Published online Jul 7, 2016. doi: 10.3748/wjg.v22.i25.5753
Peer-review started: March 12, 2016
First decision: April 14, 2016
Revised: April 26, 2016
Accepted: May 23, 2016
Article in press: May 23, 2016
Published online: July 7, 2016
Processing time: 114 Days and 22.6 Hours
Barrett’s esophagus (BE) is an important condition given its significant premalignant potential and dismal five-year survival outcomes of advanced esophageal adenocarcinoma. It is therefore suggested that patients with a diagnosis of BE undergo regular surveillance in order to pick up dysplasia at an earlier stage to improve survival. Current “gold-standard” surveillance protocols suggest targeted biopsy of visible lesions followed by four quadrant random biopsies every 2 cm. However, this method of Barrett’s surveillance is fraught with poor endoscopist compliance as the procedures are time consuming and poorly tolerated by patients. There are also significant miss-rates with this technique for the detection of neoplasia as only 13% of early neoplastic lesions appear as visible nodules. Despite improvements in endoscope resolution these problems persist. Chromoendoscopy is an extremely useful adjunct to enhance mucosal visualization and characterization of Barrett’s mucosa. Acetic acid chromoendoscopy (AAC) is a simple, non-proprietary technique that can significantly improve neoplasia detection rates. This topic highlight summarizes the current evidence base behind AAC for the detection of neoplasia in BE and provides an insight into the direction of travel for further research in this area.
Core tip: Neoplasia detection in surveillance of Barrett’s esophagus (BE) remains challenging as current gold-standard four quadrant biopsies have a high miss-rate and are poorly adhered to. Evidence to support the use of acetic acid chromoendoscopy (AAC) is growing. We discuss the current evidence of AAC in BE and the direction of travel for future research.
- Citation: Chedgy FJQ, Subramaniam S, Kandiah K, Thayalasekaran S, Bhandari P. Acetic acid chromoendoscopy: Improving neoplasia detection in Barrett's esophagus. World J Gastroenterol 2016; 22(25): 5753-5760
- URL: https://www.wjgnet.com/1007-9327/full/v22/i25/5753.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i25.5753
The incidence of esophageal cancer is increasing[1], representing the ninth most common cancer in the United Kingdom. Seven thousand and eight hundred people are diagnosed with the condition every year, and it accounts for 5% of all cancer deaths in the United Kingdom[2]. It is well recognized that Barrett’s esophagus (BE) is a significant risk factor for the development of esophageal adenocarcinoma (EAC) and is present in 1.6% of the general population[3] and in up to 20% of patients with gastroesophageal reflux[4].
BE is defined as an esophagus in which any portion of the normal distal squamous epithelial lining has been replaced by metaplastic columnar epithelium, which is clearly visible endoscopically above the gastro-esophageal junction[5]. It is universally recognized that the presence of intestinal metaplasia (IM) confers an increased risk of developing Barrett’s-related EAC and that IM is present in the vast majority of long-segment Barrett’s[6]. The development of Barrett’s EAC is postulated to occur in a progressive fashion from IM to low grade dysplasia (LGD) to high grade dysplasia (HGD) and then EAC. The annual rate of transformation into EAC in patients with non-dysplastic BE is estimated to be between 0.07% and 0.82%[7-9]. However, the annual rate of progression from LGD to HGD or EAC is as high as 8.8% as demonstrated by the recent SURF trial[10] and from HGD to EAC is 12% to 40%[11,12]. The aim of endoscopic surveillance is to alter the natural history of the disease by identifying neoplasia at an earlier stage and thus instituting curative endoscopic therapy.
Established surveillance protocols suggest taking targeted biopsies of visible lesions and random four quadrant biopsies (4QBS) every 2 cm (Cleveland protocol) which reportedly proffers the maximum yield of dysplasia in comparison with other biopsy protocols[13]. However, there are several drawbacks to this technique. With only 13% of early neoplastic lesions appearing as visible nodules[14], a significant proportion of Barrett’s neoplasia is not visible on high-definition white-light endoscopy alone, with reported sensitivity in the range 40%-64% and specificity 98%-100%[15]. These non-visible neoplastic foci can occupy areas as small as 0.5 cm2[16]. Unsurprisingly, there is a significant miss-rate with 4QBS. Studies comparing 4QBS with surgical resection specimen have shown that 41%-66% of dysplastic lesions are missed by 4QBS[17,18]. The total mucosal surface sampled with 4QBS is equivalent to 0.5 cm2 equating to sampling of only 3.5% of an average-length BE. 4QBS are notoriously poorly adhered to[19], with worse adherence for longer segments, further compounding miss-rates. In addition, 4QBS are time-consuming and poorly tolerated by patients. The cost of processing 4QBS is significant, with each cassette of tissue costing £58.90 ($90.61) to process[20].
These pitfalls in surveillance have prompted evaluation of more effective techniques to improve the diagnostic accuracy for the detection of IM and early Barrett’s neoplasia, the most promising of which is acetic acid chromoendoscopy (AAC). This review aims to summarize the current evidence for AAC in BE and provide insight into the direction of travel for further research in this area.
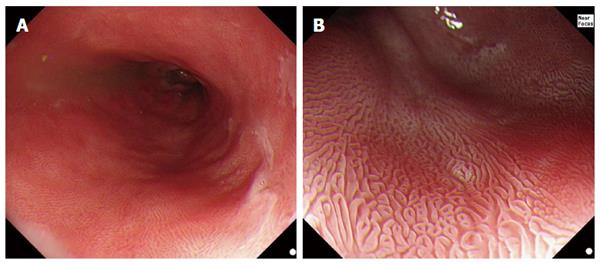
The use of acetic acid (AA) in the digestive tract was first reported by Guelrud and Herrera[21], to aid in the identification of small islands of BE following ablative therapy. The technique was derived from gynecology where AA instilled onto the cervix has been used to highlight dysplastic areas during screening for cervical intraepithelial neoplasia[22]. When AA is sprayed onto squamous epithelium, there is an acetowhitening reaction caused by masking of the submucosal capillaries and increasing opacity of the mucosal surface[23]. As AA (pH 2.5-3.0) infiltrates through the multi-layered squamous epithelium it is neutralized, which protects the subepithelial stroma and vasculature[24]. In contrast, when sprayed on Barrett’s epithelium, at low concentrations (1%-3%), AA initially eliminates the superficial mucus layer by breakage of glycoprotein disulphide bonds. The unbuffered acid then causes a reversible acetylation of cellular proteins and a change in the spatial properties of nuclear and cytoplasmic proteins, initially causing an acetowhitening reaction that highlights the surface pattern (Figure 1). With the disruption of the mucus layer, AA reaches stromal capillaries causing vascular congestion, leading to focal erythema but this is hidden under the acetowhite mucosa and only becomes visible after the loss of acetowhitening (LAW). This focal redness due to LAW was first described, by the Portsmouth group in 2010[2], as a strong predictor of neoplasia. The exact mechanism remains unclear but it is believed that the difference in acetowhitening reaction between non-neoplastic and neoplastic mucosa is due to the difference in the nucleocytoplasmic ratio between non-neoplastic and neoplastic cells. The low cytoplasmic content of neoplastic cells allows them to lose acetowhitening quicker than non-neoplastic cells. This reaction leads to focal erythema - a pathognomic sign of neoplasia with AAC.
The diagnosis of Barrett’s esophagus, according to American society guidelines[25], is defined as the presence of esophageal IM. As IM is not readily identifiable by white light endoscopy, this diagnosis is made based on histology. Efforts have been made to visually identify IM by means of enhanced endoscopy. AA coupled with magnification endoscopy has been shown to accurately identify IM[21]. Guelrud et al[21] classified the surface pattern of Barrett’s mucosa into 4 categories: (1) round pits; (2) reticular (circular or oval pits); (3) villous (fine villiform appearance without visible pits); and (4) ridged (thick villi with convoluted, cerebriform appearance without visible pits).
They found that Pattern I corresponded to fundic or cardiac type without IM and Patterns II, III, an IV each corresponded to IM with increasing sensitivities. The overall accuracy of AA with magnification endoscopy for the diagnosis of IM was 92.2%. These findings were reliably replicated by Toyoda et al[26] and Fortun et al[27].
A recent meta-analysis by Coletta et al[28] evaluated the use of AA for the detection of IM and HGD/EAC in patients with BE using histology as the reference standard. A total of 13 prospective studies (1690 patients) were included in the meta-analysis. Eight of the 13 studies, provided data on the diagnosis of IM. For the characterization of IM, the pooled sensitivity, specificity, positive likelihood ratio (LR+), and negative likelihood ratio (LR-) for all the included studies (8 studies, 516 patients) were 0.96 (95%CI: 0.83-0.99), 0.69 (95%CI: 0.54-0.81), 3.0 (95%CI: 2.0-4.7) and 0.06 (95%CI: 0.01-0.26), respectively. No significant sources of heterogeneity were identified on subgroup analysis. AA may be helpful for the exclusion of specialized IM, however, histological confirmation remains critical due to low specificity (0.69). In our view, this is clinically not relevant when dealing with long-segment BE as presence of specialized IM would not alter surveillance intervals.
Use of AA to aid identification of IM in BE is important in the stratification of surveillance intervals[5]. However, the overriding utility of AA is the identification and characterization of Barrett’s neoplasia. There is a growing body of evidence to support the use of AA in this setting.
In 2006 Réaud et al[29], furthered Guelrud’s work aiming to define the neoplastic appearances of BE following 6% AA dye spray and magnification endoscopy. In their study of 28 patients, they noted that patients with HGD on biopsy displayed mucosal architectural disorganization and hypervascularity - a phenomenon previously identified by Rey et al[30] in 2003. Using these parameters, they demonstrated a positive predictive value (PPV) of 75% for neoplasia. Camus et al[31] identified similar features when combining AA with FICE.
In their study of 62 patients in 2006, Fortun et al[27] examined whether the combination of magnification endoscopy and 3% AA could improve diagnostic accuracy in patients with BE. Patients underwent a repeat endoscopy having recently undergone surveillance endoscopy (mean 7 mo prior). Barrett’s neoplasia was identified in 9 patients: 5 LGD, 1 HGD and 3 EAC. The main drawback from this study is that the index endoscopy was used as a control, raising the question as to whether the neoplasia detected was de-novo or previously missed, with the total number of neoplasias being small. At the same time, Yagi et al[32] reported that Barrett’s EAC was associated with an irregular granular pattern or a minute grain-like pattern following 1.5% AA dye spray and magnification endoscopy.
A year later Vázquez-Iglesias et al[33] reported on their prospective study of 100 patients undergoing Barrett’s surveillance, 13 of whom had neoplasia, using 3% AA and non-magnification endoscopy. They proposed the following mucosal classification: (1) normal pattern: uniform reticulum along entire columnar-lined esophagus; and (2) abnormal pattern: rough or irregular reticulum.
Applying these characteristics, they demonstrated 100% sensitivity and 97.7% specificity (PPV 86% NPV 100%) for the detection of early neoplasia. with the false positives arising in 2 patients; one with esophagitis, the other with an esophageal ulcer.
These results were a significant improvement on those reported by Mayinger et al[34] in 2006 who reported sensitivities for neoplasia recognition in the range 55.5% to 82.4% in endoscopists trained in interpretation of AA enhanced magnification endoscopy. The same study also demonstrated extremely low inter- and intra-observer agreement for the technique.
The Wiesbaden group first reported their experiences of AAC for neoplasia detection in Barrett’s in 2007[35]. They performed a prospective randomized crossover tandem endoscopy study examining 57 patients with a history of Barrett’s neoplasia with AAC or virtual chromoendoscopy, using Fujinon Intelligent Chromoendoscopy (FICE), 4-6 wk apart. The patients had a known history of Barrett’s neoplasia (discrete mucosal alteration/ macroscopically occult lesions/ prior endoscopic treatment for neoplasia). Targeted biopsy of visible abnormalities was performed along with 4QBS. In 24 patients neoplasia was identified with the AAC achieving an 87% sensitivity. There are however, limitations with this study in that combined biopsies (targeted plus 4QBS) were used as the reference standard not surgical resection specimens. The study population was neoplasia-enriched in a tertiary center and thus results may not reflect the true performance of AAC in the community, surveillance population.
Longcroft-Wheaton et al[36] from Portsmouth reported on their cohort of patients undergoing Barrett’s examination with AAC with strikingly similar results. The study design was similar to the Wiesbaden group with 190 procedures performed in 119 patients. After esophageal cleansing, with a 50 mL solution containing 40 mL of water, 5 mL of 10% N-acetylcysteine and 5 mL of simeticone, patients underwent conventional white light endoscopy followed by 2.5% AAC. The Barrett’s segment was assessed for the following features: (1) surface pattern: ridged, villous, round, irregular; (2) vascular pattern: regular or irregular; and (3) acetowhitening reaction: No loss of acetowhitening or focal early loss of acetowhitening.
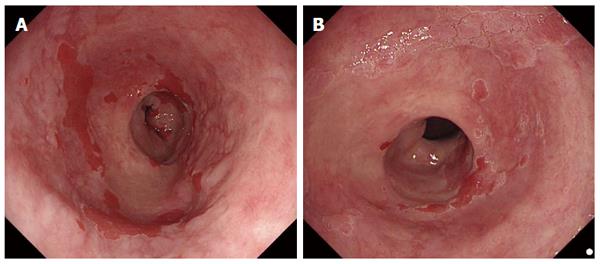
Dysplastic Barrett’s was defined endoscopically as (Figure 2): (1) irregular surface patterns AND/OR; (2) increased vascularity or irregular vessels AND/OR; and (3) focal, early loss of acetowhitening was present.
Targeted biopsies were performed followed by 4QBS (unless area already sampled with targeted biopsy). Again, the combination of targeted and 4QBS was used as the reference for final histological diagnosis.
Seventy-eight procedures were performed in patients with no prior neoplasia history (low-risk group) and 112 procedures were performed in patients referred with a history of neoplasia (high-risk group). Neoplasia was histologically confirmed in 88/190 procedures: 21/88 EAC (T1a/b), 51/88 HGD, 16/88 LGD. AAC targeted biopsy demonstrated a sensitivity of 95.5% and specificity 80% for neoplasia detection. Significant correlation between the in vivo diagnosis of neoplasia and final histology was noted (r = 0.98). There was a 2.5-fold increase in visible neoplasia detection with AAC as compared to white light alone (P = 0.001). The limitations of this study are similar to those of the Wiesbaden group: single center, expert endoscopist with a dysplasia-enriched population. What these studies cannot answer is how AAC would perform in the surveillance population where dysplasia prevalence is much lower and how AAC performs in non-expert hands.
Another factor limiting the use of AAC is the additional skills required to interpret surface and vascular patterns and their subjective nature. To that end the Portsmouth group sought to develop an objective tool using the duration of acetowhitening for the diagnosis of neoplasia[2]. One hundred and thirty-two patients underwent 2.5% AAC with targeted biopsies of neoplasia, followed by 4QBS. Time taken to lose acetowhitening effect was measured and analyzed for metaplasia, HGD and EAC. In cases of cancer, acetowhitening was lost in a median of 23 s (range 3-81 s), for HGD the median was 53 s (range 4-288 s). In non-dysplastic Barrett’s median time was 311 s (range 14-992). They proved the concept of focal loss of acetowhitening (LAW) as a very effective tool in distinguishing metaplasia from HGD and HGD from EAC. The time differences to lose acetowhitening were statistically significant (P < 0.05). In order to further refine the tool, the authors plotted a receiver operating characteristic and determined that a time of 142 s yielded the optimum sensitivity of 98% and specificity of 84%. The benefit of this tool is that it provides endoscopists an objective measure of neoplasia, avoiding subjective interpretation of mucosal and vessel patterns. This is clinically very relevant as this phenomenon can be universally applied, regardless of endoscope manufacturer or definition, and requires minimal training. Their results reach the ASGE PIVI (preservation and incorporation of valuable endoscopic innovations) criteria[37] (sensitivity ≥ 90%, NPV ≥ 98% and specificity > 80%), reaching these thresholds eliminates the need for random 4QBs.
In the meta-analysis by Coletta et al[28], 9 studies (1379 patients) looked at AAC for the diagnosis of HGD/EAC. The pooled sensitivity, specificity, LR+, LR- was 0.92 (95%CI: 0.83-0.97), 0.96 (95%CI: 0.85-0.99), 25.0(95%CI: 5.9-105.3) and 0.08 (95%CI: 0.04-0.18), respectively. Subgroup analysis did not identify significant sources of heterogeneity. The results highlight, high sensitivity 92% and specificity 96% for AAC in the diagnosis of HGD/EAC.
The advent of advanced endoscopic imaging technologies such as NBI, FICE and i-scan have improved the identification and characterization of neoplastic lesions, but these technologies require significant financial investment. Therefore, the role of AA in the surveillance population is of great interest as a potentially cost-effective, accurate and non-proprietary tool for improving dysplasia detection.
In 2010 the Wiesbaden group published a much larger AAC series[38]. In their study they enrolled 701 consecutive Barrett’s patients, 406 in a high-risk group (history of Barrett’s neoplasia) and 295 in a low-risk group (no history of neoplasia). Each patient was examined with high-resolution white light followed by 1.5% AAC. Targeted biopsy of visible lesions was performed followed by 4QBS every 1-2cm (unless area already sampled with targeted biopsy). To improve visibility during 4QBS of long segment Barrett’s the dry-biopsy technique[39] was employed - spraying 1:20000 adrenaline onto the Barrett’s segment prior to biopsy. A total of 459 targeted biopsies were taken and 5485 4QBS. One hundred and thirty-two early neoplastic lesions (HGD/EAC) were identified in 92 patients. AA was demonstrated to perform with a sensitivity of 96.7% and specificity of 66.5% overall with PPV 30.4% and NPV 99.3%. Only 3 additional patients (3.3%) with neoplasia were identified by 4QBS in the high-risk group. Their data suggested that there was minimal additional yield of 4QBS over AA targeted biopsy for the detection of dysplasia with the mean number of targeted biopsies required to yield one diagnosis of neoplasia being 5.2 vs 1828 for 4QBS. However, all HGD and EAC detected in this series were from the high-risk group, limiting applicability in the low-risk surveillance population.
Bhandari et al[20] conducted a retrospective cohort study of all AAC procedures for BE performed from 2005-2010 to examine the efficacy and cost implications of this method in the identification of neoplasia. This study was done in a tertiary-center with all procedures being performed by a single expert endoscopist. High definition white light endoscopy (HDWL) was used in all cases prior to 2.5% AAC. Targeted biopsies of all AA-enhanced visible lesions were taken, followed by 4QBS. 197 high-risk patients underwent 263 procedures. Of these, 68 patients were referred with non-visible HGD on random biopsy. Notably, there was a high proportion of high-risk neoplasia (HGD/EAC) in this cohort of patients (143/263 procedures; 54.4%). There was a twofold increase in neoplasia detection using AA (96%) as compared to HDWL (48%), P = 0.0001. HGD was missed with AA in 5/98 patients (5.1%) however, 4 of these were in the complex, post-EMR follow up group.
They performed a cost modelling exercise of 3 alternative biopsy sampling protocols incorporating AA using their mean length BE of 4.5cm (Table 1). There was a 4% neoplasia miss-rate in the AA-targeted biopsies alone group. Nevertheless, the cost saving calculated is significant in the context of the high-risk population included in this study and if applied to the usual surveillance population with a lower neoplasia prevalence rate of < 5%, cost-effectiveness increases 10 fold.
| Biopsy protocols | Cost of biopsies for cohort, n = 263 (£/$) | Cost per patient | Cost reduction vs seattle protocol |
| Seattle protocol: | £278832.60 | £1060.20 | |
| 4QBS every 1 cm individual cassettes | $428929.60 | $1630.91 | |
| Cleveland protocol: | £139416.30 | £530.10 | 50% reduction |
| 4QBS every 2 cm individual cassettes | $214464.80 | $815.46 | |
| Portsmouth protocol: | £25032.50 | £95.18 | 91% reduction |
| AA-targeted and 4QBS (2 cassettes) | $38507.62 | $146.42 | |
| Modified portsmouth protocol: | £15490.70 | £58.90 | 95% reduction |
| Visible neoplasia - AA-targeted or No neoplasia - 4QBS (1 cassette) | $23829.42 | $90.61 | |
| Futuristic protocol: | £9541.80 | £30.91 | 97% reduction |
| AA-targeted biopsies only | $14678.20 | $47.55 |
The Portsmouth group published another retrospective cohort study[40] comparing the neoplasia yield of AAC with 4QBS, in a routine BE surveillance population. Nine hundred and seventy-two patients were included in the study, with 655 (67%) undergoing 4QBS and 327 (33%) AAC. A gain in neoplasia detection was demonstrated in the AAC group on both per patient and per biopsy analysis. A significant (P = 0.0001) gain from 2% neoplasia rates in the 4QBS group to 12.5% in the AAC group was noted. When analyzed per biopsy, a 14.7-fold increase in neoplasia detection was seen in the AAC group per biopsy compared to 4QBS (0.025 vs 0.0017, P < 0.05). The number of biopsies required to detect one neoplasia was 15 times lower in the AAC cohort compared to the 4QBS cohort (40 biopsies vs 604 biopsies). This study was the first of its kind in a Barrett’s surveillance population and demonstrates a proof of concept that can be used to power a randomized controlled trial comparing 4QBS with AAC.
These data demonstrate that AAC targeted biopsy protocols are extremely cost-effective in high-risk populations and suggest even greater gains are to be expected in the surveillance population.
The Portsmouth group is currently underway with the ABBA study[41]. This is a multi-center randomized, crossover, tandem endoscopy study comparing 4QBS versus AA targeted biopsies, in a Barrett’s surveillance population. The study will also focus on training the AAC technique by a web-based training program utilizing a comprehensive and well-validated image and video library. The results of this study (expected to complete in 2016) will add to the growing evidence base on the use of AAC in the surveillance population.
The evidence for the use of AAC in the detection and characterization of Barrett’s neoplasia is compelling. The large studies from the Portsmouth and Wiesbaden groups demonstrate that experts are able to meet the ASGE PIVI criteria[40] (sensitivity ≥ 90%, NPV ≥ 98% and specificity > 80%) and are thus able to justifiably dispense with 4QBS. The technique is cheap and can be universally applied, regardless of endoscope manufacturer. However, further data from a well-powered randomized controlled trial are required before completely abandoning 4QBS and it may be that the modified Portsmouth protocol provides optimum results for cost-effective Barrett’s surveillance.
| 1. | Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 787] [Article Influence: 43.7] [Reference Citation Analysis (1)] |
| 2. | Longcroft-Wheaton G, Brown J, Basford P, Cowlishaw D, Higgins B, Bhandari P. Duration of acetowhitening as a novel objective tool for diagnosing high risk neoplasia in Barrett’s esophagus: a prospective cohort trial. Endoscopy. 2013;45:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Talley NJ, Agréus L. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 655] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 4. | Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002;287:1972-1981. [PubMed] |
| 5. | Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 911] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 6. | Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 529] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 7. | de Jonge PJ, van Blankenstein M, Grady WM, Kuipers EJ. Barrett’s oesophagus: epidemiology, cancer risk and implications for management. Gut. 2014;63:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 1003] [Article Influence: 66.9] [Reference Citation Analysis (1)] |
| 9. | Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, Fullarton G, Di Pietro M, Ravi N, Visser M. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 458] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 11. | Konda VJ, Ross AS, Ferguson MK, Hart JA, Lin S, Naylor K, Noffsinger A, Posner MC, Dye C, Cislo B. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett’s esophagus overestimated? Clin Gastroenterol Hepatol. 2008;6:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Heitmiller RF, Redmond M, Hamilton SR. Barrett’s esophagus with high-grade dysplasia. An indication for prophylactic esophagectomy. Ann Surg. 1996;224:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 213] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Fitzgerald RC, Saeed IT, Khoo D, Farthing MJ, Burnham WR. Rigorous surveillance protocol increases detection of curable cancers associated with Barrett’s esophagus. Dig Dis Sci. 2001;46:1892-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Pech O, Gossner L, Manner H, May A, Rabenstein T, Behrens A, Berres M, Huijsmans J, Vieth M, Stolte M. Prospective evaluation of the macroscopic types and location of early Barrett’s neoplasia in 380 lesions. Endoscopy. 2007;39:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Sturm MB, Wang TD. Emerging optical methods for surveillance of Barrett’s oesophagus. Gut. 2015;64:1816-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Rey JF, Inoue H, Guelrud M. Magnification endoscopy with acetic acid for Barrett’s esophagus. Endoscopy. 2005;37:583-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Falk GW, Rice TW, Goldblum JR, Richter JE. Jumbo biopsy forceps protocol still misses unsuspected cancer in Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc. 1999;49:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 193] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Edwards MJ, Gable DR, Lentsch AB, Richardson JD. The rationale for esophagectomy as the optimal therapy for Barrett’s esophagus with high-grade dysplasia. Ann Surg. 1996;223:585-589; discussion 589-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Peters FP, Curvers WL, Rosmolen WD, De Vries CE, Ten Kate FJW, Krishnadath KK, Fockens P, Bergman JJGHM. Surveillance history of endoscopically treated patients with early Barrett’s neoplasia: nonadherence to the Seattle biopsy protocol leads to sampling error. Dis Esophagus. 2008;21:475-479. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 20. | Bhandari P, Kandaswamy P, Cowlishaw D, Longcroft-Wheaton G. Acetic acid-enhanced chromoendoscopy is more cost-effective than protocol-guided biopsies in a high-risk Barrett’s population. Dis Esophagus. 2012;25:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Guelrud M, Herrera I, Essenfeld H, Castro J. Enhanced magnification endoscopy: a new technique to identify specialized intestinal metaplasia in Barrett’s esophagus. Gastrointest Endosc. 2001;53:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 171] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Van Le L, Broekhuizen FF, Janzer-Steele R, Behar M, Samter T. Acetic acid visualization of the cervix to detect cervical dysplasia. Obstet Gynecol. 1993;81:293-295. [PubMed] |
| 23. | Lambert R, Rey JF, Sankaranarayanan R. Magnification and chromoscopy with the acetic acid test. Endoscopy. 2003;35:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Canto MI. Acetic-acid chromoendoscopy for Barrett’s esophagus: the “pros”. Gastrointest Endosc. 2006;64:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV, Decker GA, Fanelli RD, Fisher DA, Foley KQ. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 26. | Toyoda H, Rubio C, Befrits R, Hamamoto N, Adachi Y, Jaramillo E. Detection of intestinal metaplasia in distal esophagus and esophagogastric junction by enhanced-magnification endoscopy. Gastrointest Endosc. 2004;59:15-21. [PubMed] |
| 27. | Fortun PJ, Anagnostopoulos GK, Kaye P, James M, Foley S, Samuel S, Shonde A, Badreldin R, Campbell E, Hawkey CJ. Acetic acid-enhanced magnification endoscopy in the diagnosis of specialized intestinal metaplasia, dysplasia and early cancer in Barrett’s oesophagus. Aliment Pharmacol Ther. 2006;23:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Coletta M, Sami SS, Nachiappan A, Fraquelli M, Casazza G, Ragunath K. Acetic acid chromoendoscopy for the diagnosis of early neoplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc. 2016;83:57-67.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Réaud S, Croue A, Boyer J. Diagnostic accuracy of magnifying chromoendoscopy with detection of intestinal metaplasia and dysplasia using acetic acid in Barrett’s esophagus. Gastroenterol Clin Biol. 2006;30:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Rey JF, Kuznetsov K. Usefulness of chromoscopy with acetic acid and magnification for Barrett’s esophagus. Gastrointest Endosc. 2003;AB91. |
| 31. | Camus M, Coriat R, Leblanc S, Brezault C, Terris B, Pommaret E, Gaudric M, Chryssostalis A, Prat F, Chaussade S. Helpfulness of the combination of acetic acid and FICE in the detection of Barrett’s epithelium and Barrett’s associated neoplasias. World J Gastroenterol. 2012;18:1921-1925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Yagi K, Nakamura A, Sekine A, Umezu H. Endoscopic diagnosis of mucosal adenocarcinomas and intestinal metaplasia of columnar-lined esophagus using enhanced-magnification endoscopy. Dig Endosc. 2006;18:21-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Vázquez-Iglesias JL, Alonso-Aguirre P, Diz-Lois MT, Vázquez-Millán MA, Alvarez A, Lorenzo MJ. Acetic acid allows effective selection of areas for obtaining biopsy samples in Barrett’s esophagus. Eur J Gastroenterol Hepatol. 2007;19:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Mayinger B, Oezturk Y, Stolte M, Faller G, Benninger J, Schwab D, Maiss J, Hahn EG, Muehldorfer S. Evaluation of sensitivity and inter- and intra-observer variability in the detection of intestinal metaplasia and dysplasia in Barrett’s esophagus with enhanced magnification endoscopy. Scand J Gastroenterol. 2006;41:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Pohl J, May A, Rabenstein T, Pech O, Nguyen-Tat M, Fissler-Eckhoff A, Ell C. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy. 2007;39:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Longcroft-Wheaton G, Duku M, Mead R, Poller D, Bhandari P. Acetic acid spray is an effective tool for the endoscopic detection of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2010;8:843-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Sharma P, Savides TJ, Canto MI, Corley DA, Falk GW, Goldblum JR, Wang KK, Wallace MB, Wolfsen HC. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett’s Esophagus. Gastrointest Endosc. 2012;76:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Pohl J, Pech O, May A, Manner H, Fissler-Eckhoff A, Ell C. Incidence of macroscopically occult neoplasias in Barrett’s esophagus: are random biopsies dispensable in the era of advanced endoscopic imaging? Am J Gastroenterol. 2010;105:2350-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Pohl J, Nguyen-Tat M, Manner H, Pech O, van Weyenberg SJ, Ell C. “Dry biopsies” with spraying of dilute epinephrine optimize biopsy mapping of long segment Barrett’s esophagus. Endoscopy. 2008;40:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Tholoor S, Bhattacharyya R, Tsagkournis O, Longcroft-Wheaton G, Bhandari P. Acetic acid chromoendoscopy in Barrett’s esophagus surveillance is superior to the standardized random biopsy protocol: results from a large cohort study (with video). Gastrointest Endosc. 2014;80:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Portsmouth Hospitals NHS Trust. Using Dilute Vinegar to Find Changes in Cells During Endoscopy for Patients With Barrett’s Oesophagus (The ABBA Study). Available from: http://ClinicalTrials.gov. |
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: United Kingdom
Peer-Review Report Classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Cho YS, Lee SH, Rimbas M, Tantau M, Yu B S- Editor: Qi Y L- Editor: A E- Editor: Wang CH