Published online Aug 7, 2016. doi: 10.3748/wjg.v22.i29.6652
Peer-review started: April 6, 2016
First decision: May 30, 2016
Revised: June 8, 2016
Accepted: June 29, 2016
Article in press: June 29, 2016
Published online: August 7, 2016
Processing time: 119 Days and 10.3 Hours
Liver fibrosis is a repair process in response to damage in the liver; however, severe and chronic injury promotes the accumulation of fibrous matrix, destroying the normal functions and architecture of liver. Hepatic stellate cells (HSCs) are quiescent in normal livers, but in damaged livers, they transdifferentiate into myofibroblastic HSCs, which produce extracellular matrix proteins. Hedgehog (Hh) signaling orchestrates tissue reconstruction in damaged livers and contributes to liver fibrogenesis by regulating HSC activation. MicroRNAs (miRNAs), endogenous small non-coding RNAs interfering with RNA post-transcriptionally, regulate various cellular processes in healthy organisms. The dysregulation of miRNAs is closely associated with diseases, including liver diseases. Thus, miRNAs are good targets in the diagnosis and treatment of various diseases, including liver fibrosis; however, the regulatory mechanisms of miRNAs that interact with Hh signaling in liver fibrosis remain unclear. We review growing evidence showing the association of miRNAs with Hh signaling. Recent studies suggest that Hh-regulating miRNAs induce inactivation of HSCs, leading to decreased hepatic fibrosis. Although miRNA-delivery systems and further knowledge of interacting miRNAs with Hh signaling need to be improved for the clinical usage of miRNAs, recent findings indicate that the miRNAs regulating Hh signaling are promising therapeutic agents for treating liver fibrosis.
Core tip: MicroRNAs (miRNAs) influence various biological responses by controlling gene expression. Recent studies investigate the roles of miRNAs in liver fibrosis, due to their potential as biomarkers and therapeutic agents. Hedgehog (Hh) signaling contributes to hepatic fibrosis. Hence, regulation of Hh signaling is one of the therapeutic strategies against liver fibrogenesis. Therefore, we introduce miRNAs relevant to Hh signaling and discuss the interaction of miRNAs with Hh signaling, with a particular focus on the anti-fibrotic effect of Hh-regulating miRNAs in liver diseases. This review suggests that miRNAs-mediating Hh signaling are the novel diagnostic and therapeutic targets for treating liver disease.
- Citation: Hyun J, Jung Y. MicroRNAs in liver fibrosis: Focusing on the interaction with hedgehog signaling. World J Gastroenterol 2016; 22(29): 6652-6662
- URL: https://www.wjgnet.com/1007-9327/full/v22/i29/6652.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i29.6652
Liver fibrosis is a major characteristic of most chronic liver diseases[1]. This is a process in which extracellular matrix (ECM) proteins are accumulated as a wound-healing response to repeated hepatic injury[1]. Although hepatocytes are quiescent in healthy livers, they re-enter cell cycles and proliferate in response to damage[2-5]. Under severe and/or persistent injury, the death rate of hepatocytes is much higher than the rate of the proliferation of hepatocytes, leading to proliferation of other types of cells, such as hepatic stellate cells (HSCs) or progenitors (also called oval cells) to compensate for the loss[4,6-8]. HSCs are quiescent in healthy livers but are activated upon liver injury and further transitioned into contractile myofibroblasts[9-11], which produce ECM proteins substituting for hepatic parenchyma, distorting the normal hepatic architecture[1]. Cirrhosis is defined as the end stage at which liver fibrosis eventually forms nodules of collagen bands, leading to hepatocellular dysfunction and portal hypertension[1,12]. Therefore, it is necessary to understand the mechanism underlying the fibrotic process and to identify a strategy for regulating the activation of HSCs.
Hedgehog (Hh) signaling was originally known for regulating cell fate decisions during developmental processes[13]. Recently, the Hh signaling pathway has been reported to be an important pathway in liver diseases, including liver fibrosis[14,15]. It promotes liver fibrosis by stimulating the activation and proliferation of HSCs[16-18]. Thus, regulation of Hh signaling in the activated HSCs has been suggested as a promising treatment against liver fibrosis. Recently, Hh inhibitors developed for treating liver cancer and neutralizing antibodies to Hh have been shown to reduce hepatic fibrosis by suppressing HSC activation[18]; however, those agents have been shown to have several limitations, such as induction of resistance, pH-dependence, and side effects on normal cells, although they effectively impair the activation and survival of HSCs[19,20]. Hence, it is necessary to develop a novel strategy that effectively and safely modulates Hh signaling in liver fibrosis.
MicroRNAs (miRNAs), about 22 nucleotides of endogenous non-coding RNAs, have emerged as clinical agents due to their biological characteristics[21]. MiRNA genes are first transcribed by RNA polymerase II (Pol II), generating the primary miRNA (pri-miRNA), and then the pri-miRNAs go through sequential processing steps to become mature miRNA duplexes by the RNase III-type endonucleases, Drosha and Dicer, in the nucleus and cytosol, respectively[22,23]. Subsequently, one strand of a miRNA duplex is loaded onto an AGO protein to form an RNA-induced silencing complex (RISC), which is capable of inducing translational repression and the decay of target mRNAs through the interaction with the translation machinery and mRNA decay factors[23,24]. MiRNAs recognize their target genes by base-pairing between a sequence of nucleotide positions 2 to 7 at their 5’ end, called the seed sequence, and the complementary sequence within the mRNA of target genes, usually the 3’ untranslated region (UTR)[25]. MiRNAs are easily detectable in various biological fluids, including serum, saliva, and urine, because they are stable outside of cells as a form contained in circulating exosomes[26-28]. Therefore, miRNAs are good candidates for biomarkers and therapeutic agents for diseases.
Despite the advances in the research of miRNAs in liver fibrosis, the manner in which miRNAs interact with Hh signaling in liver fibrogenesis is poorly understood. Herein, we introduce recent notable findings of miRNAs interacting with Hh signaling in liver fibrosis and other tissues/cells, which helps in understanding their function in liver.
Hh was first identified in Drosophila melanogaster and named based on the disorganized hair-like bristles with the appearance of hedgehog spines on the hh-null embryos[29]. Hh was identified as a secreted protein involved in the pattern formation of the adjacent cells during development[13]. There are three types of conserved Hh ligands in mammals, including sonic Hh (Shh), Indian Hh (Ihh), and desert Hh (Dhh), all with different activities in different developing organs. Hh signaling also plays a key role in liver development, including regulating the survival of hepatoblasts and the differentiation of hepatic progenitors[30,31]. In adult tissues, Hh signaling influences stem cell homeostasis, and its persistent activation is responsible for the pathogenesis of various cancers, including hepatocellular carcinoma[14,32].
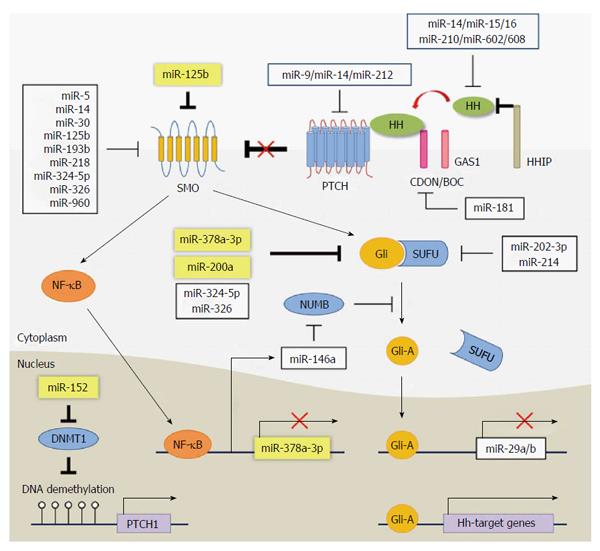
The first identified receptor of Hh was a transmembrane protein, Patched 1 (Ptch), which constitutively inhibits Hh signaling through the repression of Smoothened (Smo)[33,34]. The binding of Hh ligands with Ptch brings to the accumulation of Smo at the plasma membrane by increasing either trafficking of Smo-enriched endosomes or its stability[35-37]. Once activated, Smo is phosphorylated and undergoes a conformational change to an open form of cytoplasmic tail, which interacts with GLI-Kruppel (Gli) family[38]. There are three members of the Gli family- Gli1, Gli2, and Gli3-which have a DNA-binding domain and a C-terminal activation domain[39]. Gli2 and Gli3 also contain an N-terminal repressor domain[39]. The C-terminal-cleaved form of Gli3 dominantly acts as a repressor and reduces the expression of Gli1/2 and Gli-target genes including Pax2, Sall1, Cyclin D1 and N-myc in embryonic development, whereas the Gli1 and Gli2 function as the transcriptional activators[40,41]. In the absence of Hh, the C-terminal domain of Glis is phosphorylated and ubiquitinated for degradation[14,42]. The suppressor of a fused (SUFU) protein is a well-known negative regulator, which directly binds with Glis[43-47], inducing ubiquitination of Glis. In the presence of Hh, the active Smo inhibits the proteolytic processing of Glis, allowing Glis to act as a transcriptional activator to initiate the Hh signaling. Thus the full-length form of Gli3 as well as Gli2 activates Hh signaling[48]. In addition, the active form of Gli3 was report to be upregulated in colorectal cancer[49] and liver fibrosis[50]. The Gli-mediated transcriptions can be regulated by Smo-independent way, which is referred as non-canonical Hh signaling pathway[14,51]. The canonical Hh signaling is well-known in the primary cilium in vertebrates. Hh signaling is activated by the translocation of Smo into the primary cilium, a single, tiny, microtubule-based organelle that projects from the surface of most vertebrate cells[14,52,53]. Inherited ciliary defects, such as Bardet-Biedl syndrome and Meckel syndrome, was reported to have the disrupted Hh signaling[14,54]. In addition, ciliary dysfunction blocks the proteolytic process of full-length Gli3 to the truncated repressor because of the localized SUFU-Gli3 in the tip of cilia where proteolytic processing occurs. Therefore, it induces the aberrant activation of various Hh-target genes, causing developmental failure[14,55].
The Hh signaling pathway is inactivated in a healthy adult liver. Quiescent HSCs (Q-HSCs) and liver sinusoidal endothelial cells (LSECs) in a healthy adult liver highly express an Hh-interacting protein (Hhip), which disrupts the engagement between the Hh ligand and the receptor[14,17,18,56,57]; however, the Hh signaling pathway is reactivated when liver reconstruction is required. In the experimental model of a 70% partial hepatectomy (PHx), the expression of Shh and Ihh was known to be elevated during liver regeneration[58]. In livers of patients with nonalcoholic steatohepatitis or primary biliary cirrhosis, a higher expression of Shh and Ihh was demonstrated[56,57,59,60]. As the level of Hh ligands increase, the number of Hh-responsive cells, such as HSCs and progenitors, also increases with the decrease of Hhip expression[57,59,61]. This activation of Hh signaling is required for liver regeneration, which is supported by the evidence that Smo-inhibited mice exhibit the reduced hepatic accumulation of Hh-responsive cells in the liver, resulting in death after PHx[58]; however, the sustained expression of Hh due to persistent hepatic injury expands the population of cells responsible for the progression of chronic liver diseases, such as myofibroblastic HSCs (MF-HSCs)[59,62]. Jung et al[56] previously reported that dying hepatocytes produced Hh ligands, and Hh-responsive cells, such as progenitors and HSCs, were proliferated and activated[18]. These activated HSCs or MF-HSCs by Hh ligands released from apoptotic hepatocytes in turn produce Hh ligands and further accelerate Hh signaling in both an autocrine and a paracrine manner[17] and produce more collagen fibrils, eventually contributing to the accumulation of fibrous ECM in the liver. Chen et al[16] demonstrated that Hh signaling regulated the metabolism of HSCs during transdifferentiation into MF-HSCs. In their findings, Hh signaling induced the expression of Hif-1α and promoted glycolysis rather than gluconeogenesis and lipogenesis, leading to reprogramming the gene expression toward fibrogenesis. These findings demonstrate that Hh signaling is critically important in hepatic fibrogenesis; hence, the Hh signaling is a good target for therapeutic approaches aimed at controlling the activation of HSCs during liver fibrogenesis.
To investigate the degenerative effects of miRNAs, researchers have commonly used Dicer-knockout or -knockdown models because Dicer-mediated processing is critical to miRNA maturation[63,64]. The knockdown of the Dicer1 transcript in mice showed disrupted cortical layering of the anterior cerebellum, which resulted from the premature differentiation of granule cell precursors during neonatal development[65]. Interestingly, the expression of Hh signaling components, especially Gli2, was downregulated in the defective cerebellum, suggesting the importance of miRNAs as promoters of Hh signaling in granule cell precursor development[65]. Also, in Dicer-mutated mouse skin, the expression of Shh and Gli1 was lost by postnatal day 7 with proliferative defects of hair follicles and evagination of dermal cells into the epidermis[66]. In addition, Munoz et al[67] reported that PTCH1 protein was rarely reduced in Dicer-knockdown glioblastoma multiforme cells, indicating the inhibitory effect of PTCH1 translation. These findings suggest that the miRNAs are closely associated with the expression and function of Hh signaling during development.
MiRNAs are involved in various biological processes, including normal development, physiology, and pathogenesis[68,69]. In the liver, miRNAs regulate lipid and glucose metabolism, inflammation, cell survival, and proliferation[70]. Therefore, the dysregulation of miRNAs is closely associated with various diseases, including liver disease. Emerging evidence has shown that several miRNAs associated with Hh signaling have important functions on liver fibrosis, and they are summarized in Table 1.
| miRNA(s) | Expression | Target gene(s) | Sample type(s) | Ref. |
| miR-378a-3p | Downregulated | Gli3 | CCl4-induced mouse fibrotic liver/Primary mouse HSC | [50] |
| miR-200a | Downregulated | Gli2 | Rat HSC | [76] |
| miR-125b | Downregulated | Smo | CCl4-induced rat fibrotic liver/Primary rat HSC | [77] |
| miR-152 | Downregulated | DNMT1 | CCl4-induced mouse fibrotic liver/Primary mouse HSC | [75] |
| miR-29 | Downregulated | - | MCDE-dieted mouse liver | [71] |
The first attempt to find the relation of miRNAs with Hh signaling in liver fibrosis was performed in a mouse model with disrupted NF-κB signaling in albumin-expressing cells, including HSCs[71]. Because NF-κB signaling was reported to inhibit the expression of anti-fibrotic miR-29[72], Hyun et al[71] investigated whether miR-29 prevented hepatic fibrosis in NF-κB-defective mice fed a hepatotoxic methionine/choline-deficient diet supplemented with ethionine (MCDE diet). Although the expression of the miR-29 family was upregulated in the livers of NF-κB-defective mice fed an MCDE diet, liver fibrosis was more severe than in the livers of chow-fed mice with normal NF-κB signaling. Primary HSCs isolated from mice with impaired NF-κB showed a decreased expression of a quiescent marker but increased expressions of activation markers of HSCs compared to HSCs from normal mice. In addition, the activated HSCs were Gli2-positive in livers of MCDE-treated mice with disrupted NF-κB. These results demonstrated the essential role of Hh signaling in HSCs activation, leading to liver fibrosis, even when miR-29 was significantly up-regulated.
Hh signaling promotes epithelial-to-mesenchymal transition (EMT), which is involved in the activation of HSCs[14,73,74]. When Q-HSCs are activated into MF-HSCs, the expression of quiescent markers (e.g., PPARγ and GFAP) and epithelial genes (e.g., BMP7, desmoplakin, and E-cadherin) is downregulated but the expression of myofibroblastic markers (e.g., α-SMA, vimentin, fibronectin and Col1α1) and mesenchymal genes (e.g., Snail and Lhx2) is upregulated in MF-HSCs[73,74]. Leptin, an anti-adipogenic and pro-EMT factor, promotes the activation of HSCs by inducing the expression of Hh signaling components[74]. These findings indicate that EMT process characterizes the transdifferentiation of the Q-HSC into MF-HSC. Recently, the role of miRNAs in Hh signaling has investigated during the EMT process. Yu et al[75] suggested that miR-152 indirectly regulated Hh signaling by targeting DNA methyltransferase 1 (DNMT1), which methylated the Ptch1 gene. Although the promoter region of the Ptch1 gene was so hypermethylated in activated HSCs that it could not be expressed, Salvianolic acid B (Sal B) induced the expression of miR-152 suppressing Dnmt1, and then demethylated Ptch1 inhibited the Smo-Gli2 pathway and EMT, leading to the inactivation of HSCs in CCl4-treated mice. In another study, Yu et al[76] also reported that miR-200a directly targeted Gli2, and an overexpression of miR-200a resulted in an increase of epithelial markers, including BMP7 and Id2, but a decrease of mesenchymal markers, including Snail1 and S100a4, through Gli2 downregulation in rat HSCs, which inhibited the HSCs proliferation and activation.
MiRNA regulating HSC activation by interacting with Hh-target genes have also been reported in other studies. MiR-125b released from placenta-derived mesenchymal stem cells (PDSCs) promoted the inactivation of HSCs by inhibiting Hh signaling, contributing to reduced hepatic fibrosis[77]. In this study, Hyun et al[77] found that PDSC-derived exosomes contained a large amount of miR-125b transcripts, which was upregulated in livers of CCl4-treated rats after PDSCs-transplantation, followed by the decreased expression of Hh signaling and fibrotic markers. When the expression of miR-125b was suppressed in PDSCs, the PDSCs failed to block the expression of Hh and pro-fibrotic genes in activated HSCs. These data indicated that miR-125b-mediated Hh signaling influences liver regeneration by regulating HSC activation. MiR-378a-3p was shown to suppress the activation of HSCs by directly targeting Gli3 in the livers of CCl4-treated mice. In this study, Hyun et al[50] performed a microarray analysis of miRNA expression in normal (corn-oil-treatment: control) and chronically damaged livers (CCl4 treatment) of mice with fibrosis and found that the miR-378 family, including miR-378a-3p, miR-378b, and miR-378d, was downregulated in CCl4-treated livers compared to corn-oil-treated control livers[50]. The expression of miR-378 family members also decreased in mouse primary HSCs during activation of HSCs. Particularly, miR-378a-3p led to the inactivation of HSCs by reducing Gli2 and Gli3 expressions in activated HSCs in vitro. The expression level of Gli2 and Gli3 is upregulated in activated HSCs and CCl4-induced liver fibrosis[50,77,78]. The transcription of primary miR-378a, a transcribed form of miR-378a DNA, was repressed by an NF-κB subunit, p65, of which activity was regulated by Smo, an upstream signal of Glis. In addition, Hyun et al[50] showed that the miR-378a-3p exerted an anti-fibrotic effect on CCl4-induced liver fibrosis in mice by reintroducing the miR-378a-3p to the livers using L-tyrosine polyurethane 2a (LTU2a)-based nanoparticles. Taken together, these findings suggest that Hh signaling plays a significant role in the complex regulatory network of liver fibrosis and that Hh-regulating miRNAs are promising therapeutic agents for treating liver fibrosis.
The relationships between miRNAs and Hh signaling have also been investigated in other types of cells and experimental models. The first report of a certain miRNA interacting with Hh signaling was in 2005, and it showed that miR-196 inhibited the expression of Shh by targeting Hoxb8, an upstream positive signal of Shh, in the development of chick forelimb[79]. In adult cells, miR-125b, miR-324-5p, and miR-326 were first identified to target Smo (miR-324-5p also targeting Gli1) in differentiated granule cells, and their expressions were downregulated in human medulloblastoma with a high Gli1 level[80]. These studies are useful in understanding the pathogenesis of liver fibrosis by investigating whether these miRNAs also influence liver fibrosis by modulating Hh signaling. The miRNAs shown to directly or indirectly interact with Hh signaling in various sample types are summarized in Supplementary Table 1.
Among them, several miRNAs have already been investigated in liver fibrosis. Both miR-29a and miR-29b-1 were reported to have a Gli-binding site in their promoter region, so they were transcriptionally suppressed by Hh signaling in human cholangiocarcinoma cells[81]. In fibrotic livers of both humans and rodents, the miR-29 family was downregulated with an increase in collagen[72], and miR-29b suppressed the activation of HSCs[82]; however, in a mouse model of liver fibrosis, Hyun et al[71] demonstrated that the Hh pathway compromised the anti-fibrotic effect of the miR-29 family. Although the reason for this inconsistency remains to be elucidated, it is possible that stimulus type, pathological condition, or cell status exert an effect on the function and/or the expression of these miRNAs. For example, the expression of miR-29s was alleviated by lipopolysaccharide (LPS), but the level of collagen produced was rarely elevated[83]. In addition, miR-29s was regulated differently by NF-κB activated by interleukin-1[72]. The function and expressional regulation of miR-29s also seems to be different according to which signaling pathway is triggered in response to the damage because these effects are known to vary depending on which upstream signaling pathways are engaged[84-86]. Thus, this complicated link between Hh signaling and the miR-29 family in liver fibrosis needs to be investigated further.
In patients with chronic hepatitis C viral (HCV) infections, the miR-21 induced by TGF-β signaling negatively regulated the expression of SMAD7, inhibiting TGF-β signaling, and further enhanced the pathway of miR-21 and TGF-β similar to a positive feedback[87]. The miR-21 expression was also upregulated by Hh signaling in glioblastoma initiating cells[88]. Hh signaling was reported to be activated in chronic HCV-infected livers[89,90]. These results suggest the possibility that the expression of miR-21 might be regulated by Hh signaling in a chronic HCV-infected liver. In addition, it is possible that miR-21 enhances the Hh signaling by up-regulating TGF-β expression in the chronic liver of patients with HCV infection, because the TGF-β signaling is known to promote the expression of Gli1/2 in a Smo-independent manner in various cell types, such as skin and lung fibroblasts and pancreatic cancer cells[91,92]. These findings indicate that miR-21 is involved in the crosstalk between Hh and TGF-β signaling.
MiR-146a targeting SMAD4 was downregulated in primary MF-HSCs isolated from rat livers with fibrosis by CCl4, and the overexpression of miR-146 suppressed TGF-β-mediated proliferation and induced the apoptosis of HSCs[93]. In mouse livers of nonalcoholic steatohepatitis with fibrosis and activated HSCs, the expression of miR-146a-5p was also reduced, and the overexpression of miR-146a-5p blocked both the proliferation and activation of HSCs through targeting Wnt1 and Wnt5a[28]. Moreover, the miR-146a increased the activation of Hh signaling by targeting Numb involved in Gli1 degradation in a mouse colitis model of intestinal inflammation and primary mouse macrophages[94].
Because the Gli1 has been reported to be an oncogene, which is amplified in cancer cells and a number of human malignancies[95,96], blocking agents for the Hh pathway, such as cyclopamine and vismodegib (also called as GDC-0449), which inhibits Smo activity, and other small molecular inhibitors have been tested in treating epithelial cancers of animal models, including prostate and pancreatic cancer xenografts[97]; however, there is concern regarding the use of cyclopamine in humans because it is converted into isomers, such as veratramine or undefined isomers, causing low effectivity and side effects such as hemolysis under acidic conditions, such as in the stomach[20]. To overcome this problem, a synthetic miRNA and its corresponding temporal miRNA duplex, called Gli1-miRNA-3548 and Duplex-3548, respectively, have been engineered[98,99]. The synthetic miRNA was designed to target 3’-UTR of gli1 mRNA, and they significantly inhibited the proliferation and division of Gli1-positive pancreatic and ovarian tumor cells. The antisense and sense strands of Duplex-3548 have a sequence homology with the natural miR-361 and partially with the miR-136, respectively, suggesting that there might be naturally acting miRNAs on the regulation of gli transcripts. Vismodegib targeting Smo-dependent Hh signaling has been approved by the FDA for the treatment against advanced basal cell carcinoma[100] and it has shown the therapeutic effects on both liver fibrosis and hepatocellular carcinoma in mice[101,102]. However, vismodegib also has side effects, such as muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, diarrhea, decreased appetite, constipation, arthralgia, vomiting, ageusia, hyponatremia, pyelonephritis and presyncope[103,104]. Especially, vismodegib is not allowed to be prescribed to pregnant women due to its teratogenicity, embryotoxicity and fetotoxicity. In addition, it does not work for patients having mutations in Smo receptor[103,104]; thus the novel therapeutic strategies should be developed. A recent study reports that the co-treatment of vismodegib with miR-29b-1 targeting several pro-fibrotic genes, such as Col1α1, FN-1 and PDGF-β, regresses the hepatic injuries and fibrosis in bile duct ligated livers of mice[105]. Compared with the single treatment with miR-29b-1 or vismodegib, this combination therapy was more effective in reducing the levels of injury-related enzymes and the expression of fibrotic proteins in liver tissue, implicating the synergistic action of miRNA and small molecular inhibitor in treating liver fibrosis[105].
Recently, bioinformatics and oligonucleotides-modifying techniques have made great advances in knowledge regarding the biological functions of miRNAs in pathogenesis. Therefore, therapeutics using chemically modified oligonucleotides to target endogenous miRNAs, called the miRNA inhibitor or miRNA mimic, have been developed to modulate the expression of miRNAs in diseases[106-108]. There are two miRNA therapeutic agents in particular that are used in clinical trials for liver diseases. The miravirsen SPC3649 (Santaris Pharma, Horsholm, Denmark), which is an inhibitor of miR-122, is for patients with chronic HCV infections and is in phase 2a in clinical trials[109]. The MRX34 (Mirna Therapeutics, Inc.), a mimic of miR-34 encapsulated in a liposomal nanoparticle formulation, is the first miRNA mimic to be introduced into clinical development for hematological malignancies and solid tumors, including hepatocellular carcinoma[110]. Growing evidence also shows the significant therapeutic effects of miRNAs in vivo against liver fibrosis[50,111,112]. The ectopic expression of miR-101 targets TGF-β signaling using lentivirus attenuated CCl4-induced liver fibrosis in mice by suppressing the activation of HSCs and the apoptosis of hepatocytes[111]. Introducing the miR-142-5p inhibitor or/and miR-130a-3p mimic by intravenous injection also resulted in decreased fibrosis of CCl4-treated mouse livers by controlling the expression of pro-fibrogenic genes in macrophages[112]. Moreover, the LTU2a nanoparticle-mediated delivery of miR-378a-3p into mice with chronic liver fibrosis by CCl4 led to the inactivation of HSCs by suppressing Hh signaling[50]. These miRNAs can be promising therapeutic agents that should be developed further for clinical use.
Still, there are challenges in safe and effective systems used for delivering the therapeutic miRNAs to target cells. Several obstacles, including poor in vivo stability, inappropriate biodistribution, disruption and saturation of endogenous RNA machinery, and untoward side effects, are currently concerns[113]. In addition, therapy utilizing miRNAs is complex because miRNAs are possible to generate false positive effects by targeting multiple target genes. For example, miR-125b that directly targeted Smo in medulloblastoma[80] was shown to have an anti-fibrotic effect by regulating Hh signaling in CCl4-injured liver of rats[77]. Zhou et al[114] also reported that miR-125b directly targeted SMAD4, which inhibited EMT process in hepatocellular carcinoma cells. Because EMT is closely associated with HSC activation, it is possible that miR-125b exerts its anti-fibrotic role through targeting SMAD4 and other EMT-related genes, including Hh signaling, in CCl4-induced liver fibrosis. Therefore, baseline expression of various target genes in each patient should be carefully considered for miRNA therapy.
Viral vectors are effective carriers of miRNA-targeting agents, but they are toxic and immunogenic[115]. Therefore, non-viral synthetic materials that offer certain advantages, such as enabling the control of molecular composition, simplified manufacturing, modification and analysis, tolerance for cargo sizes, and relatively lower immunogenicity, have been developed as delivery systems[116]. In addition, the delivery efficiency of non-viral carriers can be improved by modifying particle size and surface properties. These materials include liposomes, polyethylenimine (PEI), dendrimers, poly(lactide-co-glycolide) (PLGA) particles, and naturally occurring polymers, such as chitosan, protamine, atelocollage, and peptides, derived from protein translocation domains[113]. The LTU2a nanoparticles used in an in vivo study by Hyun et al[50] were biodegradable spheres of 340 nm on average and were optimized for cellular uptake. It was confirmed that the nanoparticles were uninfluential in normal livers by assessing the changes of histology and gene expression in normal livers before and after nanoparticle treatment. The nanoparticles containing miR-378a-3p mimic in particular showed a significant anti-fibrotic effect at 3 wk after just a single intraperitoneal injection. The nanoparticles gradually enhanced the level of miR-378a-3p in the damaged liver up to a similar level of miR-378a-3p in normal livers at 3 wk. The increased expression of miR-378a-3p paralleled with the decreased level of its target, Gli3. These findings suggest that the LTU2a nanoparticle is one of the best candidates for the miRNA-delivering material in clinical use; however, it is necessary to improve delivery systems for miRNA therapeutics in clinical use because optimal delivery systems should be designed for specific types of diseases.
Overcoming chronic liver disease is a significant challenge facing modern populations, and liver fibrosis is a prominent feature of chronic liver diseases[1]. Therefore, researchers have made several efforts to reverse or to prevent the progression of liver fibrosis. Hh signaling is a good target for this goal because it increases the proliferation and viability of activated HSCs, creating an Hh-enriched microenvironment in the damaged liver[14-17]. MiRNAs have been designated for therapeutic interventions because they regulate the gene expression of disease-associated signaling pathways[106-108]. Therefore, it is meaningful to investigate miRNAs associated with Hh signaling to ameliorate hepatic fibrosis by regulating HSC activation.
In this review, we discussed previous findings regarding the miRNAs in liver fibrosis and focused on the interaction with the Hh signaling pathway. In the studies of the knockdown of the Dicer gene, it was observed that miRNAs and Hh signaling are closely related and that they influence each other in their expressions and functions. In addition, the direct or indirect interactions between certain miRNAs and Hh signaling have been shown in various animal models and human cases. Recent studies have demonstrated how miRNAs interact with Hh signaling in liver fibrosis and that modulating the expression of Hh-targeting miRNAs reduces the activation of HSCs. We depicted the components of Hh signaling pathway and their interaction with miRNAs in Figure 1.
The studies on the role of miRNAs in liver fibrosis were conducted during the past 5 years and are still in their infancy. To provide fundamental knowledge about the complex processes of liver fibrosis, the interactive roles of miRNAs with Hh should be further demonstrated in various models of liver fibrosis. Therefore, miRNAs that interact with Hh signaling could be useful biomarkers and novel therapeutic agents of personalized medicine for liver fibrosis.
| 1. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4213] [Article Influence: 200.6] [Reference Citation Analysis (11)] |
| 2. | Diehl AM. Liver regeneration. Front Biosci. 2002;7:e301-e314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 518] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 4. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1193] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 5. | Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 492] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 6. | Nevzorova YA, Hu W, Cubero FJ, Haas U, Freimuth J, Tacke F, Trautwein C, Liedtke C. Overexpression of c-myc in hepatocytes promotes activation of hepatic stellate cells and facilitates the onset of liver fibrosis. Biochim Biophys Acta. 2013;1832:1765-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (5)] |
| 7. | Yang S, Koteish A, Lin H, Huang J, Roskams T, Dawson V, Diehl AM. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Chung C, Iwakiri Y. Activated hepatic stellate cells: negative regulators of hepatocyte proliferation in liver diseases. Hepatology. 2012;56:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 397] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 10. | Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728-1734. [PubMed] |
| 11. | Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 596] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 12. | Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 534] [Article Influence: 33.4] [Reference Citation Analysis (4)] |
| 13. | Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059-3087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2300] [Cited by in RCA: 2357] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 14. | Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Choi SS, Omenetti A, Syn WK, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43:238-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, Xie G, Moylan CA, Garibaldi F, Premont R. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319-29.e1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 17. | Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, Huang J, Zdanowicz M, Camp T, Torbenson MS. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest. 2005;85:1368-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Metcalfe C, de Sauvage FJ. Hedgehog fights back: mechanisms of acquired resistance against Smoothened antagonists. Cancer Res. 2011;71:5057-5061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Wilson SR, Strand MF, Krapp A, Rise F, Petersen D, Krauss S. Hedgehog antagonist cyclopamine isomerizes to less potent forms when acidified. J Pharm Biomed Anal. 2010;52:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1784] [Cited by in RCA: 1835] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 22. | Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3513] [Cited by in RCA: 3675] [Article Influence: 159.8] [Reference Citation Analysis (0)] |
| 23. | Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3368] [Cited by in RCA: 4222] [Article Influence: 351.8] [Reference Citation Analysis (1)] |
| 24. | Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1638] [Cited by in RCA: 1821] [Article Influence: 121.4] [Reference Citation Analysis (0)] |
| 25. | Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14460] [Cited by in RCA: 16303] [Article Influence: 959.0] [Reference Citation Analysis (2)] |
| 26. | Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 879] [Article Influence: 62.8] [Reference Citation Analysis (22)] |
| 27. | Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z. miRNA in plasma exosome is stable under different storage conditions. Molecules. 2014;19:1568-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 28. | Du J, Niu X, Wang Y, Kong L, Wang R, Zhang Y, Zhao S, Nan Y. MiR-146a-5p suppresses activation and proliferation of hepatic stellate cells in nonalcoholic fibrosing steatohepatitis through directly targeting Wnt1 and Wnt5a. Sci Rep. 2015;5:16163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2958] [Cited by in RCA: 2936] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 30. | Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, Caballero M, Fair JH, Ludlow JW, McClelland RE. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859-G870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Hirose Y, Itoh T, Miyajima A. Hedgehog signal activation coordinates proliferation and differentiation of fetal liver progenitor cells. Exp Cell Res. 2009;315:2648-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 850] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 33. | Hooper JE, Scott MP. The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell. 1989;59:751-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 322] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | Nakano Y, Guerrero I, Hidalgo A, Taylor A, Whittle JR, Ingham PW. A protein with several possible membrane-spanning domains encoded by the Drosophila segment polarity gene patched. Nature. 1989;341:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 271] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Denef N, Neubüser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 432] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 36. | Xia R, Jia H, Fan J, Liu Y, Jia J. USP8 promotes smoothened signaling by preventing its ubiquitination and changing its subcellular localization. PLoS Biol. 2012;10:e1001238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Razumilava N, Bronk SF, Smoot RL, Fingas CD, Werneburg NW, Roberts LR, Mott JL. miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology. 2012;55:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Zhao Y, Tong C, Jiang J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature. 2007;450:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 39. | Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915-3924. [PubMed] |
| 40. | Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, Rosenblum ND. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development. 2006;133:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 815] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 42. | Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1167] [Cited by in RCA: 1408] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 43. | Kogerman P, Grimm T, Kogerman L, Krause D, Undén AB, Sandstedt B, Toftgård R, Zaphiropoulos PG. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999;1:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 388] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 44. | Pearse RV, Collier LS, Scott MP, Tabin CJ. Vertebrate homologs of Drosophila suppressor of fused interact with the gli family of transcriptional regulators. Dev Biol. 1999;212:323-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Stone DM, Murone M, Luoh S, Ye W, Armanini MP, Gurney A, Phillips H, Brush J, Goddard A, de Sauvage FJ. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci. 1999;112:4437-4448. [PubMed] |
| 46. | Dunaeva M, Michelson P, Kogerman P, Toftgard R. Characterization of the physical interaction of Gli proteins with SUFU proteins. J Biol Chem. 2003;278:5116-5122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Merchant M, Vajdos FF, Ultsch M, Maun HR, Wendt U, Cannon J, Desmarais W, Lazarus RA, de Vos AM, de Sauvage FJ. Suppressor of fused regulates Gli activity through a dual binding mechanism. Mol Cell Biol. 2004;24:8627-8641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Tyurina OV, Guner B, Popova E, Feng J, Schier AF, Kohtz JD, Karlstrom RO. Zebrafish Gli3 functions as both an activator and a repressor in Hedgehog signaling. Dev Biol. 2005;277:537-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Iwasaki H, Nakano K, Shinkai K, Kunisawa Y, Hirahashi M, Oda Y, Onishi H, Katano M. Hedgehog Gli3 activator signal augments tumorigenicity of colorectal cancer via upregulation of adherence-related genes. Cancer Sci. 2013;104:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Hyun J, Wang S, Kim J, Rao KM, Park SY, Chung I, Ha CS, Kim SW, Yun YH, Jung Y. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat Commun. 2016;7:10993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 51. | Grzelak CA, Martelotto LG, Sigglekow ND, Patkunanathan B, Ajami K, Calabro SR, Dwyer BJ, Tirnitz-Parker JE, Watkins DN, Warner FJ. The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury. J Hepatol. 2014;60:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (3)] |
| 52. | Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1173] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 53. | Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1170] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 54. | Quinlan RJ, Tobin JL, Beales PL. Modeling ciliopathies: Primary cilia in development and disease. Curr Top Dev Biol. 2008;84:249-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 704] [Cited by in RCA: 739] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 56. | Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, Guy CD, Diehl AM. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 57. | Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, Schuppan D, Diehl AM. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57:1275-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, Zubiaga AM, Fresnedo O, Omenetti A, Zdanowicz M. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 59. | Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, Wang J, Witek RP, Fearing CM, Pereira TA. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478-1488.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 60. | Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45:1091-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Fleig SV, Choi SS, Yang L, Jung Y, Omenetti A, VanDongen HM, Huang J, Sicklick JK, Diehl AM. Hepatic accumulation of Hedgehog-reactive progenitors increases with severity of fatty liver damage in mice. Lab Invest. 2007;87:1227-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, Choi S, Suzuki A, Diehl AM. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1990] [Cited by in RCA: 2003] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 64. | Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663-4670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1614] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 65. | Constantin L, Wainwright BJ. MicroRNAs Promote Granule Cell Expansion in the Cerebellum Through Gli2. Cerebellum. 2015;14:688-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 67. | Munoz JL, Rodriguez-Cruz V, Ramkissoon SH, Ligon KL, Greco SJ, Rameshwar P. Temozolomide resistance in glioblastoma occurs by miRNA-9-targeted PTCH1, independent of sonic hedgehog level. Oncotarget. 2015;6:1190-1201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 68. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 28193] [Article Influence: 1281.5] [Reference Citation Analysis (0)] |
| 69. | Macfarlane LA, Murphy PR. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics. 2010;11:537-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1008] [Cited by in RCA: 1369] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 70. | Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 71. | Hyun J, Choi SS, Diehl AM, Jung Y. Potential role of Hedgehog signaling and microRNA-29 in liver fibrosis of IKKβ-deficient mouse. J Mol Histol. 2014;45:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 666] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 73. | Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093-G1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 74. | Choi SS, Syn WK, Karaca GF, Omenetti A, Moylan CA, Witek RP, Agboola KM, Jung Y, Michelotti GA, Diehl AM. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010;285:36551-36560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 75. | Yu F, Lu Z, Chen B, Wu X, Dong P, Zheng J. Salvianolic acid B-induced microRNA-152 inhibits liver fibrosis by attenuating DNMT1-mediated Patched1 methylation. J Cell Mol Med. 2015;19:2617-2632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 76. | Yu F, Zheng Y, Hong W, Chen B, Dong P, Zheng J. MicroRNA200a suppresses epithelialtomesenchymal transition in rat hepatic stellate cells via GLI family zinc finger 2. Mol Med Rep. 2015;12:8121-8128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Hyun J, Wang S, Kim J, Kim GJ, Jung Y. MicroRNA125b-mediated Hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Sci Rep. 2015;5:14135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 78. | Pritchett J, Harvey E, Athwal V, Berry A, Rowe C, Oakley F, Moles A, Mann DA, Bobola N, Sharrocks AD. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology. 2012;56:1108-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 79. | Hornstein E, Mansfield JH, Yekta S, Hu JK, Harfe BD, McManus MT, Baskerville S, Bartel DP, Tabin CJ. The microRNA miR-196 acts upstream of Hoxb8 and Shh in limb development. Nature. 2005;438:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 80. | Ferretti E, De Smaele E, Miele E, Laneve P, Po A, Pelloni M, Paganelli A, Di Marcotullio L, Caffarelli E, Screpanti I. Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J. 2008;27:2616-2627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 81. | Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem. 2010;110:1155-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 82. | Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun. 2011;412:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 83. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1602] [Article Influence: 84.3] [Reference Citation Analysis (1)] |
| 84. | Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133-6140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 665] [Cited by in RCA: 678] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 85. | Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, Zhuang SM. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology. 2010;51:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 86. | Santanam U, Zanesi N, Efanov A, Costinean S, Palamarchuk A, Hagan JP, Volinia S, Alder H, Rassenti L, Kipps T. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc Natl Acad Sci USA. 2010;107:12210-12215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 87. | Marquez RT, Bandyopadhyay S, Wendlandt EB, Keck K, Hoffer BA, Icardi MS, Christensen RN, Schmidt WN, McCaffrey AP. Correlation between microRNA expression levels and clinical parameters associated with chronic hepatitis C viral infection in humans. Lab Invest. 2010;90:1727-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 88. | Fu J, Rodova M, Nanta R, Meeker D, Van Veldhuizen PJ, Srivastava RK, Shankar S. NPV-LDE-225 (Erismodegib) inhibits epithelial mesenchymal transition and self-renewal of glioblastoma initiating cells by regulating miR-21, miR-128, and miR-200. Neuro Oncol. 2013;15:691-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 89. | Choi SS, Bradrick S, Qiang G, Mostafavi A, Chaturvedi G, Weinman SA, Diehl AM, Jhaveri R. Up-regulation of Hedgehog pathway is associated with cellular permissiveness for hepatitis C virus replication. Hepatology. 2011;54:1580-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Pereira Tde A, Witek RP, Syn WK, Choi SS, Bradrick S, Karaca GF, Agboola KM, Jung Y, Omenetti A, Moylan CA. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 91. | Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A. TGF-β/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 2011;71:5606-5610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 92. | Dennler S, André J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981-6986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 93. | He Y, Huang C, Sun X, Long XR, Lv XW, Li J. MicroRNA-146a modulates TGF-beta1-induced hepatic stellate cell proliferation by targeting SMAD4. Cell Signal. 2012;24:1923-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 94. | Ghorpade DS, Sinha AY, Holla S, Singh V, Balaji KN. NOD2-nitric oxide-responsive microRNA-146a activates Sonic hedgehog signaling to orchestrate inflammatory responses in murine model of inflammatory bowel disease. J Biol Chem. 2013;288:33037-33048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 95. | Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700-15708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 97. | Stecca B, Ruiz i Altaba A. The therapeutic potential of modulators of the Hedgehog-Gli signaling pathway. J Biol. 2002;1:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Tsuda N, Ishiyama S, Li Y, Ioannides CG, Abbruzzese JL, Chang DZ. Synthetic microRNA designed to target glioma-associated antigen 1 transcription factor inhibits division and induces late apoptosis in pancreatic tumor cells. Clin Cancer Res. 2006;12:6557-6564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 99. | Tsuda N, Mine T, Ioannides CG, Chang DZ. Synthetic microRNA targeting glioma-associated antigen-1 protein. Methods Mol Biol. 2009;487:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 101. | Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, Moylan C, Venkatraman T, Feuerlein S, Syn WK. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 102. | Hirsova P, Ibrahim SH, Bronk SF, Yagita H, Gores GJ. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One. 2013;8:e70599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 103. | Sheikh A, Alvi AA, Aslam HM, Haseeb A. Hedgehog pathway inhibitors - current status and future prospects. Infect Agent Cancer. 2012;7:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Sandhiya S, Melvin G, Kumar SS, Dkhar SA. The dawn of hedgehog inhibitors: Vismodegib. J Pharmacol Pharmacother. 2013;4:4-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 105. | Kumar V, Mondal G, Dutta R, Mahato RI. Co-delivery of small molecule hedgehog inhibitor and miRNA for treating liver fibrosis. Biomaterials. 2016;76:144-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 106. | Broderick JA, Zamore PD. MicroRNA therapeutics. Gene Ther. 2011;18:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 328] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 107. | van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110:496-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 108. | van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6:851-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 490] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 109. | Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014;42:609-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 110. | Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. 2013;31:577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 111. | Tu X, Zhang H, Zhang J, Zhao S, Zheng X, Zhang Z, Zhu J, Chen J, Dong L, Zang Y. MicroRNA-101 suppresses liver fibrosis by targeting the TGFβ signalling pathway. J Pathol. 2014;234:46-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 112. | Su S, Zhao Q, He C, Huang D, Liu J, Chen F, Chen J, Liao JY, Cui X, Zeng Y. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat Commun. 2015;6:8523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 113. | Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013;172:962-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 467] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 114. | Zhou JN, Zeng Q, Wang HY, Zhang B, Li ST, Nan X, Cao N, Fu CJ, Yan XL, Jia YL. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology. 2015;62:801-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 115. | Ramamoorth M, Narvekar A. Non viral vectors in gene therapy- an overview. J Clin Diagn Res. 2015;9:GE01-GE06. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 116. | Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 758] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Mailleux AA, Morales-Ruiz M S- Editor: Yu J L- Editor: A E- Editor: Wang CH