Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.6016
Peer-review started: April 5, 2016
First decision: May 12, 2016
Revised: May 19, 2016
Accepted: June 13, 2016
Article in press: June 13, 2016
Published online: July 14, 2016
Processing time: 92 Days and 22.9 Hours
AIM: To investigate in vitro the therapeutic effect and mechanisms of silybin in a cellular model of hepatic steatosis.
METHODS: Rat hepatoma FaO cells were loaded with lipids by exposure to 0.75 mmol/L oleate/palmitate for 3 h to mimic liver steatosis. Then, the steatotic cells were incubated for 24 h with different concentrations (25 to 100 μmol/L) of silybin as phytosome complex with vitamin E. The effects of silybin on lipid accumulation and metabolism, and on indices of oxidative stress were evaluated by absorption and fluorescence microscopy, quantitative real-time PCR, Western blot, spectrophotometric and fluorimetric assays.
RESULTS: Lipid-loading resulted in intracellular triglyceride (TG) accumulation inside lipid droplets, whose number and size increased. TG accumulation was mediated by increased levels of peroxisome proliferator-activated receptors (PPARs) and sterol regulatory element-binding protein-1c (SREBP-1c). The lipid imbalance was associated with higher production of reactive oxygen species (ROS) resulting in increased lipid peroxidation, stimulation of catalase activity and activation of nuclear factor kappa-B (NF-κB). Incubation of steatotic cells with silybin 50 μmol/L significantly reduced TG accumulation likely by promoting lipid catabolism and by inhibiting lipogenic pathways, as suggested by the changes in carnitine palmitoyltransferase 1 (CPT-1), PPAR and SREBP-1c levels. The reduction in fat accumulation exerted by silybin in the steatotic cells was associated with the improvement of the oxidative imbalance caused by lipid excess as demonstrated by the reduction in ROS content, lipid peroxidation, catalase activity and NF-κB activation.
CONCLUSION: We demonstrated the direct anti-steatotic and anti-oxidant effects of silybin in steatotic cells, thus elucidating at a cellular level the encouraging results demonstrated in clinical and animal studies.
Core tip: FaO hepatic cells loaded with lipids by exposure to oleate/palmitate mixture represent a widely accepted cellular model of hepatic steatosis. FaO steatotic cells were used to investigate in vitro the possible direct effects of silybin as phytosome complex with vitamin E on lipid accumulation and metabolism and on oxidative stress. We demonstrated the ability of silybin in reducing fat accumulation and improving the oxidative imbalance caused by lipid excess. The results may elucidate at a cellular level the encouraging results demonstrated for silybin in previous clinical and animal studies.
- Citation: Vecchione G, Grasselli E, Voci A, Baldini F, Grattagliano I, Wang DQ, Portincasa P, Vergani L. Silybin counteracts lipid excess and oxidative stress in cultured steatotic hepatic cells. World J Gastroenterol 2016; 22(26): 6016-6026
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/6016.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.6016
Non-alcoholic fatty liver disease (NAFLD) is characterized by excess fat accumulation, mainly in the form of triglycerides (TGs), in hepatocytes of subjects who do not consume excess alcohol. NAFLD definition encompasses a large spectrum of liver abnormalities which range from the simple steatosis, to nonalcoholic steatohepatitis (NASH), till to cirrhosis and hepatocellular carcinoma[1,2]. NAFLD is the most common cause of abnormal liver function tests in Western countries, and evidences substantiate a strong association between NAFLD and metabolic abnormalities such as obesity, insulin resistance, and metabolic syndrome[3].
In the hepatocyte, TGs[4] are synthesized from fatty acids (FAs) deriving from three major sources: plasmatic non-esterified fatty acids (NEFAs) from adipose tissue, de novo lipogenesis, and dietary FAs[5]. Uptake of FAs by hepatocytes is related to their serum concentrations and is mediated by different classes of fatty acid transport proteins[6]. In the liver, FAs follow three different destinations: (1) oxidation, mainly in mitochondria, but also in extra-mitochondrial organelles such as peroxisomes; (2) assembly and export as very low-density lipoproteins (VLDL); and (3) storing as TGs within lipid droplets (LDs).
Storing of excess TGs in LDs is a protective mechanism against FA-induced toxicity that is mainly related to FA oxidation. Most FAs are metabolized through β-oxidation in mitochondria which is primarily regulated by carnitine palmitoyltransferase 1 (CPT-1) required for transport of long-chain fatty acids into mitochondria[7]. Over-active FA oxidation leads to over-production of reactive oxygen species (ROS) with consequent oxidative stress[8,9]. Excess ROS as well as pro-inflammatory cytokines can activate inflammatory signaling such as that sustained by the transcription factor nuclear factor kappa-B (NF-κB) which is implicated in the response to oxidative stress[10].
In light of these considerations, a reduction in liver steatosis through a stimulation of lipolytic pathways may potentially expose hepatocytes to the damaging effect of excess free FAs[11] and this point has to be considered when anti-steatotic molecules are tested for therapeutic applications.
Medicinal plants have become popular as the source of dietary supplements[12]. Flavonoids, a large class of polyphenolic products, are well-known antioxidants and free radical scavengers[13]. Silymarin, the standardized extract from milk thistle (Sylibum marianum), and its major active compound silybin, have been used for a long time as a hepatoprotective agents for the treatment of acute and chronic liver diseases[14]. Previous clinical findings evidenced the efficacy of silybin on insulin resistance and liver injury in patients with NAFLD[15]. Moreover, in a recent study on sixty four patients with NASH, silymarin helped to lower the hepatic enzymes, particularly ALT[16]. The improvement of liver histology after silybin treatment was recently reported in a multicenter randomized controlled trial[17]. However, the molecular mechanisms associated with the hepatoprotective activity of silybin remain to be elucidated.
The hepatic lipid metabolism is governed by two main families of transcription factors, the peroxisome proliferator-activated receptors (PPARs) that regulate both lipogenic and lipolytic pathways[18], and the sterol regulatory element-binding proteins (SREBPs) that stimulate sterol and fatty acid biosynthesis[19,20]. FAs are endogenous ligands of all PPAR isofoms[6]; uptake of FAs into hepatocytes and their oxidation is regulated mainly by PPARα, while their esterification and conversion to TGs by PPARγ and SREBP-1, whose expression typically increases in NAFLD[21]. Moreover, macroautophagy, a process that leads to the degradation of cellular constituents through lysosomes[22], participates in lipid metabolism through the breakdown of LDs (lipophagy). A decreased autophagic function may promote the development of hepatic steatosis and the progression of steatosis to NASH[23]. The autophagy-related protein 7 (Atg7) is considered essential for this process in mammalian cells[23].
This study aimed to clarify whether silybin as phytosome complex with vitamin E may favorably affect lipid and radical homeostasis using an in vitro model of NAFLD induced by the exposure of hepatoma FaO cells to exogenous FAs. Changes in expression of PPARs and SREBP-1c, the main regulators of lipid metabolism, as well as of CPT1, the master controller of mitochondrial FA oxidation, and Atg7, a key autophagy-promoting gene, have been assessed to investigate the pathways possibly activated by silybin. In parallel, the stimulation of defense systems against oxidative stress, such as catalase and NF-κB, were evaluated to assess the potential protective effects of silybin.
All chemicals, unless otherwise indicated, were of analytical grade and were supplied by Sigma-Aldrich Corp. (Milan, Italy).
Rat hepatoma FaO cells (European Collection of Cell Cultures, Sigma-Aldrich Corp., Milan, Italy) are a liver cell line maintaining hepatocyte specific markers[24]. Cells were grown in Coon’s modified Ham’s F12 with 10% foetal bovine serum (FBS). For treatments, cells were grown until 80% confluence, then incubated overnight in high-glucose medium with 0.25% bovine serum albumin (BSA). Steatosis was induced by exposing cells for 3 h to an oleate/palmitate mixture (2:1 molar ratio, final concentration 0.75 mmol/L). Thereafter, steatotic cells were incubated for 24 h with 0 to 100 μmol/L (final concentration) of silybin (S) as phytosome complex with vitamin E (Realsil®, Istituto Biochimico Italiano, Lorenzini S.p.a, Italy). Silybin stock (10 mmol/L) was prepared in dimethyl sulfoxide (DMSO) and then diluted with the culture medium to a final concentration 0.3 mmol/L.
The viability of FaO cells upon exposure to FAs or silybin, both as single agents or combined, was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and measured spectrophotometrically[25].
For determination of intracellular TG content, at the end of each treatment cells were scraped and centrifuged at 14000 ×g for 3 min. After cell lysis, obtained by passing cell suspension through 25 gauge needle, lipids were extracted using the chloroform/methanol (2:1) method[26] and TG content was measured by spectrophotometric analysis (“Triglycerides liquid” kit, Sentinel, Milan, Italy). Values were normalized to protein content as determined by the bicinchoninic acid (BCA) method using BSA as a standard[27]. In parallel, TG content was determined in the culture medium. Data are expressed as percent TG content relative to controls.
Cells were grown on collagen-coated glass slides (Falcon, BD, Milan, Italy); neutral lipids were visualized using the selective Oil-RedO (ORO) dye. Briefly, after fixing in 4% paraformaldehyde, cells were washed with PBS, stained with ORO 1% in triethyl phosphate 60% for 20 min and washed[28]. Slides were examined by Leica DMRB light microscope equipped with a Leica CCD camera DFC420C (Leica, Wetzlar, Germany). In parallel, the LD abundance inside the cells was assessed fluorimetrically using Nile Red (NR), a vital lipophilic dye. At the end of each treatment, cells were incubated with 0.3 μmol/L NR solution (from a stock solution of 100 μg/mL in PBS) for 30 min at 37 °C. After washing with PBS the NR fluorescence was measured by LS-50B spectrofluorimeter (Perkin Elmer, United States) at λex = 580 nm and λem = 630 nm and slit width set to 5.0[29]. All measurements were performed at 25 °C using a water-thermostated cuvette holder.
The oxidation of the cell-permeant 2’-7’ dichlorofluorescin diacetate (DCF-DA, Fluka, Germany) to 2’-7’ dichlorofluorescein (DCF) is extensively used for quantifying in situ the production of H2O2 and other ROS[30]. Stock solution of DCF-DA (10 mmol/L in DMSO) was prepared and stored at -20 °C in the dark. At the end of each treatment, cells were scraped and gently spun down (600 ×g for 10 min at 4 °C). After washing, cells were loaded with 10 μmol/L DCF-DA in PBS for 30 min at 37 °C in the dark, centrifuged and suspended in PBS. The DCF fluorescence was measured fluorometrically at λex = 495 nm and λem = 525 nm using. All measurements were performed at 25 °C using a water-thermostated cuvette holder.
In parallel, H2O2 production was assessed directly on cells grown on collagen-coated glass slides. After treatment, cells were incubated with 100 μmol/L DCF-DA for 1 h[31]. Slides were examined by Leica DMRB light microscope equipped with a Leica CCD camera DFC420C. Lipid peroxidation was determined spectrophotometrically through the thiobarbituric acid reactive substances (TBARS) assay which is based on the reaction of malondialdehyde (MDA; 1,1,3,3-tetramethoxypropane) with thiobarbituric acid (TBA)[32]. Briefly, 1 vol. of cell suspension was incubated for 45 min at 95 °C with 2 vol. of TBA solution (0.375% TBA, 15% trichloroacetic acid, 0.25 mol/L HCl. Then, 1 vol. of N-butanol was added and the organic phase was read using a Varian Cary50 spectrophotometer at 532 nm. Results were expressed as pmol MDA/mL per milligram protein.
Catalase (CAT, EC 1.11.16) activity was evaluated in both 12000 ×g supernatant and pellet of cell lysates following the consumption of H2O2 at 25 °C according to[33]. Enzyme activity (as the sum of both pellet and supernatant) was expressed as μmoles of decomposed H2O2 per min/mg protein. Protein content was determined by BCA method. All measurements were performed at 25 °C using a water-thermostated cuvette holder.
RNA was isolated using the Trizol reagent, cDNA was synthesized and quantitative real-time PCR (qPCR) was performed in quadruplicate using 1 × IQTM SybrGreen SuperMix and Chromo4TM System apparatus (Biorad, Milan, Italy)[34]. The relative quantity of target mRNA was calculated by the comparative Cq method using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as housekeeping gene, and expressed as fold induction with respect to controls[35]. Primer pairs were designed ad hoc starting from the coding sequences of Rattus norvegicus available on the GenBank database (http://www.ncbi.nlm.nih.gov/Genbank/GenbankSearch.html) and synthesized by TibMolBiol custom oligosynthesis service (Genova, Italy). Primers are listed in Table 1.
| Primer name | Primer sequence (5’→3’) | Annealing temperature (°C) | Product lenght (bp) | Accession ID |
| GAPDH-F | GACCCCTTCATTGACCTCAAC | 60 | 136 | DQ403053 |
| GAPDH- R | CGCTCCTGGAAGATGGTGATGGG | |||
| PPARα-F | CCCCACTTGAAGCAGATGACC | 60 | 139 | NM_013196 |
| PPARα-R | CCCTAAGTACTGGTAGTCCGC | |||
| PPARδ-F | AATGCCTACCTGAAAAACTTCAAC | 60 | 96 | AJ306400 .1 |
| PPARδ-R | TGCCTGCCACAGCGTCTCAAT | |||
| PPARγ-F | CGGAGTCCTCCCAGCTGTTCGCC | 60 | 116 | Y12882 |
| PPARγ-R | GGCTCATATCTGTCTCCGTCTTC | |||
| CPT1-F | CCGCTCATGGTCAACAGCA | 60 | 105 | NM_031559 |
| CPT1-R | CAGCAGTATGGCGTGGATGG | |||
| Atg7-F | CCTCAGCGGATGTATGGACC | 60 | 160 | NM_001012097.1 |
| Atg7-R | AGCCACATTACACCCCAAGG |
Both cellular and nuclear homogenates were processed by Western blot analysis to assess the protein levels of SREBP1-c and NF-κB/p65, respectively. In fact, activation of the NF-κB transcription factor is associated with its phosphorylation and nuclear translocation of the p65 component of the complex. Cells were lysed on ice in lysis buffer (NaCl 150 mmol/L, Tris HCl pH 7.4, 50 mmol/L, SDS 0.33%) as described elsewhere[34]. For nuclear extraction, the cellular pellet was suspended in 400 μL ice-cold Buffer A (20 mmol/L Tris HCl pH 7.8, 50 mmol/L KCl, 10 μg/mL Leupeptin, 0.1 mmol/L Dithiothreitol-DTT, 1 mmol/L phenylmethanesulfonyl fluoride-PMSF); then 400 μL Buffer B (Buffer A plus 1.2% Nonindet P40) was added. The suspension was vortex-mixed for 10 sec; after centrifugation (14000 ×g for 30 s, 4 °C) the supernatant was discarded and the nuclear pellet was washed with 400 μL Buffer A and centrifuged. Next, the nuclear pellet was suspended in 100 μL Buffer B, mixed thoroughly in ice for 15 min and finally centrifuged (14000 ×g for 20 min, 4 °C). The supernatant containing the nuclear extracts was collected and the protein content was measured by BCA method. About 30-50 μg proteins were electrophoresed on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)[36]. Membrane was blocked for 1 h in 5% fat-free milk/PBS (pH 7.4) and probed using mouse anti–human SREBP-1 (SC-13551) or rabbit anti-human NF-κB p65 (SC-109) antibodies supplied by Santa Cruz Biotechnology (DBA, Milan, Italy). Membranes were incubated overnight at 4 °C with primary antibody in PBST buffer (PBS with 0.1% Tween 20)[37], washed and incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (Sigma-Aldrich), as a secondary antibody in PBST for 1 h at room temperature. Immune complexes were visualized using an enhanced chemiluminescence Western blotting analysis system (Bio-Rad ChemiDoc XRS System). Films were digitized and band optical densities were quantified against the actin band using a computerized imaging system and expressed as Relative Optical Density (ROD, arbitrary units). ROD of each band was expressed as percentage respect to control.
RNA and protein data are expressed as mean ± SD of at least four independent experiments in triplicate. Statistical analysis was performed using ANOVA with Tukey’s post-test (GraphPad Software, Inc., San Diego, CA, United States).
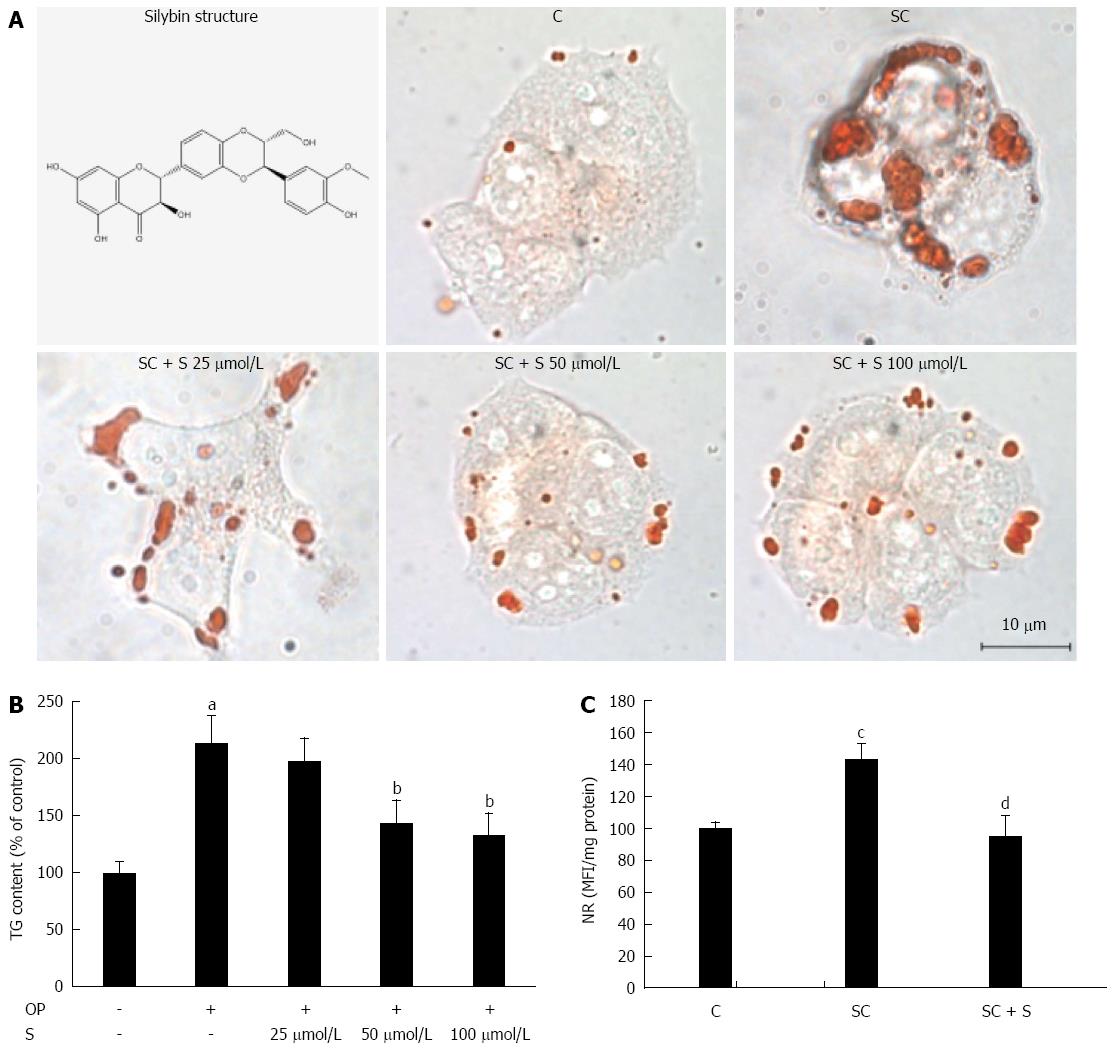
Lipid accumulation was visualized in situ by optical microscopy of ORO-stained cells in control (C) and steatotic hepatocytes incubated for 24 h in the absence (SC) or in the presence (SC + S) of increasing concentrations of silybin (25, 50, and 100 μmol/L). The number and size of LDs increased markedly in steatotic cells, and decreased dose-dependently with silybin incubation (Figure 1A). Also TG content was significantly higher in steatotic than in control cells (+113%; P≤ 0.001) (Figure 1B). Incubation with silybin 50 μmol/L and 100 μmol/L decreased significantly the TG content by -33% and by -38%, respectively, (P≤ 0.001 for both doses vs untreated steatotic cells). Silybin did not affect the lipid content of control cells (data not shown).
Following these pilot experiments, silybin at a concentration of 50 μmol/L was used for all further experiments. Using NR fluorescence we measured LD accumulation as a function of the treatments. The average content of LDs increased significantly by +43% (P≤ 0.01) in steatotic cells compared to control, and decreased by -33% (P≤ 0.01) with silybin 50 μmol/L compared to steatotic cells (Figure 1C). Cell viability was not significantly affected by FA and/or silybin treatments as single agents or combined (data not shown).
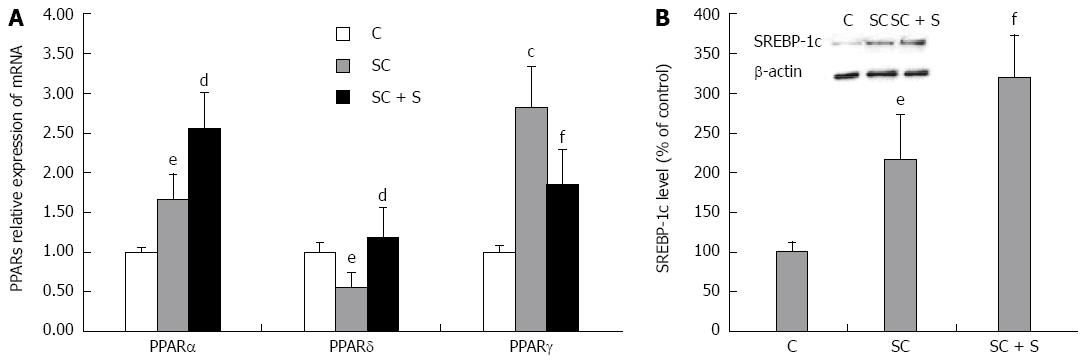
Lipid metabolism pathways are controlled by PPARS, a family of FA-regulated transcription factors. In rat hepatocytes, we have previously showed the following abundance of the three PPAR transcripts: PPARα > PPARδ > PPARγ[38]. The present results show that expression of PPARα mRNA was 1.7 fold higher in steatotic cells compared to control (P≤ 0.05), and increased further upon incubation with silybin 50 μmol/L (+54% with respect to steatotic cells; P≤ 0.01) (Figure 2A). A significant increase was also observed in PPARγ mRNA expression upon lipid-loading (2.8 fold induction with respect to control; P≤ 0.01), but, in in this case, silybin 50 μmol/L reduced the up-regulation (-34% with respect to steatotic cells, P≤ 0.05) (Figure 2A). By contrast, mRNA expression of PPARδ decreased upon lipid-loading (about 0.55 fold induction with respect to control; P≤ 0.05) and silybin 50 μmol/L restored PPARδ expression to values similar to control (+115% compared to steatotic cells, P≤ 0.01) (Figure 2A).
SREBP-1c is a PPARα-target gene which regulates expression of lipogenic enzymes. Activation of SREBP-1c increased in steatotic hepatocytes (+116%; P≤ 0.05) with respect to control and further increased upon exposure to silybin 50 μmol/L (+48% compared to steatotic cells; P≤ 0.05) (Figure 2B).
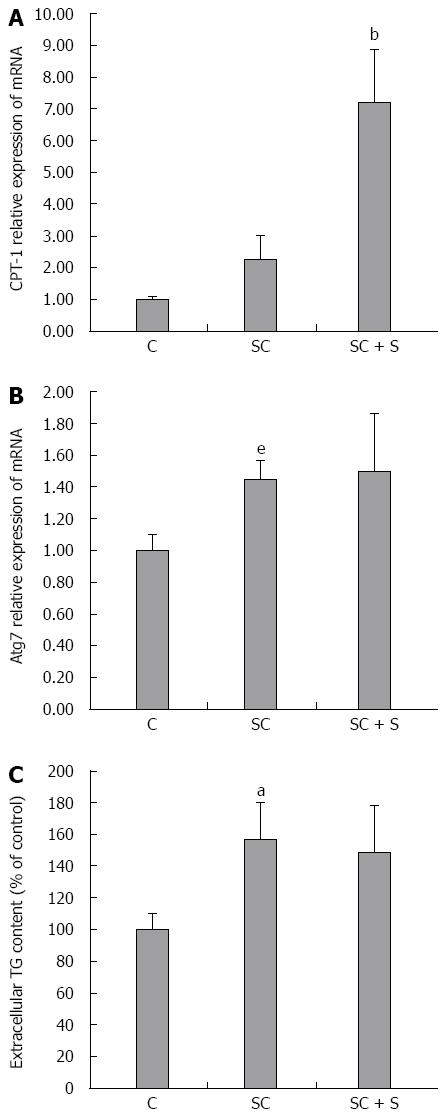
A reduced TG accumulation in hepatocytes might be sustained by both stimulation of oxidative and/or secretory pathways. CPT-1, the main regulator of mitochondrial β-oxidation of FAs, is significantly up-regulated in steatotic cells upon incubation with silybin 50 μmol/L (+217% with respect to steatotic cells; P≤ 0.001) (Figure 3A). At the same time, the mRNA expression of Atg7, an autophagy-promoting gene in hepatocytes, was significantly increased in steatotic cells (1.45 fold induction with respect to control; P≤ 0.05), but it did not change upon treatment with silybin 50 μmol/L (Figure 3B). On the other hand, steatotic cells showed an increased TG secretion into the culture medium compared to control cells (+57%; P≤ 0.001), but incubation with silybin 50 μmol/L did not further stimulate TG secretion as compared to steatotic cells (Figure 3C).
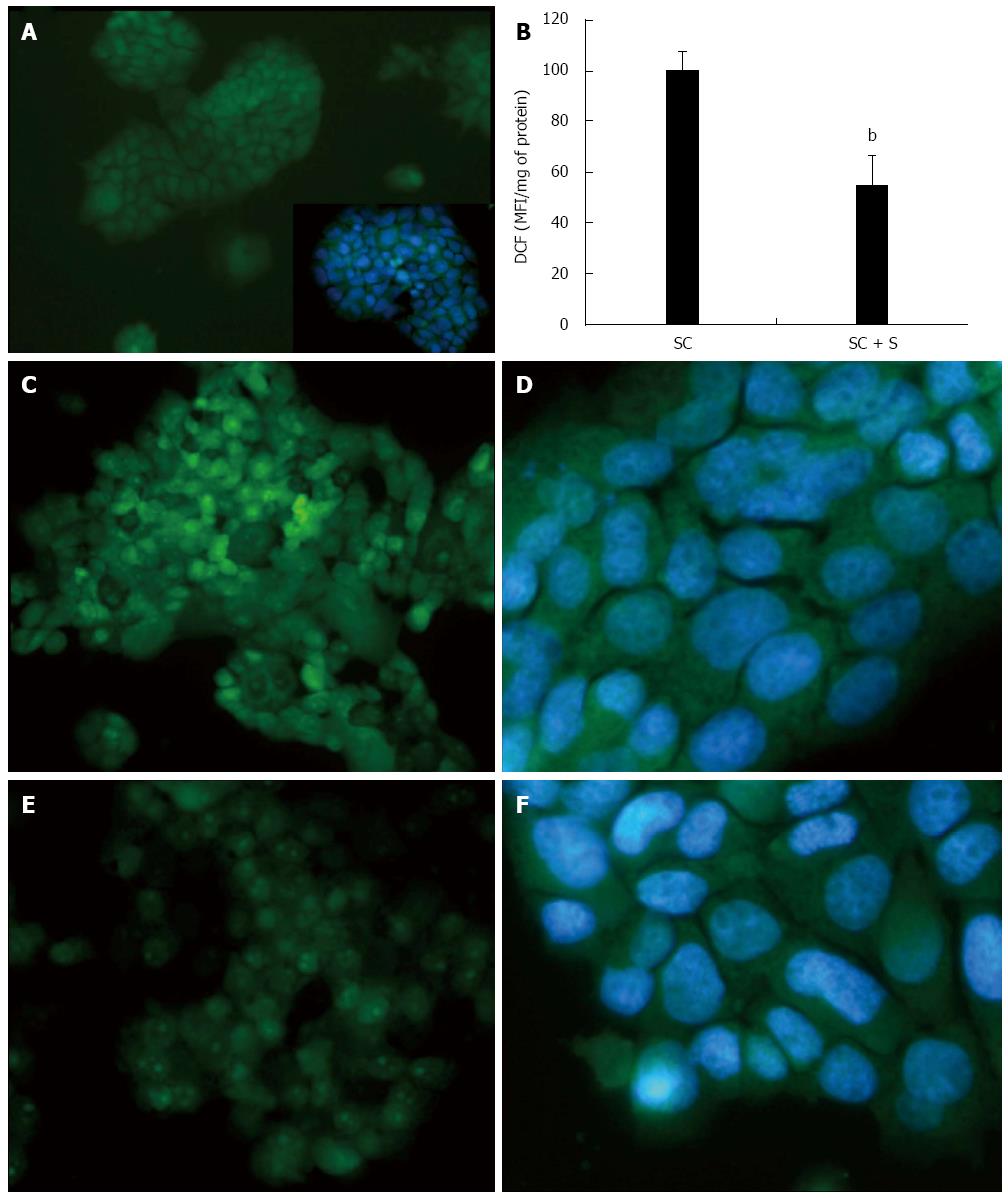
The intracellular production of ROS, mainly hydrogen peroxide (H2O2), was visualized in situ by fluorescence microscopy of DCF-stained cells (Figure 4). Higher and diffuse DCF fluorescence was observed in steatotic cells (Figure 4C, D) and it was lower in cells treated with silybin 50 μmol/L (Figure 4E, F). DCF fluorescence was almost null in control cells (Figure 4A). These changes were quantified by spectrofluorometer readings (Figure 4B) that showed a significant DCF decrease (-45%; P≤ 0.001) in silybin-treated cells with respect to steatotic cells used as control.
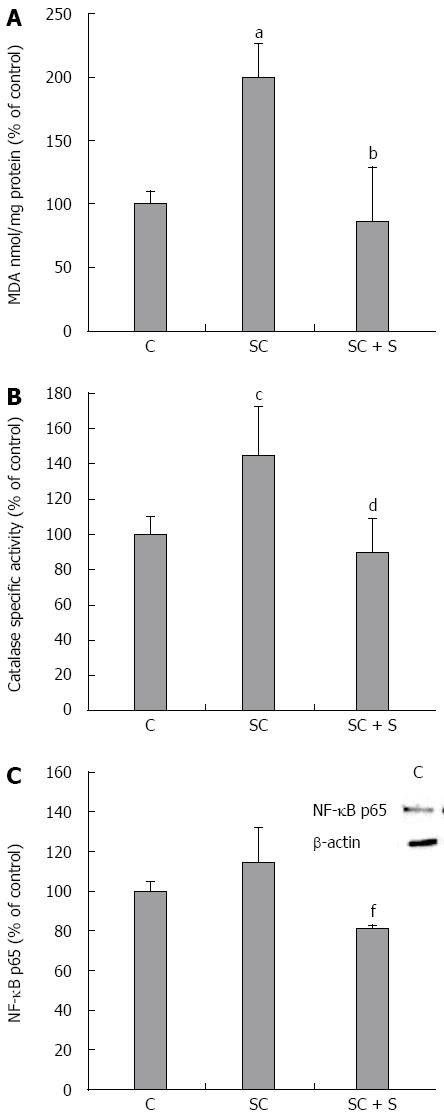
Also MDA level was greater in steatotic than in control cells (+100%; P≤ 0.001) and decreased with silybin 50 μmol/L (-57% with respect to steatotic cells; P≤ 0.001) (Figure 5A). The analyses of antioxidant defence mechanisms showed increased catalase activity in steatotic cells with respect to control (+45% vs control, P≤ 0.01) that decreased with silybin 50 μmol/L (-38% with respect to steatotic cells, P≤ 0.01) (Figure 5B). Also NF-κB activation showed a trend to an increase in steatotic cells and a significant reduction upon exposure to silybin 50 μmol/L (-29% with respect to steatotic cells; P≤ 0.05) (Figure 5C).
The burden of nonalcoholic liver steatosis is rapidly increasing worldwide and is exposing the populations to the risk of liver-related complications[1]. Ultimate therapeutic approaches are lacking so far, besides diet and physical exercise. The nutraceutic silybin has shown preliminary encouraging results either in clinical and animal studies[39,40]. The present study provides a deeper characterization of some pathophysiologically relevant mechanisms of action of silybin administered as phytosome complex with vitamin E directly to fatty hepatocytes mimicking a steatosis condition in vitro. In this study, silybin disclosed direct anti-steatotic and anti-oxidant properties in steatotic cells, thus extending previous observations carried out by our group in studies with patients suffering with liver steatosis[17], and in animal models fed a steatogenic diet[41].
Accumulation of LDs within hepatocytes is the first step in the development of NAFLD[42], while oxidative stress causing membrane lipid peroxidation and necro-inflammatory changes represents the second step leading to NASH[43,44]. The exposure of hepatoma cells to exogenous FAs mimicked the first “hit” of NAFLD and led to TG accumulation within large cytosolic LDs that we observed by microscopic, fluorimetric and spectrophotometric analyses.
At the molecular level, TG accumulation in LDs seems to be sustained by the up-regulation of PPARα that promotes FA transportation inside the cells, and by a larger up-regulation of PPARγ, the lipogenic isoform that promotes TG synthesis from FAs. It has to be underlined that, although PPARα is mostly known for its ability to induce FA oxidation, growing evidence points to its role in regulation of lipogenesis. In fact, PPARα regulates many aspects of hepatic lipid metabolism including FA uptake through membranes, intracellular FA trafficking and oxidation, TG storage and lipolysis. In the present study, the changes in PPARα mRNA expression are paralleled by those in the protein levels of SREBP-1c, that in fact is a PPARα target gene[45]. Moreover, PPARα up-regulation can explain both the increase in lipophagy, TG secretion and in CPT-1 expression observed in steatotic cells. In fact, PPARα typically promotes lipid mobilization from the cytosolic stores by stimulating both LD autophagy, lipid secretion and FA oxidation in mitochondria.
The activation of all these pathways represents an attempt of the cell to reduce excess fat accumulation, but are usually accompanied by increased ROS production. Indeed, the present data show that excess fat accumulation is accompanied by increased oxidative stress. To assess cellular oxidative stress, we measured: (1) content of ROS and hydrogen peroxide and activity of catalase, the main scavenger of H2O2; (2) lipid peroxidation, one of the most common indicators of free radical formation and a key indicator of oxidative stress; and (3) activation of NF-κB, the master transcription factor in the control of molecular pathways related to oxidative stress. All these indices were increased in steatotic cells.
Incubation of steatotic cells with silybin 50 μmol/L significantly reduces TG accumulation likely by promoting lipid catabolism pathways, as suggested by the up-regulation of PPARα and PPARδ, and by inhibiting lipogenic pathways, as suggested by the down-regulation of PPARγ. In fact, PPARα and PPARδ stimulation is known to promote hepatic fatty acid oxidation, and one of the strategies for improving lipid metabolism in metabolic diseases is to use a molecule which can simultaneously activate these two PPARs[46], action that seems to be played by silybin. The stimulation of mitochondrial catabolism of FAs by silybin is also indicated by the marked up-regulation of CPT-1, which is the first component and rate-limiting step of mitochondrial β-oxidation as it allows long chain fatty acids (LCFA) to enter the mitochondria. Many studies indeed indicate that the activity of CPT1 determines the rate of LCFA oxidation and that over-expression of CPT1a in cultured cells increases fatty acid oxidation[47,48]. On the other hand, silybin-dependent TG reduction occurred without a significant stimulation of lipophagic pathways and of TG release into the culture medium, as indicated by the unaltered levels of Atg7 transcripts and extracellular TG content. It is noteworthy that this fact could be important for possible therapeutic applications.
In general, when TG storage inside LDs is inhibited, the extent of liver steatosis decreases, but liver damage might even increase due to excess of reactive free FAs produced by fat oxidation reactions. In fact, Teissier et al[49]. found that PPAR agonists induce ROS production by stimulating NADPH oxidase activity. By contrast, in our study, the reduction in fat accumulation exerted by silybin in the steatotic hepatocytes is associated with the improvement of the oxidative unbalance caused by lipid excess as demonstrated by the reduction in lipid peroxidation (MDA levels, namely), catalase activity and NF-κB activation.
Taken together, our results suggest that the antioxidant capacity of silybin effectively counteracts the possible damaging effects of a high rate of fat catabolism which is stimulated in the attempt to reduce excess FA accumulation. This step occurs by neutralizing the free radicals produced by fat oxidation reactions, thus rendering unnecessary the TG synthesis as a protection against toxic and proapoptotic effects of excess FAs[50].
In conclusion, the present study points to the cellular modulatory and protective effect of silybin with respect to lipid accumulation, expression of lipid metabolism genes, TG secretion, and FA-driven oxidative stress. This study fits with prior studies investigating the beneficial effects of silybin on liver steatosis in animal models[41], on inflammation and fibrosis in human hepatic stellate cells[51] and in patients with NAFLD and metabolic disorders[17]. Therefore silybin could represent a promising molecule for therapy of NAFLD and maybe of NASH. The prosecution of our work will be focused on testing the possible efficacy of silybin in NASH.
A special tank to Professor Gabriella Gallo for the constant support and encouragement, and to Francesca Baldini and Maddalena Ghelardoni for their help with cell treatments.
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of abnormal liver function tests in western countries, and evidences substantiate a strong association between NAFLD and metabolic abnormalities such as obesity, insulin resistance, and metabolic syndrome. Silymarin, the standardized extract from milk thistle (Sylibum marianum), and its major active compound silybin, have been used for a long time as a hepatoprotective agents for the treatment of acute and chronic liver diseases. However, the exact mechanisms of actions of this compound are still not completely clear.
The identification of compounds which could improve the oxidative stress condition and counteract the liver damages may represent a useful instrument for the treatment of NAFLD to prevent its progression. This research field represents one of the most important challenge of the next few years for the scientific society.
Previous clinical and animal studies on silybin have found encouraging results showing its ability to improve liver disorders. This study demonstrates that silybin as phytosome complex with vitamin E favorably affects lipid and radical homeostasis through a direct action on hepatic cells. To this aim, the authors used an in vitro model of NAFLD induced by the exposure of hepatoma FaO cells to exogenous fatty acids. The intracellular triglyceride (TG) accumulation is mediated by increased levels of peroxisome proliferator-activated receptors (PPARs) and sterol regulatory element-binding protein-1c (SREBP-1c). The lipid imbalance is associated with higher production of reactive oxygen species (ROS) leading to increased lipid peroxidation, stimulation of catalase activity and activation of nuclear factor kappa-B (NF-κB). The use of silybin significantly reduces TG accumulation likely by promoting lipid catabolism and by inhibiting lipogenic pathways, as suggested by the changes in carnitine palmitoyltransferase 1 (CPT-1), PPAR and SREBP-1c levels. The reduction in fat accumulation exerted by silybin in the steatotic cells is associated with the improvement of the oxidative imbalance caused by lipid excess as demonstrated by the reduction in ROS content, lipid peroxidation, catalase activity and NF-κB activation.
This study indicates that silybin plays direct anti-steatotic and anti-oxidant effects in steatotic hepatocytes, thus supporting a potentially therapeutic use of this compound for preventing and or improving NAFLD progression.
NAFLD refers to a group of conditions where there is accumulation of excess fat in the liver of people who drink little or no alcohol. Steatosis consist of excess content of triglycerides in hepatocytes. NAFLD definition encompasses a large spectrum of liver abnormalities which range from the simple steatosis, to nonalcoholic steatohepatitis, till to cirrhosis and hepatocellular carcinoma. Silybin is the major active constituent of silymarin, a standardized extract of the milk thistle seeds, containing a mixture of flavonolignans consisting of silibinin, isosilibinin, silicristin, silidianin and others.
The manuscripts describes that ability of silybin to counteract lipid excess and oxidative stress in cultured steatotic hepatic cells. This study presents interesting findings on the therapeutic effect of silybin, a flavolignan molecule of increasing interest for its beneficial effects. Both the problems and objectives of the manuscript are appropriated.
| 1. | Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA, Rinella ME. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 635] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 2. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1804] [Article Influence: 164.0] [Reference Citation Analysis (5)] |
| 3. | Portincasa P, Grattagliano I, Palmieri VO, Palasciano G. Nonalcoholic steatohepatitis: recent advances from experimental models to clinical management. Clin Biochem. 2005;38:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2112] [Cited by in RCA: 2672] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 6. | Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 611] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 7. | Ramsay RR, Gandour RD, van der Leij FR. Molecular enzymology of carnitine transfer and transport. Biochim Biophys Acta. 2001;1546:21-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (3)] |
| 8. | Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67:101-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 459] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 9. | Seifert EL, Estey C, Xuan JY, Harper ME. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J Biol Chem. 2010;285:5748-5758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 509] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 11. | Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 798] [Article Influence: 42.0] [Reference Citation Analysis (2)] |
| 12. | Chang J. Medicinal herbs: drugs or dietary supplements? Biochem Pharmacol. 2000;59:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Kandaswami C, Middleton E. Free radical scavenging and antioxidant activity of plant flavonoids. Adv Exp Med Biol. 1994;366:351-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 229] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 430] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 15. | Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, Del Vecchio Blanco C, Loguercio C. A new silybin-vitamin E-phospholipid complex improves insulin resistance and liver damage in patients with non-alcoholic fatty liver disease: preliminary observations. Gut. 2006;55:901-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Solhi H, Ghahremani R, Kazemifar AM, Hoseini Yazdi Z. Silymarin in treatment of non-alcoholic steatohepatitis: A randomized clinical trial. Caspian J Intern Med. 2014;5:9-12. [PubMed] |
| 17. | Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, Chiaramonte M, Cursaro C, Danila M, de Sio I, Floreani A. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med. 2012;52:1658-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Huang YY, Gusdon AM, Qu S. Nonalcoholic fatty liver disease: molecular pathways and therapeutic strategies. Lipids Health Dis. 2013;12:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Ferré P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12 Suppl 2:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 553] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 20. | Shimano H. Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res. 2001;40:439-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 580] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 21. | Eberlé D, Hegarty B, Bossard P, Ferré P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1163] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 22. | Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 695] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 23. | Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1755] [Cited by in RCA: 1961] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 24. | Clayton DF, Weiss M, Darnell JE. Liver-specific RNA metabolism in hepatoma cells: variations in transcription rates and mRNA levels. Mol Cell Biol. 1985;5:2633-2641. [PubMed] |
| 25. | Grasselli E, Cortese K, Voci A, Vergani L, Fabbri R, Barmo C, Gallo G, Canesi L. Direct effects of Bisphenol A on lipid homeostasis in rat hepatoma cells. Chemosphere. 2013;91:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Grasselli E, Voci A, Pesce C, Canesi L, Fugassa E, Gallo G, Vergani L. PAT protein mRNA expression in primary rat hepatocytes: Effects of exposure to fatty acids. Int J Mol Med. 2010;25:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Wiechelman KJ, Braun RD, Fitzpatrick JD. Investigation of the bicinchoninic acid protein assay: identification of the groups responsible for color formation. Anal Biochem. 1988;175:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 527] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 28. | Trunkey DD. Priorities in trauma management. Mil Med. 1990;155:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965-973. [PubMed] |
| 30. | Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1665] [Cited by in RCA: 1582] [Article Influence: 71.9] [Reference Citation Analysis (11)] |
| 31. | Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci USA. 1996;93:12553-12558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 241] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Iguchi H, Kojo S, Ikeda M. Lipid peroxidation and disintegration of the cell membrane structure in cultures of rat lung fibroblasts treated with asbestos. J Appl Toxicol. 1993;13:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15211] [Cited by in RCA: 15753] [Article Influence: 375.1] [Reference Citation Analysis (0)] |
| 34. | Grasselli E, Voci A, Canesi L, Salis A, Damonte G, Compalati AD, Goglia F, Gallo G, Vergani L. 3,5-diiodo-L-thyronine modifies the lipid droplet composition in a model of hepatosteatosis. Cell Physiol Biochem. 2014;33:344-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24144] [Cited by in RCA: 26585] [Article Influence: 1063.4] [Reference Citation Analysis (0)] |
| 36. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185380] [Cited by in RCA: 189396] [Article Influence: 3382.1] [Reference Citation Analysis (1)] |
| 37. | Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350-4354. [PubMed] |
| 38. | Grasselli E, Voci A, Canesi L, Goglia F, Ravera S, Panfoli I, Gallo G, Vergani L. Non-receptor-mediated actions are responsible for the lipid-lowering effects of iodothyronines in FaO rat hepatoma cells. J Endocrinol. 2011;210:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Reina M, Martínez A. Silybin and 2,3-Dehydrosilybin Flavonolignans as Free Radical Scavengers. J Phys Chem B. 2015;119:11597-11606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Rosso N, Marin V, Giordani A, Persiani S, Sala F, Cavicchioli L, Rovati LC, Tiribelli C. The Pros and the Cons for the Use of Silybin-Rich Oral Formulations in Treatment of Liver Damage (NAFLD in Particular). Curr Med Chem. 2015;22:2954-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Grattagliano I, Diogo CV, Mastrodonato M, de Bari O, Persichella M, Wang DQ, Liquori A, Ferri D, Carratù MR, Oliveira PJ. A silybin-phospholipids complex counteracts rat fatty liver degeneration and mitochondrial oxidative changes. World J Gastroenterol. 2013;19:3007-3017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (1)] |
| 43. | Aragno M, Tomasinelli CE, Vercellinatto I, Catalano MG, Collino M, Fantozzi R, Danni O, Boccuzzi G. SREBP-1c in nonalcoholic fatty liver disease induced by Western-type high-fat diet plus fructose in rats. Free Radic Biol Med. 2009;47:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? Gut. 2002;50:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Fernández-Alvarez A, Alvarez MS, Gonzalez R, Cucarella C, Muntané J, Casado M. Human SREBP1c expression in liver is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem. 2011;286:21466-21477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Ament Z, West JA, Stanley E, Ashmore T, Roberts LD, Wright J, Nicholls AW, Griffin JL. PPAR-pan activation induces hepatic oxidative stress and lipidomic remodelling. Free Radic Biol Med. 2016;95:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res. 2002;41:197-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 48. | Rubí B, Antinozzi PA, Herrero L, Ishihara H, Asins G, Serra D, Wollheim CB, Maechler P, Hegardt FG. Adenovirus-mediated overexpression of liver carnitine palmitoyltransferase I in INS1E cells: effects on cell metabolism and insulin secretion. Biochem J. 2002;364:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Teissier E, Nohara A, Chinetti G, Paumelle R, Cariou B, Fruchart JC, Brandes RP, Shah A, Staels B. Peroxisome proliferator-activated receptor alpha induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-alpha activation properties. Circ Res. 2004;95:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 501] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 51. | Trappoliere M, Caligiuri A, Schmid M, Bertolani C, Failli P, Vizzutti F, Novo E, di Manzano C, Marra F, Loguercio C. Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J Hepatol. 2009;50:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
P- Reviewer: Deminice R, Pan TL S- Editor: Yu J L- Editor: A E- Editor: Ma S