Published online Aug 7, 2016. doi: 10.3748/wjg.v22.i29.6726
Peer-review started: March 25, 2016
First decision: May 12, 2016
Revised: May 25, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: August 7, 2016
Processing time: 127 Days and 6.1 Hours
AIM: To hypothesize that in patients with colon cancer showing heavy intestinal wall invasion without distant metastasis (T4bN0-2M0), small tumor size would correlate with more aggressive tumor behaviors and therefore poorer cancer-specific survival (CSS).
METHODS: We analyzed T4bN0-2M0 colon cancer patients in the Surveillance, Epidemiology and End Results (SEER) database. A preliminary analysis of T4bN0-2M0 colon cancer patients at the Fudan University Shanghai Cancer Center is also presented.
RESULTS: A total of 1734 T4bN0-2M0 colon cancer patients from the SEER database were included. Kaplan-Meier analysis revealed decreasing CSS with decreasing tumor size (P < 0.001). Subgroup analysis showed a significant association between poorer CSS with smaller tumor size in T4bN0 patients (P = 0.024), and a trend of association in T4bN1 (P = 0.182) and T4bN2 patients (P = 0.191). Multivariate analysis identified tumor size as an independent prognostic factor for CSS in T4bN0-2M0 patients (P = 0.024). Preliminary analysis of Fudan University Shanghai Cancer Center samples suggested the 5-year CSS was 50.0%, 72.9% and 77.1% in patients with tumors ≤ 4.0 cm, 4.0-7.0 cm and ≥ 7.0 cm.
CONCLUSION: Smaller tumor size is associated with poorer CSS in the T4bN0-2M0 subset of colon cancer, particularly in the T4bN0M0 subgroup.
Core tip: In contrast to the association of larger tumor size with poor cancer-specific survival (CSS) in colon cancer patients overall, this study suggested that smaller tumor size was associated with poor CSS in the T4bN0-2M0 subpopulation, particularly in the T4bN0M0 subgroup.
- Citation: Huang B, Feng Y, Mo SB, Cai SJ, Huang LY. Smaller tumor size is associated with poor survival in T4b colon cancer. World J Gastroenterol 2016; 22(29): 6726-6735
- URL: https://www.wjgnet.com/1007-9327/full/v22/i29/6726.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i29.6726
Locally advanced colorectal cancer, including T4b colorectal cancer, accounts for 5%-22% of all colorectal cancer cases[1]. Several prognostic factors have been identified for locally advanced colorectal cancer, including pN+[1,2], pT4[1-3], poor tumor differentiation[2], high serum CEA[2,4], and lymphovascular or perineural invasion[1,5]. T4b lesions (previously classified as T4) are associated with poorer prognosis when they are invasive or when they adhere to other organs or structures[3,6,7].
Standard surgical treatment for such patients is multivisceral resection (MVR), which refers to en bloc removal of the tumor and infiltrated organs in order to achieve margin-negative (R0) resection[8,9]. This procedure is associated with increased incidence of a wide variety of complication[9-11], and it is often impossible to distinguish malignant invasion from inflammatory adhesions intraoperatively[12]. Thus, identifying novel risk factors in such patients might allow a more appropriate adjuvant treatment planning and a better assessment of the long-term prognosis after the MVR.
Smaller tumor size is generally associated with greater cancer-specific survival (CSS) in patients with colon cancer[13-15]. However, analysis of subgroups of colon cancer patients has produced surprising results. In a study of 610 Japanese colon cancer patients, Takahashi et al[16] found CSS to be lower with smaller tumors: patients with tumors 4-8 cm in size showed an HR of 0.45 (95%CI: 0.293-0.696, P < 0.001) relative to patients with tumors < 4 cm. Similarly, a study of stage IIA colon cancer[17] found decreased CSS with smaller tumor size.
We hypothesized that small tumor size would be associated with more aggressive tumor behavior and thus lower CSS in the subgroup of colon cancer patients with heavy intestinal wall invasion (T4bN0-2) without distal metastasis (M0). To address this hypothesis, we analyzed patients with T4bN0-2M0 colon cancer in the US Surveillance, Epidemiology and End Results (SEER) database. Subgroup analysis was conducted separately for patients in the T4bN0, T4bN1, and T4bN2 stages. We also carried out a preliminary analysis of the 101 patients with T4bN0-2M0 colon cancer receiving surgical treatment at the Fudan University Shanghai Cancer Center (FUSCC).
The SEER database is a population-based cancer registry that collects and publishes cancer incidence and survival data from 18 population-based cancer registries covering approximately 26% of the US population. Cases of invasive colon cancer from January 1988 to December 2003 were extracted from the database (http://seer.cancer.gov, April 2013 release). For inclusion in our study, subjects had to meet the following criteria: (1) age between 18 and 75 years at time of diagnosis; (2) diagnosis of adenocarcinoma, mucinous adenocarcinoma or signet-ring carcinoma of the colon in AJCC stages T4bN0-2; (3) known maximum tumor diameter, depth of invasion, and lymph node status; (4) at least 12 harvested lymph nodes; (5) colon cancer surgically resected; (6) confirmation of diagnosis based on pathology of a surgical specimen, rather than based on death certificate or autopsy; (7) non-metastatic (AJCC stage M0); (8) known survival time and cause of death; and (9) colon cancer as the only malignant tumor. Subjects who underwent neoadjuvant chemoradiotherapy (CRT) or only local tumor excision were excluded.
The FUSCC colorectal cancer database was established in January 2006, and prospectively collects data of colorectal cancer patients receiving treatment at FUSCC. Data were extracted for T4bN0-2M0 colon cancer patients treated between 2006 and 2012 with radical surgery and 6 mo of adjuvant chemotherapy involving either the combination of oxaliplatin and fluorouracil/folinic acid or the combination of oxaliplatin and oral capecitabine. The inclusion and exclusion criteria were identical to that in the data selection from the SEER database. The FUSCC Ethical Committee and Institutional Review Board approved the research protocol, and all subjects provided written informed consent.
We collected data on the following variables from the SEER database: gender, race, patient age at diagnosis (with 60 years applied as a cut-off in analyses), primary site, number of primary tumors, year of diagnosis, histological type, pathology grade, tumor size, TNM stage, number of lymph nodes harvested (with 18 applied as the cut-off in analyses), number of metastatic lymph nodes (N0, N1, or N2), radiation sequence with surgery, survival time and cause of death. The same data were collected from the FUSCC dataset, from which we also collected information on lymphovascular invasion and perineural invasion. All cases were restaged according to the 7th edition of the AJCC Cancer Staging Manual.
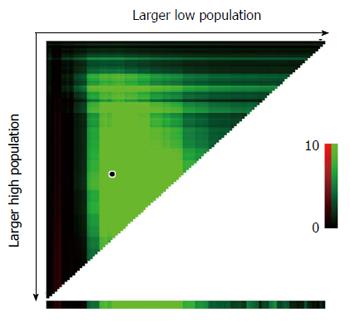
Tumor size was defined as the maximal tumor diameter obtained from pathology reports on resected cancer specimens. Tumor size cut-off points to assign patients to high-, medium-, or low-risk groups were identified using X-tile software (http://www.tissuearray.org/rimmlab)[18]. In X-tile, data are shown in a triangular grid, with each point in the grid representing a potential cut-off point. The intensity of the color in the grid represents the strength of the association between tumor size and CSS. The primary study outcome was CSS. CSS was calculated from the date of diagnosis to the date of colon cancer-specific death. Cases were censored if patients died from other causes or were alive at the time of last follow-up.
Cut-off points for CSS were determined based on minimal P values from log-rank χ2 statistics using X-tile. Groups with different tumor sizes, defined using the cut-off points determined by X-tile, were compared in terms of patient- and disease-related characteristics using χ2 tests. Survival data were plotted as Kaplan-Meier curves and compared using log-rank tests. A multivariate Cox regression model was used to analyze risk factors for survival outcomes. All computed P values were 2-tailed, and statistical significance was accepted at P < 0.05. All analyses were performed using SPSS 20.0.
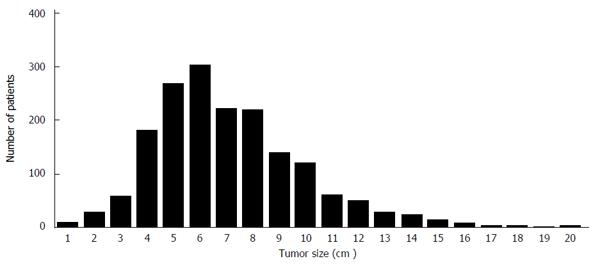
A total of 1734 patients (857 men and 877 women) in the SEER database were included in the final analysis, of whom 978 (56.4%) were recorded as having died from colon cancer. Patient demographics and pathology features stratified by tumor size are shown in Table 1. Median follow-up time was 43 mo (interquartile range, 15-107 mo). Most subjects were Caucasian (80.0%), followed by African-Americans (11.2%). Histology types included adenocarcinoma (77.9%), mucinous cancer (19.7%), and signet ring cell cancer (2.4%). Lymph node status included N0 (no lymph node involvement), 41.8%; N1 (1-3 metastasized lymph nodes), 26.5%; and N2 (> 3 metastasized lymph nodes), 31.7%. The proportions of patients with lymph node metastasis (N1 or N2) were 66.0% in the subgroup with tumors ≤ 4.0 cm, 61.0% in the subgroup with tumors 4.0-7.0 cm, and 53.3% in the subgroup with tumors ≥ 7.0 cm (P = 0.001). The distribution of patients with T4bN0-2M0 colon cancer among the tumor size subgroups is shown in Figure 1.
| Characteristic | Tumor size (cm) | P value | |||
| Total (n = 1734) | ≤4.0 (n = 274) | 4.0-7.0 (n = 653) | ≥7.0 (n = 807) | ||
| Median follow-up (mo) | 43 | 33 | 43 | 50 | |
| IQR | 15-107 | 12-92 | 17-105 | 14-114 | |
| Sex | 0.395 | ||||
| Male | 857 (49.4) | 131 (47.8) | 313 (47.9) | 413 (51.2) | |
| Female | 877 (50.6) | 143 (52.2) | 340 (52.1) | 394 (48.8) | |
| Year of diagnosis | 0.867 | ||||
| 1988-1999 | 856 (49.4) | 137 (50.0) | 317 (48.5) | 402 (49.8) | |
| 2000-2003 | 878 (50.6) | 137 (50.0) | 336 (51.5) | 405 (50.2) | |
| Age at diagnosis (yr) | 0.330 | ||||
| ≤ 60 | 785 (45.3) | 130 (47.4) | 281 (43.0) | 374 (46.3) | |
| > 60 | 949 (54.7) | 144 (52.6) | 372 (57.0) | 433 (53.7) | |
| Primary site | 0.187 | ||||
| Right colon | 1103 (63.6) | 176 (64.2) | 398 (60.9) | 529 (65.6) | |
| Left colon | 631 (36.4) | 98 (35.8) | 255 (39.1) | 278 (34.4) | |
| Race | 0.145 | ||||
| White | 1386 (80.0) | 212 (77.4) | 530 (81.2) | 644 (79.8) | |
| Black | 195 (11.2) | 41 (15.0) | 61 (9.3) | 93 (11.5) | |
| Other1 | 153 (8.8) | 21 (7.6) | 63 (9.5) | 69 (8.7) | |
| Histological type | 0.016 | ||||
| Adenocarcinoma | 1351 (77.9) | 223 (81.4) | 525 (80.4) | 603 (74.7) | |
| Mucinous adenocarcinoma | 341 (19.7) | 42 (15.3) | 118 (18.1) | 181 (22.4) | |
| Signet-ring cell carcinoma | 42 (2.4) | 9 (3.3) | 10 (1.5) | 23 (2.9) | |
| Pathology grade | 0.001 | ||||
| High/Moderate | 1158 (66.8) | 189 (69.0) | 466 (71.4) | 503 (62.3) | |
| Poor/Undifferentiated | 576 (33.2) | 85 (31.0) | 187 (28.6) | 304 (37.7) | |
| N stage | 0.001 | ||||
| N0 | 725 (41.8) | 93 (34.0) | 255 (39.0) | 377 (46.7) | |
| N1 | 460 (26.5) | 74 (27.0) | 188 (28.8) | 198 (24.5) | |
| N2 | 549 (31.7) | 107 (39.0) | 210 (32.2) | 232 (28.8) | |
| LNH | 0.013 | ||||
| ≤ 18 | 938 (54.1) | 157 (57.3) | 375 (57.4) | 406 (50.3) | |
| > 18 | 796 (45.9) | 117 (42.6) | 278 (42.6) | 401 (49.7) | |
Several Kaplan-Meier analyses of T4bN0-2M0 patients were conducted using tumor size cut-off points ranging from 3 to 10 cm. In all analyses, patients with smaller tumor size showed poorer CSS than those with larger tumor size at each cut-off point tested. As the cut-off point increased, so did the survival rate of patients with tumors smaller than that cut-off (Table 2). X-tile analysis identified 4.0 and 7.0 cm as optimal cut-off values (Figure 2).
| Cut-off | n | 5-yr CSS | Log-rank χ2 | P value |
| ≤ 3 | 94 | 35.9% | 3.710 | 0.054 |
| > 3 | 1640 | 48.7% | ||
| ≤ 4 | 274 | 37.1% | 15.214 | < 0.001 |
| > 4 | 1460 | 50.0% | ||
| ≤ 5 | 542 | 40.8% | 18.235 | < 0.001 |
| > 5 | 1192 | 51.2% | ||
| ≤ 6 | 845 | 43.3% | 14.118 | < 0.001 |
| > 6 | 889 | 52.4% | ||
| ≤ 7 | 1066 | 45.1% | 7.303 | 0.007 |
| >7 | 668 | 52.5% | ||
| ≤ 8 | 1284 | 46.3% | 3.877 | 0.049 |
| > 8 | 450 | 52.5% | ||
| ≤ 9 | 1422 | 46.9% | 2.270 | 0.132 |
| > 9 | 312 | 52.8% | ||
| ≤ 10 | 1541 | 47.0% | 5.061 | 0.024 |
| > 10 | 193 | 55.8% |
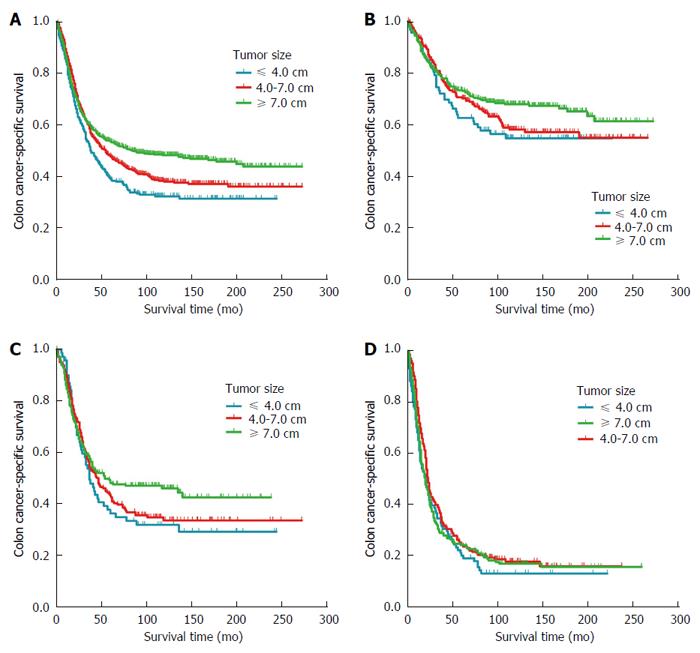
Using 4.0 and 7.0 cm as cut-off values, Kaplan-Meier analysis showed decreasing CSS with decreasing tumor size (P < 0.001): 5-year CSS was 37.1% in patients with tumors ≤ 4.0 cm, 47.0% in patients with tumors 4.0-7.0 cm and 52.5% in those with tumors ≥ 7.0 cm (Figure 3A). Univariate analysis of all subjects suggested the following risk factors: age at diagnosis (P < 0.001), histology type (P < 0.001), pathology grade (P < 0.001), primary site (P = 0.001), tumor size (P < 0.001), number of lymph nodes harvested (P = 0.001) and N stage (P < 0.001). Multivariate Cox proportional modeling identified the following independent prognostic factors: age at diagnosis (P < 0.001), pathology grade (P = 0.002), tumor size (P = 0.024), number of lymph nodes harvested (P = 0.009), and N stage (P < 0.001). Relative to patients with tumors ≤ 4.0 cm, patients with tumors 4.0-7.0 cm were more likely to show greater CSS (HR = 0.820, 95%CI: 0.683-0.985, P = 0.034), as were patients with tumors ≥ 7.0 cm (HR = 0.775, 95%CI: 0.645-0.932, P = 0.007) (Table 3).
| Variable | Univariateanalysis | Multivariateanalysis | |||
| 5-yr CSS | P value | HR | 95%CI | P value | |
| Sex | 0.404 | NI | |||
| Male | 46.7% | ||||
| Female | 49.1% | ||||
| Year of diagnosis | 0.339 | NI | |||
| 1988-1999 | 49.4% | ||||
| 2000-2003 | 46.6% | ||||
| Race | 0.184 | NI | |||
| White | 48.9% | ||||
| Black | 41.1% | ||||
| Other1 | 48.6% | ||||
| Age at diagnosis (yr) | < 0.001 | < 0.001 | |||
| ≤ 60 | 51.7% | 1.000 | reference | ||
| > 60 | 44.8% | 1.309 | 1.148-1.492 | ||
| Primary site | 0.001 | 0.061 | |||
| Right colon | 45.1% | 1.000 | reference | ||
| Left colon | 52.9% | 0.875 | 0.761-1.006 | ||
| Histological type | < 0.001 | 0.100 | |||
| Adenocarcinoma | 48.5% | 1.000 | reference | ||
| Mucinous adenocarcinoma | 49.7% | 1.008 | 0.848-1.197 | ||
| Signet-ring cell carcinoma | 17.2% | 1.518 | 1.037-2.222 | ||
| Pathology grade | < 0.001 | 0.002 | |||
| High | 67.8% | 1.000 | reference | ||
| Moderate | 51.4% | 1.530 | 1.127-2.077 | ||
| Poor | 38.6% | 1.729 | 1.258-2.376 | ||
| Undifferentiated | 25.0% | 2.526 | 1.389-4.596 | ||
| N stage | < 0.001 | < 0.001 | |||
| N0 | 69.5% | 1.000 | reference | ||
| N1 | 43.7% | 1.857 | 1.559-2.212 | ||
| N2 | 22.5% | 3.600 | 3.056-4.239 | ||
| LNH | 0.001 | 0.009 | |||
| ≤ 18 | 43.5% | 1.000 | reference | ||
| > 18 | 53.2% | 0.841 | 0.738-0.959 | ||
| Tumor size, cm | < 0.001 | 0.024 | |||
| ≤ 4.0 | 37.1% | 1.000 | reference | ||
| 4.0-7.0 | 47.0% | 0.820 | 0.683-0.985 | 0.034 | |
| ≥ 7.0 | 52.5% | 0.775 | 0.645-0.932 | 0.007 | |
Kaplan-Meier analysis based on N stage showed decreasing CSS with increasing N stage (P < 0.001), the 5-year CSS was 69.5% in T4bN0 patients, 43.7% in T4bN1 patients and 22.6% in T4bN2 patients. Subgroup analysis confirmed the inverse relationship between tumor size and CSS in T4bN0 patients (P = 0.024; Figure 3B). The 5-year CSS was 58.4% in patients with tumors ≤ 4.0 cm, 69.3% in patients with tumors 4.0-7.0 cm and 72.4% in those with tumors ≥ 7.0 cm. In contrast, the corresponding 5-year CSS rates were 35.8%, 42.5% and 47.9% in the subgroup of T4bN1 patients, suggesting that tumor size was not significantly associated with CSS (P = 0.182; Figure 3C). Similar results were obtained in the subgroup of T4bN2 patients, for which the corresponding 5-year CSS rates were 19.1%, 23.7% and 23.3% (P = 0.191; Figure 3D).
Univariate analysis of data from the subgroup of T4bN0M0 subjects identified the following factors as associated with CSS: tumor size (P = 0.024), number of lymph nodes harvested (P = 0.029) and age at diagnosis (P = 0.002). Multivariate analysis identified tumor size (P = 0.048) and age at diagnosis (P = 0.004) as independent prognostic factors. Relative to patients with tumors ≤ 4.0 cm, patients with tumors 4.0-7.0 cm were not likely to have a significantly different CSS (HR = 0.790, 95%CI: 0.554-1.127; P = 0.194), while patients with tumors ≥ 7.0 cm were more likely to show higher CSS (HR = 0.656, 95%CI: 0.464-0.926; P = 0.017) (Table 4).
| Variable | Univariateanalysis | Multivariateanalysis | |||
| 5-yr CSS | P value | HR | 95%CI | P value | |
| Sex | 0.443 | NI | |||
| Male | 67.7% | ||||
| Female | 71.2% | ||||
| Year of diagnosis | 0.473 | NI | |||
| 1988-1999 | 71.9% | ||||
| 2000-2003 | 66.7% | ||||
| Race | 0.388 | NI | |||
| White | 70.0% | ||||
| Black | 62.2% | ||||
| Other1 | 73.0% | ||||
| Primary site | 0.819 | NI | |||
| Right colon | 70.1% | ||||
| Left colon | 68.6% | ||||
| Histological type | 0.401 | NI | |||
| Adenocarcinoma | 69.0% | ||||
| Mucinous adenocarcinoma | 72.1% | ||||
| Signet-ring cell carcinoma | 40.0% | ||||
| Pathology grade | 0.552 | NI | |||
| High | 80.6% | ||||
| Moderate | 68.5% | ||||
| Poor | 66.0% | ||||
| Undifferentiated | 66.7% | ||||
| Age at diagnosis (yr) | 0.002 | 0.004 | |||
| ≤ 60 | 74.1% | 1.000 | reference | ||
| > 60 | 65.5% | 1.441 | 1.127-1.841 | ||
| LNH | 0.029 | 0.063 | |||
| ≤ 18 | 64.3% | 1.000 | reference | ||
| > 18 | 75.1% | 0.795 | 0.624-1.013 | ||
| Tumor size, cm | 0.024 | 0.048 | |||
| ≤ 4.0 | 58.4% | 1.000 | reference | ||
| 4.0-7.0 | 69.3% | 0.790 | 0.554-1.127 | 0.194 | |
| ≥ 7.0 | 72.4% | 0.656 | 0.464-0.926 | 0.017 | |
This analysis included 101 patients (59 men and 42 women; Table 5). Median follow-up time was 47 mo (interquartile range, 33-62 mo). The distribution of histology types was adenocarcinoma, 87.1%; mucinous cancer, 9.9%; and signet ring cell cancer, 3.0%. The distribution of lymph node status was N0, 42.6%; N1, 33.7%; and N2, 23.8%. The rate of lymphovascular invasion was 55.6% for tumor at ≤ 4.0 cm, 30.8% and 34.1% for tumor at 4.0-7.0 cm and ≥ 7.0 cm.
| Characteristic | Tumor size (cm) | P value | |||
| Total (n = 101) | ≤4.0 (n = 18) | 4.0-7.0 (n = 39) | ≥7.0 (n = 44) | ||
| Median follow-up (mo) | 49 | 46 | 51 | 44 | |
| IQR | 33-62 | 27-72 | 31-61 | 33-62 | |
| Sex | 0.582 | ||||
| Male | 59 (58.4) | 9 (50.0) | 22 (56.4) | 28 (63.6) | |
| Female | 42 (41.6) | 9 (50.0) | 17 (43.6) | 16 (36.4) | |
| Age at diagnosis (yr) | 0.245 | ||||
| ≤ 60 | 33 (32.7) | 3 (16.7) | 13 (33.3) | 17 (38.6) | |
| > 60 | 68 (67.3) | 15 (83.3) | 26 (66.7) | 27 (61.4) | |
| Primary site | 0.668 | ||||
| Right colon | 50 (49.5) | 8 (44.4) | 18 (46.2) | 24 (54.5) | |
| Left colon | 51 (50.5) | 10 (55.6) | 21 (53.8) | 20 (45.5) | |
| Histology type | 0.262 | ||||
| Adenocarcinoma | 88 (87.1) | 16 (88.9) | 35 (89.7) | 37 (84.1) | |
| Mucinous adenocarcinoma | 10 (9.9) | 1 (5.6) | 2 (5.1) | 7 (15.9) | |
| Signet-ring cell carcinoma | 3 (3.0) | 1 (5.6) | 2 (5.1) | 0 (0.0) | |
| Pathology grade | 0.567 | ||||
| High/Moderate | 72 (71.3) | 11 (61.1) | 29 (74.4) | 32 (72.7) | |
| Poor/Undifferentiated | 29 (28.7) | 7 (38.9) | 10 (25.6) | 12 (27.3) | |
| N stage | 0.210 | ||||
| N0 | 43 (42.6) | 4 (22.2) | 20 (51.3) | 19 (43.2) | |
| N1 | 34 (33.7) | 10 (55.6) | 10 (25.6) | 14 (31.8) | |
| N2 | 24 (23.8) | 4 (22.2) | 9 (23.1) | 11 (25.0) | |
| LNH | 0.109 | ||||
| ≤ 18 | 56 (55.4) | 14 (77.8) | 20 (51.3) | 22 (50.0) | |
| > 18 | 45 (44.6) | 4 (22.2) | 19 (48.7) | 22 (50.0) | |
| Lymphovascular invasion | 0.176 | ||||
| Positive | 37 (36.6) | 10 (55.6) | 12 (30.8) | 15 (34.1) | |
| Negative | 64 (63.4) | 8 (44.4) | 27 (69.2) | 29 (65.9) | |
| Perineural invasion | 0.906 | ||||
| Positive | 24 (23.8) | 5 (27.8) | 9 (23.1) | 10 (22.7) | |
| Negative | 77 (76.2) | 13 (72.2) | 30 (76.9) | 34 (77.3) | |
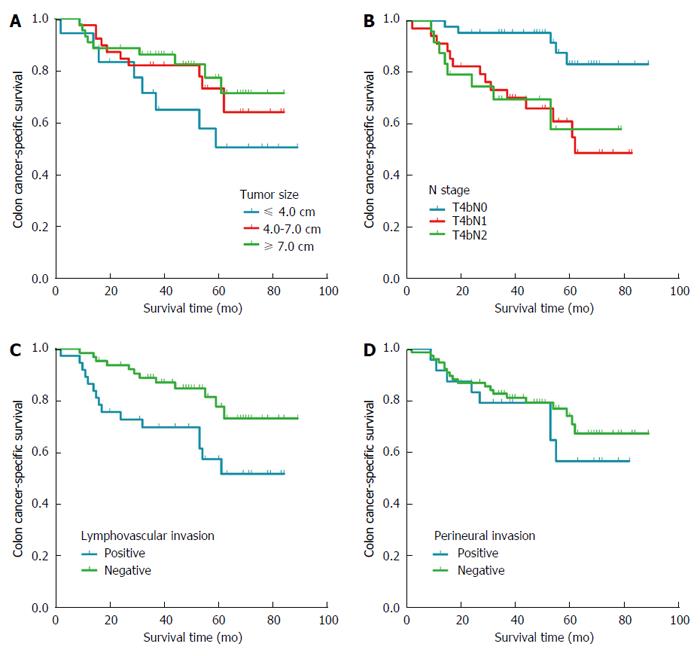
The 5-year CSS across all N subtypes was 50.0% for patients with tumors ≤ 4.0 cm, 72.9% for those with tumors 4.0-7.0 cm and 77.1% for those with tumors ≥ 7.0 cm (Figure 4A). The 5-year CSS for patients was 83.0% in the T4bN0 subgroup, 60.8% in the T4bN1 subgroup and 56.8% in the T4bN2 subgroup (Figure 4B). Across all N subtypes and tumor sizes, 5-year CSS was lower in the presence of lymphovascular invasion (57.3%) than in its absence (77.5%), and it was lower in the presence of perineural invasion (55.5%) than in its absence (73.9%) (Figure 4C and D).
The TNM staging system, as recommended by the American Joint Committee on Cancer (AJCC), is instrumental in the diagnosis and prognosis prediction in many malignancies. In colorectal cancer, “T” in the TNM staging is distinct and reflects the depth of local invasion rather than absolute tumor size. In several studies, it has been proposed that tumor size can be a useful addition to the TNM staging system for the sake of higher prognostic accuracy in colorectal cancer[19,20].
In the cohort of patients with T4bN0-2M0 colon cancer, small tumor size was associated with poor prognosis, particularly in T4bN0M0 patients, as well as with lymph node metastasis. Analysis of the FUSCC dataset suggested a possible link between small tumor size and lymphovascular invasion. Since lymph node involvement and lymphovascular invasion were associated with poor survival, our results suggest that small tumor size in T4b patients may reflect a more biologically aggressive phenotype. The association between tumor size and CSS appeared as a non-significant trend in the subgroup of T4bN1 patients, which may reflect the small sample size. Similarly, this association was not significant in the subgroup of T4bN2 patients, which may reflect the extensive involvement of lymph nodes, which likely influences patient survival to a greater extent than tumor size. Another factor that may explain our observed associations is that clinicians may be more likely to treat large tumors more aggressively: multivisceral resection is associated with increased tumor size[10]. This more aggressive treatment may result in greater survival.
Multivisceral resection can improve survival, but a recent population-based study revealed that most United States patients with locally advanced adherent CRC do not undergo this procedure[21]. Our present results lead us to recommend more aggressive excision in patients with T4bN0-2M0 colon cancer whose tumors are small. We also recommend the use of multimodality therapy in patients with T4b colon cancer involving small tumors. Multimodality therapy which comprises neoadjuvant CRT, total mesorectal excision surgery, and adjuvant chemotherapy is now widely used to treat patients with locally advanced rectal cancer[22-25]. Studies suggest that neoadjuvant CRT can downstage locally advanced rectal cancer, resulting in increased resectability, improved sphincter preservation, reduced local recurrence rates, and improved survival[26,27]. Even though official guidelines do not currently recommend neoadjuvant CRT for colon cancer. Nevertheless, studies suggest that neoadjuvant CRT followed by multivisceral resection can be used to treat selected patients with locally advanced adherent colon cancer[28,29]. We advocate more extensive surgery and multimodality therapy to improve the poor survival currently associated with T4b colon cancer involving small tumors.
To our knowledge, this is the first published study to focus on the relationship between tumor size and CSS in T4bN0-2M0 colon cancer patients. Primary data came from the large SEER database, and they gave similar results as our own data from the FUSCC database. The fact that our results for a heterogeneous cohort of US patients drawn from populations covering a quarter of the country were similar to those for a much smaller cohort of Chinese patients from a single large cancer hospital suggests that our findings are likely to be reliable.
At the same time, our study has several limitations. Despite the large size of SEER, our stratification by tumor size and N stage led to relatively small subgroups, reducing statistical power to detect small differences. This may help explain why we failed to detect significant associations between tumor size and CSS in the subgroups of T4bN1 patients. Second, the SEER database does not include information on adjuvant therapy, comorbidities, performance status, surgical margins or pathology techniques. This limited our ability to correlate tumor size with other patient and disease characteristics. Third, our analysis did not include T4b colon cancer patients who had unresectable tumors or who refused surgical intervention, so our results may not be generalizable to those groups.
Our results provide the first evidence that small tumor size in patients with T4bN0-2M0 colon cancer, particularly T4bN0M0 cancer, is associated with worse prognosis than large tumor size. Further colon cancer studies should look specifically at T4bN0-2M0 patients to understand the underlying genetic and molecular mechanisms that may make small tumors more dangerous in this subgroup than in other subgroups of colon cancer patients.
The authors would like to thank SEER program for access to the database.
Smaller tumor size is generally associated with greater cancer-specific survival (CSS) in patients with colon cancer, but this association may not hold for specific subgroups of colon cancer patients. The authors hypothesized that in patients with cancer showing heavy intestinal wall invasion without distant metastasis (T4bN0-2M0), small tumor size would correlate with more aggressive tumor behavior and therefore lower CSS.
Some researchers found CSS to be lower with smaller tumors, patients with tumors 4-8 cm in size showed greater CSS relative to patients with tumors < 4 cm. Similarly, a study of stage IIA colon cancer found decreased CSS with smaller tumor size.
These results provide the first evidence that small tumor size in patients with T4bN0-2M0 colon cancer, particularly T4bN0M0 cancer, is associated with worse prognosis than large tumor size.
The association between small tumor size and poor prognosis in T4bN0-2M0 patients might improve decision-making about the potential harms and benefits of the multivisceral resection.
This article is well written and illustrates an interesting observation. The numbers are good and it has been statistically analyzed well.
| 1. | Campos FG, Calijuri-Hamra MC, Imperiale AR, Kiss DR, Nahas SC, Cecconello I. Locally advanced colorectal cancer: results of surgical treatment and prognostic factors. Arq Gastroenterol. 2011;48:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Koca D, Binicier C, Oztop I, Yavuzsen T, Ellidokuz H, Yilmaz U. Prognostic factors affecting recurrence and survival in patients with locally advanced rectal cancer. J BUON. 2012;17:291-298. [PubMed] |
| 3. | An MS, Yoo JH, Kim KH, Bae KB, Choi CS, Hwang JW, Kim JH, Kim BM, Kang MS, Oh MK. T4 stage and preoperative anemia as prognostic factors for the patients with colon cancer treated with adjuvant FOLFOX chemotherapy. World J Surg Oncol. 2015;13:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Søreide K, Søreide JA, Kørner H. Prognostic role of carcinoembryonic antigen is influenced by microsatellite instability genotype and stage in locally advanced colorectal cancers. World J Surg. 2011;35:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Huh JW, Lee JH, Kim HR, Kim YJ. Prognostic significance of lymphovascular or perineural invasion in patients with locally advanced colorectal cancer. Am J Surg. 2013;206:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Garcia-Granero E, Frasson M, Pous S, Cervantes A. T4a and t4b colorectal cancer: what does this mean nowadays? Dis Colon Rectum. 2012;55:e367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 446] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 8. | Mohan HM, Evans MD, Larkin JO, Beynon J, Winter DC. Multivisceral resection in colorectal cancer: a systematic review. Ann Surg Oncol. 2013;20:2929-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Croner RS, Merkel S, Papadopoulos T, Schellerer V, Hohenberger W, Goehl J. Multivisceral resection for colon carcinoma. Dis Colon Rectum. 2009;52:1381-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Nakafusa Y, Tanaka T, Tanaka M, Kitajima Y, Sato S, Miyazaki K. Comparison of multivisceral resection and standard operation for locally advanced colorectal cancer: analysis of prognostic factors for short-term and long-term outcome. Dis Colon Rectum. 2004;47:2055-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Park S, Lee YS. Analysis of the prognostic effectiveness of a multivisceral resection for locally advanced colorectal cancer. J Korean Soc Coloproctol. 2011;27:21-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Chen YG, Liu YL, Jiang SX, Wang XS. Adhesion pattern and prognosis studies of T4N0M0 colorectal cancer following en bloc multivisceral resection: evaluation of T4 subclassification. Cell Biochem Biophys. 2011;59:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Kornprat P, Pollheimer MJ, Lindtner RA, Schlemmer A, Rehak P, Langner C. Value of tumor size as a prognostic variable in colorectal cancer: a critical reappraisal. Am J Clin Oncol. 2011;34:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Saha S, Shaik M, Johnston G, Saha SK, Berbiglia L, Hicks M, Gernand J, Grewal S, Arora M, Wiese D. Tumor size predicts long-term survival in colon cancer: an analysis of the National Cancer Data Base. Am J Surg. 2015;209:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Moghimi-Dehkordi B, Safaee A, Zali MR. Prognostic factors in 1,138 Iranian colorectal cancer patients. Int J Colorectal Dis. 2008;23:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Takahashi T, Kato T, Kodaira S, Koyama Y, Sakabe T, Tominaga T, Hamano K, Yasutomi M, Ogawa N. Prognostic factors of colorectal cancer. Results of multivariate analysis of curative resection cases with or without adjuvant chemotherapy. Am J Clin Oncol. 1996;19:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Zhuo C, Shi D, Zheng H, Xu Y, Gu W, Cai S, Cai G. Unfavorable effect of small tumor size on cause-specific survival in stage IIA colon cancer, a SEER-based study. Int J Colorectal Dis. 2015;30:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 3081] [Article Influence: 146.7] [Reference Citation Analysis (8)] |
| 19. | Poritz LS, Sehgal R, Hartnett K, Berg A, Koltun WA. Tumor volume and percent positive lymph nodes as a predictor of 5-year survival in colorectal cancer. Surgery. 2011;150:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Balta AZ, Özdemir Y, Sücüllü İ, Derici ST, Bağcı M, Demirel D, Akın ML. Can horizontal diameter of colorectal tumor help predict prognosis? Ulus Cerrahi Derg. 2014;30:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Govindarajan A, Fraser N, Cranford V, Wirtzfeld D, Gallinger S, Law CH, Smith AJ, Gagliardi AR. Predictors of multivisceral resection in patients with locally advanced colorectal cancer. Ann Surg Oncol. 2008;15:1923-1930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Rau B, Hohenberger P, Gellermann J, Hünerbein M, Hildebrandt B, Schneider U, Riess H, Wust P, Schlag PM. [T4 rectal carcinoma. Surgical and multimodal therapy]. Chirurg. 2002;73:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 586] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 24. | Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab HR. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 514] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 25. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4555] [Article Influence: 207.0] [Reference Citation Analysis (7)] |
| 26. | Ortholan C, Francois E, Thomas O, Benchimol D, Baulieux J, Bosset JF, Gerard JP. Role of radiotherapy with surgery for T3 and resectable T4 rectal cancer: evidence from randomized trials. Dis Colon Rectum. 2006;49:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Rödel C, Grabenbauer GG, Schick C, Papadopoulos T, Hohenberger W, Sauer R. Preoperative radiation with concurrent 5-fluorouracil for locally advanced T4-primary rectal cancer. Strahlenther Onkol. 2000;176:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Hallet J, Zih FS, Lemke M, Milot L, Smith AJ, Wong CS. Neo-adjuvant chemoradiotherapy and multivisceral resection to optimize R0 resection of locally recurrent adherent colon cancer. Eur J Surg Oncol. 2014;40:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Cukier M, Smith AJ, Milot L, Chu W, Chung H, Fenech D, Herschorn S, Ko Y, Rowsell C, Soliman H. Neoadjuvant chemoradiotherapy and multivisceral resection for primary locally advanced adherent colon cancer: a single institution experience. Eur J Surg Oncol. 2012;38:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chow CFK S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S