Published online Jul 28, 2016. doi: 10.3748/wjg.v22.i28.6469
Peer-review started: March 24, 2016
First decision: May 12, 2016
Revised: May 21, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 28, 2016
Processing time: 120 Days and 17.8 Hours
Hepatocellular carcinoma (HCC), chronic hepatitis B (CHB) and chronic hepatitis C (CHC) are characterized by exhaustion of the specific CD8+ T cell response. This process involves enhancement of negative co-stimulatory molecules, such as programmed cell death protein-1 (PD-1), cytotoxic T-lymphocyte antigen-4 (CTLA-4), 2B4, Tim-3, CD160 and LAG-3, which is linked to intrahepatic overexpression of some of the cognate ligands, such as PD-L1, on antigen presenting cells and thereby favouring a tolerogenic environment. Therapies that disrupt these negative signalling mechanisms represent promising therapeutic tools with the potential to restore reactivity of the specific CD8+ T cell response. In this review we discuss the impressive in vitro and in vivo results that have been recently achieved in HCC, CHB and CHC by blocking these negative receptors with monoclonal antibodies against these immune checkpoint modulators. The article mainly focuses on the role of CTLA-4 and PD-1 blocking monoclonal antibodies, the first ones to have reached clinical practice. The humanized monoclonal antibodies against CTLA-4 (tremelimumab and ipilimumab) and PD-1 (nivolumab and pembrolizumab) have yielded good results in testing of HCC and chronic viral hepatitis patients. Trelimumab, in particular, has shown a significant increase in the time to progression in HCC, while nivolumab has shown a remarkable effect on hepatitis C viral load reduction. The research on the role of ipilimumab, nivolumab and pembrolizumab on HCC is currently underway.
Core tip: In certain types of chronic diseases, such as hepatocellular carcinoma and chronic viral hepatitis, disease curation involves restoration of the specific cytotoxic T cell response. Chronic hepatotropic viruses and tumoural cells develop mechanisms to induce exhaustion of the specific CD8+ T cells in order to escape immune destruction. One hallmark of this dysfunction is the overexpression of negative co-stimulatory molecules. Blockade of these negative co-stimulatory pathways, a process known as immune checkpoint modulation, is a promising novel therapy that could improve the treatment of liver diseases that feature T cell exhaustion.
- Citation: Moreno-Cubero E, Larrubia JR. Specific CD8+ T cell response immunotherapy for hepatocellular carcinoma and viral hepatitis. World J Gastroenterol 2016; 22(28): 6469-6483
- URL: https://www.wjgnet.com/1007-9327/full/v22/i28/6469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i28.6469
Specific CD8+ T cells have a central role in pathogenesis of hepatocellular carcinoma (HCC) as well as control of infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) because these cells are able to recognise infected/tumoural cells and destroy them[1-11]. Nevertheless, in chronic viral infections and tumoural diseases that feature high-grade and persistent antigenemia, the adaptive immune system has to surrender in order to diminish tissue damage[12-16]. This is the case for HCC and chronic viral hepatitis, wherein tumoural cells and HBV/HCV viruses modulate common mechanisms to induce specific T cell exhaustion. Among such viral and tumoural strategies, the induction of negative co-stimulatory molecules stands out.
Unfortunately, the on-going lack of effective treatments for HCC[17], for achieving complete HBV clearance[18] and for preventing HCV relapse after direct-acting antiviral (DAA) agent failure[19] has led to an urgent need for developing new therapeutic approaches, such as immunotherapy focused on specific cytotoxic T cell restoration[20]. Modulation of negative co-stimulatory signalling molecules expressed on these cells could have a substantial impact when developed as a therapeutic tool. In this review we discuss the specific CD8+ T cell response during HCC and chronic hepatitis B and C (commonly known as CHB and CHC respectively), focusing on the disease mechanisms used by tumoural cells and hepatotropic viruses to induce T cell exhaustion and on the potential therapeutic strategies to modulate co-stimulatory pathways in order to restore specific T cell reactivity.
CD8+ T cell activation depends on physical interaction between the T cell receptor and the major histocompatibility complex I (MHC I)/epitope complex, as well as that between co-stimulatory molecules with their ligands in an adequate cytokine milieu[21]. Upon completion of their effector tasks, primed specific T cells switch-off their effector activity by expressing negative co-stimulatory molecules, generating a sustained memory T cell population[22]. Thus, the balance between positive and negative co-stimulation determines the status of CD8+ T cell activation and the intensity of the accompanying immune response[23].
During tumoural and persistent viral infections - characterized by high-grade and persistent antigenemia - the adaptive immune system is tuned down in order to avoid host-induced tissue damage. Tumoural cells and persistent viruses have developed mechanisms that induce early expression of negative co-stimulatory molecules so as to favour T cell exhaustion before the effector T cells are able to control the disease[24-26]. This phenomenon could represent an evolutionary advantage for the chronic persistence of these diseases.
T cell exhaustion is characterized by a lack of effector cell capacity that is linked to overexpression of negative co-stimulatory pathways. Upon binding to their respective ligands, these negative co-stimulatory molecules act to disrupt the processes of T cell proliferation as well as secretion of type-I cytokines and development of cytolytic functions, creating an environment that allows for tumour persistence and virus evasion[13,27-29]. Among these negative co-stimulatory pathways, the most advanced in the pipeline for clinical use are cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1), as will be discussed later[30,31].
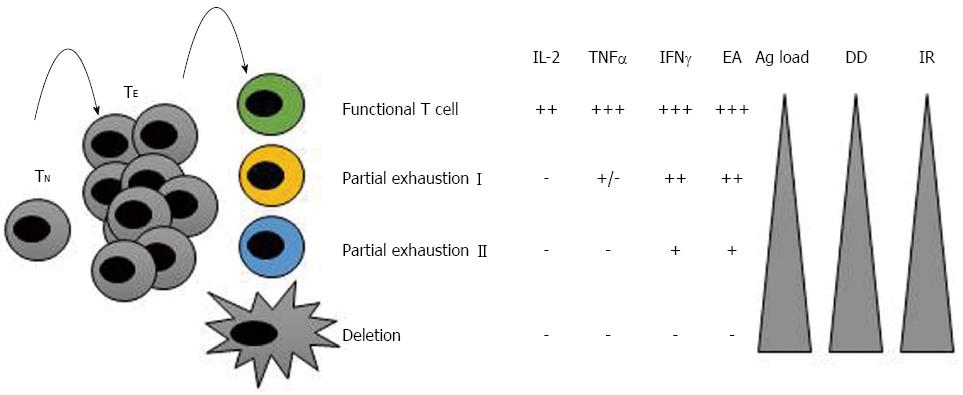
Exhausted CD8+ T cells were first identified during chronic lymphocoriomeningitis virus (LCMV) infection and defined as virus-specific CD8+ T cells that did not produce antiviral cytokines and were ineffective at controlling the infection[32]. Since that time, subsequent research has provided descriptions of T cell exhaustion in different human chronic infections and cancers[33-35]. Loss of functionality occurs in a hierarchical manner throughout the process of exhaustion. It has been noted that the greater the antigen load or the duration of the disease, the greater the extent of exhaustion. Usually, functions such as interleukin (IL)-2 production, high expansion ability and ex vivo killing are lost first; this stage is named “partial exhaustion I”. In the next stage of exhaustion, “partial exhaustion II”, these cells lose their ability to produce tumour necrosis factor (TNF)-α, and their expansion ability and antigen-induced production of interferon (IFN)-γ become impaired. The final stage of exhaustion is the deletion of these cells by apoptosis[32,36,37] (Figure 1). A detailed understanding of the mechanism underlying this process may aid in development of efficacious therapies that restore the function of these cells and - from a practical point of view - the modulation of negative co-stimulatory pathways.
As previously described, one reason why specific cytotoxic T cells become exhausted in HCC, CHB and CHC is related to the strategies developed by the pathogen/tumour itself; yet, the host contributes to the exhaustion process as well, due to the particular liver features that are described below.
Bowen et al[38] elegantly showed that activation of primary CD8+ T cells within the lymph nodes leads to an efficient response, whereas activation of primary CD8+ T cells within the liver commits T cells to the development of an immunotolerant state. This divergent response is related to the liver’s intense tolerogenic properties, which are in line with this organ’s role in dealing with a massive load of foreign antigens from the gastrointestinal tract. For this reason, in order to develop new immunotherapeutic approaches to treat viral hepatitis and HCC it is first necessary to understand how intrahepatic immunity is regulated. An important feature to consider is that liver can support primary T cell activation independently of secondary lymphoid tissues and involvement of dendritic cells (DCs). Moreover, the ligands expressed by resident liver cells could favour exhaustion of specific liver-infiltrating T cells after antigen recognition. These two conditions could definitely impair the quality of T cell response[39,40].
Several liver cell types (listed below) can work as antigen-presenting cells (APCs) to activate naïve CD8+ T cells.
Hepatocytes represent about two-thirds of the total cell population in the liver. Antigen presentation by hepatocytes is the most relevant mechanism of infection with hepatotropic viruses. Naïve CD8+ T cells can directly interact with hepatocytes via liver sinusoidal endothelial cell (LSEC) fenestrations[41]. Although hepatocytes have been demonstrated as capable of promoting rapid activation and proliferation of CD8+ T cells in vivo[39,40], they do not express positive co-stimulatory molecules, such as CD80 and CD86; therefore, because of this they could fail to induce functional CD8+ T cells in in vivo conditions[42,43]. Besides, one of the ligands of the negative co-stimulatory molecule PD-1 (PD-L1) can be expressed by hepatocytes[44], and its interaction with PD-1 on the hepatocyte-activated CD8+ T cell contributes to its functional suppression[45].
Kupffer cells (KCs) are the resident macrophages in the liver and represent the largest population of resident tissue macrophages in the entire body[46]. KCs are localized mainly in the periportal area, where they serve to clear endotoxins and phagocytose debris and microorganisms. These cells can also pass through the space of Dissé, coming into contact with hepatocytes and phagocytosing any with apoptotic features[47,48]. KCs express Fas-ligand[49] and PD-L1[50], leading to apoptosis and functional exhaustion of CD8+ T cells respectively. In addition, the KCs can secrete immunosuppressive cytokines, such as IL-10 and tumour growth factor (TGF)-β, both of which can contribute to T cell exhaustion[51].
LSECs can express MHC and co-stimulatory molecules and are capable of presenting antigen to CD8+ T cells by at least two pathways, thereby promoting tolerance. Firstly, these cells express PD-L1 even at low antigen concentration[52] and, secondly, they can secrete IL-10 and TGF-β[53], which could impair CD8+ T cell activation, as previously commented on.
Hepatic stellate cells (HSCs), located at the space of Dissé, represent the major cell type involved in liver fibrosis, but they are also involved in antigen presentation[54]. TGF-β secreted by the HSCs contributes both to liver fibrosis and to the exhaustion of CD8+ T cells[55].
Resident hepatic DCs are predominantly immature cells, prone to capturing and processing of antigens[56]. Because IL-10 and TGF-β are secreted by KCs and LSECs, the uninfected liver provides a unique cytokine environment that may render a tolerogenic state for the resident DCs[56-58]. Moreover, resting DCs can induce peripheral CD8+ T cell tolerance through up-regulation of PD-1 and CTLA-4[59].
Consequently, CD8+ T cells that are activated by these liver APCs are not optimally primed and fail to exert effector functions, thus promoting tolerance and T cell exhaustion. This situation can represent a survival advantage for hepatotropic viruses and HCC, since the specific cytotoxic T cells that are capable of recognising viral and tumoural antigens can become exhausted easily, due to the tolerogenic liver status. Such an environment features high-level expression of negative co-stimulatory ligands on the resident liver cells as well as induction of negative co-stimulatory receptors on the specific T cells, as related to the liver cytokine milieu.
Following our above introduction of the concept of T cell exhaustion in HCC, CHB and CHC, as well as of the mechanisms involved in this process, we will next highlight the current evidence showing why specific cytotoxic T cell response restoration could impact HCC, HCV and HBV treatment.
Worldwide rates of liver cancer classify it as the fifth most common cancer in men and the seventh in women. Infection with HBV and HCV, chronic alcoholism and fatty liver disease, among others, are major risk factors for HCC[60]. Once diagnosed, HCC usually has a poor prognosis, due to lack of efficacy of the available treatments. Therefore, novel effective therapies are urgently needed to treat patients with this type of tumour, particularly for those in advanced stages for whom the most efficacious of the current treatments are still only suboptimal.
Immune evasion is a general strategy of cancers, but much is still unknown about it. Most of the research on this phenomenon has focused on devising ways to directly destroy the tumoural cell, while the role that immune system restoration may play in resisting or eradicating the tumour formation and its progression has been largely, if not completely, overlooked[61]. Adaptive immune response, especially the cytotoxic response, is known to play a crucial role in the control of solid tumours[8]. Several lines of evidence have been reported that support the importance of CD8+ T cells during HCC. Firstly, the presence of a high number of tumour-infiltrating T cells in HCC tissue suggests a role in HCC pathogenesis[9]. Secondly, the quantity of tumour-infiltrating T cells is considered a good prognosis marker of HCC[10]. Finally, adoptive immunotherapy could protect against HCC, diminishing the recurrence risk after surgical treatment[11].
Mizukoshi et al[62] analysed immune responses against various HCC epitopes in peripheral blood mononuclear cells from patients with HCC. After radiofrequency ablation (RFA), the authors noted an improvement of these responses in two-thirds of the patients; interestingly, those patients with a detectable response also experienced longer survival[62]. Flecken et al[12] recently described some tumour-associated antigen (TAA)-specific CD8+ T cell responses in HCC. In that elegant study, the authors applied overlapping peptides to a large cohort of HCC patients and showed that a variety of TAAs can induce CD8+ responses against α-fetoprotein (AFP), glypican-3 (GPC-3), melanoma-associated antigen-1 (MAGE-1) and New York-oesophageal squamous cell carcinoma-1 (NY-ESO-1). The authors also showed a positive correlation between either the quantity of TAA-specific CD8+ T cells or the number of TAA targets and the survival of these patients. Finally, they also demonstrated that TAA-specific CD8+ T cells were able to proliferate, but not able to produce IFN-γ after antigen encounter[12]. Therefore, HCC features CD8+ T cells that are able to recognise tumoural neo-antigens; although, these cells display an exhausted behaviour. Interestingly, PD-1 was found to be up-regulated in these cells, a feature which could represent a base for immunotherapy by blocking this negative co-stimulatory molecule. Consequently, one possible approach for HCC treatment could be to restore the effector capabilities of these cells and one option towards achieving this end could be the modulation of negative co-stimulatory pathways, such as PD-1, as will be discussed below.
HCV was first cloned in 1989 as a non-A non-B hepatitis virus[63]. Since then, substantial progress has been made in our understanding of both the virus and its interactions with the host system. The final result of this intense research effort has been the generation of DAAs that show curative effect on HCV infection in approximately 95% of the CHC patients[64-66].
In the last two decades, we have learnt several important lessons about the strategies that the HCV employs to avoid the immune system in order to persist in the host. HCV-specific CD8+ T cells play an essential role in controlling HCV during acute infection[1-3], based upon their abilities to both recognize and destroy the infected cell through cytolytic and non-cytolitic mechanisms[4,67]. However, in approximately 70% of primo-infections, the virus is able to persist in the host, leading to chronic infection[68,69]. Viral escape mutations are the first mechanism used by HCV to avoid immune control, exploiting the lack of a proofreading function by the viral polymerase[70]. The second mechanism involves overwhelming the immune response. Because of the persistent antigenemia that accompanies HCV infection, the HCV-specific CD8+ T cell response becomes exhausted and fails to control infection, featuring loss of effector capacities and overexpression of negative-regulation pathways[13,14].
Thus, although DAAs are very effective treatments, continued research in HCV immunotherapy is still necessary because of the existence of DAA non-responders and to develop it as a strategy to boost other anti-viral treatments (both established and new) and to support development of a therapeutic vaccine. Moreover, since these HCV-specific CD8+ T cells up-regulate negative co-stimulatory molecules, blocking the interaction of these receptors with their ligands could be considered as a potential therapeutic strategy.
HBV is a hepatotropic non-cytopathic DNA virus and member of the family Hepadnaviridae[71]. Approximately 2 billion people worldwide have been infected by HBV, and it is estimated that more than 350 million of these individuals are persistent carriers of the virus. Most HBV infections occur via vertical transmission. Around 5%-10% of patients infected during adulthood develop CHB, with 10%-30% of those patients progressing to liver cirrhosis and/or HCC. Ultimately, however, 1-2 million HBV-related deaths are reported annually[72].
The primary treatment of CHB is based on two kinds of drugs currently: pegylated IFN-α and nucleoside/nucleotide analogues[73]. Nevertheless, complete HBV eradication, with clearance of the covalently closed circular DNA, is rarely achieved[18], making it necessary to develop new effective therapies for this major public health problem. The potential benefit of immunotherapy in HBV is highlighted by several important aspects of the infection itself. Firstly, in subjects who spontaneously clear the HBV infection, viral control is determined by the development of a strong, polyclonal and multi-specific CD8+ T cell response[5-7]; this usually happens during adulthood, when nearly 90% of the infected subjects are able to control the virus and in contrast to individuals (children) who obtain the virus through vertical transmission and in who the HBV persists due to immunotolerance induction[74]. Secondly, CHB resolution occurs in some bone marrow transplantation recipients who received tissue from a donor with natural immunity to HBV[75]. Finally, those individuals with natural immunity to HBV maintain immunological memory through HBV-specific CD8+ T cells that can last decades after the primo-infection that is capable of controlling virus at trace amounts[76]. Nevertheless, in chronically infected patients, the HBV-specific CD8+ T cell response is weak (barely detectable) and exhausted in both the peripheral blood and the liver[15,16], and this feature is accompanied by up-regulated expression of negative co-stimulatory molecules, as will be discussed in the following paragraphs. Thus, taking into account these facts, immunotherapy based on modulation of the co-stimulatory pathway could be a promising approach to improve HBV chronic infection treatment.
The cytotoxic T cell response is essential to eradication of tumoural and virus-infected cells. In patients with chronic viral hepatitis and HCC this response is impaired and, theoretically, its restoration could help in disease control. As previously commented, one of the strategies used by tumoural and virus-infected cells to induce exhaustion of the CD8+ T cells is up-regulation of negative co-stimulatory molecules. Therefore, therapeutic blockade of various inhibitory receptors, a process also referred to as “checkpoint blockade”, has begun to provide very promising results in the treatment of different diseases; these will be summarised hereafter.
The first proof of concept of the efficacy of this kind of treatment was reported for “ipilimumab”, a human monoclonal antibody against the negative co-stimulatory molecule CTLA-4 that was approved by the United States’ Food and Drug Administration in 2011; this drug is currently in clinical use for treating metastatic melanoma[77]. Since its introduction, this antibody (and others) against different co-stimulatory molecules has entered testing for other malignancies and various viral infections. There are several completed, on-going and planned clinical trials for investigating treatment of chronic hepatitis and HCC with single-agent inhibitors, as well as with combinations of inhibitors targeting multiple checkpoints or adding other therapies to this blockade. In the next lines, we will review the mechanism of action of these immune checkpoints, and the effect of blockade as determined in pre-clinical and clinical studies.
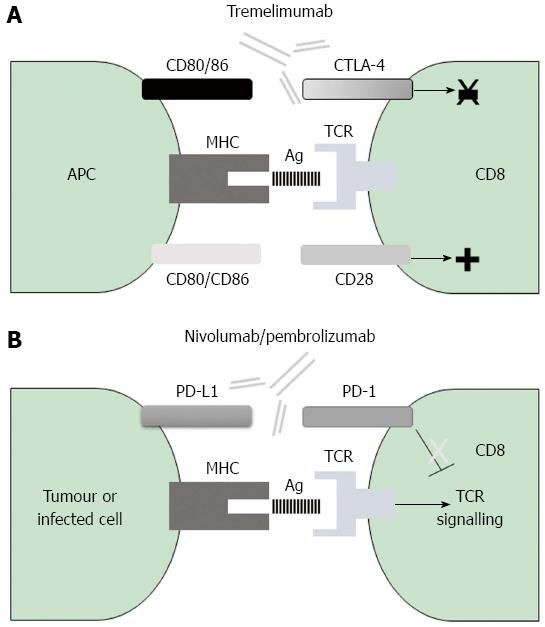
Mechanism of action of CTLA-4: Since its discovery in 1987[78], research has determined that CTLA-4 is expressed only on T cells, where it regulates early immune activation. This negative co-stimulatory molecule counteracts the activity of the positive co-stimulatory molecule CD28[79-82]. After antigen encounter, the CD28 co-signal triggers the T cell receptor (TCR) signal that activates T cells[83]. CTLA-4 and CD28 share the same APC-expressed ligands, namely CD80 and CD86. CTLA-4 displays at least two different ways by which it can inhibit T cell activation; in the first, it inhibits positive signalling of CD28 according to the feature that CTLA-4 has more affinity for CD80 and CD86 than the positive co-stimulatory molecule CD28[84] and in the second, CTLA-4 directly inhibits TCR signalling[85,86] (Figure 2).
The central function of CTLA-4 is regulation of the access of CD28 to its shared ligands in order to protect against autoimmunity and to switch-off a normal immune response after antigen control has been achieved. The vital importance of CTLA-4 was demonstrated in two different studies that were based on Ctla-4 knock-out mice. The CTLA-4-deficient mice present a profound immune dysregulation and autoimmune disease that leads to massive lymphoproliferation and fatal multi-organic tissue destruction[87,88]. Nevertheless, the inadequate induction of CTLA-4 under viral infection and tumour conditions and disrupted effects on specific CD8+ T cells could favour early exhaustion of these cells and consequently allow persistence of the disease.
Thus, considering the known mechanism of action of CTLA-4, enhancement of the CD8+ T cell response by CTLA-4 blockade could represent a satisfactory approach to treating diseases that feature persistent antigenemia, such as viral hepatitis and HCC.
CTLA-4 blockade (pre-clinical): Nakamoto et al[27] discovered that CTLA-4 is overexpressed in PD-1+ intrahepatic mononuclear cells of patients with CHC (Table 1). In addition, when the authors blockaded these inhibitory receptors individually they found no restoration of intrahepatic HCV-specific CD8+ T cell response. Surprisingly, however, when they blocked both inhibitory receptors simultaneously, the effector ability of these cells was restored, indicating the existence of a synergic effect between both receptors[27]. CTLA-4 blockade alone, however, could be sufficient to restore specific cytotoxic T cell response in persistent HBV infection. Schurich et al[28] showed that CTLA-4 blockade is able to restore the expansion ability of HBV-specific CD8+ T cells in both the intrahepatic and peripheral compartments of patients with CHB. It is unfortunate, though, that to date the research in HCC has only involved in vitro investigations of the blockade of CTLA-4 in peripheral blood mononuclear cells from HCC patients and that the results have shown no restoration of the ability of IFN-γ secretion by GPC-3-specific CD8+ T cells[29]. However, a study of a mouse model carried out by Leach et al[89] showed that in vivo administration of antibodies against CTLA-4 can result in regression of certain types of tumours, specifically those that are more immunogenic. These last data are consistent with the results obtained in a recent clinical trial that will be commented on later in this review.
| NCT | Status | Disease | Agent | Result | Ref. |
| 01008358 | Completed | HCC and CHC | Tremelimumab | TTP: 6.48 m | [30] |
| 01853618 | Recruiting | HCC | Tremelimumab | TTP: 7.4 m | [111] |
| 00703469 | Completed | CHC | Nivolumab | 15% significant reduction of viral load | [31] |
| 02658019 | Not yet recruiting | HCC | Pembrolizumab | NA | [112] |
| 01658878 | Recruiting | HCC | Nivolumab | NA | [113] |
| Ipilimumab |
Mechanism of action of PD-1: PD-1 was first identified in 1992 by Ishida et al[90] as a negative co-stimulatory molecule that belongs to the CD28 immunoglobulin superfamily of transmembrane proteins[91]. PD-1 is inducibly expressed on T cells, B cells and monocytes, upon their activation[92]. The PD-1 ligands, PD-L1 and PD-L2, are members of the B7 co-stimulatory molecules family[93,94]. APCs and non-lymphoid tissues, including the liver, express PD-L1, while DCs and macrophages can up-regulate PD-L2 expression[34,44,94-97] (Table 1). Interaction of PD-1 with its ligands leads to inhibition of proliferation through a cell cycle arrest at G0/G1 and also impairs IL-2 secretion by T cells[98]. In addition, interaction between PD-1 and PD-L1 or PD-L2 promotes apoptosis and secretion of the immunosuppressive cytokine IL-10[99-101] (Figure 2).
The immunoregulatory properties of PD-1 are reflected by the Pdl-1 knockout mouse model, which presents with severe autoimmune disease[102]. Thus, PD-1 is considered to play an important role in controlling the cellular immune response and in switching-off cells after they have completed their tasks in order to avoid autoimmune disorders; yet, its early expression can induce T cell exhaustion. Several studies have reported a positive correlation between exhaustion and PD-1 up-regulation[24,103,104]. Therefore, the blockade of PD-1 and its ligands could represent an efficient therapeutic approach by which to restore an effector T cell response against HCC and viral hepatitis.
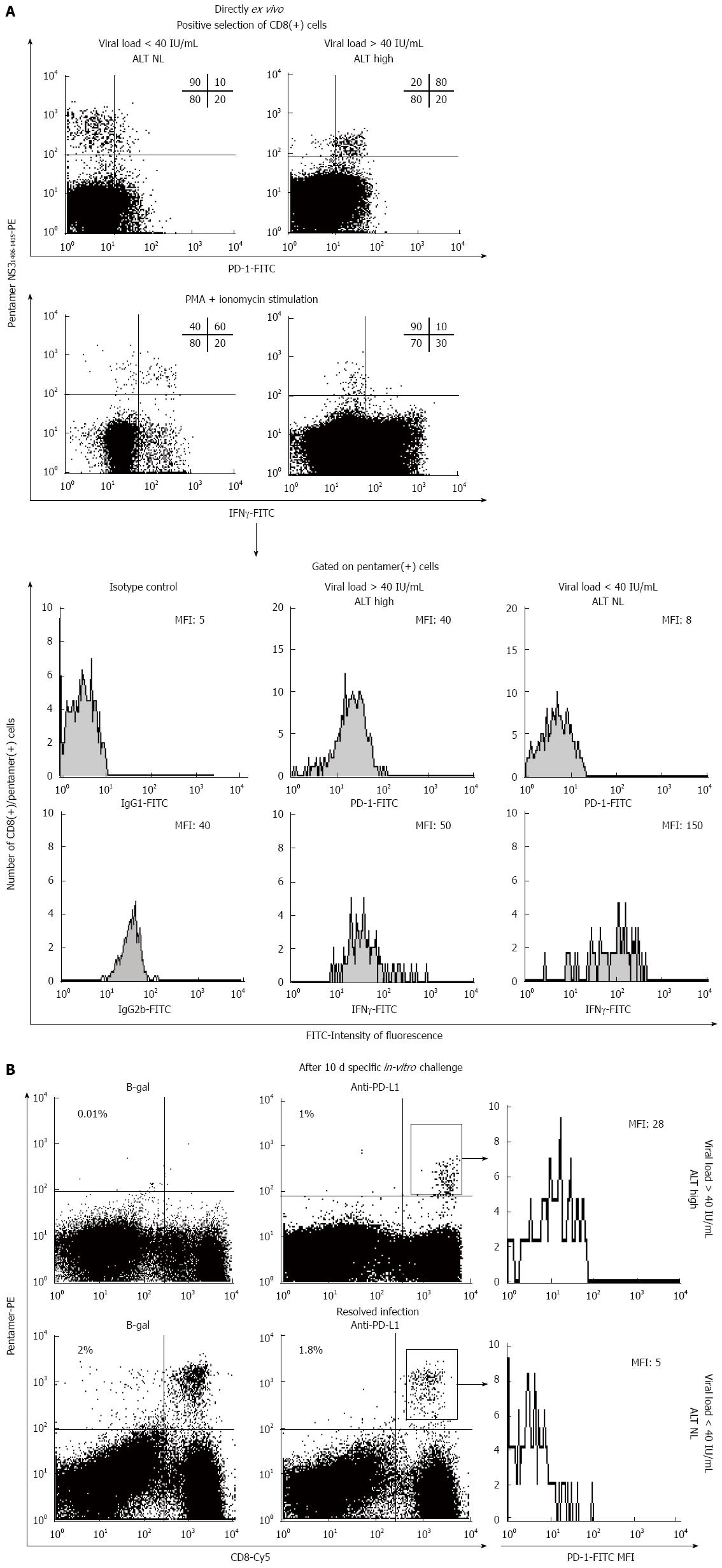
PD-1/PD-L1 blockade (pre-clinical): Several studies have been carried out to evaluate the effect of blocking the PD-1/PD-L1 pathway under conditions of viral hepatitis and HCC. Studies focusing on CHC, by Penna et al[105] and Radziewicz et al[106], along with data obtained by our own group, have shown that up-regulation of PD-1 affects HCV-specific CD8+ T cells in peripheral blood and in the intrahepatic compartment during chronic HCV infection (Table 1). Besides, blockade of the PD-1/PD-L1 interaction was shown to improve the expansion ability of and IFN-γ secretion from HCV-specific CD8+ T cells[24,105,106]. Moreover, Fuller et al[107] elegantly showed how PD-1 blockade could control HCV replication in a chimpanzee model of CHC. Interestingly, the chimpanzee with controlled infection also presented a broader base-line immunity response than the cohort animals that were non-responders, suggesting that anti-PD-1 treatment may be useful in only those cases with a critical threshold of pre-existing HCV-specific CD8+ T cells.
Figure 3 shows an example of HCV-specific CD8+ T cell restoration achieved by use of anti-PD-1 monoclonal antibody. In a study of CHB infection carried out by Peng et al[25], PD-1 up-regulation on HBV-specific CD8+ T cells was shown in blood samples of patients with chronic infection vs the controls (Table 1); moreover, blockade of the PD-1/PD-L1 pathway was shown to significantly enhance the expansion ability of and the IFN-γ production from HBV-specific CD8+ T cells after antigen encounter[25]. Fisicaro et al[108] further demonstrated that HBV-specific CD8+ T cells from the mononuclear cell population of livers infected with hepatitis virus express higher levels of PD-1 than those in the peripheral compartment; additionally, blockade of the PD-1/PD-L1 pathway was shown to improve the effector capacity of these intrahepatic cells, as evidenced by measure of their expansion ability and production of the Tc1 cytokines, such as IL-2 and IFN-γ[108]. Tzeng et al[109] studied T cell exhaustion affecting the intrahepatic infiltrating T cells, using a mouse model of persistent HBV infection; the authors discovered that PD-1 was up-regulated on the HBV-specific CD8+ T cells and that blockade of the interaction between PD-1 and PD-L1 results in restoration of the capacity of these cells to produce IFN-γ[29]. Furthermore, Gao et al[26] showed that overexpression of the PD-1 ligand, PD-L1, in HCC is associated with tumour aggressiveness, providing the rationale for developing a new therapy based on blockade of the PD-1/PD-L1 pathway (Table 1). Finally, Shi et al[110] demonstrated that the HCC-infiltrating CD8+ T cells have a drastic increase in PD-1 expression. Taken together, these data support the rationale to set-up clinical trials to analyse the usefulness of blocking the PD-1/PD-L1 pathway in HCC and HBV/HCV chronic infections.
Taking into account all the previously discussed known characteristics and features of the specific CD8+ T cell response in HCC and chronic viral hepatitis, a number of clinical trials have been designed to analyse the effect of PD-1 and CTLA-4 pathway blocking (Table 2). Here, we will describe the completed, on-going and planned clinical trials investigating checkpoint modulation as a therapeutic approach for treating HCC and chronic viral hepatitis.
Regarding CTLA-4, Sangro et al[30] performed the first pilot clinical trial to address the potential anti-tumour and anti-viral effects of a monoclonal anti-CTLA-4 antibody in CHC and HCC. For this purpose, the authors used “tremelimumab”, a fully humanized IgG2 monoclonal antibody that blocks CTLA-4. The study cohort was a small population of patients with HCC superimposed on CHC. Tremelimumab showed an acceptable safety profile, as well as both anti-tumour and anti-viral activities. The anti-tumour effect was particularly encouraging as it was characterized by a significant increase in the time to progression (TTP), up to 6.48 mo, with respect to the control group. For its anti-viral activity, the drug was shown to induce a significant decrease in viral load, with 15% of the patients achieving sustained virological response without any other anti-HCV treatment. This anti-viral efficacy may appear low, but it could represent a difference in patients who are non-responders to DAA, in who a DAA + anti-PD-1 treatment combination could improve the sustained virologic response rate. Moreover, these outcomes may be improved by use of a higher dose of the drug[30].
Other studies with the same anti-CTLA-4 molecule are currently in progress. Greten et al[111], for example, are currently recruiting patients to participate in their clinical trial to test the safety and effectiveness of tremelimumab in combination with transarterial chemoembolization or RFA in advanced liver cancer. These authors presented their preliminary results during the 2015 American Society of Clinical Oncology Annual Meeting, stating that the combination was safe, feasible and effective, increasing TTP up to 7.4 mo[18].
Similar to the results of the studies on CTLA-4, the current research data from the studies of PD-1/PD-L1 blockade are encouraging. Gardiner et al[31] described, for the first time and as proof-of-concept, their findings from an evaluation of anti-PD-1 antibody treatment in patients with CHC, in which the fully human anti-PD-1 monoclonal IgG4 antibody “nivolumab” was administered as a single dose. The treatment did not produce any significant side effects, indicating a sufficient safety, and one-third of the patients experienced significant reductions in viral load. Perhaps, if the authors had combined blockade of the PD-1 pathway with modulation of other co-stimulatory molecules, they could have achieved better results. However, the importance of this pilot clinical trial is that it highlights the significant role of the PD-1 pathway during CHC[31] and shows that it could represent an adjuvant treatment for DAA regimes.
Feun et al[112] have already designed a clinical trial to determine the anti-tumour effect of anti-PD-1 antibody treatment in patients with advanced, unresectable HCC using another humanized monoclonal IgG4 antibody that binds to PD-1, which is called “pembrolizumab”. The primary objective of this study is to assess therapeutic efficacy, but the researchers also plan to evaluate the expression of PD-L1 in tumour tissue, with the aim of gaining insights into which cases may benefit most from this type of treatment.
After realising that blocking either CTLA-4 or PD-1 pathways could be useful to treat HCC, it becomes apparent that one option to increase anti-tumoural effectiveness could be a treatment combination. A clinical trial is currently in progress to study such an alternative, namely the role of combining the fully humanized anti-CTLA-4 IgG1 antibody called “ipilimumab” in conjunction with the previously introduced anti-PD-1 “nivolumab”[113]. The first part of that study will evaluate the safety profile of nivolumab in HCC patients, after which the efficacy of nivolumab will be compared with that of sorafenib. Finally, the researchers plan to address the safety and efficacy profiles of the combination nivolumab and ipilimumab for treatment of advanced HCC[86]. In the near future, hepatologists and oncologists should know if this hopeful combination represents a bona fide treatment option for patients with advanced HCC.
Although CTLA-4 and PD-1 are the best characterized inhibitory receptors to date, there are other negative co-stimulatory molecules involved in CD8+ T cell exhaustion that deserve to be studied. Most of the work carried out on the role of these other co-stimulatory molecules has been done in the conditions of HVB and HCV infections, while information in the condition of HCC remains scarce.
LAG-3 was identified in 1990 by Triebel et al[114]. Even though its main ligand is MHC class II, it also helps PD-1 to maintain CD8+ T cell exhaustion during chronic viral infections[115]. Chen et al[116] demonstrated that LAG-3 was overexpressed on HCV-specific CD8+ T cells in patients with CHC and that LAG-3 blockade restored effector capacity of these cells, as evidenced by measure of their expansion ability and cytokine production (Table 1). Kennedy et al[117] demonstrated different expression patterns of LAG-3 on HBV-specific CD8+ T cells during the natural history of CHB, whereby LAG-3 up-regulation occurs progressively, suggesting that HBV infection induces a progressive status of T cell exhaustion over time. Li et al[118] studied LAG-3 expression in patients with HBV-related HCC and discovered an overexpression of LAG-3 on the HBV-specific CD8+ T cells from liver tissue, as compared with those in the peripheral blood. Taking these collective data into account, LAG-3 appears to be another immune checkpoint to bear in mind during consideration of diseases affected by T cell exhaustion.
The 2B4 was first identified in 1993 by Valiante et al[119]. Its ligand, CD48, has 5-10 times stronger affinity for 2B4 than CD2, a molecule necessary for T cell activation[120,121]. This competitive advantage of 2B4 for binding to CD48 could impair T cell activation if 2B4 is induced early. Bengsch et al[13] and Schlaphoff et al[122] studied 2B4 expression on HCV-specific CD8+ T cells in the blood of patients with chronic hepatitis infections and demonstrated overexpression in PD-1+-HCV-specific CD8+ T cells, which was further linked to an exhausted behaviour. Similarly, Kroy et al[123] showed an up-regulation of 2B4 in HCV-specific CD8+ intrahepatic lymphocytes, as compared with peripheral lymphocytes, during CHC. Bengsch et al[13] studied the effect of blockade of several inhibitory receptors, including 2B4, in the functionality of HBV-specific CD8+ T cells during CHB and found that the response to blockade was primarily mediated by PD-1 (Table 1). Considering all of these data, 2B4 up-regulation seems to be linked with PD-1 overexpression, and its modulation could have a less robust effect on T cell restoration than other negative co-stimulatory molecules.
T-cell immunoglobulin and mucin-domain containing (Tim)-3 was first identified in 2002 by Monney et al[124]. Tim-3 is another immune checkpoint receptor that limits the duration and magnitude of T cell responses[125-127]. McMahan et al[128] demonstrated that Tim-3 was up-regulated on HCV-specific CD8+ T cells in patients with chronic hepatitis infection, as compared to those who had been able to control the infection; in addition, remarkably, Tim-3 blockade restored the expansion ability of these cells. Wu et al[129] studied Tim-3 expression on HBV-specific CD8+ T cells during CHB and discovered that Tim-3 overexpression was related to disease progression; the authors also demonstrated that blockade of the Tim-3 pathway restored the effector capacity of the HBV-specific CD8+ T cells[130] (Table 1). Interestingly, a lack of Tim-3 has thus far not been found to be associated with autoimmune diseases[131], making Tim-3 a very attractive therapeutic target.
CD160 was discovered in 1998 by Anumanthan et al[132] and is another negative co-stimulatory molecule associated with T cell exhaustion[133]. Bengsch et al[13] and Schlaphoff et al[122] also studied CD160 expression on HCV-specific CD8+ T cells obtained from patients with chronic hepatitis infections and demonstrated an overexpression of this protein that was related with exhaustion. Nevertheless, Kroy et al[123] showed that there was no overexpression of this molecule in intrahepatic HCV-specific CD8+ T cells in CHC, as compared to the peripheral cells. Another study from Viganò et al[134] investigated the expression of CD160 on HCV-specific CD8+ T cells during CHC and found functional impairment in these cells, which was independent of PD-1 expression; moreover, the blockade of CD160/CD160L in this study restored the expansion ability of the HCV-specific CD8+ T cells[134] (Table 1). Nevertheless, due to the current contradictory data more studies are needed to gain an adequate understanding of this pathway before it can be considered as a potential therapeutic target.
Immune checkpoint modulation can resolve CD8+ T cell exhaustion in vitro and in vivo in chronic infections with hepatoviruses and in tumoural diseases. This therapy could represent a promising treatment option for patients with advanced HCC and chronic viral hepatitis who are non-responders to the current standard of care. The use of humanized monoclonal antibodies against negative co-stimulatory molecules can reverse the exhaustion state of CD8+ T cells, thereby making them able to control either the tumour or the virus. In the upcoming years, we will likely see the results of combining these agents with current HCC and anti-viral therapies. While the current results from clinical studies remain modest, they could be the base for encouraging development and implementation of novel therapeutic strategies. Clearly, we must expand our basic and clinical knowledge about how modulating and finely tuning these receptors will help to avoid adverse events, and how appropriate patient selection will help to define the groups most likely to respond to this kind of treatment. We must also learn how to best use these drugs in combination with other therapies in order to obtain the maximum clinical benefit. Fortunately, the field of immunotherapy highlights the fact that translational medicine can improve the health of our patients, bringing the results obtained by basic research to the bedside in a short period.
| 1. | Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1003] [Cited by in RCA: 1004] [Article Influence: 38.6] [Reference Citation Analysis (1)] |
| 2. | Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, Houghton M, Parham P, Walker CM. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 294] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, Rosen HR. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol. 2008;82:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Larrubia JR, Moreno-Cubero E, Lokhande MU, García-Garzón S, Lázaro A, Miquel J, Perna C, Sanz-de-Villalobos E. Adaptive immune response during hepatitis C virus infection. World J Gastroenterol. 2014;20:3418-3430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 5. | Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, Larrubia JR, Webster GJ, McMichael AJ, Ferrari C. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117:1386-1396. [PubMed] |
| 6. | Gallimore A, Glithero A, Godkin A, Tissot AC, Plückthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383-1393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 644] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 7. | Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, Brown D, Amlot PL, Williams R, Vergani D. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 312] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2996] [Article Influence: 199.7] [Reference Citation Analysis (0)] |
| 9. | Yoong KF, McNab G, Hübscher SG, Adams DH. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. J Immunol. 1998;160:3978-3988. [PubMed] |
| 10. | Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 305] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 12. | Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 303] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 13. | Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 14. | Wood NA, Linn ML, Bowen DG. Exhausted or just sleeping: awakening virus-specific responses in chronic hepatitis C virus infection. Hepatology. 2011;54:1879-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 666] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 16. | You CR, Lee SW, Jang JW, Yoon SK. Update on hepatitis B virus infection. World J Gastroenterol. 2014;20:13293-13305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 17. | El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 834] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 18. | Bitton Alaluf M, Shlomai A. New therapies for chronic hepatitis B. Liver Int. 2016;36:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 658] [Article Influence: 54.8] [Reference Citation Analysis (2)] |
| 20. | Romano E, Romero P. The therapeutic promise of disrupting the PD-1/PD-L1 immune checkpoint in cancer: unleashing the CD8 T cell mediated anti-tumor activity results in significant, unprecedented clinical efficacy in various solid tumors. J Immunother Cancer. 2015;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Choudhuri K, Kearney A, Bakker TR, van der Merwe PA. Immunology: how do T cells recognize antigen? Curr Biol. 2005;15:R382-R385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 572] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 23. | Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2385] [Article Influence: 183.5] [Reference Citation Analysis (0)] |
| 24. | Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, González-Praetorius A, Albertos S, García-Garzón S, Lokhande M, Parra-Cid T. Bim-mediated apoptosis and PD-1/PD-L1 pathway impair reactivity of PD1(+)/CD127(-) HCV-specific CD8(+) cells targeting the virus in chronic hepatitis C virus infection. Cell Immunol. 2011;269:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 666] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 27. | Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 28. | Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 29. | Xu Y, Li H, Gao RL, Adeyemo O, Itkin M, Kaplan DE. Expansion of interferon-gamma-producing multifunctional CD4+ T-cells and dysfunctional CD8+ T-cells by glypican-3 peptide library in hepatocellular carcinoma patients. Clin Immunol. 2011;139:302-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 771] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 31. | Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang KM, Sulkowski M, Marro SO, Anderson J. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8:e63818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 32. | Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1578] [Cited by in RCA: 1613] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 33. | Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 903] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 34. | Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C. World J Gastroenterol. 2009;15:5129-5140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Larrubia JR, Moreno-Cubero E, Miquel J, Sanz-de-Villalobos E. Hepatitis C virus-specific cytotoxic T cell response restoration after treatment-induced hepatitis C virus control. World J Gastroenterol. 2015;21:3480-3491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 980] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 37. | Larrubia JR, Lokhande MU, García-Garzón S, Miquel J, González-Praetorius A, Parra-Cid T, Sanz-de-Villalobos E. Persistent hepatitis C virus (HCV) infection impairs HCV-specific cytotoxic T cell reactivity through Mcl-1/Bim imbalance due to CD127 down-regulation. J Viral Hepat. 2013;20:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Bertolino P, Bowen DG, McCaughan GW, Fazekas de St Groth B. Antigen-specific primary activation of CD8+ T cells within the liver. J Immunol. 2001;166:5430-5438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Morimoto J, Tan X, Teague RM, Ohlén C, Greenberg PD. Induction of tolerance in CD8+ T cells to a transgenic autoantigen expressed in the liver does not require cross-presentation. J Immunol. 2007;178:6849-6860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology. 2006;44:1182-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 42. | Bertolino P. Impaired function of dendritic cells translocating the liver sinusoids: a veto effect contributing to intrahepatic tolerance? Eur J Immunol. 2008;38:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Holz LE, Benseler V, Bowen DG, Bouillet P, Strasser A, O’Reilly L, d’Avigdor WM, Bishop AG, McCaughan GW, Bertolino P. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology. 2008;135:989-997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, Schölmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 45. | Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1: PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007;178:2714-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 46. | Mackay IR. Hepatoimmunology: a perspective. Immunol Cell Biol. 2002;80:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Gale RP, Sparkes RS, Golde DW. Bone marrow origin of hepatic macrophages (Kupffer cells) in humans. Science. 1978;201:937-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | MacPhee PJ, Schmidt EE, Groom AC. Intermittence of blood flow in liver sinusoids, studied by high-resolution in vivo microscopy. Am J Physiol. 1995;269:G692-G698. [PubMed] |
| 49. | Sun Z, Wada T, Maemura K, Uchikura K, Hoshino S, Diehl AM, Klein AS. Hepatic allograft-derived Kupffer cells regulate T cell response in rats. Liver Transpl. 2003;9:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067-8075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 51. | Zhang M, Xu S, Han Y, Cao X. Apoptotic cells attenuate fulminant hepatitis by priming Kupffer cells to produce interleukin-10 through membrane-bound TGF-β. Hepatology. 2011;53:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Schurich A, Berg M, Stabenow D, Böttcher J, Kern M, Schild HJ, Kurts C, Schuette V, Burgdorf S, Diehl L. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J Immunol. 2010;184:4107-4114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | von Oppen N, Schurich A, Hegenbarth S, Stabenow D, Tolba R, Weiskirchen R, Geerts A, Kolanus W, Knolle P, Diehl L. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology. 2009;49:1664-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Viñas O, Bataller R, Sancho-Bru P, Ginès P, Berenguer C, Enrich C, Nicolás JM, Ercilla G, Gallart T, Vives J. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Li Y, Kim BG, Qian S, Letterio JJ, Fung JJ, Lu L, Lin F. Hepatic Stellate Cells Inhibit T Cells through Active TGF-β1 from a Cell Surface-Bound Latent TGF-β1/GARP Complex. J Immunol. 2015;195:2648-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Lau AH, Thomson AW. Dendritic cells and immune regulation in the liver. Gut. 2003;52:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | Khanna A, Morelli AE, Zhong C, Takayama T, Lu L, Thomson AW. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J Immunol. 2000;164:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur J Immunol. 1997;27:1848-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 59. | Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 421] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 60. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3126] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 61. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48711] [Article Influence: 3247.4] [Reference Citation Analysis (12)] |
| 62. | Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, Yamashita T, Fushimi K, Kaneko S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 63. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [PubMed] |
| 64. | Zhang X. Direct anti-HCV agents. Acta Pharm Sin B. 2016;6:26-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Attar BM, Van Thiel DH. Hepatitis C virus: A time for decisions. Who should be treated and when? World J Gastrointest Pharmacol Ther. 2016;7:33-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | González-Grande R, Jiménez-Pérez M, González Arjona C, Mostazo Torres J. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (4)] |
| 67. | Jo J, Aichele U, Kersting N, Klein R, Aichele P, Bisse E, Sewell AK, Blum HE, Bartenschlager R, Lohmann V. Analysis of CD8+ T-cell-mediated inhibition of hepatitis C virus replication using a novel immunological model. Gastroenterology. 2009;136:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 68. | Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (1)] |
| 69. | Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 752] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 70. | Chang KM, Rehermann B, McHutchison JG, Pasquinelli C, Southwood S, Sette A, Chisari FV. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 241] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 602] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 72. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 988] [Article Influence: 54.9] [Reference Citation Analysis (1)] |
| 73. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2411] [Article Influence: 172.2] [Reference Citation Analysis (1)] |
| 74. | Balmasova IP, Yushchuk ND, Mynbaev OA, Alla NR, Malova ES, Shi Z, Gao CL. Immunopathogenesis of chronic hepatitis B. World J Gastroenterol. 2014;20:14156-14171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 75. | Ilan Y, Nagler A, Adler R, Tur-Kaspa R, Slavin S, Shouval D. Ablation of persistent hepatitis B by bone marrow transplantation from a hepatitis B-immune donor. Gastroenterology. 1993;104:1818-1821. [PubMed] |
| 76. | Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 638] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 77. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 12025] [Article Influence: 751.6] [Reference Citation Analysis (0)] |
| 78. | Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 930] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 79. | Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065-1068. [PubMed] |
| 80. | Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2054] [Cited by in RCA: 2044] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 81. | Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 678] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 82. | Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 2015;36:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 83. | Poggi A, Bottino C, Zocchi MR, Pantaleo G, Ciccone E, Mingari C, Moretta L, Moretta A. CD3+ WT31- peripheral T lymphocytes lack T44 (CD28), a surface molecule involved in activation of T cells bearing the alpha/beta heterodimer. Eur J Immunol. 1987;17:1065-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793-801. [PubMed] |
| 85. | Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 487] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 86. | Xiao Y, Qiao G, Tang J, Tang R, Guo H, Warwar S, Langdon WY, Tao L, Zhang J. Protein Tyrosine Phosphatase SHP-1 Modulates T Cell Responses by Controlling Cbl-b Degradation. J Immunol. 2015;195:4218-4227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541-547. [PubMed] |
| 88. | Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985-988. [PubMed] |
| 89. | Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734-1736. [PubMed] |
| 90. | Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895. [PubMed] |
| 91. | Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765-772. [PubMed] |
| 92. | Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779-782. [PubMed] |
| 93. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [PubMed] |
| 94. | Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2292] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 95. | Grakoui A, John Wherry E, Hanson HL, Walker C, Ahmed R. Turning on the off switch: regulation of anti-viral T cell responses in the liver by the PD-1/PD-L1 pathway. J Hepatol. 2006;45:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 419] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 97. | Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 520] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 98. | Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 633] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 99. | Selenko-Gebauer N, Majdic O, Szekeres A, Höfler G, Guthann E, Korthäuer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637-3644. [PubMed] |
| 100. | Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 101. | Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 102. | Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327-336. [PubMed] |
| 103. | Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2815] [Cited by in RCA: 3239] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 104. | Barathan M, Gopal K, Mohamed R, Ellegård R, Saeidi A, Vadivelu J, Ansari AW, Rothan HA, Ravishankar Ram M, Zandi K. Chronic hepatitis C virus infection triggers spontaneous differential expression of biosignatures associated with T cell exhaustion and apoptosis signaling in peripheral blood mononucleocytes. Apoptosis. 2015;20:466-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 105. | Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 106. | Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 107. | Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, Sharpe AH, Dustin LB, Rice CM, Grakoui A. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1). Proc Natl Acad Sci USA. 2013;110:15001-15006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 108. | Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, Cavallo MC, Silini EM, Andreone P, Missale G. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682-693, 693.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 402] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 109. | Tzeng HT, Tsai HF, Liao HJ, Lin YJ, Chen L, Chen PJ, Hsu PN. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS One. 2012;7:e39179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 110. | Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 111. | ClinicalTrials ; National Cancer Institute (NCI). Tremelimumab With Chemoembolization or Ablation for Liver Cancer. gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2013; Available from: https://clinicaltrials.gov/ct2/show/NCT01853618. |
| 112. | ClinicalTrials ; University of Miami. Pembrolizumab (Keytruda) in Advanced Hepatocellular Carcinoma. gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2016; Available from: https://clinicaltrials.gov/ct2/show/study/NCT02658019#contacts. |
| 113. | Squibb BM. A Study to Evaluate the Effectiveness, Safety and Tolerability of Nivolumab and the Combination Nivolumab Plus Ipilimumab in Patients With Advanced Liver Cancer. gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2012; [cited 2016 May 18] Available from: https://clinicaltrials.gov/ct2/show/NCT01658878. |
| 114. | Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 703] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 115. | Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1703] [Cited by in RCA: 1679] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 116. | Chen N, Liu Y, Guo Y, Chen Y, Liu X, Liu M. Lymphocyte activation gene 3 negatively regulates the function of intrahepatic hepatitis C virus-specific CD8+ T cells. J Gastroenterol Hepatol. 2015;30:1788-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Kennedy PT, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan AT, Naik S, Foster GR, Bertoletti A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology. 2012;143:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 118. | Li FJ, Zhang Y, Jin GX, Yao L, Wu DQ. Expression of LAG-3 is coincident with the impaired effector function of HBV-specific CD8(+) T cell in HCC patients. Immunol Lett. 2013;150:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 119. | Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178:1397-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 120. | Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med. 1998;188:2083-2090. [PubMed] |
| 121. | Latchman Y, McKay PF, Reiser H. Identification of the 2B4 molecule as a counter-receptor for CD48. J Immunol. 1998;161:5809-5812. [PubMed] |
| 122. | Schlaphoff V, Lunemann S, Suneetha PV, Jaroszewicz J, Grabowski J, Dietz J, Helfritz F, Bektas H, Sarrazin C, Manns MP. Dual function of the NK cell receptor 2B4 (CD244) in the regulation of HCV-specific CD8+ T cells. PLoS Pathog. 2011;7:e1002045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 123. | Kroy DC, Ciuffreda D, Cooperrider JH, Tomlinson M, Hauck GD, Aneja J, Berger C, Wolski D, Carrington M, Wherry EJ. Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors. Gastroenterology. 2014;146:550-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 124. | Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1330] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 125. | Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1637] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 126. | Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187-2194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1692] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 127. | Liu Y, Gao LF, Liang XH, Ma CH. Role of Tim-3 in hepatitis B virus infection: An overview. World J Gastroenterol. 2016;22:2294-2303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 128. | McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546-4557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 129. | Wu W, Shi Y, Li J, Chen F, Chen Z, Zheng M. Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection. Virol J. 2011;8:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 130. | Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, Chen Z. Blockade of Tim-3 signaling restores the virus-specific CD8⁺ T-cell response in patients with chronic hepatitis B. Eur J Immunol. 2012;42:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 131. | Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 467] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 132. | Anumanthan A, Bensussan A, Boumsell L, Christ AD, Blumberg RS, Voss SD, Patel AT, Robertson MJ, Nadler LM, Freeman GJ. Cloning of BY55, a novel Ig superfamily member expressed on NK cells, CTL, and intestinal intraepithelial lymphocytes. J Immunol. 1998;161:2780-2790. [PubMed] |
| 133. | Kared H, Saeed S, Klein MB, Shoukry NH. CD127 expression, exhaustion status and antigen specific proliferation predict sustained virologic response to IFN in HCV/HIV co-infected individuals. PLoS One. 2014;9:e101441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 134. | Viganò S, Banga R, Bellanger F, Pellaton C, Farina A, Comte D, Harari A, Perreau M. CD160-associated CD8 T-cell functional impairment is independent of PD-1 expression. PLoS Pathog. 2014;10:e1004380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 135. | Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, Li XF, Zheng L. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 136. | Wenjin Z, Chuanhui P, Yunle W, Lateef SA, Shusen Z. Longitudinal fluctuations in PD1 and PD-L1 expression in association with changes in anti-viral immune response in chronic hepatitis B. BMC Gastroenterol. 2012;12:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Spain
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Abdel-Razik A, Lleo A, Otsuka M S- Editor: Ma YJ L- Editor: A E- Editor: Ma S