Published online Jun 28, 2016. doi: 10.3748/wjg.v22.i24.5548
Peer-review started: March 11, 2016
First decision: April 14, 2016
Revised: April 26, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: June 28, 2016
Processing time: 102 Days and 16 Hours
AIM: To determine risk factors for early neurologic complications (NCs) after liver transplantation from perspective of recipient, donor, and surgeon.
METHODS: In all, 295 adult recipients were enrolled consecutively between August 2001 and February 2014 from a single medical center in Taiwan. Any NC in the first 30 d post-liver transplantation, and perioperative variables from multiple perspectives were collected and analyzed. The main outcome was a 30-d NC. Generalized additive models were used to detect the non-linear effect of continuous variables on outcome, and to determine cut-off values for categorizing risk. Risk factors were identified using multiple logistic regression analysis.
RESULTS: In all, 288 recipients were included, of whom 142 (49.3%) experienced at least one NC, with encephalopathy being the most common 106 (73%). NCs prolonged hospital stay (35.15 ± 43.80 d vs 20.88 ± 13.58 d, P < 0.001). Liver recipients’ age < 29 or ≥ 60 years, body mass index < 21.6 or > 27.6 kg/m2, Child-Pugh class C, history of preoperative hepatoencephalopathy or mental disorders, day 7 tacrolimus level > 8.9 ng/mL, and postoperative intra-abdominal infection were more likely associated with NCs. Novel risk factors for NCs were donor age < 22 or ≥ 40 years, male-to-male gender matching, graft-recipient weight ratio 0.9%-1.9%, and sequence of transplantation between 31 and 174.
CONCLUSION: NCs post- liver transplantation occurs because of factors related to recipient, donor, and surgeon. Our results provide a basis of risk stratification for surgeon to minimize neurotoxic factors during transplantation.
Core tip: The study uses generalized additive models and logistic regression in statistics to control confounders in the case-control study. We identified 11 risk factors for early neurologic complication after transplantation. From liver recipients’ perspective, age < 29 or ≥ 60 years, body mass index < 21.6 or > 27.6 kg/m2, Child-Pugh class C, history of preoperative hepatoencephalopathy or mental disorders, day 7 tacrolimus level > 8.9 ng/mL, and postoperative intra-abdominal infection were at risk. From donors’ perspective, age < 22 or ≥ 40 years, male-to-male gender matching, graft-recipient weight ratio 0.9%-1.9% was at risk. From surgeons’ perspective, sequence of transplantation between 31 and 174 were at risk.
- Citation: Wu SY, Chen TW, Feng AC, Fan HL, Hsieh CB, Chung KP. Comprehensive risk assessment for early neurologic complications after liver transplantation. World J Gastroenterol 2016; 22(24): 5548-5557
- URL: https://www.wjgnet.com/1007-9327/full/v22/i24/5548.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i24.5548
Neurologic complications (NCs) are common after solid organ transplantation, especially liver transplantation[1]. While mortality rates decreased with advances in surgical technique and postoperative care in recent years, NCs continue to affect more than one-third of transplanted patients and cause significant morbidity, mortality, and prolonged hospital stay[2,3]. The liver recipient is vulnerable to NCs since many patients have preoperative hepatic encephalopathy, which is a well-known risk factor[3-6]. Moreover, the unfavorable condition of recipients including metabolic, nutritional, and electrolyte imbalances may predispose them to NCs[7,8]. Generally, NCs develop early, with more than 80% of patients developing NCs within 30 d after liver transplantation[6,9]. Encephalopathy is the most frequent etiology[3,5,9-12]. As it is multifactorial in nature, the transplant team should identify patients at risk and avoid predisposing variables during the perioperative period to minimize its incidence.
In the last decade, the risk factors and mechanism of NCs have been investigated in several studies, most of which were retrospective[3,6,9-12]. The majority of these focused on recipients’ factors depending on timing of transplant, such as presence of hepatic encephalopathy, etiology of cirrhosis before transplant, hyponatremia, cerebrovascular insult during transplant, immunosuppressant toxicity and central nervous system infection after transplant. However, the nature of liver graft, donor-recipient matching, and experience of surgeon and transplant team also impact outcome, and few studies have investigated NCs after liver transplantation from these perspectives. No study showed how the accumulated experience of the transplant team influences NC development. Besides, the non-linear effect of certain continuous variables, such as age, body mass index (BMI) and graft-recipient weight ratio (GRWR), was not considered in the statistical analysis of prior studies. Our study aimed to identify new risk factors of 30-d NCs after liver transplantation from multiple perspectives. In particular, we performed statistical analyses to explore how the non-linear effect of certain continuous variables increase NC risk.
From August 2001 to February 2014, 295 consecutive adult liver transplantation surgeries were performed in the Tri-Service General Hospital (Taipei, Taiwan). All causes of mortality were included in the study except for surgical mortality unrelated to NCs. All procedures were performed after approval of the Ethics Committee. The transplant team included 2 qualified transplant surgeons, 2 fellows and 2 senior residents. The immunosuppressant induction protocol consisted of intravenous methylprednisolone, starting with a large bolus dose during the anhepatic phase, followed by daily tapering off until postoperative day 5, and triple oral immunosuppressants (tacrolimus, mycophenolate mofetil, steroids). Serum trough levels of tacrolimus were checked daily in the first postoperative week to maintain a level of 6-10 ng/mL. If basiliximab was included, the first 20 mg were given during anhepatic phase and the second dose on postoperative day 4, along with halving the steroid dose. Diagnoses of NCs were primarily made by the transplant team based on clinical examination, and ancillary examinations such as computed tomography, magnetic resonance imaging, and electroencephalography. Neurologists were consulted for major NCs. Only new onset of postoperative neurologic disorders was regarded as NCs. The clinical data and patients’ outcomes were retrospectively reviewed from medical charts. The final follow-up was conducted until March 31, 2014.
The medical records and transplant database were reviewed after approval of the Institutional Review Board I of Tri-Service General Hospital, National Defense Medical Center (TSGHIRB No.100-05-220). Data were collected from 4 major perspectives: recipient, donor, donor-recipient match, and surgeon. The recipient-related variables were demographic details, comorbidities other than liver diseases, primary liver disease requiring liver transplantation, preoperative Child-Pugh and Model for End-Stage Liver Disease (MELD) scores, any complications of end-stage liver disease, such as ascites, hepatic encephalopathy, variceal bleeding and hepatorenal syndrome. In addition, any mental disorders related to alcoholism or encephalopathy were recorded. The perioperative variables included intraoperative blood loss, length of the procedure, complex vascular anatomy, and splenectomy. The postoperative variables included dose and serum level of tacrolimus, inclusion of basiliximab, and blood chemistry parameters (ammonia, sodium, and magnesium) on postoperative day 7. In addition, any postoperative complications as acute rejection, intra-abdominal infection, or kidney injury requiring dialysis were recorded. The donor and donor-recipient matching-related variables were graft’s type and weight, gender match, GRWR, and ABO compatibility. The surgeon variables were surgeon in charge, and the chronological sequence of transplantations.
The primary outcome was occurrence of any NCs within 30 d after liver transplantation. We adapted the NC classification from Dhar et al[3] as follows: (1) encephalopathy: delirium, psychosis, or alteration of conscious level diagnosed after excluding specific lesions of the central nervous system; (2) seizures; (3) drug neurotoxicity: symptoms subsiding after reduction or discontinuation of immunosuppressants, with severity varying from tremors to imaging-confirmed posterior reversible encephalopathy syndrome; (4) cerebrovascular insults; (5) central nervous system infection; and (6) central pontine myelinolysis: acute para- or quadriparesis, dysphagia, dysarthria, diplopia, loss of consciousness with evident change in serum sodium levels. Patients experiencing “locked-in syndrome”, that is, awake but unable to move or communicate, were categorized into “central pontine myelinolysis” if rapid changes in serum sodium level coexisted. If the definite etiology could not be determined, they were recorded separately.
Other outcome data were hospital days and complications other than NCs during the first month. In addition, the complications were classified into minor and major according to the clinical finding and its severity. Minor complications included those that improved spontaneously without sequela within 1 mo, while the others causing functional deficit, brain damage or death were considered as major complications.
Statistical analysis was performed using the R 3.1.0 software (R Foundation for Statistical Computing, Vienna, Austria). A P-value of < 0.05 was considered statistically significant.
Descriptive statistics were used to express data as mean ± SD for continuous variables, and frequency and percentage for categorical variables. Differences between the two groups, patients developing NCs and those who did not, were analyzed using Wilcoxon’s rank-sum test for continuous variables, and Fisher’s exact test for categorical variables.
Some continuous variables had non-linear effect, such as age and BMI. The regression model may have described poor correlation between these variables and the outcome since linearity was usually assumed during the analysis. Generalized additive models (GAMs)[13] for binary response (patients developing NCs and those who did not) were fitted to detect the potential nonlinear effects of continuous covariates; if nonlinearity existed, appropriate cut-off point(s) for discretizing the continuous covariate were selected in the GAM plots. This procedure was carried out using the vgam function (with the default values of smoothing parameters) of the VGAM package in R.
Multiple logistic regression analysis was conducted by fitting a generalized linear model to estimate the effects of predictors on the occurrence of NCs. First, all variables, donor-recipient gender match combinations, and new categorical variables obtained from previous cut-off points for discretizing continuous variables by GAM were selected. Next, a step-wise variable selection procedure went through iteration between the forward and backward steps with both the significant levels for entry and for stay set to 0.15 for being conservative. Then, the best candidate final logistic regression model was identified manually by dropping the covariates with P value > 0.05 one at a time until all regression coefficients were significantly different from 0. The final fitted logistic model was assessed with 3 goodness of fit measures: the estimated area under the receiver operating characteristic curve (≥ 0.7 acceptable), adjusted generalized R2 (≥ 0.30 acceptable), and the Hosmer-Lemeshow test (P≥ 0.05, larger means better fit). In addition, the variance inflation factor was checked for multicollinearity (no more than 10 for continuous covariates and 2.5 for categorical covariates).
Among the 295 patients, 7 with surgical mortality unrelated to NCs were excluded: 2 failed to awake due to irreversible brain damage secondary to fulminant hepatitis, and 5 experienced primary graft failure. Finally, 288 patients were enrolled in this study, with 95 receiving deceased donor liver transplantation (DDLT) and 193 receiving living donor liver transplantation (LDLT). Baseline characteristics of the study population are shown in Table 1.
| Recipient variables | |
| Demographics | |
| Age (yr) | 52.3 ± 9.81 |
| Gender (M/F) | 213/75 |
| BMI (kg/m2) | 24.4 ± 3.72 |
| Primary diagnosis, n (%) | |
| Hepatitis B | 175 (60.8) |
| Hepatitis C | 73 (25.3) |
| Alcoholic liver disease | 77 (26.7) |
| HCC | 138 (47.9) |
| Severity of liver disease | |
| Child-Pugh score | 10.0 ± 2.49 |
| MELD score | 15.0 ± 9.01 |
| Complication, n (%) | |
| Hepatic encephalopathy | 132 (45.8) |
| Ascites | 189 (65.6) |
| Variceal bleeding | 114 (39.6) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 103 (35.7) |
| Hypertension | 52 (18.1) |
| Uremia | 14 (4.9) |
| Mental disorders | 99 (34.4) |
| Blood test before transplant | |
| Glucose (mg/dL) | 129 ± 76.07 |
| Albumin (g/dL) | 3.08 ± 0.66 |
| Creatinine (mg/dL) | 1.22 ± 1.53 |
| INR | 1.62 ± 0.99 |
| T. bilirubin (mg/dL) | 7.78 ± 11.19 |
| Platelet (× 103/μL) | 85.4 ± 53.85 |
| Ammonia (μg/dL) | 138.0 ± 97.05 |
| Perioperative variables | |
| Blood loss (mL) | 2896 ± 3409 |
| Operation time (min) | 575 ± 122.7 |
| Splenectomy, n (%) | 87 (30.2) |
| Complex vascularity, n (%) | 75 (26.0) |
| Postoperative variables | |
| Tacrolimus dose (mg/d) | 3.4 ± 1.67 |
| Basiliximab, n (%) | 51 (18) |
| Serum level - Day 7 | |
| Tacrolimus level (ng/mL) | 7.4 ± 6.24 |
| Ammonia (μg/dL) | 69.6 ± 130.8 |
| Sodium (mmol/L) | 136 ± 4.92 |
| Magnesium (mEq/L) | 1.73 ± 0.34 |
| Donor variables | |
| Age (yr) | 33 ± 11.21 |
| Male gender, n (%) | 171 (59.4) |
| Graft weight (g) | 872 ± 357.88 |
| Graft type | 95/47/142/4 |
| (whole/left/right/S67), n (%) | (33.0/16.3/49.3/1.4) |
| Donor-recipient matching | |
| GRWR (%) | 1.34 ± 0.57 |
| ABO incompatible, n (%) | 9 (3.1) |
| Gender match (MM/MF/FM/FF), n (%) | 128/44/86/30 (44.4/15.3/29.9/10.4) |
| Surgeon variables | |
| Surgeon A/B/C, n (%) | 234/51/3 (81/18/1) |
| Sequence of transplantation | 1-288 |
The outcome data are shown in Table 2. One hundred and forty two patients experienced 145 events of NCs, with an overall incidence of 49.3%. The most frequent events were encephalopathy (n = 106, 73%), including delirium (n = 75, 52%), conscious change (n = 26, 18%) and psychosis (n = 5, 4%). The average hospital stay was significantly longer in those with postoperative NCs (35.15 ± 43.80 d vs 20.88 ± 13.58 d, P < 0.001).
| Event | Minor/major | |
| Neurologic complications | 145 (100) | 97 (67)/48 (33) |
| Encephalopathy | 106 (73) | 88 (83)/18 (17) |
| Delirium | 75 (52) | 64 (85)/11 (15) |
| Psychosis | 5 (4) | 4 (80)/1 (20) |
| Change in consciousness | 26 (18) | 20 (77)/6 (23) |
| Seizures | 10 (7) | 0 (0)/10 (100) |
| Cerebrovascular events | 5 (4) | 0 (0)/5 (100) |
| Drug neurotoxicity | 10 (7) | 5 (50)/5 (50) |
| Locked-in syndrome | 12 (8) | 4 (33)/8 (67) |
| Central pontine myelinolysis | 2 (1) | 0 (0)/2 (100) |
| Other complications | ||
| Acute kidney injury | 1 | |
| Intra-abdominal infection | 9 | |
| Graft failure | 2 | |
| Reoperation | 31 | |
| Acute rejection | 61 | |
| Hepatitis B recurrence | 10 | |
| Tuberculosis infection | 8 | |
| Cytomegalovirus infection | 7 | |
| Total complications | 85 |
The differences between the patients with NC or not are shown in Table 3. Both groups were similar in demographics of recipient and donor, liver graft type, surgeon and sequence of transplantation. For primary diagnosis, alcoholic liver cirrhosis was more common in the NC group (P = 0.001), while hepatocellular carcinoma was prevalent in the control group (P < 0.001). The NC group included patients with more severe liver disease before transplant, more preoperative mental disorders and hepatic encephalopathy, more intraoperative complex vascular anatomy, higher day 7 tacrolimus level, more postoperative complications of acute rejection, intra-abdominal infection, and kidney injury requiring dialysis.
| NC | No NC | P value | |
| (n = 142) | (n =146) | ||
| Recipient variables | |||
| Preoperative | |||
| Age (yr) | 51.75 ± 10.51 | 52.77 ± 9.09 | 0.567 |
| Gender (M/F) | 109/33 | 104/42 | 0.347 |
| BMI (kg/m2) | 24.65 ± 4.25 | 24.26 ± 3.11 | 0.617 |
| Hepatitis B | 86 | 89 | 1.000 |
| Hepatitis C | 30 | 43 | 0.136 |
| Alcoholic liver disease | 50 | 27 | 0.001 |
| HCC | 51 | 87 | < 0.0001 |
| Child-Pugh score | 10.26 ± 2.26 | 8.27 ± 2.32 | < 0.0001 |
| MELD score | 20.3 ± 9.4 | 14.0 ± 7.4 | < 0.0001 |
| Hepatic encephalopathy | 91 | 41 | < 0.0001 |
| Variceal bleeding | 51 | 63 | 0.229 |
| Ascites | 106 | 83 | 0.002 |
| Mental disorder | 66 | 33 | < 0.0001 |
| Serum Albumin (g/dL) | 2.95 ± 0.57 | 3.21 ± 0.72 | 0.01 |
| Serum T. bilirubin (mg/dL) | 10.91 ± 13.16 | 4.74 ± 7.79 | < 0.0001 |
| Perioperative | |||
| Blood loss (mL) | 3329 ± 3953 | 2474 ± 2728 | 0.081 |
| Operation time (min) | 558.98 ± 99.63 | 579.9 ± 127.0 | 0.160 |
| Complex vascularity | 45 | 30 | 0.033 |
| Postoperative | |||
| Day 7 tacrolimus level (ng/mL) | 8.23 ± 7.42 | 6.54 ± 4.69 | 0.023 |
| Acute rejection | 38 | 23 | 0.030 |
| Intra-abdominal infection | 32 | 12 | < 0.0001 |
| Kidney injury requiring dialysis | 18 | 7 | 0.021 |
| Donor variables | |||
| Donor age (yr) | 32.5 ± 11.4 | 33.6 ± 11.0 | 0.234 |
| Donor gender (M/F) | 89/53 | 82/64 | 0.282 |
| Graft type (whole/left/right/S67) | 45/21/74/2 | 50/26/68/2 | 0.827 |
| Donor-recipient matching | |||
| GRWR | 1.33 ± 0.56 | 1.35 ± 0.57 | 0.726 |
| Surgeon variables | |||
| Surgeon A/B/C | 116/25/1 | 118/26/2 | 0.855 |
| Sequence of transplantation | 95.35 ± 69.66 | 108.12 ± 74.41 | 0.186 |
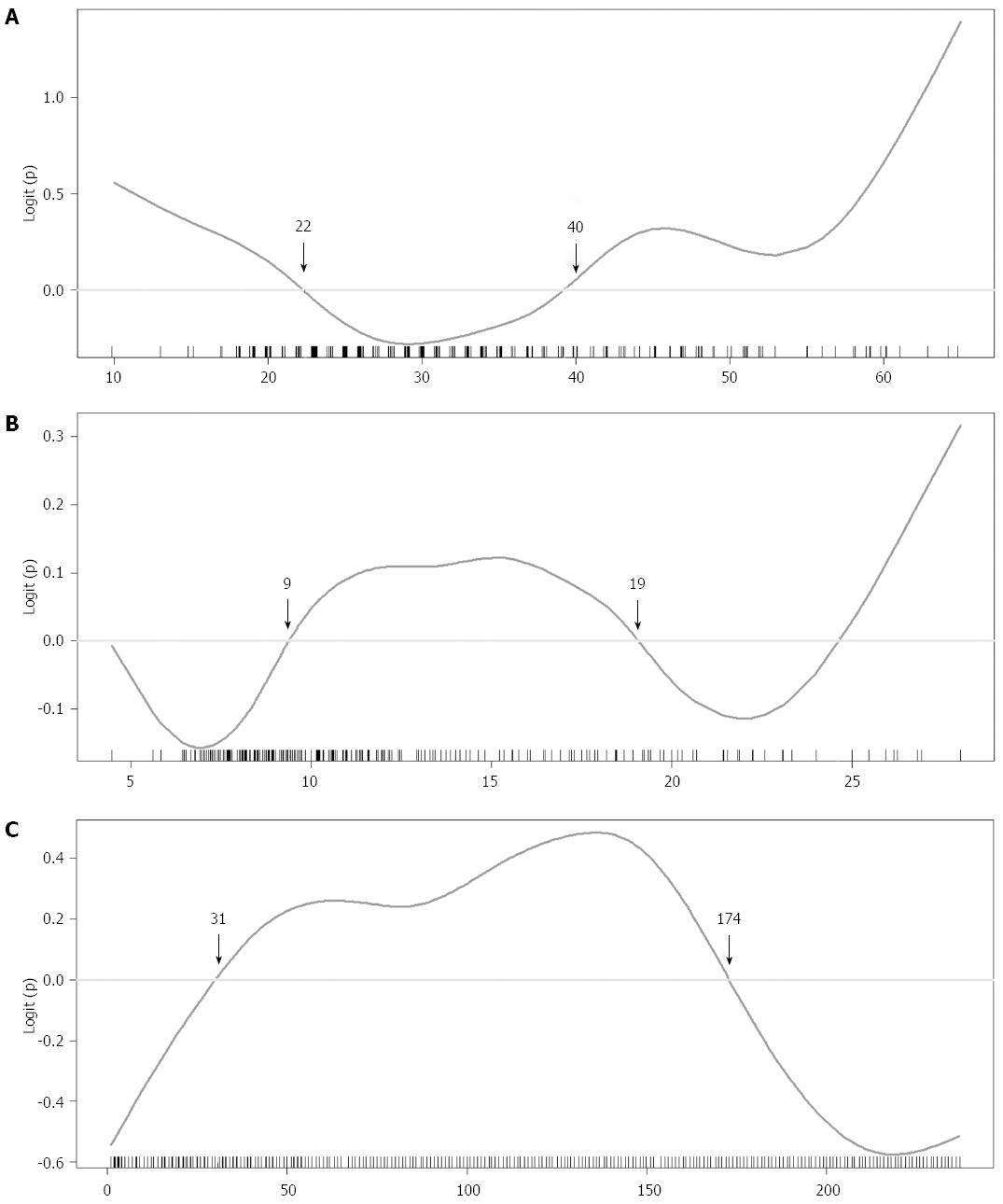
GAMs were fitted to continuous variables with potential non-linear effect on outcome. The selected GAM plots for continuous variables on the NC group are shown in Figure 1. According to the GAM plots, the following scales of variables were associated with higher probability of NCs: age < 29 or ≥ 60, recipient BMI < 21.6 or > 27.6 kg/m2, Child-Pugh score > 9, MELD score between 11 and 20, serum glucose > 8 mmol/L, serum albumin between 2.9 and 4.2 g/dL, serum creatinine between 0.6-1.6 mg/dL, the logarithm of INR > 0.3, platelet count < 70 × 103/μL, serum ammonia > 268 μg/dL, day 7 tacrolimus level > 9 ng/mL, day 7 serum ammonia level > 98 μg/dL, day 7 serum sodium level > 143 mmol/L, day 7 serum magnesium level > 1.8 mEq/L, donor age < 22 or ≥ 40 years, GRWR between 0.9% and 1.9%, and sequence of transplantation between 31 and 174. New categorical variables for regression analysis were obtained after grouping these continuous variables.
After stepwise multiple logistic regression analysis, 12 independent variables were identified as significant in the final logistic regression model (Table 4). Eleven positive predictors of NCs were: recipient age < 29 or ≥ 60 years, BMI < 21.6 or > 27.6 kg/m2, Child-Pugh score > 9, preoperative hepatic encephalopathy, history of mental disorder, day 7 tacrolimus level > 8.9, postoperative intra-abdominal infection, donor age < 22 or ≥ 40 years, male-to-male gender match, GRWR between 0.9% and 1.9%, and sequence of transplantation between 31 and 174. On the other hand, recipients with history of variceal bleeding were less likely to develop NCs (OR = 0.431; 95%CI: 0.221-0.821). The assessment of final logistic regression model showed fair goodness-of-fit (area under the receiver operating characteristic curve = 0.8553 > 0.7 with 95%CI: 0.8119-0.8988; adjusted R2 = 0.471 > 0.3; Hosmer-Lemeshow goodness-of-fit test P = 0.1446 > 0.05). Furthermore, variance inflation factors for each covariate in the selected final logistic regression model were between 1.075 and 1.582, indicating no multicollinearity.
| OR | 95%CI | P value | |
| Recipient variables | |||
| Preoperative | |||
| Age < 29 or ≥ 60 yr | 2.071 | 1.024-4.272 | 0.045 |
| BMI < 21.6 or > 27.6 kg/m2 | 1.877 | 1.007-3.552 | 0.049 |
| Child-Pugh score > 9 (Class C) | 1.509 | 1.288-1.790 | < 0.001 |
| Hepatic encephalopathy | 2.432 | 1.232-4.860 | 0.011 |
| Variceal bleeding | 0.431 | 0.221-0.821 | 0.012 |
| Mental disorder | 2.517 | 1.279-5.064 | 0.008 |
| Postoperative | |||
| Day 7 tacrolimus level > 8.9 ng/mL | 1.131 | 1.068-1.205 | < 0.001 |
| Intra-abdominal infection | 5.193 | 2.114-13.67 | < 0.001 |
| Donor variable | |||
| Donor age < 22 or ≥ 40 yr | 2.245 | 1.207-4.271 | 0.012 |
| Donor-recipient matching | |||
| Male to male gender match | 2.36 | 1.266-4.506 | 0.008 |
| 0.9% < GRWR < 1.9% | 1.95 | 1.069-3.624 | 0.032 |
| Surgeon variable | |||
| 31 ≤ Sequence of transplantation < 174 | 2.773 | 1.479-5.363 | 0.002 |
Our results confirmed the prevalence of NCs following liver transplantation, with three-quarters of patients developing encephalopathy. The risk factors were not only from recipient’s perspective, but also from perspectives of donor, donor-recipient match and surgeon. Since randomization of subjects is hardly possible in critical conditions as those requiring liver transplantation, studies in this area almost have case-control design. The novelty we have in this study came from better control of confounding effects by flexible statistical tools. The GAM plots enabled more proper stratification of continuous variables on outcome, and the regression analysis controlled the confounding bias by considering multiple covariates all at a time.
The incidence of NCs in the study was relatively higher than in other reports (49.3% vs 20%-30%) because of more diagnoses of minor encephalopathy (88/145, 60.7% of overall NC events). In fact, the diagnostic criteria for encephalopathy are not universal among physicians, especially for minor degrees. In our institution we tend to broaden the diagnostic criteria to include any transient delirium, psychosis or consciousness’ level change. This enables us to correct metabolic disorders and use immunosuppressants more properly. Our categorical system here is limited by not differentiating anoxic, septic or metabolic etiologies[14] accounting for intra-abdominal infection as an independent risk factor (OR = 5.193, 95%CI: 2.114-13.67).
Preoperative hepatic encephalopathy and mental disorders significantly increased the risk of NCs, with an OR 2.432 and 2.517, respectively. Hepatic encephalopathy, both episodic and active before the operation, is a well-known risk factor[3,4,6,12,14]. It is hypothesized that excess serum ammonia in end stage liver disease interferes with cerebral metabolism, and the condition is not immediately reversed after transplantation. Several investigators have emphasized the importance of comprehensive preoperative neurologic examinations including neuropsychiatric and neuromuscular assessments.
In addition to hepatic encephalopathy, chronic alcohol abuses also contribute to neurotoxicity, which impairs cognitive function especially memory. Alcoholism also renders patients at risk for thiamine deficiency. A prospective study that performed neuropsychological assessments before and after liver transplantation found that recipients with alcoholic etiology had poorer cognitive indexes in memory; in addition, a multivariate analysis determined that alcohol etiology, diabetes mellitus, and hepatic encephalopathy were predictors of poor global cognitive function after transplantation[15]. However, in line with the result of recent studies on LDLT[10,14,16], the current study, using regression analysis adjusted by other covariates, did not find alcoholic liver disease to be a predictor of NCs. The discrepancy may be related to the multifactorial nature of neurologic complications, and it is difficult to attribute to a specific cause of NC in patients with alcoholic liver disease. As a result, clinicians believe that hepatic encephalopathy, which is prevalent in cirrhotic patients, could be the most common cause of NC. A controlled study with the exact definition of alcohol abuse, complete neurologic examination before and after transplantation, standard postoperative care, and exclusion of postoperative complications confounding the result is needed to answer the question.
On the other hand, recipients with history of variceal bleeding were not likely to have NCs after controlling of potential confounders in the regression model. It was somewhat difficult to explain. One possible reason was that our recipients with variceal bleeding tended to receive living donor liver transplantation earlier than those without did, which enabled transplant proceeding under less severe liver disease. Another reason might be related to the shunt ligation procedure which we often done during transplantation blocking portosystemic encephalopathy.
Moreover, patients with NCs had more severe liver disease before transplant. Child-Pugh class C was an independent risk factor, with an odds ratio of 1.509 (95%CI: 1.288-1.790), while MELD score was not when adjusted by serum creatinine level and other factors in regression analysis. These results were consistent with data obtained in previous studies. In a retrospective study by Dhar et al[3] investigating factors associated with NCs, preoperative Child-Pugh Class C was a significant variable in the univariate analysis, while only active preoperative hepatic encephalopathy was significant in the multivariate analysis. Another retrospective study by Kanwal et al[17] used age, sex and era-matched control group to identify risk factors of post-transplant mental status change. MELD score > 15 was an independent risk factor in both univariate and multivariate analyses and was one of the four factors included in the prediction model. These results are likely to be related to encephalopathy precipitated by higher serum level of endogenous neurotoxic substances (including ammonia) in patients with severe liver disease. Besides, the frequent changes in electrolyte levels, malnutrition and metabolic disorders in decompensated cirrhosis may result in an unfavorable postoperative environment, rendering liver recipients vulnerable to postoperative neurologic disorders.
Age < 29 or ≥ 60 years and BMI < 21.6 or ≥ 27.6 kg/m2 showed increased probability of NCs in the GAM plot, and both were independent predictors in multiple logistic regression model, with odds ratios of 2.071 and 1.877, respectively (Table 4). Advanced age, preoperative cognitive impairment and multiple medical comorbidities were known risk factors for postoperative delirium after various procedures in several studies[18-20]. We believe that age ≥ 60 years had greater impact on NCs since the mean recipients’ age in our study was 52.3 ± 9.81 years, without pediatric recipients.
Underweight and overweight liver recipients had a significant risk for NCs. Extreme BMI values (< 18.5 and ≥ 40 kg/m2) are known risk factors for mortality after liver transplantation[21]. Patients are also more likely to develop infectious complications owing to malnutrition, as well as prolonged treatment time, and NCs secondary to of vitamins’ and trace elements’ deficiencies[22]. Early diagnosis and prompt treatment of nutritional deficits before transplantation may ameliorate encephalopathy and optimize transplant outcome[22,23].
In the GAM plot for postoperative variables, we found that day 7 tacrolimus level > 8.9 ng/mL, serum ammonia level > 98 μg/dL, serum sodium level > 143 mmol/L, serum magnesium level > 1.8 mEq/L were associated with higher probabilities for NCs. In the final logistic regression model, only day 7 tacrolimus level was an independent variable. This is in line with prior study[3].
Neurotoxicity during induction of immunosuppressants, mostly induced by calcineurin inhibitors (CNIs), is common in the early postoperative period. Clinical manifestations may vary from tremors, headache, and visual disturbances to altered mental status. In addition, seizures after transplantation are most often caused by drug neurotoxicity. The incidence is associated with high serum levels of CNIs while the occurrence is not excluded by normal serum CNI level. Coexisting hypomagnesemia and hypocholesterolemia are risk factors. Rarely, patients using CNIs have posterior reversible encephalopathy syndrome, a radiographic diagnosis characterized by reversible vasogenic edema of the white matter involving the posterior circulation territory on serial magnetic resonance imaging. It is likely to occur in patients with concomitant alcoholic liver disease and infection/sepsis. Management is mainly dose reduction and shift to an immunosuppressant with another mechanism of action; however, these patients may be at risk of acute rejection from the lower maintenance dose[24]. The process of optimizing immunosuppression, aiming to minimize graft rejection and avoid neurotoxicity, may need multidisciplinary teamwork and accumulated experience.
From the donor perspective, variables of graft type, donor age, donor gender, and GRWR were not significantly different between the NC and control groups. While age and GRWR had a biphasic effect on the GAM plots (Figure 1), we adjusted the cut-off values (donor age < 22 or > 40 years, and 0.9% < GRWR < 1.9%) and found that both variables were predictors in the regression analysis. In the literature, few studies investigated the influence of donor and donor-recipient match factors on NCs. Study from the SRTR database have shown an increased risk of graft failure with donor age > 40 years[25]. Prior studies on NC risk factors including donor age and GRWR showed no significant difference of these variables[5,11]. In contrast, our study indicated that risk of NC depends on either extreme donor age, or improper GRWR. In the GAM plots, we could see the probability of NCs decreasing when GRWR was above 1.9, which could suggest better detoxification capabilities of larger grafts. Besides, small size grafts are not likely to increase NC risk, suggesting non-inferiority of small graft when graft inflow is properly controlled[26].
Male-to-male gender match was another independent risk factor for NCs in this study. The association between donor-gender match and neurologic complications has not been reported previously. The impact of gender mismatch on the outcome of liver transplantation is still controversial. Several studies demonstrated that female-to-male gender match is associated with negative outcomes in kidney, heart, lung, and liver transplantations, possibly due to the effect of estrogen and the relative small-for-size of female grafts[27-30]. It is unclear whether other confounding variables specific to male gender play a role, such as prevalent alcoholic liver diseases. Further prospective, controlled studies are needed to confirm the gender match effect on NCs.
The sequence of transplantation was a notable finding. The risk of NCs decreased after 174 transplantations by the team, and the order between 31 and 174 was an independent risk factor for NC development. The lower risk in the first 30 cases could be related to strict patient selection and relatively conservative strategy of immunosuppression. Learning curve effect in liver transplantation has been described, which is affected by the volume of the center or the year of transplant[31-34]. For successful liver transplantation, multidisciplinary team work is needed. The improvement is important, not only in the surgical technique but also in donor selection, timely decision for surgery, standardized postoperative care and optimal immunosuppression. Compared to graft survival rate commonly used in studies with a learning curve, NCs are more likely to be related to non-operative factors[35,36]. It is the environment influenced by perioperative risk factors from all perspectives that results in complications. To avoid NCs, the transplant team may need risk stratification during patient selection and donor matching, correcting risk factors before the operation as much as possible, and should remain watchful during the perioperative period. Ideal immunosuppression aims at minimizing acute rejection and avoids neurotoxicity. It is crucial for the transplant surgeon to recognize this process of development and to conduct interdisciplinary learning as well as continuously improving the patient care quality of the team.
This study has limitations owing to its retrospective design. First, the complications included were those that were identified and reported by the clinician. While serious complications are rarely excluded, clinicians may have missed minor complications that resolved spontaneously without treatment. Second, NCs often manifests as one or more signs, but it is possible that only the most serious ones were documented, leading to misclassification. Third, patients in the control group might have shared features and exposures with those in the NC group. Even if we have considered as many potential important variables as we could, residual confounders are still possible. Despite these limitations, the risk factors identified in the study could be subjective for validation in further prospective, randomized studies.
In conclusion, NCs after liver transplantation occur frequently and affect almost half of liver recipients, with encephalopathy being the most common (73%). In this study, we identified 11 risk factors for neurologic complications. From the recipient’s perspective, age < 29 or ≥ 60 years along with BMI < 21.6 or > 27.6 kg/m2 significantly increased NC risk, and Child-Pugh score > 9, hepatic encephalopathy, mental disorder, 7-d tacrolimus level > 8.9 ng/mL, intraabdominal infection were complementary to previous studies. Patients with history of variceal bleeding were less likely to develop NCs. Novel risk factors from donor’s and surgeon’s perspective were donor age < 22 or ≥ 40 years, male-to-male gender match, GRWR between 0.9 and 1.9 and sequence of transplantation between 31 and 174. Our results provide a basis for risk stratification, which would enable transplant surgeons to weigh the risk during patient selection, control unfavorable factors before operation, avoid neurotoxicity during perioperative period, and perform active surveillance after operation. The manner in which the experience of transplant team influences NCs should also be taken into account by the team leader or health care manager who would allow the conduction of interdisciplinary education as well as strategies for continuous quality improvement.
The authors are grateful for the statistical support provided by Dr. Fu-Chang Hu.
Neurologic complications (NCs) are common after liver transplantation and result in significant morbidity and mortality. Encephalopathy, the most frequent etiology, is multifactorial in nature. Surgeons should identify patients at risk and avoid predisposing variables during the perioperative period to minimize their incidence. However, known risk factors have been mainly investigated from the recipient’s perspective.
The authors have considered various factors from recipient, donor, donor-recipient matching, and surgeon perspectives that had potential impact on early NC after liver transplantation. They had better control of confounders from the case-control study design using more flexible statistical tools, including generalized additive models for variables, which had a nonlinear effect on outcome.
In addition to risk factors from the recipient perspective that were complementary to those in the literature, novel risk factors discovered in this study were donor age (< 22 or ≥ 40 years), male-to-male gender matching, graft-recipient weight ratio 0.9%-1.9%, and sequence of transplantation between 31 and 174.
The results provide a basis for risk stratification for surgeons to minimize neurotoxic factors during transplantation. In addition, the transplant team leader should be aware of the higher risk of neurologic complication during the team’s earlier experiences to conduct interdisciplinary education and quality improvement program.
The manuscript is clearly written to describe the results with pretty good discussion. The method section is really well written as a clinical report and can be one of the greatest examples for physician-scientists.
| 1. | Senzolo M, Ferronato C, Burra P. Neurologic complications after solid organ transplantation. Transpl Int. 2009;22:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 2. | Zivković SA. Neurologic complications after liver transplantation. World J Hepatol. 2013;5:409-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Dhar R, Young GB, Marotta P. Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care. 2008;8:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Kim KM, Kim GS, Ko JS, Gwak MS, Lee SK, Son MG. Factors associated with consciousness recovery time after liver transplantation in recipients with hepatic encephalopathy. Transplant Proc. 2014;46:712-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Vizzini G, Asaro M, Miraglia R, Gruttadauria S, Filì D, D’Antoni A, Petridis I, Marrone G, Pagano D, Gridelli B. Changing picture of central nervous system complications in liver transplant recipients. Liver Transpl. 2011;17:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Saner FH, Gensicke J, Olde Damink SW, Pavlaković G, Treckmann J, Dammann M, Kaiser GM, Sotiropoulos GC, Radtke A, Koeppen S. Neurologic complications in adult living donor liver transplant patients: an underestimated factor? J Neurol. 2010;257:253-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Amodio P, Biancardi A, Montagnese S, Angeli P, Iannizzi P, Cillo U, D’Amico D, Gatta A. Neurological complications after orthotopic liver transplantation. Dig Liver Dis. 2007;39:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Busuttil BW, Klintmalm GK. Transplantation of the Liver. Philadelphia, Pennsylvania: Saunders 2005; 1029-1036. |
| 9. | Bronster DJ, Emre S, Boccagni P, Sheiner PA, Schwartz ME, Miller CM. Central nervous system complications in liver transplant recipients--incidence, timing, and long-term follow-up. Clin Transplant. 2000;14:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Lewis MB, Howdle PD. Neurologic complications of liver transplantation in adults. Neurology. 2003;61:1174-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Kim BS, Lee SG, Hwang S, Park KM, Kim KH, Ahn CS, Moon DB, Ha TY, Song GW, Kim DS. Neurologic complications in adult living donor liver transplant recipients. Clin Transplant. 2007;21:544-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Klintmalm GB, Davis GL, Teperman L, Netto GJ, Washburn K, Rudich SM, Pomfret EA, Vargas HE, Brown R, Eckhoff D. A randomized, multicenter study comparing steroid-free immunosuppression and standard immunosuppression for liver transplant recipients with chronic hepatitis C. Liver Transpl. 2011;17:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 13. | Yee TW, Wild C. Vector generalized additive models. J Roy Statist Soc Ser B. 1996;58:481-493. |
| 14. | Pujol A, Graus F, Rimola A, Beltrán J, Garcia-Valdecasas JC, Navasa M, Grande L, Galofré J, Visa J, Rodés J. Predictive factors of in-hospital CNS complications following liver transplantation. Neurology. 1994;44:1226-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Garcia-Martinez R, Rovira A, Alonso J, Jacas C, Simón-Talero M, Chavarria L, Vargas V, Córdoba J. Hepatic encephalopathy is associated with posttransplant cognitive function and brain volume. Liver Transpl. 2011;17:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Buis CI, Wiesner RH, Krom RA, Kremers WK, Wijdicks EF. Acute confusional state following liver transplantation for alcoholic liver disease. Neurology. 2002;59:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Kanwal F, Chen D, Ting L, Gornbein J, Saab S, Durazo F, Yersiz H, Farmer D, Ghobrial RM, Busuttil RW. A model to predict the development of mental status changes of unclear cause after liver transplantation. Liver Transpl. 2003;9:1312-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54:1578-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 326] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 892] [Cited by in RCA: 1033] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 20. | Yoshimura Y, Kubo S, Shirata K, Hirohashi K, Tanaka H, Shuto T, Takemura S, Kinoshita H. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World J Surg. 2004;28:982-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Dick AA, Spitzer AL, Seifert CF, Deckert A, Carithers RL, Reyes JD, Perkins JD. Liver transplantation at the extremes of the body mass index. Liver Transpl. 2009;15:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Bemeur C. Neurological complications post-liver transplantation: impact of nutritional status. Metab Brain Dis. 2013;28:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Antar R, Wong P, Ghali P. A meta-analysis of nutritional supplementation for management of hospitalized alcoholic hepatitis. Can J Gastroenterol. 2012;26:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Cruz RJ, DiMartini A, Akhavanheidari M, Iacovoni N, Boardman JF, Donaldson J, Humar A, Bartynski WS. Posterior reversible encephalopathy syndrome in liver transplant patients: clinical presentation, risk factors and initial management. Am J Transplant. 2012;12:2228-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1526] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 26. | Feng AC, Fan HL, Chen TW, Hsieh CB. Hepatic hemodynamic changes during liver transplantation: a review. World J Gastroenterol. 2014;20:11131-11141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 27. | Rodríguez-Castro KI, De Martin E, Gambato M, Lazzaro S, Villa E, Burra P. Female gender in the setting of liver transplantation. World J Transplant. 2014;4:229-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Burra P, De Martin E, Gitto S, Villa E. Influence of age and gender before and after liver transplantation. Liver Transpl. 2013;19:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Li C, Wen TF, Yan LN, Li B, Yang JY, Wang WT, Xu MQ, Wei YG. Predictors of patient survival following living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2011;10:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Marino IR, Doyle HR, Aldrighetti L, Doria C, McMichael J, Gayowski T, Fung JJ, Tzakis AG, Starzl TE. Effect of donor age and sex on the outcome of liver transplantation. Hepatology. 1995;22:1754-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Adam R, Cailliez V, Majno P, Karam V, McMaster P, Caine RY, O’Grady J, Pichlmayr R, Neuhaus P, Otte JB. Normalised intrinsic mortality risk in liver transplantation: European Liver Transplant Registry study. Lancet. 2000;356:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Broering DC, Kim JS, Mueller T, Fischer L, Ganschow R, Bicak T, Mueller L, Hillert C, Wilms C, Hinrichs B. One hundred thirty-two consecutive pediatric liver transplants without hospital mortality: lessons learned and outlook for the future. Ann Surg. 2004;240:1002-1012; discussion 1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Morioka D, Egawa H, Kasahara M, Ito T, Haga H, Takada Y, Shimada H, Tanaka K. Outcomes of adult-to-adult living donor liver transplantation: a single institution’s experience with 335 consecutive cases. Ann Surg. 2007;245:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Li C, Mi K, Wen Tf, Yan Ln, Li B, Yang Jy, Xu Mq, Wang WT, Wei Yg. A learning curve for living donor liver transplantation. Dig Liver Dis. 2012;44:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Campagna F, Biancardi A, Cillo U, Gatta A, Amodio P. Neurocognitive-neurological complications of liver transplantation: a review. Metab Brain Dis. 2010;25:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Teperman LW. Impact of pretransplant hepatic encephalopathy on liver posttransplantation outcomes. Int J Hepatol. 2013;2013:952828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kita K, Rydzewski A S- Editor: Qi Y L- Editor: A E- Editor: Ma S