Published online Jun 7, 2016. doi: 10.3748/wjg.v22.i21.4999
Peer-review started: February 13, 2016
First decision: March 31, 2016
Revised: April 8, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: June 7, 2016
Processing time: 109 Days and 1.8 Hours
AIM: To investigate the side effects of phosalone on intestinal cells and to evaluate benefits of ellagic acid (EA) as a remedy.
METHODS: In order to conduct an in vivo study, a rat model was used. The rats were divided into ten groups based on the materials used in the experiment and their dosage. The first group was fed normally. The second group was administered EA through gavage. Next Four groups were given (1/3, 1/5, 1/10, 1/20) LD50 phosalone; an organophosphorus compound. The last four groups received (1/3, 1/5, 1/10, 1/20) LD50 phosalone and of EA. After one month, the rats were sacrificed and their colon cells were examined to evaluate the level of inflammation, proteins and oxidative stress markers.
RESULTS: The results of this research show that phosalone elevates oxidative stress and changes the level of tumor necrosis factor-a (TNF-α), interlukin-6β (IL-6β) and nuclear factor (NF)-κB proteins. EA administration reduced phosalone toxicity and changed oxidative stress and inflammatory markers for all phosalone doses. Overall changes in reduction of TNF-α (230.47 ± 16.55 pg/mg protein vs 546.43 ± 45.24 pg/mg protein, P < 0.001), IL-6β (15.85 ± 1.03 pg/mg protein vs 21.55 ± 1.3 pg/mg protein, P < 0.05), and NF-κB (32.47 ± 4.85 pg/mg protein vs 51.41 ± 0.71 pg/mg protein, P < 0.05) manifest that the efficacy of EA is more viable for 1/3 LD50 dose of phosalone. Furthermore, EA is effective to counteract the negative outcomes of oxidative stress. When EA was used to treat 1/3 LD50 of phosalone’s side effects, it improved the level of AChE activity (48.5% ± 6% vs 25% ± 7%, P < 0.05), TTM (0.391 ± 0.008 mmol/L vs 0.249 ± 0.032 mmol/L, P < 0.05), FRAP (46.04 ± 5.005 μmol/L vs 18.22 ± 1.9 μmol/L, P < 0.01) and MPO (0.222 ± 0.019 U/mg protein vs 0.387 ± 0.04 U/mg protein, P < 0.05).
CONCLUSION: This research highlights that EA is effective to alleviate the side effects of phosalone by reducing the level of oxidative stress and inflammatory proteins.
Core tip: This research uses a rat model to evaluate the colon related side effects of phosalone which is a member of the organophosphorus family. After feeding different dosages of phosalone to the rats for one month, the colon tissue of the rats were studied using oxidative stress and pathology tests. Both tests show that the higher doses of phosalone elevate reactive oxygen species (ROS), tumor necrosis factor-α, interlukin-6β and nuclear factor-κB proteins which result in more inflammation. In our study, ellagic acid (EA) which is a strong antioxidant reduced phosalone-induced side effects. The oxidative stress and pathology results concluded that EA helps reducing inflammation and ROS.
- Citation: Ghasemi-Niri SF, Maqbool F, Baeeri M, Gholami M, Abdollahi M. Phosalone-induced inflammation and oxidative stress in the colon: Evaluation and treatment. World J Gastroenterol 2016; 22(21): 4999-5011
- URL: https://www.wjgnet.com/1007-9327/full/v22/i21/4999.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i21.4999
Pesticides are substances used in agriculture to kill pests and also as a domestic insect killer[1]. Although they are significant in agriculture use but they may also enter human body through inhalation via air born particles. Farmers may inhale such chemicals when they use them for pest control[2]. General public is prone to pesticide after eating agricultural products which are not washed properly. Over-usage of pesticides may cause plants to absorb them directly or indirectly through soil. In such case, washing may not completely cleanse the pesticides and their consumers are vulnerable to the resultant side effects[3,4].
Phosalone [O,Odiethyl-S-(6-chloro-2-oxobenzoxazolin-3-yl-methyl)-phosphorodithioate] is a member of the organophosphorus (OP) family, which is used extensively as a pesticide in agriculture and as a domestic insect killer[5]. As compared to Dicoloro Di Three ethane (DDT), phosalone has less severe side effects on human and environment and because of this reason it has replaced DDT for pest control. Regardless of the fact, that phosalone is safer than DDT, but its toxicity has been one of the important research topics in toxicology. The most important known toxicity of phosalone is related to human nervous system. The mechanism of such damage is extremely toxic and phosalone can inhibit neural cholinesterase (ChE) activity, which elevates the level of acetylcholine thus therefore prevents neural signal pathway in the nervous system[6]. Furthermore, like the other members of the OP family, phosalone increases reactive oxygen species (ROS) in the human body tissues thus reduces the level and activity of anti-oxidant enzymes. Higher amount of ROS increases lipid peroxidation (LPO) in the membrane of cells, resulting in membrane damage and disturbance in the cell functional balance[7]. The final repercussions of ROS are faster cell aging and higher chances of DNA and RNA changes, subsequently leading toward cancer and gene mutations[8,9].
The main route through which OP enters the body is mucosa in intestinal cells, where OP can pass through membrane barrier and enter blood. Human cardiovascular system distributes OP to other organs and results in nervous system and ROS related damages[10,11]. Furthermore, the effect of OP on micro flora in intestinal and gastrointestinal enzymes elevate neutrophil infiltration and pro-inflammatory proteins[12,13]. The consequence of such effects is the migration of several immune cells such as neutrophils, monocytes, lymphocytes, macrophages and chemokines then adhesion molecules move toward mucosal tissue. The final outcome of such damage is intestinal inflammation[14,15].
This research elaborates ROS related side effects of phosalone and proposes a material to reduce and potentially eliminate such side effects. The proposed material should be able to offset free-radicals. This research shows that ellagic acid (EA) can be a remarkable candidate to considerably suppress the side effects of phosalone. EA is an important natural occurring substance, which has phenol components[16]. EA is present in numerous fruits and vegetables such as grapes, nuts, strawberries, black currents, raspberries, green tea, pomegranates, and the stem and bark of Eucalyptus globulus, Eucalyptus maculatu and nuts. The international chemical name of EA is 2,3,7,8-tetrahydroxy-chromeno[5,4,3-cde] chromene-5,10-dione[17].
The biological activities of EA has been investigated in several in vivo and in vitro studies and have shown that EA has anti-cancer, anti-inflammatory and anti-oxidant properties and in addition it has beneficial therapeutic effect on colon, skin, breast cancer and inflammatory bowel disease (IBD)[18]. EA can also improve mucosa production in goblet cells in colon; reduce pro-inflammatory proteins COX-2 and iNOS over expression and neutrophil infiltration[19]. The anti-oxidant effect of EA stem is clear from the fact, that EA can scavenge free radical, nitrogen reactive species, and ROS, including hydroxyl radicals, peroxyl radicals, NO2 radicals, and peroxynitrite and therefore EA reduce DNA and cell damages[20]. Additionally, EA can potentially shield DNA and protect it from ROS, free radical and chelation of metal ions attack.
Regarding other effects of EA, some studies have reported that EA can affect cytochrome C in mitochondria which increases BAX/Bcl2, regulates cell division and apoptosis[21]. Also through stimulating the immune system, EA plays a positive role in intercellular complex signaling systems such as mitogen activated protein kinases (MAPKs) and/or the transcription factor nuclear factor κB (NF-κB)[22]. An in-depth study of these effects is presented in this paper.
In our study, we evaluate effect of phosalone on inflammation and oxidative stress with four doses as well as subsequent effect of EA on colon cells.
Acetylthiocholine iodide, 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) from Merck (Germany), trichloroacetic acid (TCA), Tris base, 1,1,3,3’-tetraethoxypropane (MDA), 2-thiobarbituric acid (TBA), n-butanol, 2,4,6-tripyridyl-s-triazine (TPTZ), n-butanol, acetic acid, FeCl3-6H2O, benzethonium chloride, 5,5′-Dithiobis(2-nitrobenzoic acid), Trizma® base, EA, o-Dianisidine dihydrochloride, phosphate buffer from Sigma-Aldrich (Germany), n-butanol, hexadecyl tri-methyl ammonium bromide (HETAB), ethylene diamine tetra acetic acid (EDTA), hydrochloric acid (HCL), acetic acid, sodium acetate, hydrogen peroxide (H2O2), O-dianisidine hydrochloride, ferric chloride (FeCl3-6H2O), Coomassie reagent, bovine serum albumin (BSA), sodium sulphate (Na2SO4), sulphuric acid (H2SO4), phosphoric acid (H3PO4), potassium dihydrogen phosphate (KH2PO4), potassium hydrogen diphosphate (K2HPO4), sodium carbonate (Na2CO3), cupric sulphate (CuSO4-5H2O) from Merck. Rat-specific tumor necrosis factor-α (TNF-α), interlukin-6 (IL-6) and NF-κB ELISA kits from (Bender MedSystems GmbH, Austria), analytical grade form of phosalone from local pesticide manufacturing companies (Agroxir) and were used in this study.
In our study, male Wistar rats weighing 180-200 g were selected according to the regulations of the ethics committee of Tehran University of Medical Sciences (TUMS) approved with code number of 93-02-45-26666. Animals were housed separately in standard polypropylene cages with a wire mesh top, kept under standard conditions, including temperature (23 °C ± 1 °C), relative humidity (55% ± 10%) and 12/12 h light/dark cycle, and fed a standard pellet diet and water ad libitum. All ethical themes of studies on animals were considered carefully.
Animals were divided into ten groups based on the materials used in the experiment and their dosage, with six rats in each group. The first group was fed normally. The second group was administered EA (10 mL/kg) through gavage. Next Four groups were given different dosage of phosalone (1/3 LD50: 40 mg/kg, 1/5 LD50: 20 mg/kg, 1/10 LD50: 12 mg/kg and 1/20 LD50: 6 mg/kg), which is a member of organophosphorus family, through gavage. The last four groups received both phosalone (1/3 LD50: 40 mg/kg, 1/5 LD50: 20 mg/kg, 1/10 LD50: 12 mg/kg and 1/20 LD50: 6 mg/kg) and EA (10 mL/kg). After one month, the rats were sacrificed and their colon cells were examined to evaluate the level of oxidative stress factors.
After 30 d, all rats were anesthetized (40% Ketamine 1000, 25% Xylazine 2%, 0.1 mL/100 g body weight) and after that all of animals were humanly sacrificed and colonic tissues were immediately separated. Isolated segments were rinsed with normal saline and then placed in an ice bath throughout the procedure. Colonic tissue was divided into two pieces. The first piece was weighed and kept in 10 mL of formalin 10%, as a fixator for the purpose of histopathological evaluation. The second piece was weighed and homogenized in 10 volumes of ice cold potassium phosphate buffer (50 mmol, pH = 7.4) and then stored at -20 °C for 24 h. The sample was then sonicated and centrifuged for 30 min at 3500 g, and the supernatant was transferred to a micro tube. Then sample was kept at -80 °C until biomarker analyses.
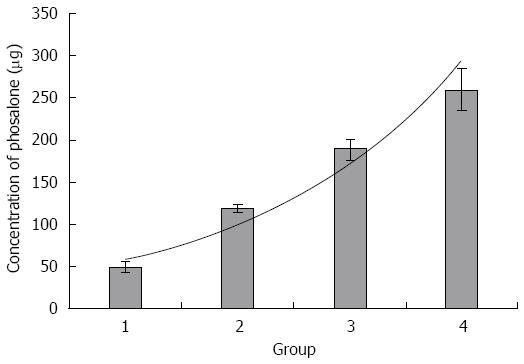
An lethal dose (LD50) of phosalone is a standard measurement of toxicity that is stated in milligrams (mg) of phosalone per kilogram (kg) of body weight at which 50% of rats are killed. For finding the LD50 of phosalone, we performed a study on Wistar rats. We divided five groups of rats and administrated with different doses of phosalone. One of group was control that didn’t receive phosalone. But four groups received different doses of phosalone, like 50 mg/kg, 120 mg/kg, 190 mg/kg and 260 mg/kg. After two days we compared all groups and found LD50 was between 120 mg/kg to 190 mg/kg. After that we analyzed all data and 120 mg/kg was LD50, used for phosalone in animal model in our study (Figure 1).
AChE activity: AChE activity of erythrocytes was measured according to method of Ellman method using acetylthiocholine iodide as the substrate and 5-5-bis dithionitrobenzoic acid (DTNB). Briefly, 10 μL of sample was added to 3 mL of solution containing 25 mmol/L DTNB in 75 mmol/L phosphate buffer. Then 10 μL of 3 mmol/L acetylcholine iodide was added and absorbance changes were measured at 412 nm in a two-fold rays spectrophotometer[23].
MPO activity was determined by a dianisidine-H2O2 method, modified for 96-well plates. Briefly, plasma samples (10 μg protein) were added in triplicate to 0.53 mmol/L o-dianisidine dihydrochloride (Sigma) and 0.15 mmol/L H2O2 in 50 mmol/L potassium phosphate buffer (pH 6.0). After incubation for 5 min at room temperature, the reaction was stopped with 30% sodium azide. The absorbance was measured at 460 nm (ε = 11300 M-1·cm-1) spectrophotometrically (Shimadzu 160A UV-VIS spectrophotometer). Results were expressed as units of MPO/mg protein, whereby 1 unit of MPO was defined as the amount of enzyme degrading 1 nmol H2O2 per min at 25 °C[24].
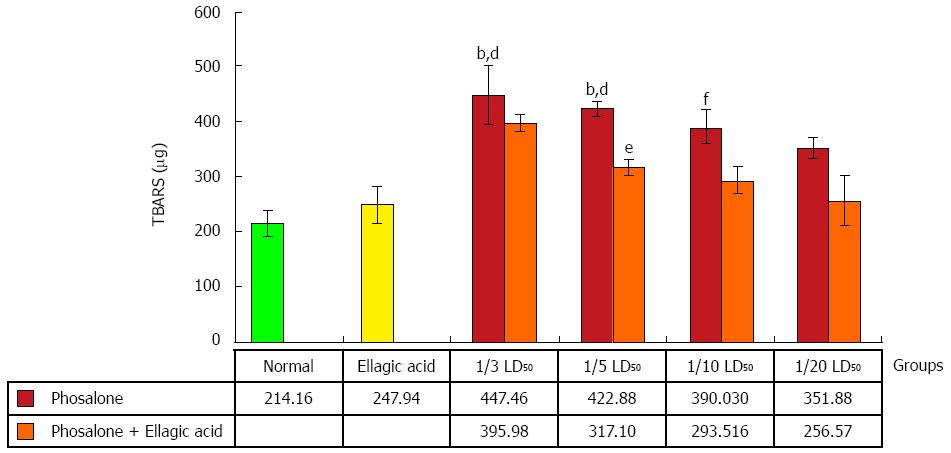
To measure LPO, thiobarbituric acid-reaction substances (TBARS) were assessed in colon tissue. TBA reacts with lipid peroxides in the samples producing a measurable pink color that has absorbance at 532 nm by a double beam spectrophotometer. Concentration of TBARS is recorded as μg[25].
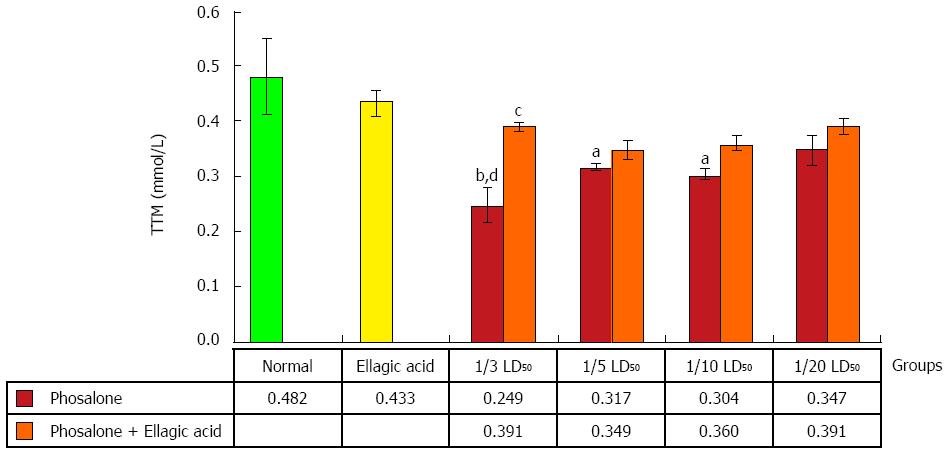
To determine TTM in the control and test groups, 0.6 mL Tris-EDTA buffer (Tris base 0.25 mol/L, ethylene diamine tetra acetic acid 20 mmol/L, pH 8.2) was added to 0.2 mL of supernatant, and after quick vortex mixing, 40 μL 5-5’-dithiobis-2-nitrobenzoic acid (10 mmol/L in pure methanol) was added. The final volume of this mixture was made up to 4.0 mL by an extra addition of pure methanol. After 15 min incubation at room temperature, the samples were centrifuged at 3000 g for 10 min and ultimately the absorbance of the supernatant was measured at 412 nm. Data are shown as mmol/L[26].
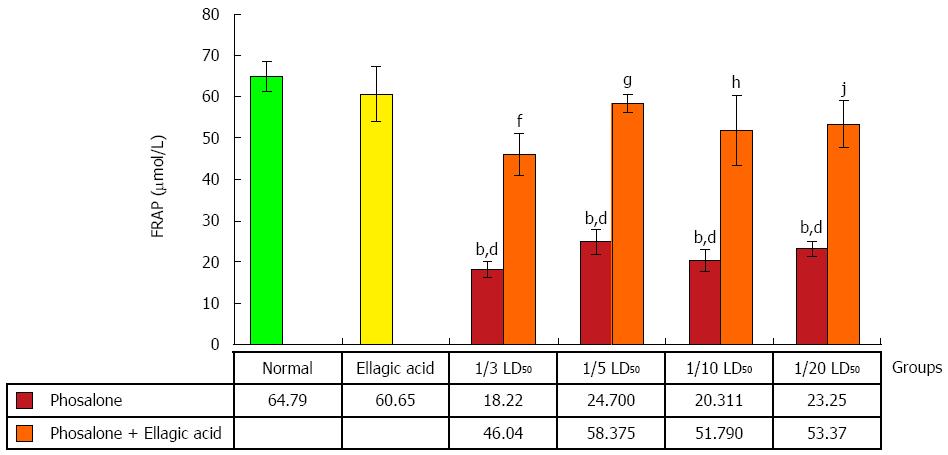
Antioxidant power of plasma was evaluated by measuring its ability to reduce of Fe3+ tripyridyltriazine (TPTZ) complex (colorless) to Fe2+ TPTZ (blue colored) formed by the action of electron donating antioxidants at low pH. The ferric reducing antioxidant power (FRAP) reagent was prepared by mixing 300 mmol/L acetate buffer, 10 mL TPTZ in 40 mmol/L HCl and 20 mmol/L FeCl3 in the proportion of 10:1:1 at 37 °C. 10 μL of the H2O diluted sample was then added to 300 mL freshly prepared reagent warmed at 37 °C. An intense blue color complex was formed when Fe3+ TPTZ complex was reduced to Fe2+ form. The complex between Fe2+ and TPTZ gives a blue color with absorbance at 593 nm. Data are shown as μmol/L[27].
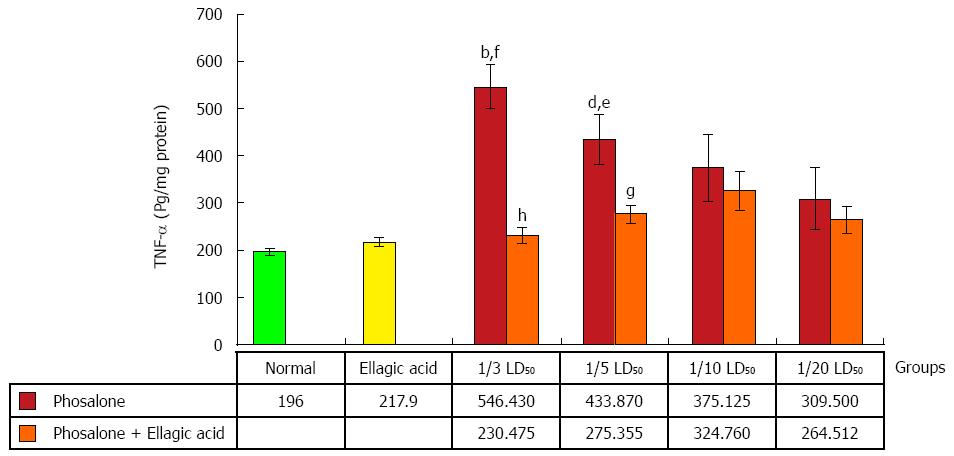
A human specific ELISA kit (BenderMed System) was used to quantify TNF-α and IL-6 in the supernatant of colon tissue. To assess the amount of TNF-α, the absorbance of sample was measured in 450 nm as the primary wavelength and 620 nm as the reference wavelength by ELIZA reader as described in the kit brochure. TNF-α and IL-6β levels were expressed as pg/mg protein of tissue[28].
The amount of NF-κB in colon cells extracts was measured by using NF-κB ELISA kits (BenderMed System) according to the manufacturer’s instructions. The levels of NF-κB in nuclear extracts were calculated using the standard curve and expressed as pg/mg protein[29].
The concentration of protein in the colon homogenate was measured by the Bradford method using BSA as the standard. The absorbance was measured by the spectrophotometer at 595 nm after 5 min. The bovine serum albumin was used as standard[30].
At least four independent experiments in repetition were carried away. Data are presented as mean ± SE. One-way ANOVA and Tukey’s multi-comparison trials were held out by Stats-Direct 3.0.169 software to determine the statistical differences while the degree of significance had been set at (P < 0.05).
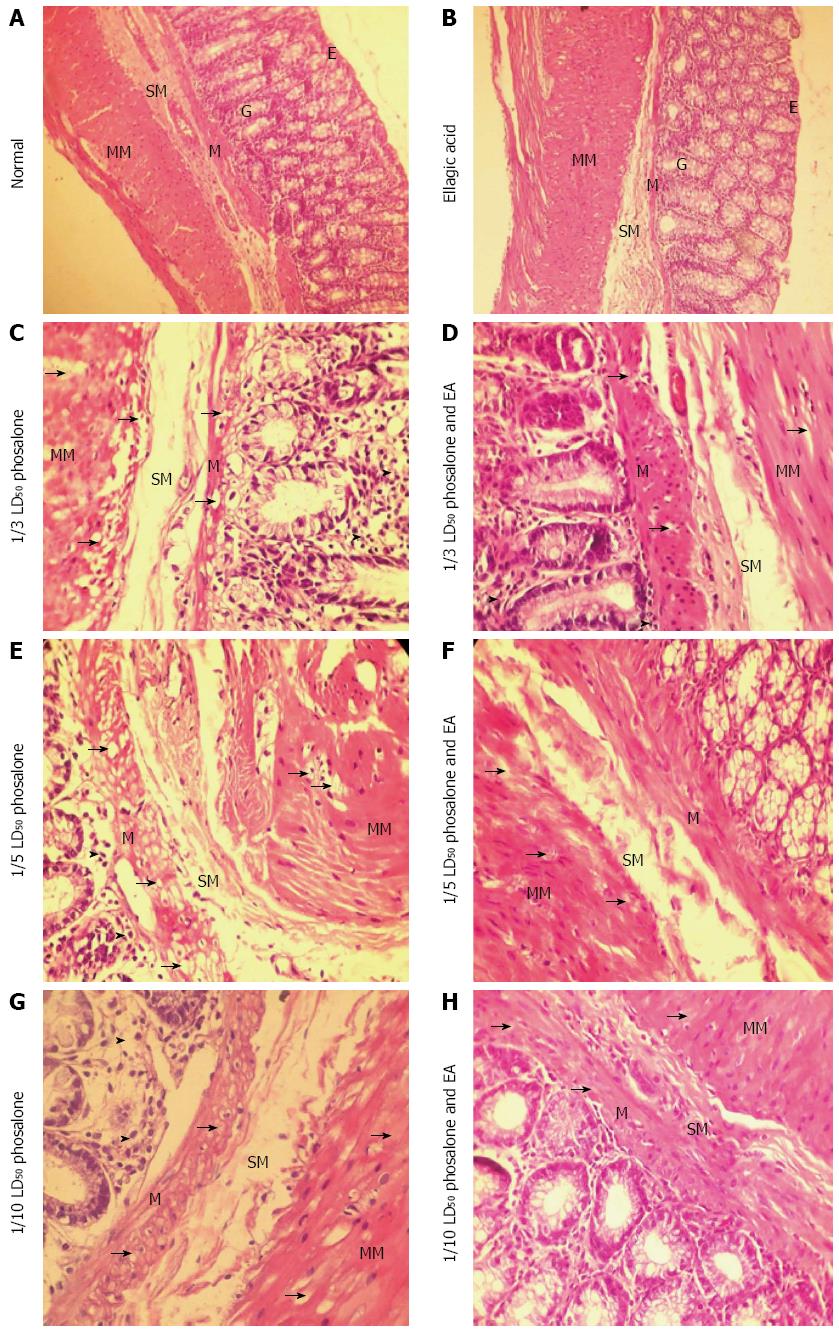
As shown in Figure 2, histopathological examination in normal group shows that there was no ulceration, no necrosis, no adhesions, no wall thickening and mucosal/submucosal polymorphonuclear (PMN) leukocyte infiltration. In EA group there was no blood and ulcer in mucosal/submucosal of the colon tissue. In the 1/3 LD50 phosalone group, it was observed in some areas infiltration, adhesions, with no any overlying blood and serous adhesion.
The 1/3 LD50 phosalone and EA group showed improvement in muscles and mucosa, a reduction inflammation in colon tissue and low lymphocytes infiltration in submucosal layer. The mucosal glands are normal but mild degeneration of mucosal muscle cells and muscle layers is observable. The level of degeneration and inflammation is less than 1/3 LD50 phosalone group. Histological examination of 1/5 LD50 phosalone and EA group showed improvement in mucosa with the reduction lymphocytes in submucosa region. The mucosal glands are normal but mild degeneration of mucosal muscle cells and muscle layers is observable. The level of degeneration is less than 1/5 LD50 phosalone group. In the 1/10 LD50 phosalone and 1/20 LD50 phosalone with EA groups, the mild degeneration of mucosal muscle cells and muscle layers were observed. In 1/10 LD50 phosalone and EA was seen a very mild inflammation due to lymphocytes infiltration between mucosal glands. But in 1/20 LD50 phosalone and EA, there was no inflammation in different layers.
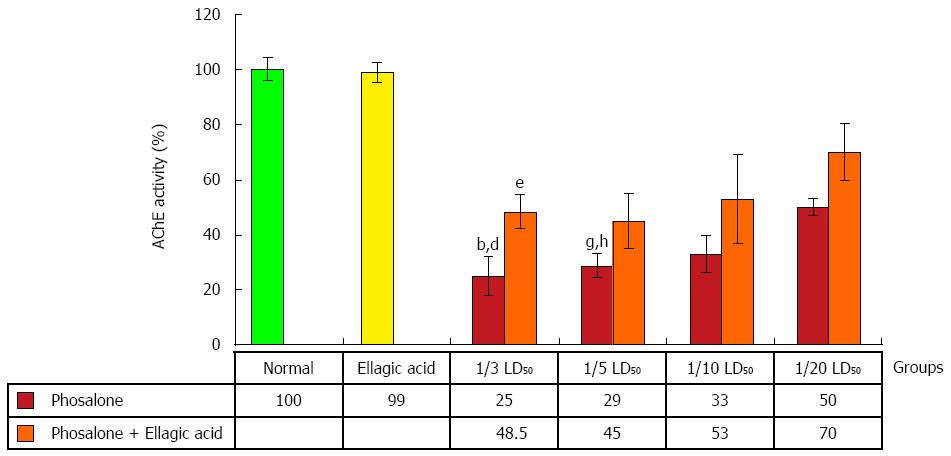
After pathological examination, the first step was the evaluation of EA through measurement of AChE activity. AChE activity was reduced in colon cells of groups receiving 1/3 and 1/5 LD50 of phosalone in comparison to normal group (P < 0.01). In two groups (1/3 and 1/5) LD50 phosalone, AChE activity was significantly decreased in comparison to EA group (P < 0.05). EA restored the activity of AChE which was suppressed by phosalone. Among different phosalone doses, such AChE activity retrieval was more significant for 1/3 LD50 (P < 0.05) (Figure 3).
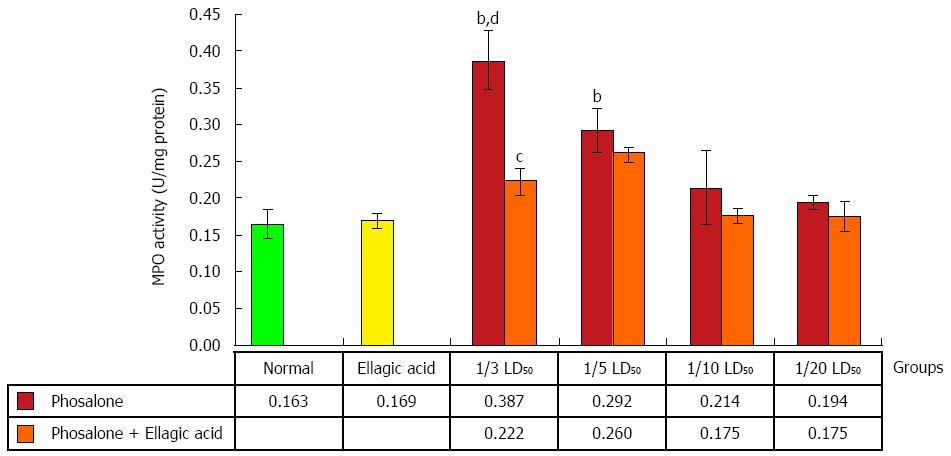
Colonic myeloperoxidase (MPO) activity in 1/3 LD50 phosalone group was noticeably higher than that of the normal and EA groups (P < 0.01). Data showed a remarkable difference between 1/5 LD50 phosalone and EA group (P < 0.01). Also, the group of animals which received EA and 1/3 LD50 phosalone, showed a reduction of MPO activity (by 26%, P < 0.05) in comparison to 1/3 LD50 phosalone group (Figure 4).
Inflammation in colon referred as over-activity of oxidative stress was found high in 1/3 and 1/5 LD50 phosalone groups as compared to normal and EA groups (P < 0.01). Colonic lipid peroxidation in 1/10 LD50 phosalone group was noticeably higher than that of the normal group (P < 0.01). Although, EA decreased oxidative stress in all doses of phosalone, it down-regulated oxidant formation significantly in 1/5 LD50 phosalone (P < 0.05) (Figure 5).
An obvious reduction in TTM was observed in 1/3 LD50 phosalone group as compared to normal and EA groups (P < 0.01). 1/5 and 1/10 LD50 phosalone groups significantly decreased TTM in comparison with normal group (P < 0.05). EA restored significantly the TTM which was suppressed by 1/3 LD50 phosalone (Figure 6).
Less ability in overcoming the oxidative stress in all doses of phosalone groups was reported in contrast to normal and EA groups (P < 0.001). FRAP value in 1/3 LD50 phosalone was significantly less than EA and 1/3 LD50 phosalone group (P < 0.01). Amount of FRAP in 1/5 LD50 phosalone was markedly lower than its normal content in EA and 1/5 LD50 phosalone group (P < 0.001). The amount of FRAP increased significantly in EA and 1/10 LD50 phosalone group compared to 1/10 LD50 phosalone group (P < 0.001). A significant increase in FRAP was seen in EA and 1/20 LD50 phosalone group when compared to 1/20 LD50 phosalone (P < 0.01) (Figure 7).
An obvious rise in TNF-α level was observed in (1/3 and 1/5) LD50 phosalone groups as compared to normal group (P < 0.01). In (1/3 and 1/5) LD50 phosalone groups showed a significant increase in TNF-α level in comparison with EA group (P < 0.05). A noticeable improve in TNF-α content was seen in EA and 1/3 LD50 phosalone group when compared with 1/3 LD50 phosalone group (P < 0.001). In EA and 1/5 LD50 phosalone group as shown in Figure 8, EA prevented more secretion of TNF-α when compared to 1/5 LD50 phosalone group (P < 0.05) (Figure 8).
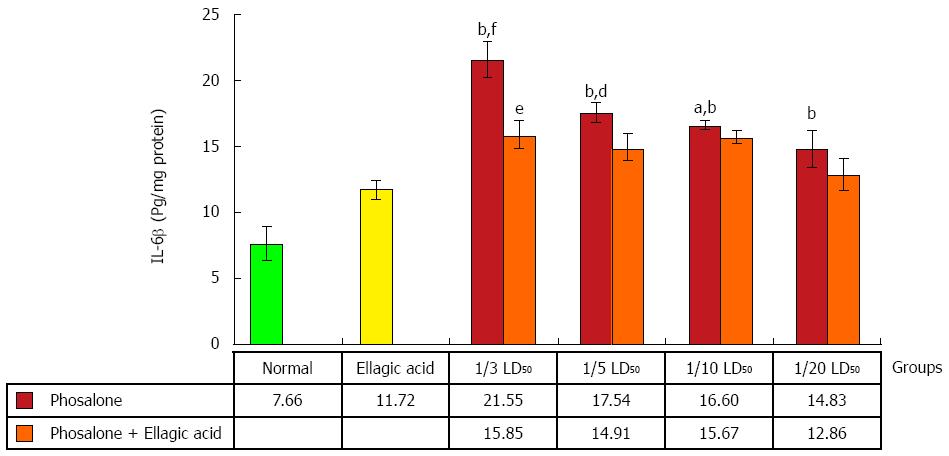
All doses of phosalone groups showed a notable elevation in IL-6β level in comparison to normal group (P < 0.001). The EA and 1/3 dose of phosalone group differed from 1/3 LD50 phosalone group remarkably (P < 0.05). IL-6β level in 1/3 LD50 phosalone group was noticeably higher than that of the EA group (P < 0.001). There was significant variation between EA, and EA and 1/5 LD50 phosalone groups (P < 0.01), while EA and 1/10 LD50 phosalone group had a less potency in decreasing IL-6β level when compared to EA group (P < 0.05) (Figure 9).
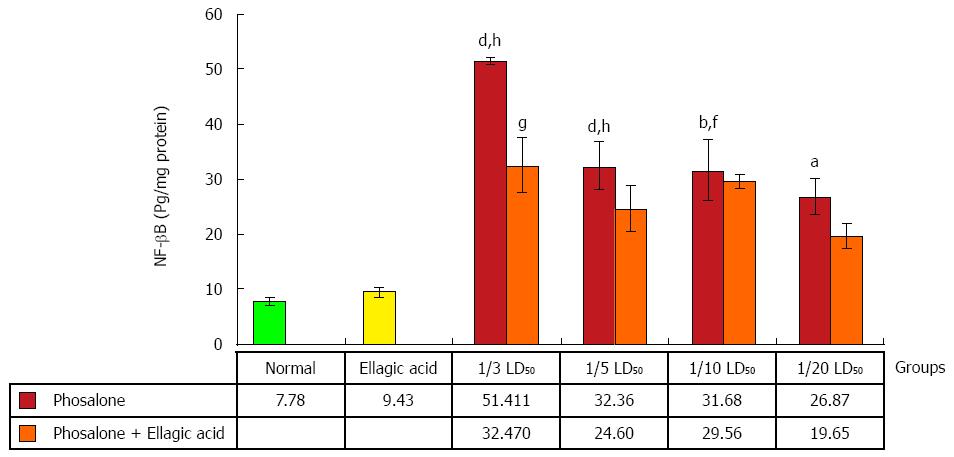
As seen in Figure 10, NF-κB production was significantly elevated in the (1/3 and 1/5) LD50 phosalone groups when compared with normal group (P < 0.001). A significant increase in NF-κB was seen in (1/10 and 1/20) LD50 phosalone groups when compared with normal (P < 0.05). The EA and 1/3 LD50 phosalone group showed more reduction in NF-κB when compared with 1/3 LD50 phosalone (P < 0.05). The (1/3, 1/5 and 1/10) LD50 phosalone groups which were treated with EA showed an apparent increase in NF-κB level when compared with EA group (P < 0.01).
In our study we succeeded to achieve our main hypothesis: to find phosalone toxicity in colonic tissues of rats as well as protective effects of EA, during subchronic exposure. Phosalone is type of OP pesticide that could affect different organs in daily and produce various toxicities[31]. As a result of phosalone exposure in rats, increase in oxidative stress and inflammatory markers were observed. In our experiment, EA was used to reduce colon injury induced by phosalone as a protective agent, which showed substantial decrease in oxidative stress and inflammatory markers. EA that is kind of polyphenol derived from different plants or fruits has already been reported to have sort of protective effects in different diseases[32,33]. As indicated in the present study, AChE activity was reduced with pronounced effect in colon cells of groups receiving (1/3 and 1/5) LD50 phosalone in comparison to both normal group and EA group. In previous studies, the same inhibition of AChE was observed during behavioral studies in phosalone-treated rats brain cells[34,35]. AChE inhibition is among best indicator of toxicity induced by any xenobiotic or chemical that initiates other signaling pathways. Despite of little effect in other groups, EA considerably reversed the activity of AChE, suppressed by phosalone in group receiving 1/3 LD50 phosalone. It has been already published that, by exposure of OPs elevated level of ACh via ChE inhibition could interfere with cholinergic receptors within hypothalamus and potentiate release of adrenocorticotropic hormone (ACTH)[36]. The present study proves that phosalone inhibits AChE activity that is associated with colon inflammation and EA could reverse its effect.
Increased production and decreased ability of ROS and antioxidant defense mechanism respectively can damage various signaling pathways, as well as cell constituents, including DNA, lipids and proteins. Induction of such oxidative impairment via redox signaling mechanisms can cause different human diseases[37]. In our experiment, biochemical assays showed that phosalone elevated oxidative stress via elevation of MPO activity and TBARS concentration, whereas in groups with combined EA administration; reduction in MPO activity and TBARS concentration has been observed. Irrespective of our study on phosalone, number of previous studies and literature demonstrate a close relation between exposure of pesticides and occurrence of various health problems via induction of oxidative stress[38,39]. A study conducted on humans via in vitro setup concluded that: oxidative stress and ROS were increased due to OPs exposure[40]. On other hand a pronounced effect of EA against free radical formation can be seen in groups receiving (1/3 and 1/5) LD50 phosalone. However, it has been reported that both MPO and TBARS are indicators of oxidative stress and colon inflammation[41,42]. In addition to this, in our study, body’s antioxidant defense mechanism was targeted by phosalone, which caused reduction in TTM and FRAP concentration as compared to normal and EA groups. A significant effect of EA as antioxidant has been observed in all groups receiving both EA and phosalone in different doses. Protective and beneficial effects of EA in oxidative stress has been previously evidenced in many studies[43,44]. It can be derived from current biochemical tests that colon tissues of rats are prone to OPs like Phosalone and thus EA can better treat colon tissue damage via different mechanisms, while further studies can be conducted for treatment of IBD (inflammatory bowel disease). Oxidative stress and its balance is the most significant feature of normal physiology, in case of high toxicity it can initiate many signaling pathways that can lead to cell death. Our study shows that, EA play its role as protective agent in oxidative impairment, against free radical production and colitis due to phosalone exposure. So in colon inflammation induced by pesticides, EA can be used as anti-inflammatory, anticancer and antioxidant.
Furthermore we evaluated the effect of phosalone on different inflammatory markers along with protective effect of EA. Our concept regarding toxic mechanisms in colon inflammation is growing and general overview is that T cells secrete IL-2, IL-1B and Interferon-c (IFN-c) that can excite macrophages to release extra TNF-α and IFN-c, ROS and other inflammatory mediators. Occurrence of TNF-α, IL-1B, ROS and some antigens target other signaling pathways which ultimately result in synthesis of cytokines[45]. TNF-α employs its action through elevating the synthesis of inflammatory mediators like IL-1 and IL-6[46]. Our recent study shows that phosalone increase TNF-α and EA well treat such condition in colon inflammation, which require further research regarding IBD. Phosalone caused increase in TNF-α level in all groups with significant change in group 1/3 LD50 phosalone and 1/5 LD50 phosalone as compared to normal and EA groups. EA showed prominent effect as protective agent to reduce TNF-α level in all groups with significant change in group 1/3 LD50 phosalone and 1/5 LD50 phosalone as compared to others.
In case of biomarker IL-6β, our study showed consistent finding and phosalone caused increase in IL-6β in all groups significantly as compared to normal and EA groups, whereas EA reversed its effect in all groups with significant change in group 1/3 LD50 phosalone as compared to EA group. It is common belief that NF-κB shows its significant function in expression of various inflammatory mediators. NF-κB controls transcriptional activity involved in inflammatory and immune process via binding to specific DNA sequences in inflammatory genes[47]. In the same pattern, NF-κB was increased in all groups receiving phosalone with significant change. Contrary to this EA reduced its concentration in almost all groups with significant effect of group receiving 1/3 LD50 phosalone. In parallel to our current study same effects of EA as anti-inflammatory agent has been observed in previous experiment[48]. It is clear from our results that how EA and phosalone target different biochemical pathways of toxicity. These distinct properties make NF-κB a promising target in novel treatment plans. There are new techniques that directly target NF-κB in inflammatory conditions including antioxidants, antisense DNA targeting, and proteasome inhibitors. Parallel to our study, a previous study also demonstrated that antioxidant effect may also give boost to anti-inflammatory actions[49]. However, EA’s mechanism of actions to offset phosalone toxicity can be further studied regarding signaling pathways and gene expressions. Our research outcomes can give new directions, regarding novel treatment plans of colitis as well as awareness of phosalone toxicity in colon tissues.
Our data correlate well with the other studies and demonstrate that phosalone is among one of causative agents to induce colon inflammation and EA is an ideal antioxidant and anti-inflammatory compound in rat modeling studies which has extraordinary effects on oxidant and inflammation systems. Anyhow, additional investigation for in vivo and human studies is required. It may indicate a new way toward the development of antioxidant therapy for colon inflammation.
Pesticides are chemical agents which are used to kill agricultural and domestic insects. Some of the pesticides are based on Organophosphorus (OP) compounds which are also harmful for human and can lead to early aging and cancer. Understanding the mechanism of action of OPs in human body is of prime importance in recent years. Such understanding will lead to finding the means to counteract the side effects resulted from OP exposure. Phosalone is an OP compound used in this study.
Prior researches have shown that OP exposure causes inflammation and oxidative stress in the body. The previous and on-going research efforts report serious damages to DNA, RNA and cell cycle due to OP agents.
This research confirms the side effects of OP in colon cells in a rat model. Such side effects are the elevated level of inflammation and oxidative stress. The research results shows that among four dosages of phosalone, highest dosage leads to the most significant and serious level of inflammation and oxidative stress. To alleviate such deteriorative side effects, this research proposes utilizing ellagic acid (EA) which is a strong antioxidant. When rats were given EA along with phosalone, the level of inflammation and oxidative stress reduced significantly for the highest dose of phosalone.
The results of this research can initiate appropriate warnings and precautions to all individuals including farmers who are exposed excessively to OP compounds. Such individuals can be directed to include EA in their diet through taking EA tablets or eating the fruits and vegetable which are rich source of antioxidants and EA like strawberries, grapes and green tea.
Reactive oxygen species (ROS) is a physiological process which happens when the body defense system gets triggered due to inflammation and oxidative stress. ROS leads to variety damages to DNA, RNA and cells.
This study is very significant and interesting. The authors have done standard measurements of toxicity, and demonstrated EA can be used to reduce oxidative stress and regulate the level of inflammatory proteins. EA maybe a good candidate which can help treat and alleviate the side effects induced by OP compounds.
| 1. | Mostafalou S, Karami-Mohajeri S, Abdollahi M. Environmental and population studies concerning exposure to pesticides in iran: a comprehensive review. Iran Red Crescent Med J. 2013;15:e13896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Jaga K, Dharmani C. Ocular toxicity from pesticide exposure: A recent review. Environ Health Prev Med. 2006;11:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10:RA141-RA147. [PubMed] |
| 4. | Malekirad AA, Faghih M, Mirabdollahi M, Kiani M, Fathi A, Abdollahi M. Neurocognitive, mental health, and glucose disorders in farmers exposed to organophosphorus pesticides. Arh Hig Rada Toksikol. 2013;64:1-8. [PubMed] |
| 5. | O’Malley MA, McCurdy SA. Subacute poisoning with phosalone, an organophosphate insecticide. West J Med. 1990;153:619-624. [PubMed] |
| 6. | Alizadeh A, Talebi-Jahromi K, Hosseininaveh V, Ghadamyari M. Toxicological and biochemical characterizations of AChE in phosalone-susceptible and resistant populations of the common pistachio psyllid, Agonoscena pistaciae. J Insect Sci. 2014;14:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Kaya H, Çelik EŞ, Gürkan M, Yılmaz S, Akbulut M. Effects of subchronic exposure to phosalone on oxidative stress and histopathological alterations in common carp (Cyprinus carpio, L., 1758). J Toxicol Environ Health A. 2013;76:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Dizdaroglu M. Oxidatively induced DNA damage and its repair in cancer. Mutat Res Rev Mutat Res. 2015;763:212-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Rezvanfar MA, Rezvanfar MA, Shahverdi AR, Ahmadi A, Baeeri M, Mohammadirad A, Abdollahi M. Protection of cisplatin-induced spermatotoxicity, DNA damage and chromatin abnormality by selenium nano-particles. Toxicol Appl Pharmacol. 2013;266:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Pakzad M, Fouladdel S, Nili-Ahmadabadi A, Pourkhalili N, Baeeri M, Azizi E, Sabzevari O, Ostad SN, Abdollahi M. Sublethal exposures of diazinon alters glucose homostasis in Wistar rats: Biochemical and molecular evidences of oxidative stress in adipose tissues. Pestic Biochem Physiol. 2013;105:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Proskocil BJ, Bruun DA, Thompson CM, Fryer AD, Lein PJ. Organophosphorus pesticides decrease M2 muscarinic receptor function in guinea pig airway nerves via indirect mechanisms. PLoS One. 2010;5:e10562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Vejares SG, Sabat P, Sanchez-Hernandez JC. Tissue-specific inhibition and recovery of esterase activities in Lumbricus terrestris experimentally exposed to chlorpyrifos. Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Pond AL, Chambers HW, Chambers JE. Organophosphate detoxication potential of various rat tissues via A-esterase and aliesterase activities. Toxicol Lett. 1995;78:245-252. [PubMed] |
| 14. | Mozaffari S, Abdollahi M. Melatonin, a promising supplement in inflammatory bowel disease: a comprehensive review of evidences. Curr Pharm Des. 2011;17:4372-4378. [PubMed] |
| 15. | Di Sabatino A, Lenti MV, Giuffrida P, Vanoli A, Corazza GR. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract. Autoimmun Rev. 2015;14:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Rahman MA, Abdullah N, Aminudin N. Antioxidative Effects and Inhibition of Human Low Density Lipoprotein Oxidation In Vitro of Polyphenolic Compounds in Flammulina velutipes (Golden Needle Mushroom). Oxid Med Cell Longev. 2015;2015:403023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Ramírez de Molina A, Vargas T, Molina S, Sánchez J, Martínez-Romero J, González-Vallinas M, Martín-Hernández R, Sánchez-Martínez R, Gómez de Cedrón M, Dávalos A. The ellagic acid derivative 4,4’-di-O-methylellagic acid efficiently inhibits colon cancer cell growth through a mechanism involving WNT16. J Pharmacol Exp Ther. 2015;353:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Rosillo MA, Sanchez-Hidalgo M, Cárdeno A, de la Lastra CA. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem Pharmacol. 2011;82:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Rosillo MA, Sanchez-Hidalgo M, Cárdeno A, de la Lastra CA. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem Pharmacol. 2011;82:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | El-Shitany NA, El-Bastawissy EA, El-desoky K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int Immunopharmacol. 2014;19:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Edderkaoui M, Odinokova I, Ohno I, Gukovsky I, Go VL, Pandol SJ, Gukovskaya AS. Ellagic acid induces apoptosis through inhibition of nuclear factor kappa B in pancreatic cancer cells. World J Gastroenterol. 2008;14:3672-3680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | González-Sarrías A, Larrosa M, Tomás-Barberán FA, Dolara P, Espín JC. NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr. 2010;104:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88-95. [PubMed] |
| 24. | Ghazanfari G, Minaie B, Yasa N, Nakhai LA, Mohammadirad A, Nikfar S, Dehghan G, Boushehri VS, Jamshidi H, Khorasani R. Biochemical and histopathological evidences for beneficial effects of satureja khuzestanica jamzad essential oil on the mouse model of inflammatory bowel diseases. Toxicol Mech Methods. 2006;16:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Astaneie F, Afshari M, Mojtahedi A, Mostafalou S, Zamani MJ, Larijani B, Abdollahi M. Total antioxidant capacity and levels of epidermal growth factor and nitric oxide in blood and saliva of insulin-dependent diabetic patients. Arch Med Res. 2005;36:376-381. [PubMed] |
| 26. | Vakilian K, Ranjbar A, Zarganjfard A, Mortazavi M, Vosough-Ghanbari S, Mashaiee S, Abdollahi M. On the relation of oxidative stress in delivery mode in pregnant women; a toxicological concern. Toxicol Mech Methods. 2009;19:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Moeinian M, Ghasemi-Niri SF, Mozaffari S, Abdolghaffari AH, Baeeri M, Navaea-Nigjeh M, Abdollahi M. Beneficial effect of butyrate, Lactobacillus casei and L-carnitine combination in preference to each in experimental colitis. World J Gastroenterol. 2014;20:10876-10885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Pedram S, Mohammadirad A, Rezvanfar MA, Navaei-Nigjeh M, Baeeri M, Abdollahi M. On The Protection by The Combination of CeO2 Nanoparticles and Sodium Selenite on Human Lymphocytes against Chlorpyrifos-Induced Apoptosis In Vitro. Cell J. 2015;17:361-371. [PubMed] |
| 29. | Esmaily H, Vaziri-Bami A, Miroliaee AE, Baeeri M, Abdollahi M. The correlation between NF-κB inhibition and disease activity by coadministration of silibinin and ursodeoxycholic acid in experimental colitis. Fundam Clin Pharmacol. 2011;25:723-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Farzaei MH, Ghasemi-Niri SF, Abdolghafari AH, Baeeri M, Khanavi M, Navaei-Nigjeh M, Abdollahi M, Rahimi R. Biochemical and histopathological evidence on the beneficial effects of Tragopogon graminifolius in TNBS-induced colitis. Pharm Biol. 2015;53:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268:157-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 673] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 32. | Farzaei MH, Abdollahi M, Rahimi R. Role of dietary polyphenols in the management of peptic ulcer. World J Gastroenterol. 2015;21:6499-6517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (2)] |
| 33. | Farzaei MH, Rahimi R, Abdollahi M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr Pharm Biotechnol. 2015;16:196-210. [PubMed] |
| 34. | Chetan PS, Kumar RR, Mohan PM. Phosalone-induced changes in regional cholinesterase activities in rat brain during behavioral tolerance. African Research Review. 2009;3:20-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Rohlman DS, Anger WK, Lein PJ. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology. 2011;32:268-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Spassova D, White T, Singh AK. Acute effects of acephate and methamidophos on acetylcholinesterase activity, endocrine system and amino acid concentrations in rats. Comp Biochem Physiol C Toxicol Pharmacol. 2000;126:79-89. [PubMed] |
| 37. | Ahmad R, Tripathi AK, Tripathi P, Singh R, Singh S, Singh RK. Studies on lipid peroxidation and non-enzymatic antioxidant status as indices of oxidative stress in patients with chronic myeloid leukaemia. Singapore Med J. 2010;51:110-115. [PubMed] |
| 38. | Grosicka-Maciąg E. [Biological consequences of oxidative stress induced by pesticides]. Postepy Hig Med Dosw (Online). 2011;65:357-366. [PubMed] |
| 39. | Soltaninejad K, Abdollahi M. Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Med Sci Monit. 2009;15:RA75-RA90. [PubMed] |
| 40. | Altuntas I, Delibas N, Doguc DK, Ozmen S, Gultekin F. Role of reactive oxygen species in organophosphate insecticide phosalone toxicity in erythrocytes in vitro. Toxicol In Vitro. 2003;17:153-157. [PubMed] |
| 41. | Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE, Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci. 2004;49:1752-1757. [PubMed] |
| 42. | D’Odorico A, Bortolan S, Cardin R, D’Inca’ R, Martines D, Ferronato A, Sturniolo GC. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289-1294. [PubMed] |
| 43. | Galano A, Francisco Marquez M, Pérez-González A. Ellagic acid: an unusually versatile protector against oxidative stress. Chem Res Toxicol. 2014;27:904-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Chao PC, Hsu CC, Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab (Lond). 2009;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680-6684. [PubMed] |
| 46. | Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119-133. [PubMed] |
| 47. | Bai AP, Ouyang Q, Xiao XR, Li SF. Probiotics modulate inflammatory cytokine secretion from inflamed mucosa in active ulcerative colitis. Int J Clin Pract. 2006;60:284-288. [PubMed] |
| 48. | Allahverdi TD, Allahverdi E, Yayla S, Deprem T, Merhan O, Vural S. The comparison of the effects of ellagic acid and diclofenac sodium on intra-abdominal adhesion: an in vivo study in the rat model. Int Surg. 2014;99:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Koriyama Y, Nakayama Y, Matsugo S, Sugitani K, Ogai K, Takadera T, Kato S. Anti-inflammatory effects of lipoic acid through inhibition of GSK-3β in lipopolysaccharide-induced BV-2 microglial cells. Neurosci Res. 2013;77:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fujino Y, Liu B S- Editor: Ma YJ L- Editor: A E- Editor: Ma S