Published online Jul 28, 2016. doi: 10.3748/wjg.v22.i28.6559
Peer-review started: April 8, 2016
First decision: May 27, 2016
Revised: June 7, 2016
Accepted: June 28, 2016
Article in press: June 28, 2016
Published online: July 28, 2016
Processing time: 105 Days and 8.7 Hours
Mastocytosis is a clonal neoplastic disorder of the mast cells (MC) that can be limited to the skin (cutaneous mastocytosis) or involve one or more extracutaneous organs (systemic mastocytosis). The clinical manifestations of mastocytosis are heterogeneous ranging from indolent disease with a long-term survival to a highly aggressive neoplasm with survival of about 6 mo. Although liver involvement in aggressive systemic mastocytosis (ASM) is relatively common, the development of portal hypertension with or without cirrhosis is rare. We report a case of ASM without skin involvement in a 72-year-old caucasian male who presented with non-cirrhotic portal hypertension based on clinical, analytical, imagiological and endoscopic findings. Given the hematological picture, the correct diagnosis was established based on ancillary tests for MC using bone marrow aspirates and biopsy. Extensive involvement of the liver and gastrointestinal tract was histologically documented. The disease progressed rapidly and severe pancytopenia and recurrent upper gastrointestinal bleeding became the dominant problem. This case illustrates the challenge in establishing a diagnosis of ASM especially when the clinical picture is atypical and without skin involvement. Gastroenterologists should consider infiltrative disease, particularly systemic mastocytosis, as a differential diagnosis in a clinical case of portal hypertension of unknown etiology.
Core tip: This clinical case describes an interesting and uncommon case of aggressive systemic mastocytosis with hepatic and gastrointestinal involvement. It illustrates not only a rare cause of non-cirrhotic portal hypertension, but also an atypical gastrointestinal involvement. The aim of this case report is to demonstrate the challenge in establishing a diagnosis of systemic mastocytosis especially when the clinical picture is atypical and without skin involvement, and alert Gastroenterologists to consider infiltrative diseases, as differential diagnosis in a clinical case of portal hypertension of unknown etiology.
- Citation: Martins C, Teixeira C, Ribeiro S, Trabulo D, Cardoso C, Mangualde J, Freire R, Gamito &, Alves AL, Cremers I, Alves C, Neves A, Oliveira AP. Systemic mastocytosis: A rare cause of non-cirrhotic portal hypertension. World J Gastroenterol 2016; 22(28): 6559-6564
- URL: https://www.wjgnet.com/1007-9327/full/v22/i28/6559.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i28.6559
Mastocytosis, a myeloproliferative neoplasm characterized by an abnormal proliferation of MC, is an uncommon, heterogeneous and progressive disease[1].
The vast majority of cases are confined to the skin (urticaria pigmentosa), but approximately 10% involve extracutaneous sites, most commonly bone, liver, spleen, gastrointestinal tract and lymph nodes[1,2]. The clinical manifestations are related to the release of MC mediators (e.g., facial flushing, urticaria, itching, diarrhea) and to uncontrolled growth and infiltration of clonal MC in different organs (e.g., hepatomegaly, splenomegaly, pancreatic insufficiency). Although most of the symptoms are not life-threatening, they may induce a significant impairment of quality of life. Nevertheless, when infiltration results in organ function impairment, the disease is considered aggressive and decrease life expectancy.
A diagnosis of mastocytosis has to fulfill the World Health Organization (WHO) diagnostic criteria[3] in which the demonstration of neoplastic MC infiltrates is the sine qua non condition. SM is diagnosed when the major and at least one minor or three minor criteria are satisfied (Table 1). Its signs and symptoms are divided into two groups: B symptoms (“borderline benign - be watchful”) and C symptoms (“consider cytoreductive therapy”). The diagnosis of ASM can be made when one or more “C” findings are present. “C” findings include anemia (Hb < 10 g/dL), thrombocytopenia (< 100000/mm3), neutropenia, hepatopathy with portal hypertension or ascitis, splenomegaly with hypersplenism, malabsorption with weight loss and osteolysis with pathological bone fractures.
| Major Criteria |
| Multifocal dense infiltrates of mast cells (> 15 mast cells in aggregates) at bone marrow biopsy and/or in sections of other extracutaneous organ(s) |
| Minor Criteria |
| > 25% of mast cells in bone marrow are atypical cells or spindle- shaped mast cells in infiltrates detected on sections of extracutaneous organ(s) |
| c-kit point mutation at codon 816 in the bone marrow or in another extracutaneous organ(s) |
| Mast cells in the bone marrow or in the another extracutaneous organ(s) expressing CD2 and/or CD25 with CD117 |
| Serum tryptase levels > 200 ng/mL |
Overlapping symptoms and heterogeneous clinical scenarios make early diagnosis extremely difficult. An absence of skin lesions at the time of diagnosis has been reported in up to 40%-50% of patients with ASM[5]. The liver is frequently involved, but only a minor percentage of ASM patients develop portal hypertension and/or cirrhosis.
In this paper we report a rare case of ASM without skin lesions who presented with non-cirrhotic portal hypertension. Bone, liver and gastrointestinal involvements were observed and histologically documented. The extensive bone marrow and gastrointestinal infiltration with the development of severe pancytopenia and recurrent upper gastrointestinal bleeding, respectively, were responsible for the poor prognosis and fatal outcome.
We report a 72-year-old caucasian male referred to our hospital due to severe anemia. The patient presented with a 3-mo clinical picture of significant involuntary weight loss, anorexy, astenia, night sweats, low-grade fever and, more recently, melena. The remaining patient’s history was uneventful. He reported no history of smoking, alcohol or drugs consumption. Physical examination showed pallor, painless hepatomegaly and splenomegaly. Skin lesions, superficial lymphadenopathy, ascitis and jaundice were absent.
Initial laboratory tests showed normocytic and normochromic anemia (hemoglobin 6.1 g/dL), normal total white blood cells count (7.1 × 109/L) but with monocytosis (18%) and eosinophilia (10%), mild thrombocytopenia (141 × 109/L) and normal prothrombin time. Liver tests were normal except for high alkaline phospatase (274 U/L). Laboratorial study of anemia revealed a multifactorial etiology with ferropenic and non-autoimmune hemolytic components.
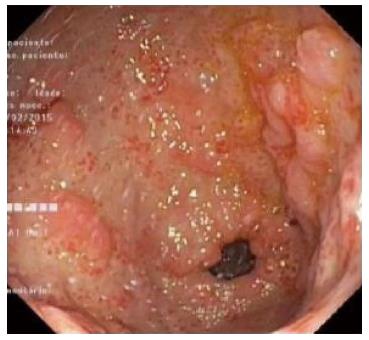
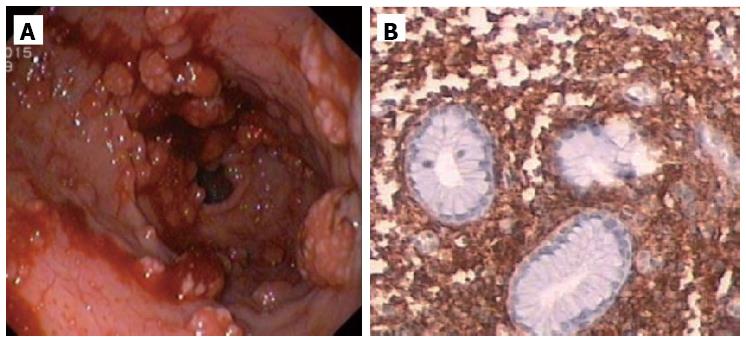
Abdominal ultrassound showed homogeneous hepatosplenomegaly. Doppler ultrassonography of the liver revealed portal vein dilation (14 mm), decreased portal flow velocity, hepatofugal portal flow and high hepatic artery resistance indexes. Upper gastrointestinal endoscopy showed small esophageal varices and gastric antral vascular ectasia (GAVE) treated with argon plasma coagulation (Figure 1). Total colonoscopy was normal.
In order to clarify the etiology of portal hypertension additional investigations were performed. Serum protein electrophoresis revealed low albumin but normal gammaglobulin levels. Serological testing for hepatitis B and C virus, HIV and autoimmune markers were negative. Serum copper, ceruloplasmin and alpha-fetoprotein were normal. Ferritin was moderately high (497 ng/mL).
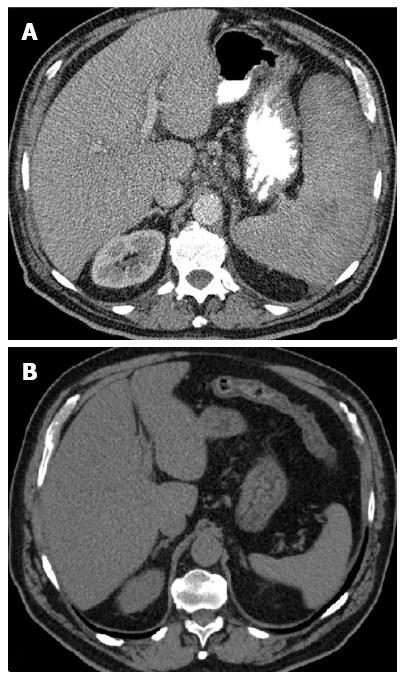
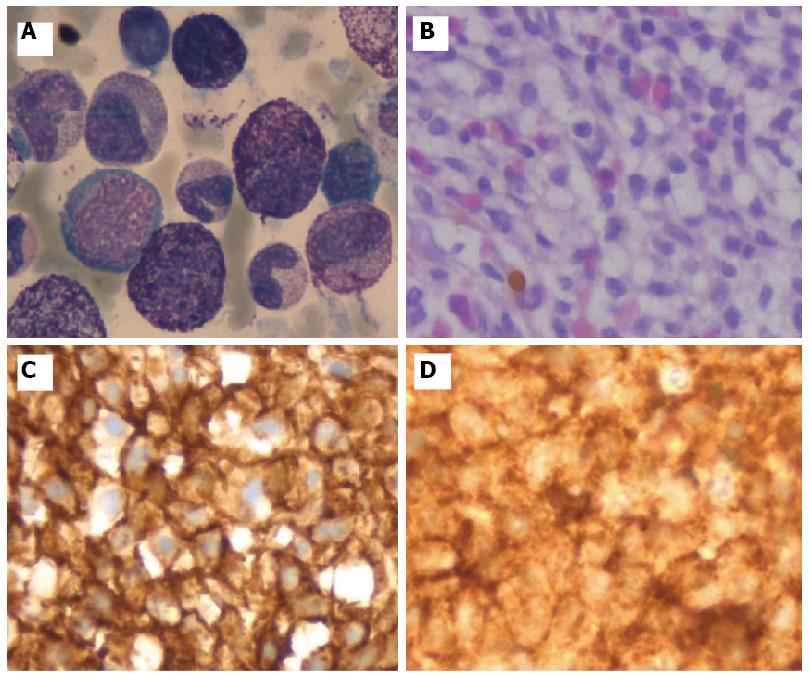
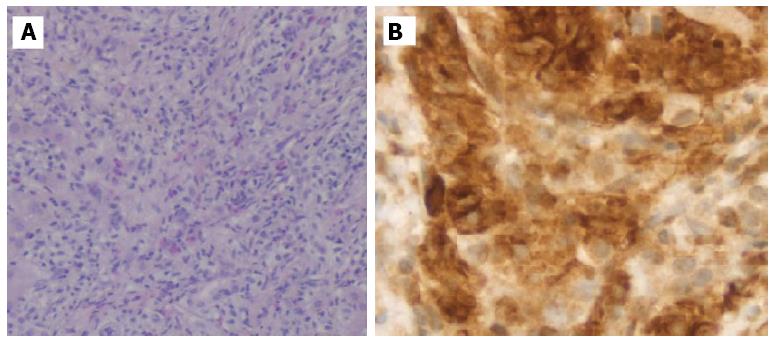
Given the hematologic changes in a patient with wasting syndrome and a non-cirrhotic portal hypertension, to exclude hematologic disease, he underwent a thoraco-abdominopelvic computed tomography (CT) scan which confirmed homogeneous hepatosplenomegaly and showed no lymphadenopathy (Figure 2); a bone marrow aspiration and biopsy, which revealed multifocal infiltration of atypical MC (> 15 aggregates) with a spindle-shape, representing 31% of total cellularity; immunohistochemistry study identified positive staining for CD117 and CD25 (Figure 3). Serum tryptase levels were high (200 mcg/L). Liver biopsy confirmed massive infiltration by atypical MC and mild portal fibrosis but without evidence of cirrhosis (Figure 4).
These findings were fully consistent with SM fulfilling the WHO diagnostic criteria for this entity, namely the presence of the major and three minor criteria: > 25% of atypical MC in bone marrow and liver, coexpression of CD25 and CD117 by atypical MC, and high serum tryptase levels.
The patient was referred to the Hematology Department and began chemotherapy with cladribine with initial partial response. However, the disease became non-responsive to cladribine and progressed, with development of severe pancytopenia and recurrent upper gastrointestinal bleeding due to extensive involvement of the stomach (Figure 5), refractory to medical and endoscopic treatment. The patient died after one year.
Mastocytosis is a rare disorder with several studies reporting an incidence of 5-10 cases/106 people/year. It is recognized as a myeloproliferative neoplasm by the 2008 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues and is defined as an accumulation of clonal MC in one or more organs. The skin is involved in most patients (80%-90%), commonly presented as pigmentosa urticaria. SM is characterized by clonal MC accumulation in bone marrow and other extracutaneous organs.
ASM is an uncommon subtype of SM (12%) and occurs generally in adults. It is defined by the presence of at least one “C” finding, associated with constitutional symptoms and organomegaly, particularly of liver, spleen and lymph nodes.
Regarding hematologic abnormalities in SM, the most common is mild-to-moderate anemia, which occurs in up to 50% of patients[6]. Normally, MC is not found in the circulation except in those with mast cell leukemia. Eosinophilia occurs in 25% of patients and monocytosis, when present in patients with ASM, represent a signal of advanced disease[7].
Liver involvement in ASM is common and may include abnormal liver tests, hepatomegaly, portal hypertension, fibrosis, cirrhosis and liver failure. Hepatomegaly is found in 41%-72% of patients. MC infiltrate the liver diffusely with or without portal fibrosis but rarely with cirrhosis or portal hypertension (4%). The development of portal hypertension and the lack of skin involvement are poor prognosis factors. Yoshida et al[8] reported a case of mast cell leukemia, a rare form of SM, complicated with rapidly progressing non-cirrhotic portal hypertension due to blocking of sinusoidal and venous flow. Despite significant hepatic involvement, liver tests are usually normal, except for alkaline phosphatase which is increased due to bone involvement[9]. Furthermore, hepatic lesion severity is not correlated with the size or the results of liver function tests[9].
Gastrointestinal involvement is seen in 70%-80% of patients with SM. Abdominal pain (51%), diarrhea (43%) and nausea or vomiting (28%) are the most common GI complaints[10]. Gastrointestinal bleeding, usually due to peptic ulcer disease, occurs in 11% of SM[10]. Other causes of upper gastrointestinal bleeding, such as GAVE, portal hypertensive gastropathy and esophageal varices, are very rare. The most common upper endoscopic features in SM patients are peptic esophagitis, peptic ulcers, thickened gastric or duodenal folds and nodular mucosa[10].
Patients with ASM are candidates for MC cytoreductive therapies, which may include interferon-alpha-2b, cladribine, glucocorticoides, tyrosine kinase inhibitors or hydroxyurea[11]. The clinical course of patients with ASM is variable, with some experiencing a rapid decline over 12 to 24 mo, while others follow a slower course with several years of survival[12].
In this case, the initial approach revealed a clinical picture of non-cirrhotic portal hypertension. The lack of skin manifestations delayed the diagnosis. The high suspicion of hematological disease due to wasting syndrome, B symptoms, hepatosplenomegaly and bicytopenia, led to the diagnosis, based on bone marrow aspirate and biopsy findings, which revealed infiltration by atypical MC with positive staining for CD117 and CD25. The liver biopsy confirmed massive infiltration by atypical MC but without evidence of cirrhosis. The diagnosis of ASM was established based on WHO 2008 criteria and the presence of “C” findings, particularly, anemia, hepatopathy with portal hypertension and splenomegaly with hypersplenism.
Besides the rare clinical presentation, this report describes an uncommon form of gastrointestinal involvement in SM. Unlike peptic ulcer disease, this patient showed atypical upper endoscopic findings, namely, esophageal varices, GAVE and diffuses gastric polypoid lesions. The last two were responsible for recurrent and refractory upper gastrointestinal bleeding.
In this patient, there were several poor prognosis factors, such as, older age at onset, weight loss, lack of skin involvement, severe anemia, trombocytopenia, monocytosis, hepatosplenomegaly, portal hypertension and gastrointestinal bleeding.
This report describes an interesting case of SM as a rare cause of non-cirrhotic portal hypertension with an atypical gastrointestinal involvement. Needless to say, patients with obvious dermatological findings are diagnosed by clinicians more quickly as having SM. Unfortunately, patients lacking skin manifestations have a delayed diagnosis even though they often have more aggressive disease. Therefore, Gastroenterologists should consider infiltrative disease, particularly ASM, as differential diagnosis in a clinical case of portal hypertension of unknown etiology. Presently there is no cure for SM and cytoreductive therapy and a multidisciplinary team approach are recommended.
A 72-year-old male presented with a clinical picture of non-cirrhotic portal hypertension.
Aggressive systemic mastocytosis with liver and gastrointestinal involvement.
Viral hepatitis, alcoholic liver disease, infiltrative diseases (lymphoma, other myeloprolipherative disorders and amyloidosis) granulomatous diseases (sarcoidosis and tuberculosis), genetic disorders (Wilson disease and hemochromatosis) and vascular diseases such as portal vein thrombosis, among others.
Normocytic and normochromic anemia, monocytosis, eosinophilia, thrombocytopenia, high alkaline phospatase and high serum tryptase levels.
Abdominal ultrasound with Doppler ultrassonography of the liver revealed hepatosplenomegaly, portal vein dilation, decreased portal flow velocity, hepatofugal portal flow and high hepatic artery resistance indexes. Upper endoscopy showed esophageal varices and gastric antral vascular ectasia.
A bone marrow aspiration and biopsy revealed multifocal infiltration of atypical mast cells (> 15 aggregates) with positive staining for CD117 and CD25; liver biopsy confirmed massive infiltration by atypical MC but without cirrhosis.
Chemotherapy with cladribine.
Aggressive systemic mastocytosis without skin involvement is uncommon. Given the hematological picture, the correct diagnosis was established based on ancillary tests for mast cells using bone marrow aspirates and biopsy. The extensive involvement of the liver and gastrointestinal tract was histologically documented.
Infiltrative diseases, particularly systemic mastocytosis, should be considered as a differential diagnosis in a clinical case of portal hypertension of unknown etiology.
This paper shows a rare case of systemic mastocytosis with non-cirrhotic portal hypertension. It illustrates the challenge in establishing a diagnosis of aggressive systemic mastocytosis especially when the clinical picture is atypical and without skin involvement.
| 1. | Patnaik MM, Rindos M, Kouides PA, Tefferi A, Pardanani A. Systemic mastocytosis: a concise clinical and laboratory review. Arch Pathol Lab Med. 2007;131:784-791. [PubMed] |
| 2. | Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Horny HP, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L, Valent P. Mastocytosis (mast cell disease). World Health Organization (WHO) classification of tumours. Pathology Genetics. Tumours of haematopoietic and lymphoid tissues. Lyon, France: International agency for research on cancer 2008; 54-63. |
| 4. | Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, Marone G, Nuñez R, Akin C, Sotlar K. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 797] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 5. | Kirsch R, Geboes K, Shepherd NA, de Hertogh G, Di Nicola N, Lebel S, Mickys U, Riddell RH. Systemic mastocytosis involving the gastrointestinal tract: clinicopathologic and molecular study of five cases. Mod Pathol. 2008;21:1508-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Lawrence JB, Friedman BS, Travis WD, Chinchilli VM, Metcalfe DD, Gralnick HR. Hematologic manifestations of systemic mast cell disease: a prospective study of laboratory and morphologic features and their relation to prognosis. Am J Med. 1991;91:612-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Horny HP, Ruck M, Wehrmann M, Kaiserling E. Blood findings in generalized mastocytosis: evidence of frequent simultaneous occurrence of myeloproliferative disorders. Br J Haematol. 1990;76:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Yoshida M, Nishikawa Y, Yamamoto Y, Doi Y, Tokairin T, Yoshioka T, Omori Y, Watanabe A, Takahashi N, Yoshioka T. Mast cell leukemia with rapidly progressing portal hypertension. Pathol Int. 2009;59:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Addada J, Lloyd J, Bain B. Teaching cases from the Royal Marsden and St Mary’s Hospitals: Case 27, ascites and oedema in a patient with systemic mastocytosis. Leuk Lymphoma. 2004;45:1713-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Jensen RT. Gastrointestinal abnormalities and involvement in systemic mastocytosis. Hematol Oncol Clin North Am. 2000;14:579-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Lim KH, Pardanani A, Butterfield JH, Li CY, Tefferi A. Cytoreductive therapy in 108 adults with systemic mastocytosis: Outcome analysis and response prediction during treatment with interferon-alpha, hydroxyurea, imatinib mesylate or 2-chlorodeoxyadenosine. Am J Hematol. 2009;84:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Casassus P, Caillat-Vigneron N, Martin A, Simon J, Gallais V, Beaudry P, Eclache V, Laroche L, Lortholary P, Raphaël M. Treatment of adult systemic mastocytosis with interferon-alpha: results of a multicentre phase II trial on 20 patients. Br J Haematol. 2002;119:1090-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Manuscript Source: Unsolicited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Portugal
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fujino Y, Papamichael KX S- Editor: Qi Y L- Editor: A E- Editor: Ma S