirtzi K, Pothoulakis C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm Bowel Dis. 2012;18:1550-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 105. | Weakley FL, Turnbull RB. Recognition of regional ileitis in the operating room. Dis Colon Rectum. 1971;14:17-23. [PubMed] |
| 106. | Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn’s disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955-958. [PubMed] |
| 107. | Ponemone V, Keshavarzian A, Brand MI, Saclarides T, Abcarian H, Cabay RJ, Fletcher E, Larsen B, Durstine LJ, Fantuzzi G. Apoptosis and inflammation: role of adipokines in inflammatory bowel disease. Clin Transl Gastroenterol. 2010;1:e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Lim H, Kim HJ, Hong SJ, Kim S. Nutrient intake and bone mineral density by nutritional status in patients with inflammatory bowel disease. J Bone Metab. 2014;21:195-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Dumitrescu G, Mihai C, Dranga M, Prelipcean CC. Bone mineral density in patients with inflammatory bowel disease from north-eastern Romania. Rev Med Chir Soc Med Nat Iasi. 2013;117:23-28. [PubMed] |
| 110. | Miznerova E, Hlavaty T, Koller T, Toth J, Holociova K, Huorka M, Killinger Z, Payer J. The prevalence and risk factors for osteoporosis in patients with inflammatory bowel disease. Bratisl Lek Listy. 2013;114:439-445. [PubMed] |
| 111. | Kim HJ, Hong SJ, Jeon YW, Han JP, Han SH, Kang JH, Tae JW, Lim HS, Kim HK, Ko BM. The early onset of disease may be a risk factor for decreased bone mineral density in patients with inflammatory bowel disease. Clin Endosc. 2013;46:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | Miheller P, Gesztes W, Lakatos PL. Manipulating bone disease in inflammatory bowel disease patients. Ann Gastroenterol. 2013;26:296-303. [PubMed] |
| 113. | Adriani A, Pantaleoni S, Luchino M, Ribaldone DG, Reggiani S, Sapone N, Sguazzini C, Isaia G, Pellicano R, Astegiano M. Osteopenia and osteoporosis in patients with new diagnosis of inflammatory bowel disease. Panminerva Med. 2014;56:145-149. [PubMed] |
| 114. | Abraham BP, Prasad P, Malaty HM. Vitamin D deficiency and corticosteroid use are risk factors for low bone mineral density in inflammatory bowel disease patients. Dig Dis Sci. 2014;59:1878-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 115. | Gupta S, Wu X, Moore T, Shen B. Frequency, risk factors, and adverse sequelae of bone loss in patients with ostomy for inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 116. | Targownik LE, Bernstein CN, Nugent Z, Johansson H, Oden A, McCloskey E, Kanis JA, Leslie WD. Inflammatory bowel disease and the risk of fracture after controlling for FRAX. J Bone Miner Res. 2013;28:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 117. | Targownik LE, Bernstein CN, Nugent Z, Leslie WD. Inflammatory bowel disease has a small effect on bone mineral density and risk for osteoporosis. Clin Gastroenterol Hepatol. 2013;11:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 118. | Wong SC, Catto-Smith AG, Zacharin M. Pathological fractures in paediatric patients with inflammatory bowel disease. Eur J Pediatr. 2014;173:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Schmidt S, Mellström D, Norjavaara E, Sundh V, Saalman R. Longitudinal assessment of bone mineral density in children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2012;55:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 120. | Targownik LE, Bernstein CN, Leslie WD. Risk factors and management of osteoporosis in inflammatory bowel disease. Curr Opin Gastroenterol. 2014;30:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | Pichler J, Hanslik A, Huber WD, Aufricht C, Bidmon-Fliegenschnee B. Paediatric patients with inflammatory bowel disease who received infliximab experienced improved growth and bone health. Acta Paediatr. 2014;103:e69-e75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 122. | van Sommeren S, Janse M, Karjalainen J, Fehrmann R, Franke L, Fu J, Weersma RK. Extraintestinal manifestations and complications in inflammatory bowel disease: from shared genetics to shared biological pathways. Inflamm Bowel Dis. 2014;20:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 123. | Billiet T, Vermeire S. Differences between adults and children: genetics and beyond. Expert Rev Gastroenterol Hepatol. 2015;9:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 124. | Terzoudis S, Zavos C, Koutroubakis IE. The bone and fat connection in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:2207-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 125. | Veit LE, Maranda L, Fong J, Nwosu BU. The vitamin D status in inflammatory bowel disease. PLoS One. 2014;9:e101583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 126. | Kini GP, Young B, Herbison P, Schultz M. Does seasonal level of serum 25-OH vitamin D correlate with the activity of Crohn’s disease? N Z Med J. 2014;127:51-59. [PubMed] |
| 127. | Dumitrescu G, Mihai C, Dranga M, Prelipcean CC. Serum 25-hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania. World J Gastroenterol. 2014;20:2392-2396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 128. | Tan B, Li P, Lv H, Li Y, Wang O, Xing XP, Qian JM. Vitamin D levels and bone metabolism in Chinese adult patients with inflammatory bowel disease. J Dig Dis. 2014;15:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 129. | Mouli VP, Ananthakrishnan AN. Review article: vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;39:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 130. | Garg M, Rosella O, Lubel JS, Gibson PR. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2634-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 131. | Hlavaty T, Krajcovicova A, Koller T, Toth J, Nevidanska M, Huorka M, Payer J. Higher vitamin D serum concentration increases health related quality of life in patients with inflammatory bowel diseases. World J Gastroenterol. 2014;20:15787-15796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 132. | Rogler G, Vavricka S. Anemia in inflammatory bowel disease: an under-estimated problem? Front Med (Lausanne). 2014;1:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 133. | Filmann N, Rey J, Schneeweiss S, Ardizzone S, Bager P, Bergamaschi G, Koutroubakis I, Lindgren S, Morena Fde L, Moum B. Prevalence of anemia in inflammatory bowel diseases in european countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 134. | Goldenberg BA, Graff LA, Clara I, Zarychanski R, Walker JR, Carr R, Rogala L, Miller N, Bernstein CN. Is iron deficiency in the absence of anemia associated with fatigue in inflammatory bowel disease? Am J Gastroenterol. 2013;108:1392-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 135. | Sikora SK, Spady D, Prosser C, El-Matary W. Trace elements and vitamins at diagnosis in pediatric-onset inflammatory bowel disease. Clin Pediatr (Phila). 2011;50:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 136. | Alkhouri RH, Hashmi H, Baker RD, Gelfond D, Baker SS. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 137. | Ringstad J, Kildebo S, Thomassen Y. Serum selenium, copper, and zinc concentrations in Crohn’s disease and ulcerative colitis. Scand J Gastroenterol. 1993;28:605-608. [PubMed] |
| 138. | Geerling BJ, Badart-Smook A, Stockbrügger RW, Brummer RJ. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr. 2000;54:514-521. [PubMed] |
| 139. | Ojuawo A, Keith L. The serum concentrations of zinc, copper and selenium in children with inflammatory bowel disease. Cent Afr J Med. 2002;48:116-119. [PubMed] |
| 140. | Antunes CV, Hallack Neto AE, Nascimento CR, Chebli LA, Moutinho IL, Pinheiro Bdo V, Reboredo MM, Malaguti C, Castro AC, Chebli JM. Anemia in inflammatory bowel disease outpatients: prevalence, risk factors, and etiology. Biomed Res Int. 2015;2015:728925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 141. | Battat R, Kopylov U, Szilagyi A, Saxena A, Rosenblatt DS, Warner M, Bessissow T, Seidman E, Bitton A. Vitamin B12 deficiency in inflammatory bowel disease: prevalence, risk factors, evaluation, and management. Inflamm Bowel Dis. 2014;20:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 142. | Yakut M, Ustün Y, Kabaçam G, Soykan I. Serum vitamin B12 and folate status in patients with inflammatory bowel diseases. Eur J Intern Med. 2010;21:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 143. | Nowak JK, Grzybowska-Chlebowczyk U, Landowski P, Szaflarska-Poplawska A, Klincewicz B, Adamczak D, Banasiewicz T, Plawski A, Walkowiak J. Prevalence and correlates of vitamin K deficiency in children with inflammatory bowel disease. Sci Rep. 2014;4:4768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 144. | Vagianos K, Bernstein CN. Homocysteinemia and B vitamin status among adult patients with inflammatory bowel disease: a one-year prospective follow-up study. Inflamm Bowel Dis. 2012;18:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 145. | Prince AC, Moosa A, Lomer MC, Reidlinger DP, Whelan K. Variable access to quality nutrition information regarding inflammatory bowel disease: a survey of patients and health professionals and objective examination of written information. Health Expect. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 146. | Semrad CE. Use of parenteral nutrition in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2012;8:393-395. [PubMed] |
| 147. | Guagnozzi D, González-Castillo S, Olveira A, Lucendo AJ. Nutritional treatment in inflammatory bowel disease. An update. Rev Esp Enferm Dig. 2012;104:479-488. [PubMed] |
| 148. | Sandhu BK, Fell JM, Beattie RM, Mitton SG, Wilson DC, Jenkins H; IBD Working Group of the British Society of Paediatric Gastroenterology, Hepatology, and Nutrition. Guidelines for the management of inflammatory bowel disease in children in the United Kingdom. J Pediatr Gastroenterol Nutr. 2010;50 Suppl 1:S1-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 149. | Papi C, Fascì-Spurio F, Rogai F, Settesoldi A, Margagnoni G, Annese V. Mucosal healing in inflammatory bowel disease: treatment efficacy and predictive factors. Dig Liver Dis. 2013;45:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 150. | Markel TA, Lou DC, Pfefferkorn M, Scherer LR, West K, Rouse T, Engum S, Ladd A, Rescorla FJ, Billmire DF. Steroids and poor nutrition are associated with infectious wound complications in children undergoing first stage procedures for ulcerative colitis. Surgery. 2008;144:540-545; discussion 545-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 151. | Tighe MP, Cummings JR, Afzal NA. Nutrition and inflammatory bowel disease: primary or adjuvant therapy. Curr Opin Clin Nutr Metab Care. 2011;14:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 152. | Wild GE, Drozdowski L, Tartaglia C, Clandinin MT, Thomson AB. Nutritional modulation of the inflammatory response in inflammatory bowel disease--from the molecular to the integrative to the clinical. World J Gastroenterol. 2007;13:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 153. | Scolapio JS. The role of total parenteral nutrition in the management of patients with acute attacks of inflammatory bowel disease. J Clin Gastroenterol. 1999;29:223-224. [PubMed] |
| 154. | McIntyre PB, Ritchie JK, Hawley PR, Bartram CI, Lennard-Jones JE. Management of enterocutaneous fistulas: a review of 132 cases. Br J Surg. 1984;71:293-296. [PubMed] |
| 155. | Ostro MJ, Greenberg GR, Jeejeebhoy KN. Total parenteral nutrition and complete bowel rest in the management of Crohn’s disease. JPEN J Parenter Enteral Nutr. 1985;9:280-287. [PubMed] |
| 156. | de Luis DA, Culebras JM, Aller R, Eiros-Bouza JM. Surgical infection and malnutrition. Nutr Hosp. 2014;30:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 157. | Ridley E, Gantner D, Pellegrino V. Nutrition therapy in critically ill patients- a review of current evidence for clinicians. Clin Nutr. 2015;34:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 158. | Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. Am J Gastroenterol. 2007;102:412-29; quiz 468. [PubMed] |
| 159. | Fevang BT, Fevang J, Lie SA, Søreide O, Svanes K, Viste A. Long-term prognosis after operation for adhesive small bowel obstruction. Ann Surg. 2004;240:193-201. [PubMed] |
| 160. | Triantafillidis JK, Vagianos C, Papalois AE. The role of enteral nutrition in patients with inflammatory bowel disease: current aspects. Biomed Res Int. 2015;2015:197167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 161. | Wagner IJ, Rombeau JL. Nutritional support of surgical patients with inflammatory bowel disease. Surg Clin North Am. 2011;91:787-803, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 162. | Diamanti A, Bizzarri C, Basso MS, Gambarara M, Cappa M, Daniele A, Noto C, Castro M. How does long-term parenteral nutrition impact the bone mineral status of children with intestinal failure? J Bone Miner Metab. 2010;28:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 163. | Hernández C, Simó R, Chacón P, Sabin P, Baena JA, Castellanos JM, Planas M. Influence of surgical stress and parenteral nutrition on serum leptin concentration. Clin Nutr. 2000;19:61-64. [PubMed] |
| 164. | Dudley J, Rogers R, Sealy L. Renal consequences of parenteral nutrition. Pediatr Nephrol. 2014;29:375-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 165. | Blackmer AB, Warschausky S, Siddiqui S, Welch KB, Horn K, Wester A, Warschausky M, Teitelbaum DH. Preliminary findings of long-term neurodevelopmental outcomes of infants treated with intravenous fat emulsion reduction for the management of parenteral nutrition-associated cholestasis. JPEN J Parenter Enteral Nutr. 2015;39:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 166. | Lawiński M, Jachnis A, Ukleja A, Pertkiewicz M. Cholelithiasis in home parenteral nutrition (Hpn) patients--complications of the clinical nutrition: diagnosis, treatment, prevention. Pol Przegl Chir. 2014;86:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 167. | Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6:172-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 777] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 168. | Bozzetti F, Mariani L, Bertinet DB, Chiavenna G, Crose N, De Cicco M, Gigli G, Micklewright A, Moreno Villares JM, Orban A. Central venous catheter complications in 447 patients on home parenteral nutrition: an analysis of over 100.000 catheter days. Clin Nutr. 2002;21:475-485. [PubMed] |
| 169. | Crispin A, Thul P, Arnold D, Schild S, Weimann A. Central venous catheter complications during home parenteral nutrition: a prospective pilot study of 481 patients with more than 30,000 catheter days. Onkologie. 2008;31:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 170. | Higuera I, Garcia-Peris P, Camblor M, Bretón I, Velasco C, Romero R, Frias L, Cuerda C. Outcomes of a general hospital-based home parenteral nutrition (HPN) program; report of our experience from a 26-year period. Nutr Hosp. 2014;30:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 171. | Crowley JJ. Vascular access. Tech Vasc Interv Radiol. 2003;6:176-181. [PubMed] |
| 172. | Wall CL, Day AS, Gearry RB. Use of exclusive enteral nutrition in adults with Crohn’s disease: a review. World J Gastroenterol. 2013;19:7652-7660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (3)] |
| 173. | Whitten KE, Rogers P, Ooi CY, Day AS. International survey of enteral nutrition protocols used in children with Crohn’s disease. J Dig Dis. 2012;13:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 174. | Brown B, Roehl K, Betz M. Enteral nutrition formula selection: current evidence and implications for practice. Nutr Clin Pract. 2015;30:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 175. | Heuschkel RB. Enteral nutrition in children with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2000;31:575. [PubMed] |
| 176. | Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn‘s disease. Cochrane Database Syst Rev. 2007;Epub ahead of print. [PubMed] |
| 177. | Fell JM, Paintin M, Arnaud-Battandier F, Beattie RM, Hollis A, Kitching P, Donnet-Hughes A, MacDonald TT, Walker-Smith JA. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2000;14:281-289. [PubMed] |
| 178. | Griga T, Voigt E, Gretzer B, Brasch F, May B. Increased production of vascular endothelial growth factor by intestinal mucosa of patients with inflammatory bowel disease. Hepatogastroenterology. 1999;46:920-923. [PubMed] |
| 179. | Fell JM. Control of systemic and local inflammation with transforming growth factor beta containing formulas. JPEN J Parenter Enteral Nutr. 2005;29:S126-S128; discussion S129-S133, S184-188. [PubMed] |
| 180. | Bannerjee K, Camacho-Hübner C, Babinska K, Dryhurst KM, Edwards R, Savage MO, Sanderson IR, Croft NM. Anti-inflammatory and growth-stimulating effects precede nutritional restitution during enteral feeding in Crohn disease. J Pediatr Gastroenterol Nutr. 2004;38:270-275. [PubMed] |
| 181. | Hartman C, Berkowitz D, Weiss B, Shaoul R, Levine A, Adiv OE, Shapira R, Fradkin A, Wilschanski M, Tamir A. Nutritional supplementation with polymeric diet enriched with transforming growth factor-beta 2 for children with Crohn’s disease. Isr Med Assoc J. 2008;10:503-507. [PubMed] |
| 182. | Wedrychowicz A, Kowalska-Duplaga K, Jedynak-Wasowicz U, Pieczarkowski S, Sladek M, Tomasik P, Fyderek K. Serum concentrations of VEGF and TGF-β1 during exclusive enteral nutrition in IBD. J Pediatr Gastroenterol Nutr. 2011;53:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 183. | Wedrychowicz A, Kowalska-Duplaga K, Pieczarkowski S, Tomasik P, Spodaryk M, Fyderek K. [Influence of enteral nutrition therapy on serum angiogenic growth factors concentrations in children]. Przegl Lek. 2010;67:31-35. [PubMed] |
| 184. | Alhagamhmad MH, Day AS, Lemberg DA, Leach ST. An update of the role of nutritional therapy in the management of Crohn’s disease. J Gastroenterol. 2012;47:872-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 185. | Lecleire S, Hassan A, Marion-Letellier R, Antonietti M, Savoye G, Bôle-Feysot C, Lerebours E, Ducrotté P, Déchelotte P, Coëffier M. Combined glutamine and arginine decrease proinflammatory cytokine production by biopsies from Crohn’s patients in association with changes in nuclear factor-kappaB and p38 mitogen-activated protein kinase pathways. J Nutr. 2008;138:2481-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 186. | Coëffier M, Marion-Letellier R, Déchelotte P. Potential for amino acids supplementation during inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 187. | Montejo JC, Zarazaga A, López-Martínez J, Urrútia G, Roqué M, Blesa AL, Celaya S, Conejero R, Galbán C, García de Lorenzo A. Immunonutrition in the intensive care unit. A systematic review and consensus statement. Clin Nutr. 2003;22:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 188. | Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34:1980-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 189. | Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double-blind randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn’s disease. J Pediatr Gastroenterol Nutr. 2000;30:78-84. [PubMed] |
| 190. | Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, Ebner C. ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin Nutr. 2006;25:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 827] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 191. | Gerasimidis K, Bertz M, Hanske L, Junick J, Biskou O, Aguilera M, Garrick V, Russell RK, Blaut M, McGrogan P. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm Bowel Dis. 2014;20:861-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 192. | Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4098] [Cited by in RCA: 4687] [Article Influence: 312.5] [Reference Citation Analysis (1)] |
| 193. | Leach ST, Mitchell HM, Eng WR, Zhang L, Day AS. Sustained modulation of intestinal bacteria by exclusive enteral nutrition used to treat children with Crohn’s disease. Aliment Pharmacol Ther. 2008;28:724-733. [PubMed] |
| 194. | Lionetti P, Callegari ML, Ferrari S, Cavicchi MC, Pozzi E, de Martino M, Morelli L. Enteral nutrition and microflora in pediatric Crohn’s disease. JPEN J Parenter Enteral Nutr. 2005;29:S173-S175; discussion S175-S178, S184-S188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 195. | Lochs H, Dejong C, Hammarqvist F, Hebuterne X, Leon-Sanz M, Schütz T, van Gemert W, van Gossum A, Valentini L, Lübke H. ESPEN Guidelines on Enteral Nutrition: Gastroenterology. Clin Nutr. 2006;25:260-274. [PubMed] |
| 196. | Akobeng AK. Crohn’s disease: current treatment options. Arch Dis Child. 2008;93:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 197. | Day AS, Whitten KE, Sidler M, Lemberg DA. Systematic review: nutritional therapy in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2008;27:293-307. [PubMed] |
| 198. | Stewart M, Day AS, Otley A. Physician attitudes and practices of enteral nutrition as primary treatment of paediatric Crohn disease in North America. J Pediatr Gastroenterol Nutr. 2011;52:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 199. | Wong S, Lemberg DA, Day AS. Exclusive enteral nutrition in the management of perianal Crohn’s disease in children. J Dig Dis. 2010;11:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 200. | Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut. 2006;55:356-361. [PubMed] |
| 201. | Rubio A, Pigneur B, Garnier-Lengliné H, Talbotec C, Schmitz J, Canioni D, Goulet O, Ruemmele FM. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment Pharmacol Ther. 2011;33:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 202. | Kappelman MD, Bousvaros A. Nutritional concerns in pediatric inflammatory bowel disease patients. Mol Nutr Food Res. 2008;52:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 203. | Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1041] [Article Influence: 65.1] [Reference Citation Analysis (1)] |
| 204. | Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465-483; quiz 464, 484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 596] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 205. | Matsui T, Sakurai T, Yao T. Nutritional therapy for Crohn’s disease in Japan. J Gastroenterol. 2005;40 Suppl 16:25-31. [PubMed] |
| 206. | Day AS, Whitten KE, Lemberg DA, Clarkson C, Vitug-Sales M, Jackson R, Bohane TD. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: a feasible and effective approach. J Gastroenterol Hepatol. 2006;21:1609-1614. [PubMed] |
| 207. | Frivolt K, Schwerd T, Werkstetter KJ, Schwarzer A, Schatz SB, Bufler P, Koletzko S. Repeated exclusive enteral nutrition in the treatment of paediatric Crohn’s disease: predictors of efficacy and outcome. Aliment Pharmacol Ther. 2014;39:1398-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 208. | Panaccione R, Ghosh S. Optimal use of biologics in the management of Crohn’s disease. Therap Adv Gastroenterol. 2010;3:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 209. | Li Y, Zuo L, Zhu W, Gong J, Zhang W, Gu L, Guo Z, Cao L, Li N, Li J. Role of exclusive enteral nutrition in the preoperative optimization of patients with Crohn’s disease following immunosuppressive therapy. Medicine (Baltimore). 2015;94:e478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 210. | Wilschanski M, Sherman P, Pencharz P, Davis L, Corey M, Griffiths A. Supplementary enteral nutrition maintains remission in paediatric Crohn’s disease. Gut. 1996;38:543-548. [PubMed] |
| 211. | Duncan H, Buchanan E, Cardigan T, Garrick V, Curtis L, McGrogan P, Barclay A, Russell RK. A retrospective study showing maintenance treatment options for paediatric CD in the first year following diagnosis after induction of remission with EEN: supplemental enteral nutrition is better than nothing! BMC Gastroenterol. 2014;14:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 212. | Hanai H, Iida T, Takeuchi K, Arai H, Arai O, Abe J, Tanaka T, Maruyama Y, Ikeya K, Sugimoto K. Nutritional therapy versus 6-mercaptopurine as maintenance therapy in patients with Crohn’s disease. Dig Liver Dis. 2012;44:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 213. | Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, Takahashi H, Takahashi S, Kinouchi Y, Hiwatashi N. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment Pharmacol Ther. 2006;24:1333-1340. [PubMed] |
| 214. | de Bie C, Kindermann A, Escher J. Use of exclusive enteral nutrition in paediatric Crohn’s disease in The Netherlands. J Crohns Colitis. 2013;7:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 215. | Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2010;105:525-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 216. | Mukai J, Miyanaga Y, Ishizaka T, Asaka K, Nakai Y, Tsuji E, Uchida T. Quantitative taste evaluation of total enteral nutrients. Chem Pharm Bull (Tokyo). 2004;52:1416-1421. [PubMed] |
| 217. | Miyanaga Y, Mukai J, Mukai T, Odomi M, Uchida T. Suppression of the bitterness of enteral nutrients using increased particle sizes of branched-chain amino acids (BCAAs) and various flavours: a taste sensor study. Chem Pharm Bull (Tokyo). 2004;52:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 218. | Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Umemura K, Iwabuchi M, Takahashi H, Takahashi S, Kinouchi Y. Quality of life of patients and medical cost of “half elemental diet” as maintenance therapy for Crohn’s disease: secondary outcomes of a randomised controlled trial. Dig Liver Dis. 2009;41:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 219. | Afzal NA, Van Der Zaag-Loonen HJ, Arnaud-Battandier F, Davies S, Murch S, Derkx B, Heuschkel R, Fell JM. Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment Pharmacol Ther. 2004;20:167-172. [PubMed] |
| 220. | Guo Z, Wu R, Zhu W, Gong J, Zhang W, Li Y, Gu L, Li N, Li J. Effect of exclusive enteral nutrition on health-related quality of life for adults with active Crohn’s disease. Nutr Clin Pract. 2013;28:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 221. | Berni Canani R, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A, D’Armiento F, Romeo EF, Cucchiara S. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis. 2006;38:381-387. [PubMed] |
| 222. | Knight C, El-Matary W, Spray C, Sandhu BK. Long-term outcome of nutritional therapy in paediatric Crohn’s disease. Clin Nutr. 2005;24:775-779. [PubMed] |
| 223. | Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr. 2000;31:8-15. [PubMed] |
| 224. | Dziechciarz P, Horvath A, Shamir R, Szajewska H. Meta-analysis: enteral nutrition in active Crohn’s disease in children. Aliment Pharmacol Ther. 2007;26:795-806. [PubMed] |
| 225. | Cameron FL, Gerasimidis K, Papangelou A, Missiou D, Garrick V, Cardigan T, Buchanan E, Barclay AR, McGrogan P, Russell RK. Clinical progress in the two years following a course of exclusive enteral nutrition in 109 paediatric patients with Crohn’s disease. Aliment Pharmacol Ther. 2013;37:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 226. | Soo J, Malik BA, Turner JM, Persad R, Wine E, Siminoski K, Huynh HQ. Use of exclusive enteral nutrition is just as effective as corticosteroids in newly diagnosed pediatric Crohn’s disease. Dig Dis Sci. 2013;58:3584-3591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 227. | Lambert B, Lemberg DA, Leach ST, Day AS. Longer-term outcomes of nutritional management of Crohn’s disease in children. Dig Dis Sci. 2012;57:2171-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 228. | Fiocchi C. Inflammatory bowel disease pathogenesis: where are we? J Gastroenterol Hepatol. 2015;30 Suppl 1:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Pierce ES, Wu DC S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH