Published online Jul 28, 2016. doi: 10.3748/wjg.v22.i28.6501
Peer-review started: March 10, 2016
First decision: April 14, 2016
Revised: May 4, 2016
Accepted: June 2, 2016
Article in press: June 2, 2016
Published online: July 28, 2016
Processing time: 134 Days and 14.6 Hours
Liver ischemia-reperfusion injury (IRI) is an inherent feature of liver surgery and liver transplantation in which damage to a hypoxic organ (ischemia) is exacerbated following the return of oxygen delivery (reperfusion). IRI is a major cause of primary non-function after transplantation and may lead to graft rejection, regardless of immunological considerations. The immediate response involves the disruption of cellular mitochondrial oxidative phosphorylation and the accumulation of metabolic intermediates during the ischemic period, and oxidative stress during blood flow restoration. Moreover, a complex cascade of inflammatory mediators is generated during reperfusion, contributing to the extension of the damage and finally to organ failure. A variety of pharmacological interventions (antioxidants, anti-cytokines, etc.) have been proposed to alleviate graft injury but their usefulness is limited by the local and specific action of the drugs and by their potential undesirable toxic effects. Polyethylene glycols (PEGs), which are non-toxic water-soluble compounds approved by the FDA, have been widely used as a vehicle or a base in food, cosmetics and pharmaceuticals, and also as adjuvants for ameliorating drug pharmacokinetics. Some PEGs are also currently used as additives in organ preservation solutions prior to transplantation in order to limit the damage associated with cold ischemia reperfusion. More recently, the administration of PEGs of different molecular weights by intravenous injection has emerged as a new therapeutic tool to protect liver grafts from IRI. In this review, we summarize the current knowledge concerning the use of PEGs as a useful target for limiting liver IRI.
Core tip: Pharmacological treatments for preventing liver ischemia reperfusion injury are limited, due to the complex pathophysiology of this condition. The drugs currently used for preventing ischemia-reperfusion injury (IRI) all have local and specific activity with potentially damaging side effects. This review focuses on the current understanding of polyethylene glycols, which are non-toxic polymers, as new emerging agents for limiting liver IRI, and proposes directions for future investigations.
- Citation: Pasut G, Panisello A, Folch-Puy E, Lopez A, Castro-Benítez C, Calvo M, Carbonell T, García-Gil A, Adam R, Roselló-Catafau J. Polyethylene glycols: An effective strategy for limiting liver ischemia reperfusion injury. World J Gastroenterol 2016; 22(28): 6501-6508
- URL: https://www.wjgnet.com/1007-9327/full/v22/i28/6501.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i28.6501
It is well known that the interruption of blood to an organ (ischemia) and its subsequent restoration (reperfusion) leads to irreversible damage which is termed ischemia reperfusion injury (IRI). IRI is inherent to liver surgical procedures such as hepatic resection and liver transplantation[1-3]. During liver resections, the damage is commonly a consequence of the vascular occlusion of the liver hilum (Pringle’s maneuver) under normothermic conditions[1]. In the case of transplantation, the damage is sustained during cold storage of the liver graft (at 4 °C) in preservation solution following explantation from the donor, and during subsequent warm reperfusion and implantation into the recipient[4].
There are several steps between organ recovery and transplantation that can exacerbate the damage to the graft. The most important are organ procurement (pre-preservation), conservation in preservation solution (cold storage), and rewarming (graft washout) before transplant (reperfusion). The cumulative injuries due to each step are determinant for the successful graft outcome after transplantation but the most significant lesions occur during cold ischemia, graft rewarming and normothemic reperfusion after transplantation.
At the cellular level, prolonged ischemia leads to ATP breakdown and provokes the accumulation of hypoxanthine, mitochondrial de-energization and ionic alterations which finally lead to liver cell necrosis. Upon oxygenation during reperfusion, reactive oxygen species (ROS) generation by uncoupled mitochondria promotes oxidative stress and a complex cascade of inflammatory mediators (nitric oxide, cytokines, adhesion molecules, chemokines, etc.) which all contribute to the spread of the damage and finally to cell death[4].
Because of the range of mechanisms involved in hepatic IRI, the choice of preventive or therapeutic strategies is very difficult[5]. Pharmacological strategies for preventing IRI focus on the use of specific agents, but the benefits of these drugs are limited because of their local actions, side effects and potential toxicity. In this situation, there is a clear need to test the use of non-toxic, water-soluble and protective agents for tissues such as PEGs as “preconditioning agents” for preventing IRI and also as potential targets for therapeutic interventions in organ transplantation.
This review is an update of the most significant advances in the use of polyethylene glycols (PEGs) as therapeutic tools for protecting the liver against IRI, placing specific emphasis on future perspectives in liver graft preservation and transplantation.
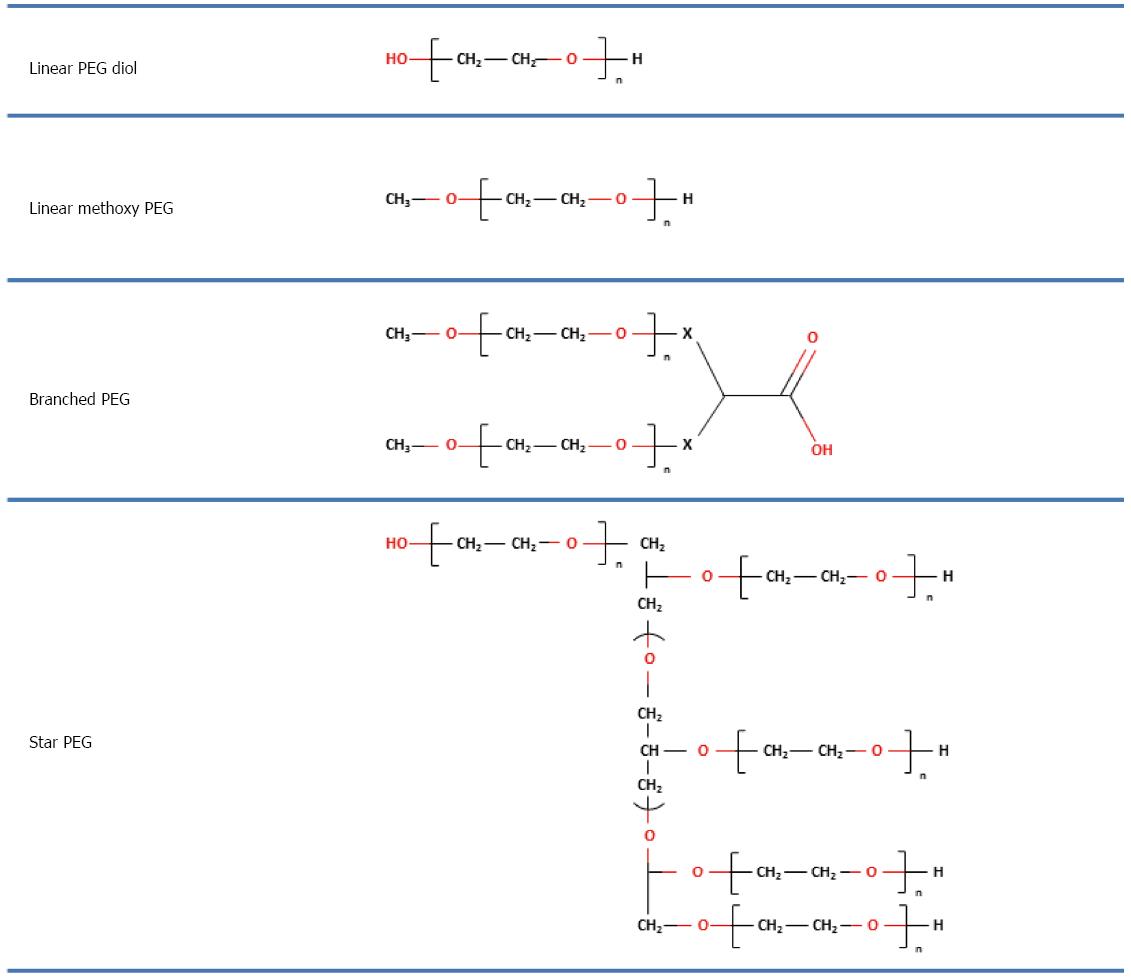
PEGs are non-immunogenic, non-toxic and water-soluble polymers which show no electric charge and no affinity for any specific organ. They are composed of repeating units of ethylene glycol which form polymers with a linear shape of different molecular weight[6-9]. PEGs with different shapes can be obtained by using different initiator molecules during the polymerization reaction (e.g., hexa-glycerin instead of methanol to form a tri PEG) or by joining different linear PEGs to create different structures, as shown in Figure 1.
PEGs are negligibly metabolized in vivo and are mainly unaltered when eliminated from the body either by the kidneys (for PEGs < 30 kDa, slowly for 30 kDa < PEGs < 40 kDa) or in the faeces (for PEGs > 20 kDa)[10]. PEGs are generally considered to have low toxicity via all routes of administration, as demonstrated by tests in many animals[11]. Due to their high flexibility, hydrophilicity, and the large number of water molecules coordinated by their chains, PEGs present a greater hydrodynamic volume than would be expected from their molecular weight, and they show high protein-rejecting properties[12].
PEGs have an apparent molecular weight which, depending on the molecular weight of the polymer, can be 5-10 times higher than a corresponding soluble protein of similar molecular mass, as shown by gel permeation chromatography[13]. The Food and Drug Administration (FDA) has approved the use of PEGs as a vehicle or a base in food, cosmetics and pharmaceuticals, including injectable and bowel solutions[14,15]. For example, low molecular weight PEGs are widely used as the basis of a number of laxatives and soft capsules, and high molecular weight PEGs have been used as components in organ preservation solutions to attenuate injury from cold perfusion in animal organs such as pancreas[16], small bowel[17], kidney[18] and liver[19]. In addition, the attachment of PEG (PEGylation) to drugs, peptides, proteins, nanoparticles, micelles, and liposomes is spreading as a technology for enhancing the bioavailability, stability, safety, and efficacy of a wide range of therapeutic agents[20,21]. Examples include PEGylated interferon alpha, which is used to treat hepatitis C[22], or PEGylated antihuman TNF-alpha for rheumatoid arthritis[23].
The use of PEGs to minimize the deleterious effects of IRI has not been studied in depth. However, PEGs have been shown to be effective in cell protection against hypoxia/oxygenation, as additives in preservation and perfusion solutions for organ transplantation and, more recently, as “preconditioning agents” for preventing IRI in heart and liver. As a result, PEGs may offer new therapeutic strategies for applications in clinical liver surgery and transplantation, as indicated below.
Current technologies can preserve livers outside the body for about 12 h using a combination of cold temperatures and a preservation solution. This has helped increase the number of successful liver transplants, but extending the time a liver can survive outside the body even further would provide many extra benefits.
The presence of PEGs has been shown to be determinant for hepatocyte preservation in hypothermic conditions[24-26]. The addition of PEG8 to the preservation solution suppressed cell swelling in cultured hepatocytes, keeping them relatively well-preserved and restoring membrane integrity[24-26]. This is consistent with the further development of a slow-cooling method that first chills rat livers at 4 °C and then drops the temperature to below freezing (named “supercooling”), allowing them to be stored in a “supercooled” but non-frozen state[27]. In this connection, a recent study by Berendsen et al[28] presented a method for extended liver storage combining supercooling and machine perfusion. An essential step in this method was the addition of PEG35 to the preservation solution. Similar results were found by the same researchers with “supercooled” hepatocytes: this addition of 5% PEG35 to the storage solution prevented cold-induced lipid peroxidation and maintained hepatocyte viability and functionality during supercooling[25-27].
The cold static preservation of solid organs using preservation solutions is the gold standard in clinical organ transplantation today. PEG35 and PEG20 have been used as oncotic agents in IGL-1 and SCOT 20 preservation solutions respectively to prevent cell swelling[29-32]. The presence of PEG35 in IGL-1 makes this solution a good alternative to UW solution (the standard goal for liver transplantation), especially in the presence of moderate to severe hepatic steatosis[33,34]. PEG20 is the basic component of the SCOT solution, which furthermore contains low K+/high Na+ concentrations. PEG20 at 15 g/L has been found to reduce alloantigen recognition after liver reperfusion in comparison to UW solution[35]. However, the use of this PEG20 in preservation solutions has not shown a greater benefit than PEG35[35].
PEG35 (at 1 g/L) plays a key role in reducing the higher vulnerability of fatty livers to IRI[33]. This is mainly due to the production of nitric oxide (NO), whose vasodilatory properties contribute to counteracting the exacerbated alterations of microcirculation in steatotic livers due to the accumulation of fat in the sinusoids, which makes it more difficult to obtain an adequate hepatic revascularization after transplantation. Moreover, the NO generated may act as a suitable scavenger for preventing the impairment of lipid peroxidation in fatty livers against reperfusion[36].
It has also been demonstrated that PEG interferes with the coagulation system and reduces platelet adhesion in vitro and in vivo[37,38] by forming a molecular barrier on the glycocalyx. This PEG barrier prevents acute platelet deposition on damaged arteries. When a relatively low molecular weight PEG (< 10 kDa) was conjugated to pericardium, it reduced the deposits of calcium and decreased platelet and leukocyte surface attachment[39,40]. Therefore, the conjugation of PEG to the surface of endothelial cells seemed to reduce inflammation and control water content. Longer PEG chains, such as that of PEG35, might be expected to interact with the surface of endothelial cells of blood vessels and/or remain in the interstitial fluid of transplanted liver, thus promoting the above mentioned beneficial effects even after the washout of the organ graft.
The presence of PEG35 in IGL-1 solution also promotes the activation of several protective cell signaling pathways during liver cold storage, as a self-response of the organ to oxygen deprivation. This leads to the induction of cytoprotective factors such as adenosine monophosphate protein kinase (AMPK), an enzyme which is involved in the glucose metabolism breakdown and modulates the energy balance towards an energy preserving state. PEG35 also activates other protective factors associated with the deprivation of oxygen supply to the organ such as the hypoxia inducible factor HIF alpha[41].
Rinse solutions help to wash the liver graft preserved in organ preservation solutions by avoiding air emboli and the secondary effects of the remnants of preservation solution, such as the excessively high concentration of intravascular potassium and metabolic waste during cold storage[42,43]. Although there is no consensus among physicians on how the graft flushes should be carried out, the most widely used solutions are Ringer Lactate and 5% human albumin[42].
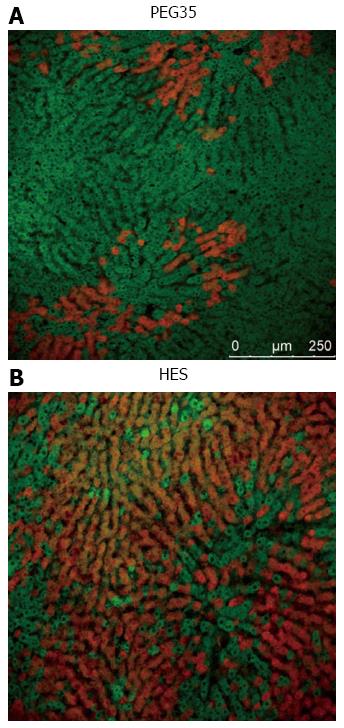
PEG35 is a suitable additive in rinse solution for an efficient liver graft washout, and also ensures additional protection against reperfusion injury[44]. Protective mechanisms induced by PEG35 against liver reperfusion injury mainly involve the preservation of liver mitochondrial status[44], as shown in Figure 2.
Rhodamine 123 cell viability marker (in green) shows the preserved membrane potential of liver mitochondria in liver grafts preserved in UW and then rinsed with PEG35 solution when compared to livers rinsed with Ringer lactate. In this case, Evans Blue labeling (in red) shows the albumin content and the disrupted mitochondrial membranes. Thus, PEG35 preserves the cytoskeleton structure and cell morphology against the effects of IRI[44,45].
Static cold preservation remains the gold standard, but the growing needs of liver transplantation oblige physicians to use machine perfusion (MP) techniques in different temperature conditions: hypothermia (HMP), normothermia (NMP) and subnormothermia (SNMP) for graft preservation purposes[46-48]. The use of PEG in MP solutions is very limited in contrast to UW-gluconate and KPS solutions[46-48]. Bessems et al[49,50] have shown that substitution of hydroxyethyl starch by PEG in Polysol perfusion solution achieved equal or better function and less damage in rat liver after 24 h of HMP, and that this Polysol-PEG solution was more efficient than UW-Gluconate perfusion solution. In rat steatotic livers, cold storage using Polysol resulted in significantly better integrity and functions of the liver[51] and thus improved the preservation quality of partial liver transplantation[52]. More recently, it was shown that addition of PEG35 to SNMP at 5 g/L using the “supercooling” technique was necessary to achieve successful liver transplantation after six days’ preservation[28,53]. Thus, PEG contributes to the rapid extension of cooling and the lower temperatures attained also contributed to preserving the membrane and cytoskeletal structure of hepatocytes during HMP[25].
Recent investigations in the heart have found that high molecular weight PEG (15-20 kDa) protected cardiac myocytes from hypoxia reoxygenation[54]. More recently, these cardioprotective benefits for PEG 15-20 were observed when it was administered just before reperfusion[55].
With this in mind, our group explored the benefits of using PEG35 to limit IRI in different experimental models of cold ischemia and warm ischemia reperfusion in rats[56,57]. Intravenous administration of PEG35 to rats before the induction of cold ischemia-reperfusion insult (a single 10 mg/kg dose) protected fatty livers from the lesions associated with IRI[56]. The prevention of liver damage was accompanied by a high protection of liver cytoskeleton and mitochondria, which was concomitant with increased phosphorylation of pro-survival protein kinase b (AKT) and the activation of cyto-protective factors such as e-NOS and AMPK respectively[56].
These investigations reveal that in vivo PEGs improve the initial conditions of organs against the cold ischemia reperfusion insult. This PEG strategy can be considered as a useful tool for multi-organ preconditioning before organ recovery and then static cold storage/machine perfusion preservation.
These protective mechanisms of PEG were also corroborated in a warm ischemia-reperfusion model in the rat[57]. Intravenous administration of PEG35 at 10 mg/kg was protective against IRI. In this case, PEG35 not only prevented mitochondria damage, but also promoted the activation of prosurvival pathways (AKT, AMPK), and reinforced the cytoskeleton structure and preservation of the hepatocytes’ morphological features[44,56]. However, the precise mechanisms by which PEGs interact with the cytoskeleton remains to be elucidated.
Many studies have been designed to prevent mitochondrial dysfunction and to increase endothelial NO generation as a tool for favoring a rapid recovery of liver graft viability after reperfusion. The use of new NO-releasing molecules covalently linked to PEG, as oncotic agents for fatty liver preservation, could help to prevent exacerbated microcirculation in steatotic liver grafts. This practice has been extended to the pharmacological preconditioning of fatty livers, with very promising results (unpublished data).
Moreover, the use of another alternative molecule similar to butanediol mononitrate, conjugated to the carboxylic groups of PEG derivatives by an ester linkage, may provide a new kind of PEG derivative obtained by preparation of PEG-dendron polymers. This may be useful for defining new PEG molecules for supercooling purposes either in combination with MP or not.
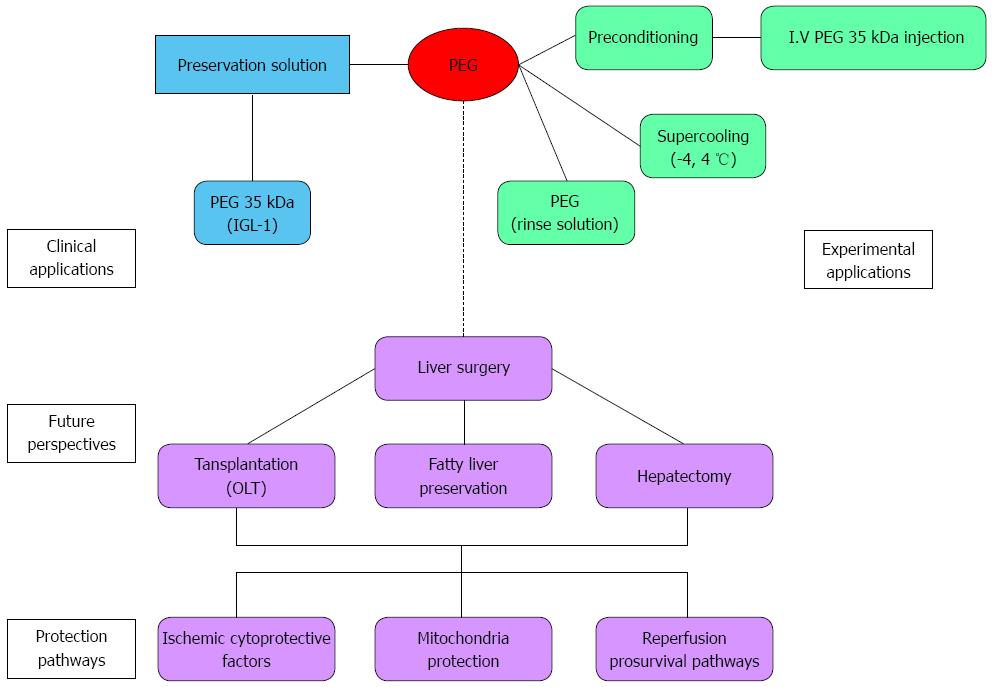
On the other hand, given the potential of PEGs as therapeutic targets for liver protection against IRI, it will be important to identify new PEG derivatives for use in liver transplantation as preconditioning agents. PEG treatment in donors and during reperfusion could minimize the deleterious effects of IRI, such as oxidative stress, cytoskeleton disruption and apoptosis. Moreover, in reduced orthotopic liver transplantation, the prophylactic administration of PEG to donor or/and recipient could contribute to a better liver regeneration of the implanted reduced graft and thus contribute to preventing the small-for-size syndrome present in living-living donor transplantation. In this particular case, the use of new derivatives based on growth factors releasing molecules covalently linked to PEG could offer potential advantages for rapid liver regeneration. Hepatic Growth Factor may be a suitable candidate. The benefits and perspectives of PEGs for limiting liver IRI are summarized in Figure 3.
The use of PEG may improve the initial conditions of organs available for transplantation, especially in the case of the most vulnerable ones such as steatotic livers. PEG is a very promising tool for limiting IRI in liver surgery (hepatectomy and transplantation) but further investigation in clinical trials is needed.
We are grateful to Michael Maudsley from the Language Advisory Service of the University of Barcelona for revising the English text.
| 1. | Weigand K, Brost S, Steinebrunner N, Büchler M, Schemmer P, Müller M. Ischemia/Reperfusion injury in liver surgery and transplantation: pathophysiology. HPB Surg. 2012;2012:176723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Bzeizi KI, Jalan R, Plevris JN, Hayes PC. Primary graft dysfunction after liver transplantation: from pathogenesis to prevention. Liver Transpl Surg. 1997;3:137-148. [PubMed] |
| 3. | Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 502] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 4. | Casillas-Ramírez A, Mosbah IB, Ramalho F, Roselló-Catafau J, Peralta C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006;79:1881-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Gurusamy KS, Gonzalez HD, Davidson BR. Current protective strategies in liver surgery. World J Gastroenterol. 2010;16:6098-6103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Mero A, Clementi C, Veronese FM, Pasut G. Covalent conjugation of poly(ethylene glycol) to proteins and peptides: strategies and methods. Methods Mol Biol. 2011;751:95-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Schiavon O, Pasut G, Moro S, Orsolini P, Guiotto A, Veronese FM. PEG-Ara-C conjugates for controlled release. Eur J Med Chem. 2004;39:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Pasut G, Veronese FM. PEG conjugates in clinical development or use as anticancer agents: an overview. Adv Drug Deliv Rev. 2009;61:1177-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 336] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 9. | Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2566] [Cited by in RCA: 2598] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 10. | Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci. 1994;83:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 530] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Fruijtier-Pölloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005;214:1-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Abuchowski A, van Es T, Palczuk NC, Davis FF. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977;252:3578-3581. [PubMed] |
| 13. | Pasut G, Veronese FM. State of the art in PEGylation: the great versatility achieved after forty years of research. J Control Release. 2012;161:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 566] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 14. | Lichtenstein G. Bowel preparations for colonoscopy: a review. Am J Health Syst Pharm. 2009;66:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Lim YJ, Hong SJ. What is the best strategy for successful bowel preparation under special conditions? World J Gastroenterol. 2014;20:2741-2745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 16. | Neuzillet Y, Giraud S, Lagorce L, Eugene M, Debre P, Richard F, Barrou B. Effects of the molecular weight of peg molecules (8, 20 and 35 KDA) on cell function and allograft survival prolongation in pancreatic islets transplantation. Transplant Proc. 2006;38:2354-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Valuckaite V, Seal J, Zaborina O, Tretiakova M, Testa G, Alverdy JC. High molecular weight polyethylene glycol (PEG 15-20) maintains mucosal microbial barrier function during intestinal graft preservation. J Surg Res. 2013;183:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Hauet T, Goujon JM, Baumert H, Petit I, Carretier M, Eugene M, Vandewalle A. Polyethylene glycol reduces the inflammatory injury due to cold ischemia/reperfusion in autotransplanted pig kidneys. Kidney Int. 2002;62:654-667. [PubMed] |
| 19. | Abbas R, Kombu RS, Dignam D, Gunning W, Stulberg JJ, Brunengraber H, Sanabria JR. Polyethylene glycol modified-albumin enhances the cold preservation properties of University of Wisconsin solution in rat liver and a hepatocyte cell line. J Surg Res. 2010;164:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22:315-329. [PubMed] |
| 21. | Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J Pharm Sci. 2016;105:460-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 519] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 22. | Kim V, Abreu RM, Nakagawa DM, Baldassare RM, Carrilho FJ, Ono SK. Pegylated interferon alfa for chronic hepatitis B: systematic review and meta-analysis. J Viral Hepat. 2016;23:154-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. BioDrugs. 2014;28 Suppl 1:S15-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Marsh DC, Lindell SL, Fox LE, Belzer FO, Southard JH. Hypothermic preservation of hepatocytes. I. Role of cell swelling. Cryobiology. 1989;26:524-534. [PubMed] |
| 25. | Stefanovich P, Ezzell RM, Sheehan SJ, Tompkins RG, Yarmush ML, Toner M. Effects of hypothermia on the function, membrane integrity, and cytoskeletal structure of hepatocytes. Cryobiology. 1995;32:389-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Dutheil D, Underhaug Gjerde A, Petit-Paris I, Mauco G, Holmsen H. Polyethylene glycols interact with membrane glycerophospholipids: is this part of their mechanism for hypothermic graft protection? J Chem Biol. 2009;2:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Puts CF, Berendsen TA, Bruinsma BG, Ozer S, Luitje M, Usta OB, Yarmush ML, Uygun K. Polyethylene glycol protects primary hepatocytes during supercooling preservation. Cryobiology. 2015;71:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Berendsen TA, Bruinsma BG, Puts CF, Saeidi N, Usta OB, Uygun BE, Izamis ML, Toner M, Yarmush ML, Uygun K. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat Med. 2014;20:790-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 29. | Bejaoui M, Pantazi E, Folch-Puy E, Baptista PM, García-Gil A, Adam R, Roselló-Catafau J. Emerging concepts in liver graft preservation. World J Gastroenterol. 2015;21:396-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Hauet T, Eugene M. A new approach in organ preservation: potential role of new polymers. Kidney Int. 2008;74:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Mosbah IB, Saidane D, Peralta C, Roselló-Catafau J, Abdennebi HB. Efficacy of polyethylene glycols in University of Wisconsin preservation solutions: a study of isolated perfused rat liver. Transplant Proc. 2005;37:3948-3950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Tabka D, Bejaoui M, Javellaud J, Roselló-Catafau J, Achard JM, Abdennebi HB. Effects of Institut Georges Lopez-1 and Celsior preservation solutions on liver graft injury. World J Gastroenterol. 2015;21:4159-4168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Ben Mosbah I, Roselló-Catafau J, Franco-Gou R, Abdennebi HB, Saidane D, Ramella-Virieux S, Boillot O, Peralta C. Preservation of steatotic livers in IGL-1 solution. Liver Transpl. 2006;12:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Adam R, Delvart V, Karam V, Ducerf C, Navarro F, Letoublon C, Belghiti J, Pezet D, Castaing D, Le Treut YP. Compared efficacy of preservation solutions in liver transplantation: a long-term graft outcome study from the European Liver Transplant Registry. Am J Transplant. 2015;15:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Gerber PC. Perio disease: target market for 90s’ GPs. Dent Manage. 1990;30:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Ben Abdennebi H, Zaoualí MA, Alfany-Fernandez I, Tabka D, Roselló-Catafau J. How to protect liver graft with nitric oxide. World J Gastroenterol. 2011;17:2879-2889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Bakaltcheva I, Ganong JP, Holtz BL, Peat RA, Reid T. Effects of high-molecular-weight cryoprotectants on platelets and the coagulation system. Cryobiology. 2000;40:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Garb LG. A new development in the provision of comprehensive medical care in Australia. A description of the Southern Memorial Hospital, Melbourne, Victoria. Am J Public Health. 1975;65:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Deible CR, Beckman EJ, Russell AJ, Wagner WR. Creating molecular barriers to acute platelet deposition on damaged arteries with reactive polyethylene glycol. J Biomed Mater Res. 1998;41:251-256. [PubMed] |
| 40. | Vasudev SC, Chandy T, Sharma CP. The antithrombotic versus calcium antagonistic effects of polyethylene glycol grafted bovine pericardium. J Biomater Appl. 1999;14:48-66. [PubMed] |
| 41. | Zaouali MA, Ben Mosbah I, Boncompagni E, Ben Abdennebi H, Mitjavila MT, Bartrons R, Freitas I, Rimola A, Roselló-Catafau J. Hypoxia inducible factor-1alpha accumulation in steatotic liver preservation: role of nitric oxide. World J Gastroenterol. 2010;16:3499-3509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Adam R, Astarcioglu I, Castaing D, Bismuth H. Ringer’s lactate vs serum albumin as a flush solution for UW preserved liver grafts: results of a prospective randomized study. Transplant Proc. 1991;23:2374-2375. [PubMed] |
| 43. | Gao WS, Takei Y, Marzi I, Lindert KA, Caldwell-Kenkel JC, Currin RT, Tanaka Y, Lemasters JJ, Thurman RG. Carolina rinse solution--a new strategy to increase survival time after orthotopic liver transplantation in the rat. Transplantation. 1991;52:417-424. [PubMed] |
| 44. | Zaouali MA, Bejaoui M, Calvo M, Folch-Puy E, Pantazi E, Pasut G, Rimola A, Ben Abdennebi H, Adam R, Roselló-Catafau J. Polyethylene glycol rinse solution: an effective way to prevent ischemia-reperfusion injury. World J Gastroenterol. 2014;20:16203-16214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Chiang ET, Camp SM, Dudek SM, Brown ME, Usatyuk PV, Zaborina O, Alverdy JC, Garcia JG. Protective effects of high-molecular weight polyethylene glycol (PEG) in human lung endothelial cell barrier regulation: role of actin cytoskeletal rearrangement. Microvasc Res. 2009;77:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Schlegel A, Kron P, Dutkowski P. Hypothermic Oxygenated Liver Perfusion: Basic Mechanisms and Clinical Application. Curr Transplant Rep. 2015;2:52-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Fontes P, Lopez R, van der Plaats A, Vodovotz Y, Minervini M, Scott V, Soltys K, Shiva S, Paranjpe S, Sadowsky D. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. 2015;15:381-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 48. | Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, Quaglia A, Holroyd D, Vogel T, Coussios CC. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transplant. 2016;16:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 381] [Article Influence: 38.1] [Reference Citation Analysis (1)] |
| 49. | Bessems M, Doorschodt BM, Hooijschuur O, van Vliet AK, van Gulik TM. Optimization of a new preservation solution for machine perfusion of the liver: which is the preferred colloid? Transplant Proc. 2005;37:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Bessems M, Doorschodt BM, Dinant S, de Graaf W, van Gulik TM. Machine perfusion preservation of the pig liver using a new preservation solution, polysol. Transplant Proc. 2006;38:1238-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Hata K, Tolba RH, Wei L, Doorschodt BM, Büttner R, Yamamoto Y, Minor T. Impact of polysol, a newly developed preservation solution, on cold storage of steatotic rat livers. Liver Transpl. 2007;13:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Yagi S, Doorschodt BM, Afify M, Klinge U, Kobayashi E, Uemoto S, Tolba RH. Improved preservation and microcirculation with POLYSOL after partial liver transplantation in rats. J Surg Res. 2011;167:e375-e383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Bruinsma BG, Berendsen TA, Izamis ML, Yeh H, Yarmush ML, Uygun K. Supercooling preservation and transplantation of the rat liver. Nat Protoc. 2015;10:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 54. | Malhotra R, Valuckaite V, Staron ML, Theccanat T, D’Souza KM, Alverdy JC, Akhter SA. High-molecular-weight polyethylene glycol protects cardiac myocytes from hypoxia- and reoxygenation-induced cell death and preserves ventricular function. Am J Physiol Heart Circ Physiol. 2011;300:H1733-H1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Xu X, Philip JL, Abdur Razzaque Md, Lloyd JW, Muller CM, Akter SA. A new strategy to limit ischemia-reperfusion injury Akhter. Thorac Cardio-vasc Surg. 2015;149:588-593. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Bejaoui M, Pantazi E, Folch-Puy E, Panisello A, Calvo M, Pasut G, Rimola A, Navasa M, Adam R, Roselló-Catafau J. Protective Effect of Intravenous High Molecular Weight Polyethylene Glycol on Fatty Liver Preservation. Biomed Res Int. 2015;2015:794287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Bejaoui M, Pantazi E, Calvo M, Folch-Puy E, Serafín A, Pasut G, Panisello A, Adam R, Roselló-Catafau J. Polyethylene Glycol Preconditioning: An Effective Strategy to Prevent Liver Ischemia Reperfusion Injury. Oxid Med Cell Longev. 2016;2016:9096549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Spain
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Machado MCC S- Editor: Qi Y L- Editor: A E- Editor: Ma S