Published online Jan 14, 2016. doi: 10.3748/wjg.v22.i2.874
Peer-review started: May 6, 2015
First decision: June 2, 2015
Revised: July 29, 2015
Accepted: November 30, 2015
Article in press: December 1, 2015
Published online: January 14, 2016
Processing time: 252 Days and 21.3 Hours
This review aims to share the lessons we learned over time during the setting of the hepatocyte transplantation (HT) program at the Hepatic Cell Therapy Unit at Hospital La Fe in Valencia. New sources of liver tissue for hepatocyte isolation have been explored. The hepatocyte isolation and cryopreservation procedures have been optimized and quality criteria for assessment of functionality of hepatocyte preparations and suitability for HT have been established. The results indicate that: (1) Only highly viable and functional hepatocytes allow to recover those functions lacking in the native liver; (2) Organs with steatosis (≥ 40%) and from elderly donors are declined since low hepatocyte yields, viability and cell survival after cryopreservation, are obtained; (3) Neonatal hepatocytes are cryopreserved without significant loss of viability or function representing high-quality cells to improve human HT; (4) Cryopreservation has the advantage of providing hepatocytes constantly available and of allowing the quality evaluation and suitability for transplantation; and (5) Our results from 5 adults with acute liver failure and 4 from children with inborn metabolic diseases, indicate that HT could be a very useful and safe cell therapy, as long as viable and metabolically functional human hepatocytes are used.
Core tip: Our aim is to share the lessons learned over time during the establishment of the hepatocyte transplantation (HT) program at our hospital and to envisage future strategies. The hepatocyte isolation and cryopreservation procedures have been optimized and, fast and sensitive criteria for assessment of functionality of hepatocyte preparations and suitability for transplantation have been set up. Neonatal hepatocytes show high-functional quality and could improve cell therapy applicability. Our results (patients with acute liver failure and inborn metabolic diseases) indicate that HT could be a safe and efficient therapy, as long as viable and high-quality, metabolically functional human hepatocytes are available.
- Citation: Ibars EP, Cortes M, Tolosa L, Gómez-Lechón MJ, López S, Castell JV, Mir J. Hepatocyte transplantation program: Lessons learned and future strategies. World J Gastroenterol 2016; 22(2): 874-886
- URL: https://www.wjgnet.com/1007-9327/full/v22/i2/874.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i2.874
Liver transplantation (LT) is the treatment of choice for end-stage liver diseases. The principal limitation of this procedure lies in the imbalance between patients requiring transplantation and organ donors, resulting in a long waiting list and increased morbidity and mortality. As a result, there is a need to find strategies that could serve as temporary or definitive alternatives to LT.
Cell-based therapies have been a particularly active research field in recent years. In particular, hepatocyte transplantation (HT) is being evaluated worldwide as a promising alternative to LT for a variety of indications, including acute liver failure (ALF) and metabolic liver diseases[1-7].
The principle behind hepatic cell therapy is based on the hypothesis that transplanted hepatocytes, when implanted into the host liver, can undertake hepatic metabolic functions, which are lacking or perform poorly in some patients[2-4]. For this purpose, only hepatocytes are used, which are totally differentiated cells capable of developing hepatic functions based on the regenerative capacity of the liver[8].
The procurement of organs donated for implantation, identification of potential candidates for this treatment and the acquisition, processing, preservation and finally, implantation of the isolated hepatocytes into the subsidiary patients are all necessary steps for this type of treatment. Since the first HT was performed in 1998 in a 10-year-old child with Crigler-Najjar disease type I[9], the indications and technical aspects of this treatment have been subjected to changes; however, the supply of good quality livers for hepatocyte isolation is the major challenge.
To perform this state-of-the-art therapy, the Hepatic Cell Therapy Unit (HCTU) was created in our hospital in 2007. Since then until now, we have come a long way, gaining more experience and tailoring the protocols according to the results obtained.
Since the HT program started in La Fe Hospital in Valencia on May 2008, 9 HT have been performed, 5 in adults and 4 in children[6,7].
The aim of this paper is to share the lessons learned over time during the HT procedure and to envisage future strategies[6,7,10-31]. The results from HT indicate that it could be a very useful and safe technique, as long as viable and high-quality, metabolically functioning human hepatocytes are available.
The use of HT as an alternative to conventional LT has been investigated during the last 30 years. The first studies were performed by Howard[32] in 1967 using animal models with metabolic liver diseases, which resulted in improved biochemistry following HT[33]. Subsequently, experimental models have been described for acute[13,34,35], chronic liver failure[35,36] and inborn hepatic metabolic disorders[37,38].
Clinical tests have been performed in patients with ALF[21,39-42] or with acute on chronic liver disease as a bridge to LT[1,7,39,43-45] and in children with congenital metabolic errors[6,9,46-52]. Over the years, the indication for HT has mainly focused on children with congenital metabolic errors, for whom the best results have been achieved. A program of adult and pediatric HT was set in our Unit obtaining poor results in the former compared to the children[6,7] and in agreement with the results published in the literature[21,39-45]. This, together with a considerable amount of hepatocytes required in the adults and the scarcity of hepatocytes sources, are the reason for our Unit to have restricted the indication for this treatment to children with metabolic disorders.
In the HCTU, the indications for HT in children have been established according to the severity, the prognosis of the metabolic disorder and the expected benefits from this therapy[6].
Obtaining human hepatocytes is a complex process due to the scarcity of suitable sources for cell isolation, the necessary infrastructure to carry out this isolation, the conservation and lability of hepatocytes’ maintenance.
Hepatocyte isolation and cryopreservation processes are performed in the HCTU at Hospital La Fe, Valencia, whose installations are accredited under the European standards EN-ISO 14.644 on clean rooms and adjoining premises; the clean rooms are classified as ISO class 6, and the adjoining rooms as ISO class 7. The isolation, characterization, and cryopreservation of human hepatic cells are performed under good manufacturing practices, in a way that the hepatocytes obtained are safe and qualified to be used in human.
The main purpose of the HCTU is to transfer basic science to the clinical practice[22-24]. Our Unit consists of a multidisciplinary team formed by the Pediatric Hepatology Unit, Liver Transplant Unit and Experimental Hepatology Unit.
The experience acquired by our research team as a result of hepatocyte isolation from surgical hepatic biopsies helped to develop a standardized protocol which has been optimized and adapted for large scale isolations from organs unsuitable for whole-organ transplantation or for liver fragments from hepatectomies[10,11,13-20,22].
Over the years, we have optimized the isolation procedures in order to obtain high functional quality human hepatocytes which are characterized according to well established quality criteria. Then, hepatocytes are cryopreserved and stored until use. Cryopreserved cells are ready to use and, additionally, extensive quality testing can be performed to determine suitability for transplantation, which are important advantages over fresh cells.
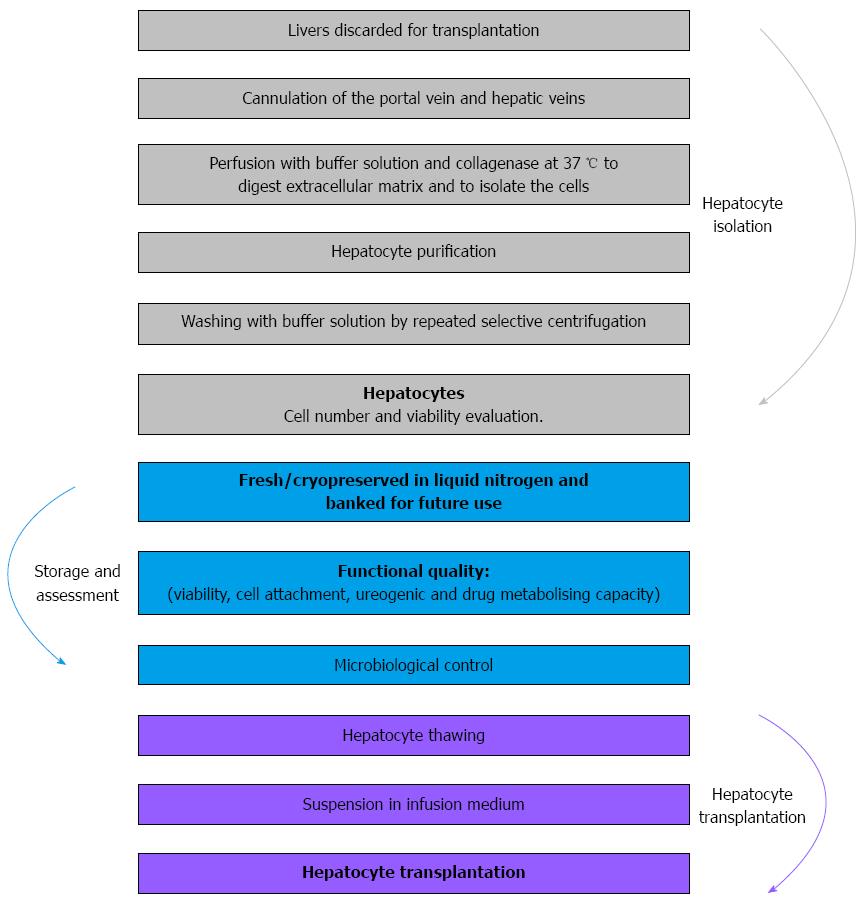
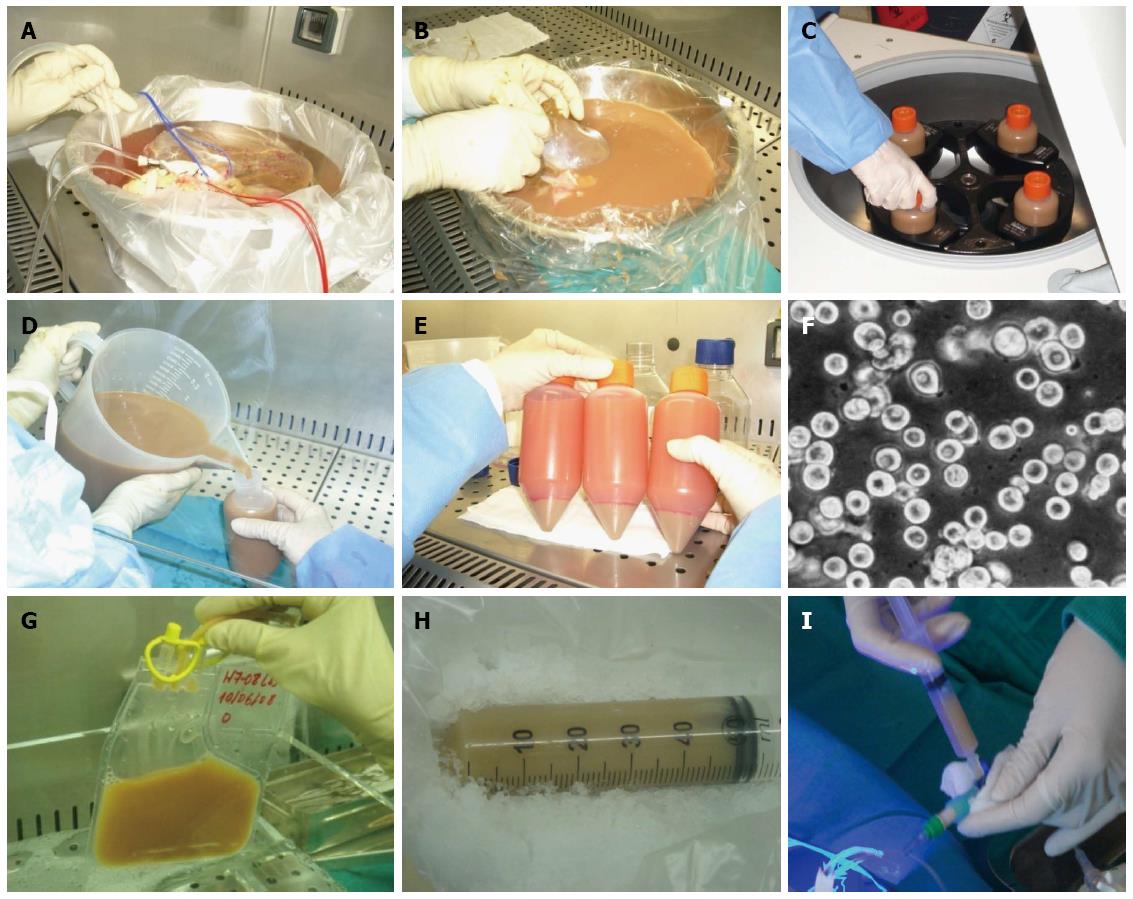
Basically, HT is made up of three phases: hepatocyte isolation from donated livers that had been rejected for whole-organ transplantation, preparation of cell suspensions, and implantation into the recipient. Following isolation, the cells can be frozen and stored (cell cryopreservation); simultaneously, these cells are analyzed for bacteriology and quality control. The implantation of the hepatocytes into the recipient is performed by infusion in the portal vein or splenic artery, with the advantage of planning this procedure if cryopreserved cells are used[10,31,53-55] (Figure 1).
As mentioned above, the main concern in HT is the lack of adequate sources for viable human hepatocytes. Current sources of liver tissue are mainly adult organs considered unsuitable for whole LT, often of marginal quality. The HCTU collaborates closely with the National Transplant Organization (NTO) to recover donated organs that have been deemed unsuitable for transplantation.
Most of the organs for hepatocyte isolation have been discarded for transplantation due to steatosis greater than 40%-50%[7,27], prolonged ischaemia time, traumatic damage to the graft[47], capsular tear[47,54] blood group incompatibility[9] and vascular or biliary lesions. Occasionally, fetal hepatocytes have been used for infusion[43].
New sources for hepatocyte isolation include liver tissue resulting from hepatic reductions, split liver grafts and segment 4 after a split-liver graft for two patients, grafts from donors after circulatory death, and donors with severe arteriosclerosis[48,49,54].
Steatotic donor livers are becoming more common with the increasing incidence of obesity in our population. For this reason, at the beginning of the program, the HCTU used to accept most of the organs discarded for transplantation, regardless of the percentage of steatosis, in order to not waste any possible source of hepatocytes[16,17,22,27,30,31]. However, based on the poor results, we decided to refuse steatotic liver grafts discarded for transplantation as a source for HT.
Most of the criteria to accept a liver for cellular isolation are similar to those required for LT donation. Appropriate liver donors for HT are all those grafts rejected for LT, with no age limit, that show no signs of sepsis or history of neoplasia, with negative serology for hepatitis C and B, HIV, HTLV, and syphilis, family consent for research purposes, steatosis less than 50%, no hepatic illnesses (cirrhosis, cholestasis, or haemophilia), and a cold ischaemia time less than 12 h when possible.
Neonatal donor: Our experience shows that this is not enough and new sources of human liver tissue for hepatocyte isolation need to be explored. An interesting alternative to adult liver is the use of unsuitable neonatal livers for LT as a potential source of differentiated hepatic cells which, to our knowledge, has been poorly explored to date[3,56,57].
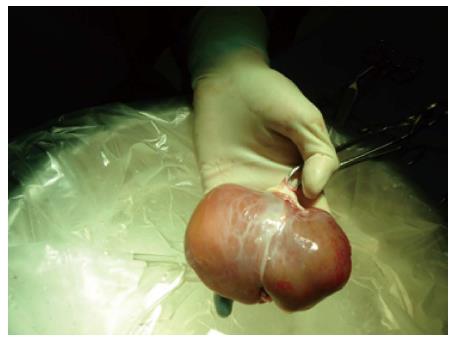
In the HCTU neonatal donors are considered a potential source of hepatocytes[29]. In 2010, a program for neonatal liver donation was set up in our center as a new source for hepatocytes after a national approval by the NTO and the ethic committee. These livers are not usually suitable for LT due to low weight, immature liver function and the technical difficulties related to the size of the vessels and the biliary tree (Figure 2). However, a high yield of good-quality hepatocytes can be isolated from neonatal liver tissue[29].
In neonatal donors, brain death certification is very difficult to verify. Despite the brain stem test in newborns, toddlers, and children being similar to that in adults, there are some differences as to the duration of the observation period and also with the instrumental tests performed, which make it much more difficult to diagnose. This inconvenience led us to consider another type of donation, such as donation after circulatory death. According to the Maastrich classification, types III and IV donors are an important source of grafts for transplantation, especially type III, in which asystole happens after the controlled withdrawal of all the therapeutic measures[58,59].
The newborn period includes the first 28 d of life. Our inclusion criteria for organ donation were patients whose pathologies did not respond to medical and/or surgical treatment and with life incompatibility: congenital anomalies, intracranial intraventricular hemorrhage grades III-IV, congenital disorders of the central nervous system with no activity in the electroencephalography (EEG), surgical pathology not amenable to treatment and incompatible with life, anencephalic, cranioencephalic trauma, premature newborns with pathologies that do not respond to therapeutic maneuvers, surgery patients with postoperative cerebral anoxia[58]. Patients with HIV-1 and 2, HBV, HCV, and sepsis with multiorgan failure were excluded.
Our laboratory works from a standardized protocol for hepatocyte isolation from surgical hepatic biopsies, which has been optimized and adapted for large scale isolations from organs unfit for whole-organ transplantation or from liver fragments from hepatectomies[10,13,15]. At the early stage of isolation, we perfused the entire organ resulting in an incomplete digestion of the hepatic tissue[22].
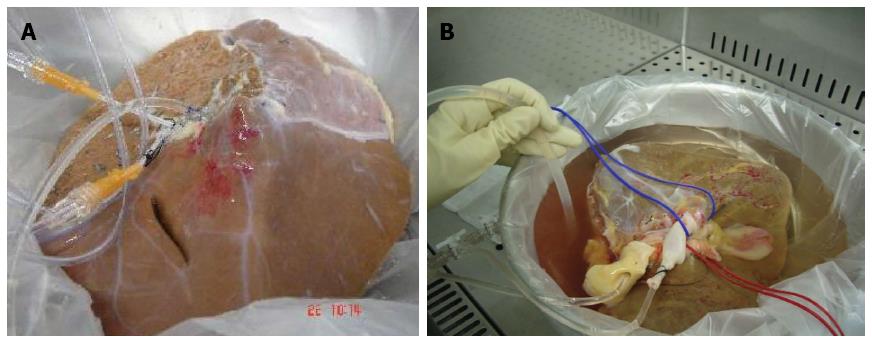
Another difficulty was to avoid digestion of the vessel cannulated to infuse the collagenase before the digestion of the parenchyma has been achieved. This spurred a modification of the perfusion system, and we divided the hepatic parenchyma into left and right lobes, performing a separate perfusion of each lobe[22,25] (Figure 3). Similar situations were described by Mitry et al[55], suggesting the use of one donor for three recipients, proposing segment IV obtained from a split, as a cellular isolation source.
In general, during the disgregation process of the liver or its lobes, a better output of the lightest samples (one lobe) was noticeable. However, splitting the liver prolonged the procedure affecting the viability and the functionality of the hepatocytes isolated due to longer cold ischaemia time. To prevent this, we performed the digestion of both lobes simultaneously in two different laminar flow hoods.
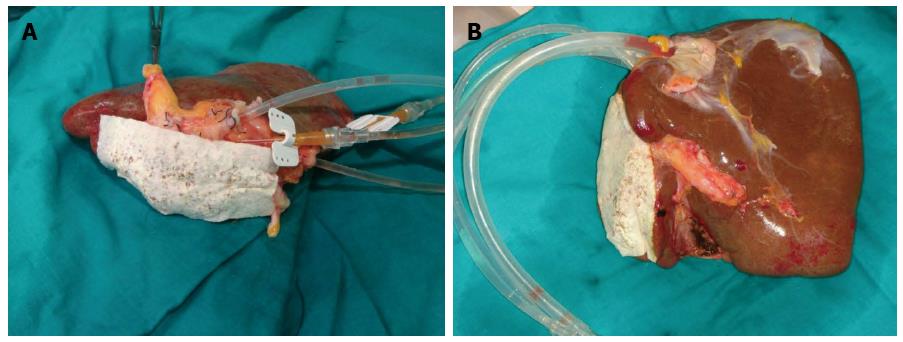
Splitting the liver created new problems in the process for hepatocytes isolation. By performing an ex situ division of the hepatic graft in right lobe and left lobe, we had to suture all the arterial, portal and suprahepatic branches in the cut surface to avoid leakage of the collagenase, leading to a tedious process due to the large amount of ligatures and stitches used, increasing graft preparation time for more than 3 h. To prevent this, we applied TachoSil®, a hemostatic sealant used during hepatectomies to stop small bleeding points and small bile leaks in the cut surface[25] (Figure 4A).
Before using the TachoSil®, we tried different biological glues, regularly used in surgical practice to seal blood vessels from the hepatic transection margin. However, the results showed that these glues were not able to tolerate collagenase pressure from the infusion pump, leading to leakage from multiple openings. As a result, we observed an incomplete digestion, which conditioned a decrease in cellular output. Moreover, we verified the presence of glue residues in the cell solution, being impossible to separate them from the cells, which disabled them for their infusion. The use of TachoSil® in human hepatocytes isolation provides us great advantages: avoids suture and ligation of arterial and venous branches with reduction in preparation time, as well as cold ischemia time of the graft, crucial factor of cellular viability and performance. Moreover, by pointing out the leak of the recirculating solution, it enables an ideal collagenase perfusion of the graft, vital step in the hepatic parenchyma digestion and, therefore, in the hepatocytes isolation[25]. In contrast with other haemostatic agents and sealants, TachoSil® has not been detected in the cell suspension infusion.
Apart from the cannulation of the portal vein, another modification was to cannulate the hepatic veins, which allowed using bigger calibre cannulas increasing the flow of the collagenase. This also contributed to a better digestion of segment 7 and 8 due to its proximity to the hepatic veins. With all these modifications, a better digestion of the liver parenchyma was achieved, improving the efficiency and the viability of the isolated hepatocytes (Figure 4B).
In the case of neonatal livers, a super rapid technique was used to procure them due to the reduced size of these organs. The hepatic veins, portal vein or umbilical vein within the umbilical cord were identified and were therefore cannulated according to vessel size. This cannula was then connected to the tissue preservation solution (Celsior® solution; Genzyme, Madrid, Spain), and liver perfusion started. The liver was placed into two or three sterile bags and transported to the laboratory while being maintained in ice-cold Celsior® solution until cell isolation[24,29]. The digestion process in these livers is usually faster (10-15 min) and uniform, with a better efficiency and viability when comparing to adult livers[29].
A standardized collagenase perfusion technique is used to digest liver tissue to obtain human hepatocytes[3,6,10,16,22,44,60]. Briefly, major liver vessels are cannulated and perfused using a peristaltic pump with buffered salt solution containing chelating agent EGTA, which removes calcium and disrupts desmosomes; the liver is subsequently perfused with collagenase solution to digest the extracellular matrix[3,10,24,59]. Initially, enzymatic perfusion is performed following cannulation of the hepatic artery and the portal vein from the donated liver tissue (Figure 5A). When feasible, we also cannulate the biliary duct[22]. During the perfusion procedure, the liver and solutions are kept at 37 °C to allow optimal enzyme activity. After digestion, the liver tissue is disrupted, and the suspended hepatocytes are purified by filtration and low-speed centrifugation (Figure 5B-F). The average hepatocyte yield is 3 × 106-20 × 106 hepatocytes per gram of liver tissue[6,13,14,22] and the conditions for tissue isolation influence the procedure results[61].
Different concerns have arised over the years in the digestion process. The first concern was to reach 37 °C in a uniform manner throughout the liver, especially in the thickest part of the right lobe corresponding to segments 7 and 8. This resulted in an incomplete digestion of the liver parenchyma on the right lobe while the left lobe was already digested. To resolve this problem, the temperature of the bath was increased up to 42 °C with the liver submerge and contained with three organ bags, which helped to preserve the sterility of the procedure and also to reach the target temperature for the collagenase to work properly.
Based on our studies with liver biopsies from surgical resections, the protocol was optimized and updated to perform hepatocytes isolation on a large scale using those livers discarded for transplantation or bigger portions of liver specimens from hepatectomies[10,13,14].
The experience gained with the results of the first hepatocyte isolation from steatotic grafts, helped us to optimize the process of hepatocyte isolation and cultivation culture from whole livers (1.5-3.5 kg) or complete lobes (0.5-1 kg) unused for transplantation (Figure 3).
We performed 8 isolations from livers discarded for transplantation because of the severe steatosis[16,17,22] (Table 1). Hepatic steatosis has become a common problem in our populations with an increased incidence of obesity. It affects about 25% of liver transplant donors and most of the steatotic donor liver are rejected for organ transplantation[27,30,62,63].
| Sample | Weight (g) | Age (yr) | Sex | Ischemia time (h) | Steatosis (%) | Viability (%) | Yield (× 106 cells/g) |
| LL | 350 | 80 | F | 12 | < 30 | 73 | 2.8 |
| WL | 3500 | 65 | F | 16 | 40-50 | 85 | 1.6 |
| WL | 1142 | 69 | F | 13 | 30 | 85 | 2.6 |
| WL | 2200 | 75 | M | 9 | 40 | 53 | 1.0 |
| RL | 1200 | 32 | M | 11 | < 30 | 86 | 2.6 |
| WL | 2032 | 26 | M | 17 | 50 | 20 | Discarded |
| WL | 1550 | 58 | M | 15 | 40 | 20 | 0.11 |
| WL | 2354 | 67 | M | 6 | 10 | 20 | Discarded |
Organs with advanced steatosis (≥ 40%) showed lower cell yields during isolation (viable hepatocytes obtained/g of tissue processed). Our experience has allowed us to demonstrate that the hepatocytes isolated from these livers are usually of low cell viability and poor function. Steatotic hepatocytes do not tolerate cryopreservation well, leading to greater cell loss, impaired metabolic function and the chances of a successful engraftment are lower, compared to cell from nonsteatotic livers[13,14,17,22,44]. According to Sagias et al[63] and based on the results, we decided to decline livers rejected for transplantation with the background of steatosis as a source for HT.
Livers from elderly donors showed tendencies of reduced viability (Table 1). In general, the disintegration process of the liver in this case, gives lower yields than those obtained through surgical biopsies of fatty liver tissue, although in some cases higher yields were observed. For this reason, livers form elderly donors are also discarded as a source of hepatocytes.
Initially, the HCTU recovered 13 donor organs deemed unsuitable for LT, but fit for HT. The hepatocytes were isolated from donors with arteriosclerosis, doubtful viral serology, donors after circulatory death, split liver grafts, fibrotic and steatotic livers[22]. All grafts were irrigated with Celsior® preservation solution during the procurement, because in our experience, its use produces a better cell viability in the human hepatocytes isolation process[14,15], compared with other preservation fluids[15].
Of the 13 donor livers (8 women, 5 men), the isolation was performed in 3 split lobes, 2 whole organs, and in the remaining 8 donor livers, the organ was divided into 2 lobes, left and right lobes to improve the digestion. The percentage of steatosis was determined by pathology studies. The cold ischaemia time remained under 4 h in all cases and the resulting cell viability was between 70% and 89% with a yield of 1 × 106-17 × 106 cells/g (Table 2)[22].
| Liver number | Sample | Weight (g) | Blood type | Age (yr) | Sex | Viability1 (%) | Yield (× 106 cells/g) | V (%) | Non-valid |
| 1 | LL | 420 | O | 53 | F | 67 | 1.3 | 78 | Split |
| 2 | WL | 420 | O | 1 | M | 68.5 | 6 | 75 | DS |
| 3 | RL | 774 | O | 14 | F | 74.7 | 6 | 75 | Split |
| 4 | LL | 260 | O | 83 | M | 63.3 | 6.7 | 89 | Liver fibrosis |
| 5 | LL | 350 | O | 32 | F | 50 | 4.9 | 79.3 | Liver fibrosis |
| RL | 832 | ||||||||
| IV | 676 | ||||||||
| 6 | LL | 376 | A | 52 | M | 37.8 | 9.9 | 87.5 | DCD |
| RL | 1154 | ||||||||
| 7 | LL | 460 | O | 15 | F | 63.8 | 5.75 | 86.5 | DCD |
| RL | 1162 | ||||||||
| 8 | LL | 444 | AB | 73 | M | NA | 3.3 | 70 | Liver fibrosis |
| 9 | LL | 325 | A | 76 | F | NA | 5.25 | 88.5 | AE |
| RL | 782 | ||||||||
| 10 | LL | 488 | O | 82 | M | 41.7 | 4.95 | 80.5 | AE |
| RL | 1140 | ||||||||
| 11 | RL | 1292 | O | 15 | F | 71 | 8.7 | 87 | Trauma,split |
| 12 | WL | 238 | A | 4d | F | 74 | 17 | 100 | No recipient |
| 13 | RL | 998 | O | 53 | F | 67 | 6.58 | 95 | CAA |
| LL | 306 |
In general, the disintegration process of the whole liver or split lobes gives lower yields than those obtained through surgical biopsies from fatty liver tissue, although we must also highlight higher yields from livers with lower weight.
As a result of this work, we created a biobank of cryopreserved hepatocytes functionally characterized and ready to be used for HT. The first HT by HCTU was performed in our hospital on May 2008 in a girl with urea cycle defect.
The clinical outcome after HT depends very much on the quality of the hepatocytes used, which depends to a large extent on the nature of the tissue utilized for cell isolation, donor factors such as age, and cell harvesting procedures[16-18,20,24,30,31,64].
In addition, from the surgical removal of tissue to the final obtaining of cell suspensions, hepatocytes are subjected to a number of factors [i.e., warm ischemia and hypoxia during surgical liver resection, cold ischemia during transportation, the cell isolation procedure, the perfusion liquid (Celsior®/University of Wisconsin (UW) solution] that can influence their viability and functionality[14,17,31]. For instance, it seems to be a negative correlation between hepatocyte viability and the duration of both warm and cold ischemia[60].
The trypan blue assay is quick and easy to perform and is the most frequently used test to evaluate cell number and viability[20,31]. However, this assay cannot detect early apoptotic cells or evaluate the metabolic function of the cell. In general, viability greater than 50%-60% is considered the minimum for clinical transplantation[3,20,59]. Simple viability measurements are not conclusive enough to predict the long-term HT outcome, and a correlation between in vitro hepatocyte viability and the graft function after transplantation is lacking[20,59]. In our experience, the hepatocytes with a initial viability below 70% generally decrease 20% of viability after cryopreservation/thawing. This does not happen in those hepatocytes isolated from neonatal donors[29].
Hepatocytes should not only be highly viable but also functionally active to support those functions lacking in the recipient liver. Therefore, other assessment criteria are needed for a better estimation of the quality of hepatocytes and to provide an objective basis for decision making.
Because we encounter frequently donor livers of poor quality, fast and sensitive procedures to evaluate the functional performance of hepatocyte preparation have been sit up in order to determine their suitability for HT[18,59]. To this end, reliable assays have been developed to assess the functional capacity of cells in the course of the isolation procedure or shortly after thawing. Key hepatic functions, such as the synthesis of plasmatic proteins, urea synthesis or ammonia removal, and drug-metabolizing capacity, have been assessed for this purpose[3,18,59,65]. In fact, a rapid quality assessment of freshly isolated hepatocytes could help to estimate their potential susceptibility to freezing/thawing-induced cell damage and the convenience of banking a particular hepatocyte preparation.
We measured cell yield and functionality through metabonomic studies of phase I and II biotransformation enzymes, ureogenesis capabilities, and total number of cells[11,12] (Figure 1).
In the HCTU we have established specific criteria for functional quality control of the hepatocytes (fresh or cryopreserved) so that we can establish their suitability for cell transplantation in a reasonable time frame (1 h). The activity of 5 major P450 cytochrome isoenzymes (CYP1A2, CYP2A6, CYP3A4, CYP2C9 and CYP2E1) is determined along with phase II conjugation enzymes [UDP, glucuronosyltranferase (UGT) and sulfotransferease] by incubation with a specific substrate cocktail recently developed in our laboratory, high performance liquid chromatography/mass spectrometry analysis[6,18-20]. In the future, we hope to use metabonomic studies and establish a useful number of cellular metabolites to characterize the quality of hepatocytes.
Innovative methods to improve cell quality have been a focal point of research. Hepatocytes perform a wide range of functions that can be individually tested after isolation. We aimed to assess the functionality of hepatocyte preparations with a view to promote customized cell preparation for each receptor to fit the metabolic defect of the recipient[6,17,24].
Neonatal hepatocytes: In our experience, neonatal hepatocytes showed better post-thawing recovery compared with adult hepatocytes, as shown by the viability values that did not differ significantly from freshly isolated cells, a higher expression of adhesion molecules (b1-integrin, b-catenin, and E-cadherin), better attachment efficiency, cell survival, and a lower number of apoptotic cells. The metabolic performance of thawed hepatocytes has been assessed by ureogenesis and drug metabolizing capability (cytochrome P450 and UGT enzymes). CYP2A6, CYP2C9, CYP2E1, and CYP3A4 activities were found in all cell preparations, while CYP1A2, CYP2B6, CYP2C19, and CYP2D6 activities were detected only in hepatocytes from a few neonatal donors. The expression of UGT1A1 and UGT1A9 (transcripts and protein) was detected in all hepatocyte preparations, while activity was measured only in some preparations, probably due to lack of maturity of the enzymes. However, isoforms UGT1A6 and UGT2B7 showed considerable activity in all preparations[27]. Compared to adult liver, the hepatocyte isolation procedure in neonatal livers also provides thawed cell suspensions with a higher proportion of hepatic progenitor cells (EpCAM+ staining), which could also participate in regeneration of liver parenchyma after transplantation[8,27]. These results could imply important advantages of neonatal hepatocytes as a source of high-quality cells to improve human HT applicability[29].
The time lag between the isolation of hepatocytes and their use causes heavy losses of many resources due to the lack of a method for conservation during prolonged periods of time. Cryopreserved cells have the advantages that they are constantly available and that extensive quality testing can be performed, whereas rapid assays are necessary for fresh cells in order to determine suitability for transplantation[4,6,22,25,65,66]. Another advantage of cryopreservation is that it allows a more effective quarantining of cells against bacterial or fungal contamination as compared with using fresh cells. For this reason, only cryopreserved cells are used in our Unit. This provides a biobank of cryopreserved cells which allows us to perform semi-elective HT with previous microbiological and functional assessment[6,7,22].
However, cryopreservation has detrimental effects on the viability and metabolic function of hepatocytes, and therefore, on thawed cells, they are often not suitable for clinical use[3,6,24,66]. Thawed cell viability and function are related to the nature of the liver tissue from which hepatocytes were isolated, including factors such as the donor’s age or condition, liver steatosis, and the duration of cold and warm ischemia times involved in procuring the liver[66]. However, hepatocytes isolated from fetal or neonatal livers can be cryopreserved without significant loss of viability or function[3,29].
Another major factor is the speed of freezing, if the velocity is too slow, the hepatocyte can suffer dehydration, but if it is too fast, the intracellular liquid fails to exit and freezes. The consequence of this process is the formation of crystals that, together with fluctuations in cellular volume, cause damage to the cellular membrane and rupture of the cell, causing cellular necrosis[67]. In recent studies, the different steps involved in the hepatocyte cryopreservation and thawing process were systematically investigated and included cell density, addition of cryoprotectants, freezing temperature program, and thawing method[26,68,69].
However, no consensus has been reached on the use of fresh or cryopreserved hepatocytes, and some centers prefer transplanting fresh hepatocytes because of better viability and function as compared to cryopreserved cells[6,7,41,43,45,46]. Despite these limitations, cryopreserved hepatocytes are often used for clinical transplantation in some patients and yield a good metabolic effect[6,7,16,46].
Another approach involves developing methods for the cold storage of hepatocytes. This has been recently demonstrated to be possible for up to two weeks using new organ preservation solutions[61].
Our protocol to cryopreserve hepatocytes has been submitted to substantial modifications over time. Cryopreservation is accomplished by controlling the freezing rate in a solution containing a permeable cytoprotectant dimethyl sulfoxide (DMSO). According to current protocols, hepatocytes are frozen in a mixture of UW solution with DMSO (10%-15%). The suspension is frozen in 250 mL bags containing only 50 mL in each with a cell density of 20 million cells/ml using a programmed temperature reduction biological freezer (CM- 2000, Metal Carbides) until reaching -140 °C. The cells are then stored in liquid nitrogen at -196 °C. For each bag prepared, 4 criovials are frozen in order to characterize the functionality of the cells before thawing the bags for infusion.
Cell thawing is performed by immersing the bags in a fixed-temperature bath at 37 °C. The cryopreservation solution is then diluted using the appropriate medium, centrifuged at 4 °C for 5 min, and the supernatant is discarded. The cellular pellet is resuspended in an appropriate volume of the medium for infusion into the patient, and cellular viability is assessed[22,26,31] (Figures 1 and 5G).
From the 13 isolations performed at the HCTU[22], cell viability, which reached 78%-89% following isolation, significantly decreased in all cases after prolonged cryopreservation storage and thawing (Table 2)[20,31].
At present, there are three generally used routes for cell transplantation: portal vein, splenic vein, and intraperitoneal duct. Injection through the portal vein (Figure 5I) should be reserved for correcting inborn metabolic errors, while the splenic artery should be considered for patients with a fibrotic liver.
Cells are most often delivered by the catheterization of the portal or inferior mesenteric vein by laparoscopic surgery and infusion[29]. The spleen can be used as an alternative transplantation site, especially in patients with liver cirrhosis. The spleen can be accessed by direct injection into the splenic artery through a catheter inserted through the femoral artery for implantation in the spleen. Cells are manually infused. Thus, during the cell infusion, a Doppler portal vein ultrasound and intraportal pressure and a repeated Doppler ultrasound after cell infusion are essential to exclude thrombosis formation.
The hepatocyte mass required to cure a liver-based metabolic disease is expected to vary according to the specific metabolic deficiency. Transplanted hepatocytes are ABO compatible with the recipient.
The hypothetical aim is to perform repeated cell infusions in order to provide an approximate total number of cells representing 5%-10% of the theoretical liver mass. This also depends on the use of fresh vs cryopreserved cells[3,9,29,30,47]. It is still unclear what constitutes the maximum number of liver cells that can be infused each time and how many infusions can be performed in all. As the average adult liver contains 2.8 × 1011 hepatocytes, no more than 1%-2% of liver mass can be transplanted in each single-cell infusion[2,70]. Nevertheless, repeated infusions can be undertaken at time intervals by monitoring portal pressure. In children, cells (up to hepatocytes 108/kg of body weight) are infused slowly (5-10 mL/kg per hour) based on the original considerations of Fox et al[9] and Horslen et al[50] for the purpose of replacing about 5% of the liver cell mass. Furthermore, cell dose and infusion rate are adjusted to patient size and clinical situation.
Cell-based therapies have been a particularly active research area in recent years. In particular, HT is being evaluated worldwide as a promising alternative to LT for a variety of indications, including ALF and metabolic liver diseases. It offers a number of potential advantages if compared with LT; the procedure is considerably less invasive with less risk of morbidity and mortality, and it can be performed repeatedly. Moreover, HT has been demonstrated to be a safe therapeutic option to improve the clinical outcome of patients with acute and chronic hepatic liver diseases, especially children with inborn metabolic-diseases. However, the clinical effectiveness of this process likely relies on the functional performance of the transplanted hepatocytes and their capability to engraft and survive in the host liver. Although considerable progress has been made in bringing HT to the bedside, the scarcity of suitable liver tissue for high-quality hepatocyte isolation is one of its main limitations. This emphasizes the need to explore other sources of tissue for hepatocyte isolation.
Hepatocytes are currently obtained from discarded organs for LT, but experience shows that what is not valid for transplanting is not suitable enough to isolate cells. Therefore, new sources for obtaining hepatocytes, such as donors after cardiac death, patients undergoing LT for metabolic disorders and the option of a domino transplant with the cells obtained have been proposed. In our Unit the use of neonatal livers as a potential source alternative to adult liver to obtain good-performing hepatic cells for HT is being successfully explored.
Cell-based therapies, including those based on stem cells or more differentiated progenitor cells, may represent the future of cell transplantation to treat metabolic liver disease. Therefore, the research of our Unit focuses on identifying alternative and reliable cell sources for transplantation that can be derived by reproducible methods. Our research interest deals with obtaining progenitor liver cells and our rationales is that the administration of mature hepatocytes with progenitor cells would combine the short-term metabolic effects of the differentiated hepatocytes with the long-term survival of the progenitor cells in the host liver.
Another related research line in our Unit is obtaining hepatocytes derived from pluripotent embryonic stem cells, mesenchymal stem cells, fat/core and from induced pluripotent stem cells which have the potential to be expanded and maintained in cell cultures. At present, remain unresolved safety issues, especially risk of neoplasia and immunogenicity, as to raise its use. Ideally, these cell lines would be highly viable preparations with a robust hepatic function and engraftment capacity, and well-characterized.
A limiting factor in hepatic cell therapy is the conservation and storage of isolated hepatic cells. It is necessary to improve hepatocytes storage both for longer periods in the cold so that they can be used as fresh material after a number of days. Therefore, one of our main goals is to create a bank of cryopreserved cells, which allows to use them in an emergency situation, and at the same time provides flexibility to scheduled this treatment. Since cryopreservation has harmful effects on the viability and metabolic function of the cells, improvements cell cryopreservation protocols are mandatory.
The general positive effect of this kind of therapy in the published reports warrants continues development of tools and technology to improve this therapy.
| 1. | Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 327] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 2. | Fitzpatrick E, Mitry RR, Dhawan A. Human hepatocyte transplantation: state of the art. J Intern Med. 2009;266:339-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, Ericzon BG. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272:201-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Puppi J, Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, Ellis EC, Nowak G, Ericzon BG, Fox IJ. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant. 2012;21:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Ribes-Koninckx C, Ibars EP, Calzado Agrasot MÁ, Bonora-Centelles A, Miquel BP, Vila Carbó JJ, Aliaga ED, Pallardó JM, Gómez-Lechón MJ, Castell JV. Clinical outcome of hepatocyte transplantation in four pediatric patients with inherited metabolic diseases. Cell Transplant. 2012;21:2267-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Pareja E, Gomez-Lechon MJ, Cortes M, Bonora-Centelles A, Castell JV, Mir J. Human hepatocyte transplantation in patients with hepatic failure awaiting a graft. Eur Surg Res. 2013;50:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1193] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 9. | Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 719] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 10. | Gómez-Lechón MJ, Donato MT, Ponsoda X, Fabra R, Trullenque R, Castell JV. Isolation, culture and use of human hepatocytes in drug research. London: Academic Press 1996; 129-153. [DOI] [Full Text] |
| 11. | Donato MT, Viitala P, Rodriguez-Antona C, Lindfors A, Castell JV, Raunio H, Gómez-Lechón MJ, Pelkonen O. CYP2A5/CYP2A6 expression in mouse and human hepatocytes treated with various in vivo inducers. Drug Metab Dispos. 2000;28:1321-1326. [PubMed] |
| 12. | Rodríguez-Antona C, Jover R, Gómez-Lechón MJ, Castell JV. Quantitative RT-PCR measurement of human cytochrome P-450s: application to drug induction studies. Arch Biochem Biophys. 2000;376:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Serralta A, Donato MT, Orbis F, Castell JV, Mir J, Gómez-Lechón MJ. Functionality of cultured human hepatocytes from elective samples, cadaveric grafts and hepatectomies. Toxicol In Vitro. 2003;17:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Donato MT, Serralta A, Jiménez N, Pérez G, Castell JV, Mir J, Gómez-Lechón MJ. Liver grafts preserved in Celsior solution as source of hepatocytes for drug metabolism studies: comparison with surgical liver biopsies. Drug Metab Dispos. 2005;33:108-114. [PubMed] |
| 15. | Serralta A, Donato MT, Martinez A, Pareja E, Orbis F, Castell JV, Mir J, Gómez-Lechón MJ. Influence of preservation solution on the isolation and culture of human hepatocytes from liver grafts. Cell Transplant. 2005;14:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Bergenfelz A, Nordén NE, Ahrén B. Intraoperative fall in plasma levels of intact parathyroid hormone after removal of one enlarged parathyroid gland in hyperparathyroid patients. Eur J Surg. 1991;157:109-112. [PubMed] |
| 17. | Bonora-Centelles A, Jover R, Donato MT, Lahoz A, Pareja E, Castell JV, Mir J, Gómez Lechón MJ. Desarrollo, análisis y optimización de modelos celulares hepáticos para estudios de fármaco-toxicología y terapia celular. An R AcadNac Farm. 2008;74:283-306. |
| 18. | Donato MT, Lahoz A, Montero S, Bonora A, Pareja E, Mir J, Castell JV, Gómez-Lechón MJ. Functional assessment of the quality of human hepatocyte preparations for cell transplantation. Cell Transplant. 2008;17:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Glineur R, Fruhling J. [Hypertrophy of the mandibular condyle: diagnostic approach (contribution of nuclear medicine)]. Acta Stomatol Belg. 1991;88:85-92. [PubMed] |
| 20. | Gómez-Lechón MJ, Lahoz A, Jiménez N, Bonora A, Castell JV, Donato MT. Evaluation of drug-metabolizing and functional competence of human hepatocytes incubated under hypothermia in different media for clinical infusion. Cell Transplant. 2008;17:887-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Pareja E, Cortes M, Bonora A, Fuset P, Orbis F, Lopez R, Mir J. New alternatives to the treatment of acute liver failure. Transplant Proc. 2010;42:2959-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Pareja E, Martínez A, Cortés M, Bonora A, Moya A, Sanjuán F, Gómez-Lechón MJ, Mir J. [Hepatic cell transplantation. Technical and methodological aspects]. Cir Esp. 2010;87:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Pareja E, Cortés M, Martínez A, Vila JJ, López R, Montalvá E, Calzado A, Mir J. [Hepatic cell transplantation: a new therapy in liver diseases]. Cir Esp. 2010;88:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Bonora-Centelles A, Donato MT, Lahoz A, Pareja E, Mir J, Castell JV, Gómez-Lechón MJ. Functional characterization of hepatocytes for cell transplantation: customized cell preparation for each receptor. Cell Transplant. 2010;19:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Pareja E, Cortes M, Bonora A, Lopez R, Moya A, Mir J. Isolation of human hepatocytes for hepatic cellular transplant: a new indication for the use of Tachosil®. Asociación Española de Cirujanos. Anuario 2010. Premios Nycomed: Ed Justim S.L 2010; . |
| 26. | Tolosa L, Bonora-Centelles A, Donato MT, Mirabet V, Pareja E, Negro A, López S, Castell JV, Gómez-Lechón MJ. Influence of platelet lysate on the recovery and metabolic performance of cryopreserved human hepatocytes upon thawing. Transplantation. 2011;91:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Tolosa L, Bonora-Centelles A, Teresa Donato M, Pareja E, Negro A, López S, Castell JV, José Gómez-Lechón M. Steatotic liver: a suitable source for the isolation of hepatic progenitor cells. Liver Int. 2011;31:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Burlev VA, Volobuev AI, Sidel’nikova VM, Oganesian AZh. [The interrelations between the uteroplacental blood flow and the indices of hormonal and metabolic activity in threatened abortion]. Vestn Akad Med Nauk SSSR. 1990;10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Tolosa L, Pareja-Ibars E, Donato MT, Cortés M, López S, Jiménez N, Mir J, Castell JV, Gómez-Lechón MJ. Neonatal livers: a source for the isolation of good-performing hepatocytes for cell transplantation. Cell Transplant. 2014;23:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Pareja E, Cortés M, Gómez-Lechón MJ, Maupoey J, San Juan F, López R, Mir J. [Current status and future perspectives of hepatocyte transplantation]. Cir Esp. 2014;92:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Gómez-Lechón MJ, Tolosa L, Pareja E, Castell JV. Cell therapies for the treatment of metabolic inborn errors. Gene and cell therapy: therapeutic mechanisms and strategies. United Kingdom: Taylor & Francis/CRC Press 2014; 1137-1150. [DOI] [Full Text] |
| 32. | Howard RB, Christensen AK, Gibbs FA, Pesch LA. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J Cell Biol. 1967;35:675-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Eiseman B. Treatment of liver failure. Colston Papers No. 19. The Liver. London: Butterworths 1967; 279-285. |
| 34. | Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery. 1977;82:124-132. [PubMed] |
| 35. | Kobayashi N, Ito M, Nakamura J, Cai J, Gao C, Hammel JM, Fox IJ. Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology. 2000;31:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Mito M, Kusano M, Sawa M. Hepatocyte transplantation for hepatic failure. Trans Reviews. 1993;73:35-43. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | De Vree JM, Ottenhoff R, Bosma PJ, Smith AJ, Aten J, Oude Elferink RP. Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology. 2000;119:1720-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Tolosa L, López S, Pareja E, Donato MT, Myara A, Nguyen TH, Castell JV, Gómez-Lechón MJ. Human neonatal hepatocyte transplantation induces long-term rescue of unconjugated hyperbilirubinemia in the Gunn rat. Liver Transpl. 2015;21:801-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Riordan SM, Williams R. Extracorporeal support and hepatocyte transplantation in acute liver failure and cirrhosis. J Gastroenterol Hepatol. 1999;14:757-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, McGavran L, Ostrowska A, Durham J. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000;6:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Bilir B, Durham JD, Kristal J. Transjugular intraportal transplantation of cryopreserved human hepatocytes in a patient with acute liver failure. Hepatology. 1996;24:728. |
| 42. | Soriano HE, WoodRP , KangDC . Hepatocellular transplantation (HCT) in children with fulminant liver failure (FLF). Hepatology. 1997;26:239A. |
| 43. | Howell SL, Tyhurst M. Barium accumulation in rat pancreatic B cells. J Cell Sci. 1976;22:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 201] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Baccarani U, Adani GL, Sanna A, Avellini C, Sainz-Barriga M, Lorenzin D, Montanaro D, Gasparini D, Risaliti A, Donini A. Portal vein thrombosis after intraportal hepatocytes transplantation in a liver transplant recipient. Transpl Int. 2005;18:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 1999;19:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 267] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 46. | Dzhioev FK, Shendrikova IA, Balanski RM. [The relationship between stomach tumor development in mice and DMBA distribution]. Vopr Onkol. 1975;21:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 365] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 47. | Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, Arya R, Wade JJ, Verma A, Heaton ND. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78:1812-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 49. | Ambrosino G, Varotto S, Strom SC, Guariso G, Franchin E, Miotto D, Caenazzo L, Basso S, Carraro P, Valente ML. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplant. 2005;14:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 50. | Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, Fox IJ. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 51. | Stéphenne X, Najimi M, Smets F, Reding R, de Ville de Goyet J, Sokal EM. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant. 2005;5:2058-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Valić F, Cigula M. Interconvertibility of asbestos fibre count concentrations recorded by three most frequent methods. Arh Hig Rada Toksikol. 1992;43:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 197] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 53. | Mitry RR, Hughes RD, Dhawan A. Progress in human hepatocytes: isolation, culture & amp; cryopreservation. Semin Cell Dev Biol. 2002;13:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Alexandrova K, Griesel C, Barthold M, Heuft HG, Ott M, Winkler M, Schrem H, Manns MP, Bredehorn T, Net M. Large-scale isolation of human hepatocytes for therapeutic application. Cell Transplant. 2005;14:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Mitry RR, Dhawan A, Hughes RD, Bansal S, Lehec S, Terry C, Heaton ND, Karani JB, Mieli-Vergani G, Rela M. One liver, three recipients: segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation. 2004;77:1614-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Meyburg J, Alexandrova K, Barthold M, Kafert-Kasting S, Schneider AS, Attaran M, Hoerster F, Schmidt J, Hoffmann GF, Ott M. Liver cell transplantation: basic investigations for safe application in infants and small children. Cell Transplant. 2009;18:777-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Marino AJ, Anwar M, Koons A, Hiatt M, Hegyi T. Prolonged low dose indomethacin for persistent ductus arteriosus. Arch Dis Child. 1991;66:1101-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 58. | Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc. 1995;27:2893-2894. [PubMed] |
| 59. | Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 60. | Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3387] [Cited by in RCA: 3610] [Article Influence: 63.3] [Reference Citation Analysis (7)] |
| 61. | Pless G, Sauer IM, Rauen U. Improvement of the cold storage of isolated human hepatocytes. Cell Transplant. 2012;21:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | ONT Results. Spanish National Transplant Organization (2014). Available from: http://www.ont.es. |
| 63. | Sagias FG, Mitry RR, Hughes RD, Lehec SC, Patel AG, Rela M, Mieli-Vergani G, Heaton ND, Dhawan A. N-acetylcysteine improves the viability of human hepatocytes isolated from severely steatotic donor liver tissue. Cell Transplant. 2010;19:1487-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Kawahara T, Toso C, Douglas DN, Nourbakhsh M, Lewis JT, Tyrrell DL, Lund GA, Churchill TA, Kneteman NM. Factors affecting hepatocyte isolation, engraftment, and replication in an in vivo model. Liver Transpl. 2010;16:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Ostrowska A, Bode DC, Pruss J, Bilir B, Smith GD, Zeisloft S. Investigation of functional and morphological integrity of freshly isolated and cryopreserved human hepatocytes. Cell Tissue Bank. 2000;1:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Terry C, Mitry RR, Lehec SC, Muiesan P, Rela M, Heaton ND, Hughes RD, Dhawan A. The effects of cryopreservation on human hepatocytes obtained from different sources of liver tissue. Cell Transplant. 2005;14:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Terry C, Dhawan A, Mitry RR, Hughes RD. Cryopreservation of isolated human hepatocytes for transplantation: State of the art. Cryobiology. 2006;53:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Calzolari F, Ceruti S, Pinna L, Tamarozzi R. Aneurysm of the azygos pericallosal artery. One case. J Neuroradiol. 1991;18:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Terry C, Dhawan A, Mitry RR, Lehec SC, Hughes RD. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transpl. 2010;16:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 70. | Selden C, Hodgson H. Cellular therapies for liver replacement. Transpl Immunol. 2004;12:273-288. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Enosawa S, Fogli L S- Editor: Yu J L- Editor: A E- Editor: Ma S