Published online Jun 7, 2016. doi: 10.3748/wjg.v22.i21.5033
Peer-review started: February 3, 2016
First decision: March 7, 2016
Revised: March 14, 2016
Accepted: March 30, 2016
Article in press: March 30, 2016
Published online: June 7, 2016
Processing time: 118 Days and 3.4 Hours
AIM: To investigate the inhibitory efficacy of 125I-labeled anti-basic fibroblast growth factor (bFGF) monoclonal antibody (mAb) in hepatocellular carcinoma (HCC).
METHODS: bFGF mAb was prepared by using the 1G9B9 hybridoma cell line with hybridization technology and extracted from ascites fluid through a Protein G Sepharose affinity column. After labeling with 125I through the chloramine-T method, bFGF mAb was further purified by a Sephadex G-25 column. Gamma radiation counter GC-1200 detected radioactivity of 125I-bFGF mAb. The murine H22 HCC xenograft model was established and randomized to interventions with control (phosphate-buffered saline), 125I-bFGF mAb, 125I plus bFGF mAb, bFGF mAb, or 125I. The ratios of tumor inhibition were then calculated. Expression of bFGF, fibroblast growth factor receptor (FGFR), platelet-derived growth factor, and vascular endothelial growth factor (VEGF) mRNA was determined by quantitative reverse transcriptase real-time polymerase chain reaction.
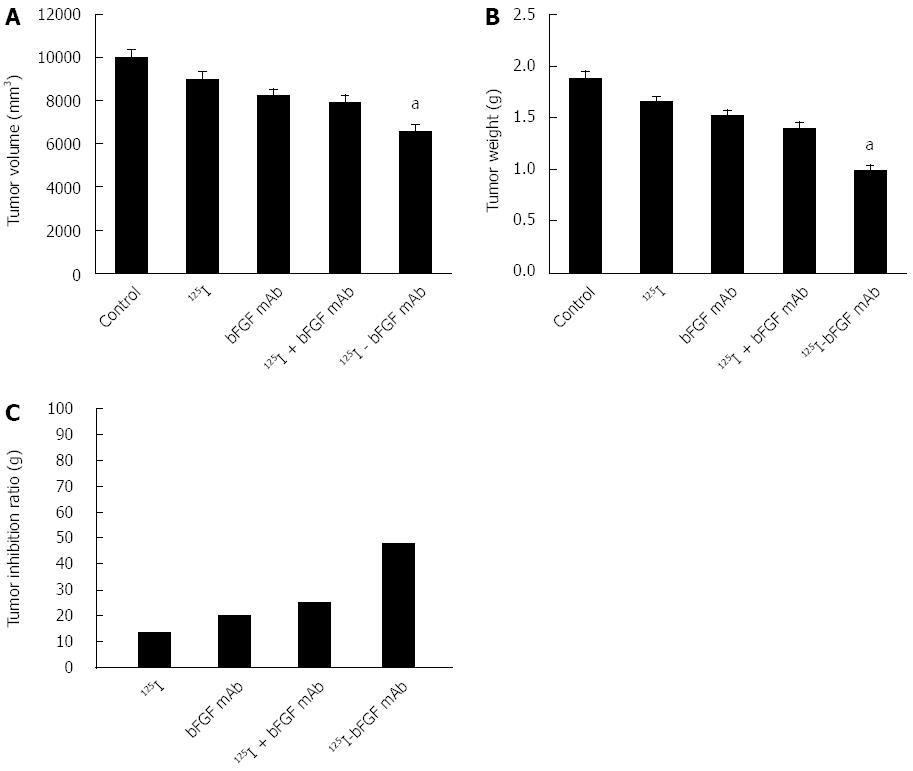
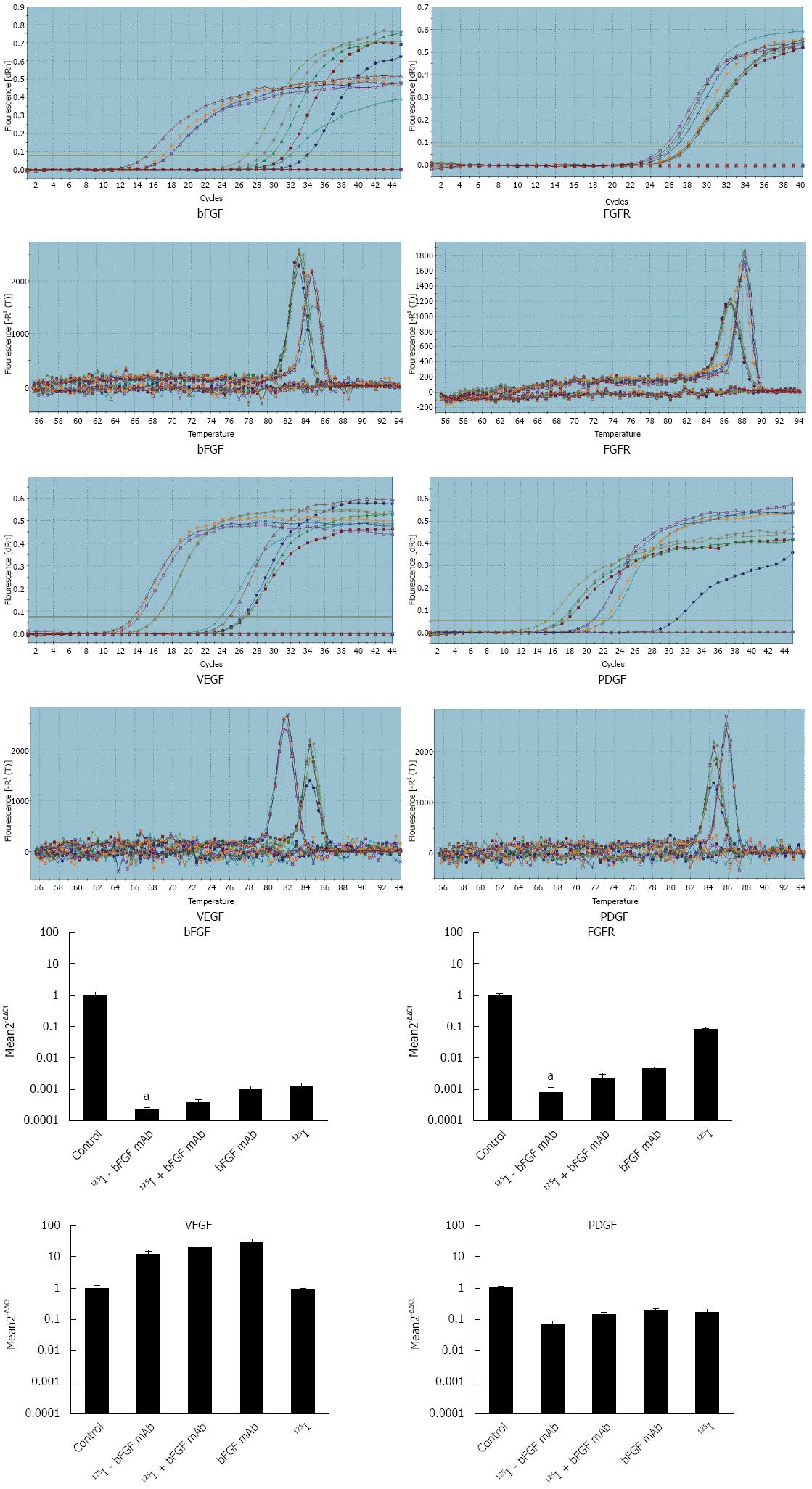
RESULTS: The purified bFGF mAb solution was 8.145 mg/mL with a titer of 1:2560000 and was stored at -20 °C. After coupling, 125I-bFGF mAb was used at a 1: 1280000 dilution, stored at 4 °C, and its specific radioactivity was 37 MBq/mg. The corresponding tumor weight in the control, 125I, bFGF mAb, 125I plus bFGF mAb, and 125I-bFGF mAb groups was 1.88 ± 0.25, 1.625 ± 0.21, 1.5 ± 0.18, 1.41 ± 0.16, and 0.98 ± 0.11 g, respectively. The tumor inhibition ratio in the 125I, bFGF mAb, 125I plus bFGF mAb, and 125I-bFGF mAb groups was 13.6%, 20.2%, 25.1%, and 47.9%, respectively. Growth of HCC xenografts was inhibited significantly more in the 125I-bFGF mAb group than in the other groups (P < 0.05). Expression of bFGF and FGFR mRNA in the 125I-bFGF mAb group was significantly decreased in comparison with other groups (P < 0.05). Groups under interventions revealed increased expression of VEGF mRNA (except for 125I group) compared with the control group.
CONCLUSION: 125I-bFGF mAb inhibits growth of HCC xenografts. The coupling effect of 125I-bFGF mAb is more effective than the concomitant use of 125I and bFGF mAb.
Core tip: The aim of this study was to investigate the inhibitory efficacy of 125I-basic fibroblast growth factor (bFGF) monoclonal antibody (mAb) in mice with hepatocellular carcinoma (HCC). 125I-bFGF mAb inhibited the growth of HCC xenografts (P < 0.05). The combination of 125I and bFGF mAb was more effective than the concomitant use of 125I and bFGF mAb. 125I-bFGF mAb also significantly reduced the expression of bFGF and fibroblast growth factor receptor (FGFR) mRNA (P < 0.05). Moreover, 125I-bFGF mAb downregulated platelet-derived growth factor mRNA and upregulated vascular endothelial growth factor mRNA.
- Citation: Hu PH, Pan LH, Wong PTY, Chen WH, Yang YQ, Wang H, Xiang JJ, Xu M. 125I-labeled anti-bFGF monoclonal antibody inhibits growth of hepatocellular carcinoma. World J Gastroenterol 2016; 22(21): 5033-5041
- URL: https://www.wjgnet.com/1007-9327/full/v22/i21/5033.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i21.5033
Hepatocellular carcinoma (HCC) ranks among the most common cancers worldwide. It is the third leading cause of cancer death, with about 700000 cases diagnosed annually[1]. It is characterized by rapid progression, metastasis, and recurrence. Surgical resection and liver transplantation are traditional therapeutic approaches for HCC. Liver transplantation offers many benefits for HCC, but shortage of donor organs and high costs constrain its application. New therapeutic methods, such as radiofrequency ablation, transcatheter arterial chemoembolization, local hyperthermia, and targeted therapy, can also be beneficial to patients with HCC[2-4].
HCC is one of the most vascularized solid tumors, and angiogenesis plays a pivotal role in its development, progression, and metastasis. Basic fibroblast growth factor (bFGF) is one of the most prominent angiogenesis-promoting agents, and its expression closely correlates with tumor angiogenesis[5]. Previous studies have revealed that bFGF stimulates proliferation of human HCC cell lines[6], and the serum bFGF levels in patients with HCC are significantly higher than those in healthy volunteers[7]. These increases in serum bFGF levels correlate closely with HCC invasion and recurrence[8,9]. These studies indicate that specific targeting of bFGF may provide a novel therapeutic strategy for HCC.
bFGF monoclonal antibody (mAb) can specifically bind to bFGF and block its growth-stimulating activity. In our previous studies, we found that bFGF mAb combined with S-1 (gimeracil and oteracil potassium) synergistically inhibited Lewis-transplanted lung cancer, which was related to its inhibition of proliferation and angiogenesis[10]. Combination of bFGF mAb and radiotherapy was shown to exert a synergistic inhibitory effect on the growth of B16-transplanted melanoma tumors, since it increases the radiosensitivity of tumor cells by reducing the expression of bFGF, decreasing angiogenesis, and promoting apoptosis[11]. bFGF mAb also inhibits the proliferation of MCF-7/ADM breast cancer cells and reverses multidrug resistance. The phenomenon may be associated with downregulation of P-glycoprotein and increased intracellular concentration of chemotherapeutic drugs[12].
125I radiotherapy enhances DNA damage, and consequently, induces liver cancer cell apoptosis and improves overall survival in HCC[13]. The use of radionuclide labels on mAbs enhances the specificity of their targeting, and increases the accuracy of evaluating therapeutic response[14]. Thus, coupling bFGF mAb with 125I was used in the present study. Our previous study demonstrated that the half-life of 125I-bFGF mAb was 81.6-90.3 h and that the radioactive counts were highly detected in the liver tissue of mice[15]. Therefore, 125I-bFGF mAb may be an attractive therapeutic modality for HCC. In this study, we aimed to investigate the feasibility and therapeutic efficacy of 125I-bFGF mAb in HCC.
We prepared the 1G9B9 hybridoma cell line, which was developed in our laboratory with hybridization technology and can secrete mAbs against bFGF. After injecting 105 hybridoma cells into each BABL/c mice with incomplete Freund’s adjuvant (Sigma-Aldrich, St Louis, MO, United States), ascites was formed in mice 7 d later. The ascites fluid was extracted and purified twice in ammonium sulfate and a Protein G Sepharose affinity column (General Electric, Fairfield, CT, United States). bFGF mAb was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The concentration and titer of purified bFGF mAb stock solution were assayed by bicinchoninic acid (BCA) standard assay kit (Pierce, Rockford, IL, United States) and indirectly by enzyme linked immunosorbent assay (ELISA), respectively. Finally, bFGF mAb stock solution was cryopreserved at -20 °C.
bFGF mAb was labeled with 125I (Amersham Biosciences, Chalfont St. Giles, United Kingdom) by using the chloramine-T method. Afterwards, 125I-bFGF mAb was purified by Sephadex G-25 column (Pharmacia, Piscataway, NJ, United States) in phosphate buffered saline (PBS) (0.05 mol/L, pH 7.5) at room temperature. The labeling efficiency and titer of 125I-bFGF mAb were tested by paper chromatography and indirectly by ELISA, respectively. In order to investigate the stability and storage temperature of 125I-bFGF mAb, assays for radiochemical purity of 125I-bFGF mAb were performed using a gamma radiation counter GC-1200 (Zhongjia Photoelectric Instrument Company, Hefei, China) in 1-8 d with variable temperatures. The radioactive counts of quality controlled samples (0.5, 5.0 and 50.0 ng/mL) were tested by gamma radiation counter GC-1200 in six replicates on three different days to evaluate the accuracy of the assay. The intra-day coefficient of variation (CV) and inter-day CV were also calculated.
We adjusted the concentration of H22 hepatoma cells to 2.5 × 106/mL during the logarithmic growth phase. Each C57BL/6 mouse was injected with 0.2 mL of cells in the armpit of the right front limb. After the tumor diameters grew to 7-8 mm, Kalium jodatum was consumed by mice for 3 d to inhibit the absorption of 125I by the thyroid gland before treatment. Twenty-five mice were randomized into five groups: control (PBS), 125I, bFGF mAb, 125I plus bFGF mAb, and 125I-bFGF mAb. The injection doses for each group per mouse were 0.2 mL PBS, 7.4 MBq Na125I, 200 μg bFGF mAb, 7.4 MBq Na125I plus 200 μg bFGF mAb, and 37 MBq/mg 125I-bFGF mAb 200 μg, respectively. The drug was given once every 3 d, five times in total (15 d). After sacrificing the mice and dissecting the tumors, the volume and weight of the tumor were measured and the ratio of tumor inhibition was calculated.
Expression of bFGF, vascular endothelial growth factor (VEGF), fibroblast growth factor receptor (FGFR), and platelet-derived growth factor (PDGF) mRNA was measured by quantitative reverse transcriptase real-time polymerase chain reaction (qRT-PCR). β-actin was used as an internal reference gene. Total RNA was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, United States). The concentration and quality of the extracted RNA were detected on the measured absorbance at 260 nm and a ratio of (A260/A280). cDNA was synthesized using Transcript High Fidelity cDNA Synthesis Kit (Fermentas, Waltham, MA, United States). The primers of DNA sequences were as follows: bFGF (5’-TAT TTC TTT GGC TGC TAC TTG-3’ and 5’-TCC AGC ATT TCG GTG TTG-3’); FGFR (5’-CCT CGT TTG GAG ACG BCT TCA-3’ and 5’-GAG CAA AGG GTG TGT GGA CTC T-3’); VEGF (5’-GAA TGT GAT TGC TTT CCT GGG TA-3’ and 5’-AGT AAA AGT GGC TGT GGT GGT CCT GA-3’); PDGF (5’-GAG ATA GAC TCC GTA GGG GCT GA-3’ and 5’-GAG CAA AGG GTG TGT GGA CTC T-3’); β-actin (5’-CAA GAT CAT TGC TCC TCC TGA-3’ and 5’-AGT CCG CCT AGA AGC ATT TG-3’). Using Light Cycler 480 SYBR Green I Master Mix (Roche, Basel, Switzerland), qPCR was performed according to the qPCR protocol. Conditions used for the qPCR amplification were shown as follows: 95 °C for 5 min, 55 cycles; 94 °C for 10 s, 62 °C for 15 s, 72 °C for 10 s, and 65 °C for 1 min. Melting curves were analyzed to detect the specificity of qPCR products. The expressions of bFGF, VEGF, FGFR, and PDGF mRNA were analyzed by Mx Pro QPCR software version 3.0, and the housekeeping gene β-actin was used as a normalized target gene.
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Laboratory Animal Center of Jinan University. The animal protocol in our experiment was designed to minimize pain and discomfort to the mice. The mice were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. Intragastric administration was carried out with conscious mice, using straight gavage needles appropriate for the animal size (15-17 g body weight: 22 gauge, 2.54 cm length, and 1.25 mm ball diameter). All mice were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for HCC xenograft collection.
The descriptive data are given as mean and standard deviation. The results were analyzed by SPSS version 16.0 (Chicago, IL, United States) with a t test. P < 0.05 was considered to be statistically significant.
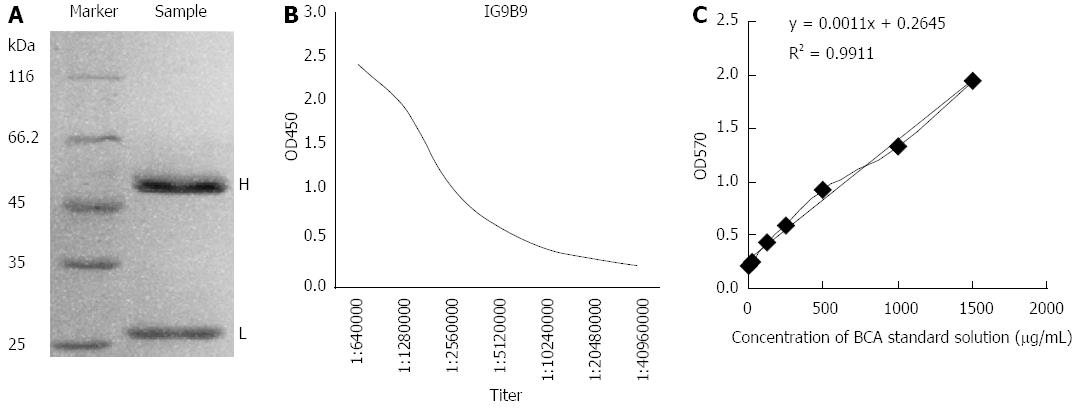
Ascites was produced after 1G9B9 hybrid tumor cells were injected into the abdominal cavity of mice for 7-12 d. Each mouse provided 1.8-2.2 mL ascites fluid, and a final volume of 30 mL was obtained. Based on SDS-PAGE of purified bFGF mAb, there were only two bFGF mAb chains, and there was no non-specific chain, indicating the high purity of bFGF mAb. The molecular weight of the heavy chain was about 50 ku while the light chain was about 25 ku (Figure 1). The titer and concentration of purified bFGF mAb solution were 1:2560000 and 8.145 mg/mL, respectively (Figure 1).
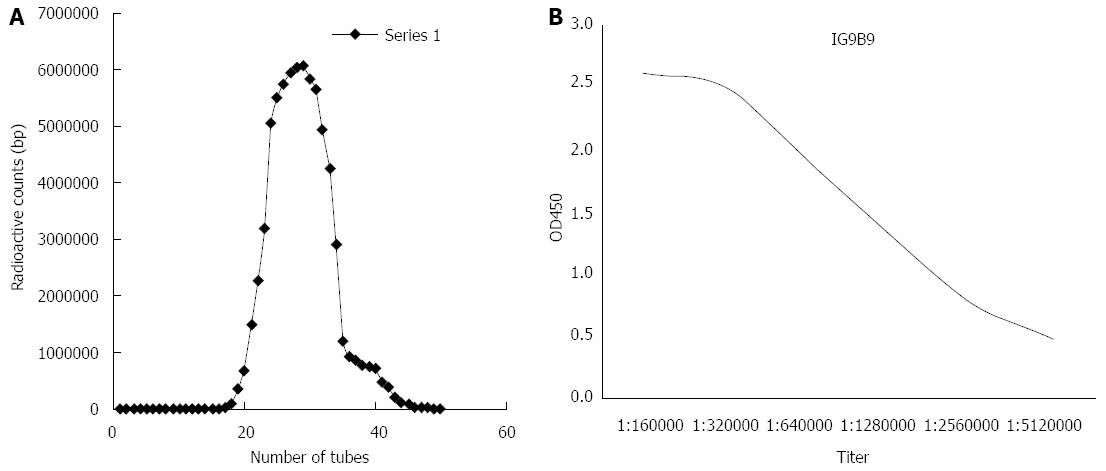
The optimal labeling conditions for the chloramine-T method in our study consisted of chloramine-T 50 μg, Na125I 3.7 MBq, Na2S2O5 100 μg, and bFGF mAb 100 μg, with a reaction time of 45 s. Gamma radiation counter GC-1200 was used to test the radioactivity of the collected tubes. From tubes 1-19, the radioactive counts were close to zero. Starting from tube 20, the radioactivity counts increased and peaked at tube 30. Subsequently, the counts began to decline and reached zero again at tube 46 (Figure 2). Formation of the radioactive peak indicated successful preparation of 125I-bFGF mAb. The remaining liquid was abandoned. The labeling efficiency of 125I-bFGF mAb was ≥ 90%, which was tested by paper chromatography. The purity of 125I-bFGF mAb became ≥ 98% after purified by Sephadex G-25 column. The titer was 1:1280000, which implicated no decrease in immunoreactivity (Figure 2). 125I-bFGF mAb was prone to denaturation at room temperature and iodine removal at -20 °C. 125I-bFGF mAb was stably maintained when stored at 4 °C as the level of radiochemical purity remained ≥ 90% over 6 d. The intra-day CV of quality controlled samples (0.5, 5.0, and 50.0 ng/mL) at the radioactive counts were 0.8%, 1.3%, and 6.8%, respectively, and the inter-day CV was 4.8%, 3.7%, and 8.5%, respectively. The specific radioactivity of the 125I-bFGF mAb used in this study was 37 MBq/mg.
The corresponding volume and weight of the tumor in the control, 125I, bFGF mAb, 125I plus bFGF mAb, and 125I-bFGF mAb groups were 9968 ± 430, 8987 ± 360, 8217 ± 301, 7927 ± 329, and 6210 ± 298 mm3 and 1.88 ± 0.25, 1.63 ± 0.21, 1.50 ± 0.18, 1.41 ± 0.16, and 0.98 ± 0.11 g, respectively. When compared with the control group, the tumor inhibition ratio in the 125I, bFGF mAb, 125I plus bFGF mAb, and 125I-bFGF mAb groups was 13.6%, 20.2%, 25.1%, and 47.9%, respectively (Figure 3). 125I-bFGF mAb effectively inhibited the growth of HCC (P < 0.05), and the tumor inhibition ratio of the 125I-bFGF mAb group was higher than that in the other groups.
qRT-PCR amplification and melt curves of β-actin, bFGF, FGFR, VEGF, and PDGF are shown in Figure 4. Expression of these genes entered the plateau of amplification. All the samples were amplified with a single product, and there was no non-specific amplification. According to the relative quantitative method of 2-ΔΔCt, the relative expression of bFGF and FGFR mRNA decreased significantly in the 125I-bFGF mAb group when compared with other treatment groups (P < 0.05). In groups with interventions, expression of PDGF mRNA decreased while VEGF mRNA was higher (except for 125I group) than that in the control group (Figure 4).
bFGF is an 18 ku non-glycosylated polypeptide consisting of 146 amino acids that was first isolated and purified from bovine pituitary and brain. It is involved in cell migration and differentiation and is a driving force of mitogenesis and angiogenesis[5]. bFGF has been shown to disrupt the balance of cell cycle progression and apoptosis[16]. By activating protein kinase B, it enhances proliferation of HCC cells via the phosphoinositide 3-kinase pathway[17]. Growth of HCC was inhibited by human sulfatase 1, a bFGF-stimulated signaling blocker[16]. Moreover, it was previously demonstrated that a novel mAb to FGF-2 alone, without radiolabeling, effectively inhibited the growth of HCC xenografts[18].
Our results showed that 125I-bFGF mAb significantly inhibited growth of HCC xenografts more than the other interventions (P < 0.05) and that the inhibition ratio of the 125I-bFGF mAb group (47.9%) was higher than that of the 125I plus bFGF mAb group (25.1%). Combining 125I and bFGF mAb was more effective than concomitant use of 125I and bFGF mAb in the treatment of HCC. The use of radionuclide labels on mAbs enhanced the specificity of cellular targeting[14]. Such augmented specificity and accuracy could allow 125I-bFGF mAb to yield greater efficacy in treating mice with HCC compared with concomitant use of 125I and bFGF mAb. Among patients with HCC, the serum levels of bFGF were increased, and elevated bFGF independently predicted poor disease-free survival preoperatively[8]. It is tempting to consider 125I-bFGF mAb as a potential clinical option for HCC therapy in the future.
125I-bFGF mAb reduced levels of FGFR and PDGF in our study. FGFR plays a pivotal role in HCC differentiation, proliferation, invasiveness, and resistance to chemotherapy[19-21]. FGFR is highly expressed in HCC and is associated with short overall survival[22]. A humanized monoclonal antibody to FGFR was reported to inhibit tumor growth in HCC xenograft models[23]. In contrast, PDGF, a proangiogenic factor, contributes to vessel maturation[24] and aids in the proliferation and metastasis of HCC[25]. Upregulation of PDGF and PDGF receptors is associated with chemoresistance of gemcitabine and poor prognosis in patients with HCC[26,27]. 125I-bFGF mAb is also a promising agent in tackling liver cancer by decreasing both FGF and PDGF. Perhaps 125I-bFGF mAb enhances therapeutic efficacy of gemcitabine when both agents are indicated in patients with HCC; this possibility requires further investigation.
Our results showed that the expression of VEGF was higher in mice treated with 125I-bFGF mAb, 125I plus bFGF mAb, and bFGF mAb in spite of the improved tumor inhibition ratios and decreased levels of bFGF, FGFR, and PDGF. Previously, increased expression of bFGF was observed in xenotransplanted squamous cell carcinoma after anti-VEGF treatment[28]. Antiangiogenic therapy may impair vessel formation but improve vascular function and tissue oxygenation[29]. Such vessel normalization may become a compensatory reaction of the tumor in response to the depletion of VEGF, leading to increased oxygenation and the observed increased bFGF[28]. In our study, application of anti-bFGF to a murine model of HCC increased VEGF, suggesting that blockade of VEGF elevates bFGF and vice versa. We speculate that vessel normalization also takes place even when anti-bFGF (an antiangiogenic agent) is used and that VEGF is increased by improved tissue oxygenation. Bevacizumab, a potent VEGF inhibitor A, was the first VEGF inhibitor approved by the United States Food and Drug Administration, and it demonstrates modest antitumor activity across a broad range of malignancies when combined with chemotherapy[30]. However, some patients are insensitive to bevacizumab. One study found that a VEGF/bFGF ratio correlated more closely with sensitivity to bevacizumab than with VEGF alone[31]. We found that 125I-bFGF mAb increased expression of VEGF in the HCC group. Therefore, we hypothesized that 125I-bFGF mAb in combination with VEGF mAb may enhance sensitivity to bevacizumab and improve efficacy in the treatment of HCC. In the future, we will determine the effect of combination 125I-bFGF mAb and bevacizumab on the treatment of HCC.
A recent study found using gefinitib-resistant cell lines that the expression of FGFR1 and bFGF was elevated and that inhibiting either bFGF or FGFR1 by small interfering RNA (siRNA) or FGFR inhibitor (PD173074) restored gefitinib sensitivity. These findings implicate activation of an FGFR autocrine loop as a mechanism of acquired resistance to epithelial growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) in non-small cell lung cancer[32]. Since 125I-bFGF mAb can decrease significantly bFGF and FGFR, combining 125I-bFGF mAb and EGFR-TKIs might enhance the therapeutic value of EGFR-TKIs.
In conclusion, 125I-bFGF mAb effectively inhibited the growth of HCC xenografts; significantly reduced expression of bFGF and FGFR; and upregulated VEGF expression. Combined 125I and bFGF mAb was more effective than concomitant use of 125I and bFGF mAb in the treatment of HCC.
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third leading cause of cancer-related death. In clinical practice, the majority of HCC patients are diagnosed at an inoperable stage, resulting in a low long-term survival rate and poor prognosis. Since basic fibroblast growth factor (bFGF) is one of the most prominent angiogenesis-related factors, angiogenesis plays an important role in HCC progression. Here, the authors investigated the biological inhibition efficacy of 125I-labeled bFGF monoclonal antibody (mAb) in mice with HCC. In the near future, these findings might be helpful to clinicians selecting individualized treatment strategies.
Targeted therapy is one of the main treatment approaches for patients with advanced HCC. New targeted drugs, such as sorafenib and sunitinib, have improved clinical efficacy. However, drug resistance and side effects of sorafenib and sunitinib constrain their clinical application. Therefore, it is necessary to investigate alternative targeting drugs, such as mAb to bFGF, for patients with advanced HCC.
To the best of our knowledge, this is the first study to label bFGF mAb with 125I for the treatment of HCC. The study revealed that 125I-bFGF mAb inhibited growth of HCC xenografts more effectively than the concomitant use of 125I and bFGF mAb. The authors also found that 125I-bFGF mAb reduced expression of bFGF, FGF receptor, and platelet-derived growth factor.
This study found that 125I-bFGF mAb inhibited growth of HCC xenografts, suggesting that it could be used to tackle liver cancer. More trials are warranted to provide evidence for other applications. 125I-bFGF mAb significantly inhibited the expression of bFGF and FGF receptor, while vascular endothelial growth factor (VEGF) expression was upregulated. Therefore, combination treatment of HCC with VEGF mAb is worthy of further investigation.
bFGF mAb is a target drug that can specifically bind to bFGF and block its growth-stimulating activity. It is widely used in laboratory research, and it can significantly inhibit growth of human HCC cell lines in vitro and in vivo. Therefore, bFGF mAb could be a promising drug in the treatment of liver cancer.
This is a well-designed and executed project on the inhibitory efficacy of 125I-bFGF mAb in HCC. The results show that 125I-bFGF mAb inhibits growth of HCC xenografts more effectively than the concomitant use of 125I and bFGF mAb. 125I-bFGF mAb may be a potential clinical option for HCC therapy in the future.
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3629] [Article Influence: 259.2] [Reference Citation Analysis (12)] |
| 2. | Song do S, Nam SW, Bae SH, Kim JD, Jang JW, Song MJ, Lee SW, Kim HY, Lee YJ, Chun HJ. Outcome of transarterial chemoembolization-based multi-modal treatment in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2015;21:2395-2404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Wang XP, Xu M, Gao HF, Zhao JF, Xu KC. Intraperitoneal perfusion of cytokine-induced killer cells with local hyperthermia for advanced hepatocellular carcinoma. World J Gastroenterol. 2013;19:2956-2962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Park JG, Park SY, Lee HW. Complete remission of advanced hepatocellular carcinoma by radiofrequency ablation after sorafenib therapy. World J Gastroenterol. 2015;21:2568-2572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Okada-Ban M, Thiery JP, Jouanneau J. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:263-267. [PubMed] |
| 6. | Ogasawara S, Yano H, Iemura A, Hisaka T, Kojiro M. Expressions of basic fibroblast growth factor and its receptors and their relationship to proliferation of human hepatocellular carcinoma cell lines. Hepatology. 1996;24:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Tsunematsu H, Tatsumi T, Kohga K, Yamamoto M, Aketa H, Miyagi T, Hosui A, Hiramatsu N, Kanto T, Hayashi N. Fibroblast growth factor-2 enhances NK sensitivity of hepatocellular carcinoma cells. Int J Cancer. 2012;130:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Poon RT, Ng IO, Lau C, Yu WC, Fan ST, Wong J. Correlation of serum basic fibroblast growth factor levels with clinicopathologic features and postoperative recurrence in hepatocellular carcinoma. Am J Surg. 2001;182:298-304. [PubMed] |
| 9. | Gao Y, Zheng DY, Cui Z, Ma Y, Liu YZ, Zhang W. Predictive value of quantitative contrast-enhanced ultrasound in hepatocellular carcinoma recurrence after ablation. World J Gastroenterol. 2015;21:10418-10426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Zhang GJ, Xu M, Zhao JF, Wang H, Xiang JJ, Deng N, Zeng SB, Wang PP. Synergistic inhibitory effects of bFGF monoclonal antibody and S-1 against proliferation of lung cancer Lewis cells and angiogenesis of transplanted tumors. Zhongguo Zhongliu Shengwu Zhiliao Zazhi. 2011;18:280-284. |
| 11. | Zheng SB, Xu M, Pan LH, Xiang JJ, Deng N, Li D, Wang PP. Synergistic inhibitory effects of bFGF monoclonal antibody combined with radio therapy on B16-transplanted tumors in mice. Zhongguo Zhongliu Shengwu Zhiliao Zazhi. 2011;18:175-180. |
| 12. | Chen WH, Xu M, Du CC, Zhao JF, Pan LH, Li HC, Xiang JJ, Deng N. Molecular mechanism of reversal effect of monoclonal antibody to basic fibroblast growth factor mediated expression of P-glycoprotein on multiple drug resistance in adriamycin-resistant human breast cancer cell line MCF-7/ADM. Basic Res. 2013;33:8-14. |
| 13. | Chen K, Chen G, Wang H, Li H, Xiao J, Duan X, He J, He K, Xiang G. Increased survival in hepatocellular carcinoma with iodine-125 implantation plus radiofrequency ablation: a prospective randomized controlled trial. J Hepatol. 2014;61:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Tolmachev V, Orlova A, Andersson K. Methods for radiolabelling of monoclonal antibodies. Methods Mol Biol. 2014;1060:309-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Pan LH, Xu M, Zheng SB, Chen WH, Li HC, Sheng LH, Zhu XH, Xiang JJ, Deng N. Study on pharmacokinetics of Monoclonal antibody to basic fibroblast growth factor in mice. Zhongguo Yaolixue Tongbao. 2011;27:1582-1585. |
| 16. | Xu G, Ji W, Su Y, Xu Y, Yan Y, Shen S, Li X, Sun B, Qian H, Chen L. Sulfatase 1 (hSulf-1) reverses basic fibroblast growth factor-stimulated signaling and inhibits growth of hepatocellular carcinoma in animal model. Oncotarget. 2014;5:5029-5039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Sun B, Xu H, Zhang G, Zhu Y, Sun H, Hou G. Basic fibroblast growth factor upregulates survivin expression in hepatocellular carcinoma cells via a protein kinase B-dependent pathway. Oncol Rep. 2013;30:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Wang L, Park H, Chhim S, Ding Y, Jiang W, Queen C, Kim KJ. A novel monoclonal antibody to fibroblast growth factor 2 effectively inhibits growth of hepatocellular carcinoma xenografts. Mol Cancer Ther. 2012;11:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Dienstmann R, Rodon J, Prat A, Perez-Garcia J, Adamo B, Felip E, Cortes J, Iafrate AJ, Nuciforo P, Tabernero J. Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors. Ann Oncol. 2014;25:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 322] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 20. | Wang J, Li J, Wang X, Zheng C, Ma W. Downregulation of microRNA-214 and overexpression of FGFR-1 contribute to hepatocellular carcinoma metastasis. Biochem Biophys Res Commun. 2013;439:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Cheng AL, Shen YC, Zhu AX. Targeting fibroblast growth factor receptor signaling in hepatocellular carcinoma. Oncology. 2011;81:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Lee HJ, Kang HJ, Kim KM, Yu ES, Kim KH, Kim SM, Kim TW, Shim JH, Lim YS, Lee HC. Fibroblast growth factor receptor isotype expression and its association with overall survival in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:60-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Bumbaca D, Wong A, Drake E, Reyes AE, Lin BC, Stephan JP, Desnoyers L, Shen BQ, Dennis MS. Highly specific off-target binding identified and eliminated during the humanization of an antibody against FGF receptor 4. MAbs. 2011;3:376-386. [PubMed] |
| 24. | Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Lu Y, Lin N, Chen Z, Xu R. Hypoxia-induced secretion of platelet-derived growth factor-BB by hepatocellular carcinoma cells increases activated hepatic stellate cell proliferation, migration and expression of vascular endothelial growth factor-A. Mol Med Rep. 2015;11:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Wu Q, Wang R, Yang Q, Hou X, Chen S, Hou Y, Chen C, Yang Y, Miele L, Sarkar FH. Chemoresistance to gemcitabine in hepatoma cells induces epithelial-mesenchymal transition and involves activation of PDGF-D pathway. Oncotarget. 2013;4:1999-2009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Wei T, Zhang LN, Lv Y, Ma XY, Zhi L, Liu C, Ma F, Zhang XF. Overexpression of platelet-derived growth factor receptor alpha promotes tumor progression and indicates poor prognosis in hepatocellular carcinoma. Oncotarget. 2014;5:10307-10317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Fruth K, Weber S, Okcu Y, Noppens R, Klein KU, Joest E, Hedrich J, Thilemann S, Pogorzelski B, Koutsimpelas D. Increased basic fibroblast growth factor release and proliferation in xenotransplanted squamous cell carcinoma after combined irradiation/anti-vascular endothelial growth factor treatment. Oncol Rep. 2012;27:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 565] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 30. | Shah MA. The development of bevacizumab in noncolorectal gastrointestinal malignancies: gastroesophageal, pancreatic, and hepatocellular carcinoma. Clin Adv Hematol Oncol. 2014;12:239-246. [PubMed] |
| 31. | Yamashita-Kashima Y, Fujimoto-Ouchi K, Yorozu K, Kurasawa M, Yanagisawa M, Yasuno H, Mori K. Biomarkers for antitumor activity of bevacizumab in gastric cancer models. BMC Cancer. 2012;12:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Terai H, Soejima K, Yasuda H, Nakayama S, Hamamoto J, Arai D, Ishioka K, Ohgino K, Ikemura S, Sato T. Activation of the FGF2-FGFR1 autocrine pathway: a novel mechanism of acquired resistance to gefitinib in NSCLC. Mol Cancer Res. 2013;11:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Giakoustidis A S- Editor: Qi Y L- Editor: Filipodia E- Editor: Ma S