INTRODUCTION
Sporadic duodenal polyps are uncommon, found in up to only 5% of patients referred for upper gastrointestinal endoscopy[1]. Sporadic duodenal polyps can be classified according to their histopathological subtype and location, as follows: nonampullary sporadic adenoma, ampullary sporadic adenoma, Brunner’s gland adenoma or harmatoma, gastric heterotopia/metaplasia, inflammatory fibroid polyp, lipoma, leiomyoma, carcinoid, gastrointestinal stromal tumors, lymphoma, and solitary Peutz-Jeghers polyps[1]. Nonampullary duodenal adenoma is relatively common in patients with familial adenomatous polyposis (FAP), but sporadic duodenal adenoma (SDA) is rare. Approximately 40% of duodenal adenomas are sporadic, and the remaining 60% present in patients with FAP[2].
The options for endoscopic resection of duodenal adenomas include snare polypectomy, endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), and argon plasma coagulation (APC) ablation[3]. EMR of duodenal neoplasms was first performed in 1992[4]. Endoscopic resection represents an attractive alternative to surgical resection in appropriately selected patients, with lower morbidity and mortality rates[5]. Surgical options include laparoscopic-assisted endoluminal surgery, laparoscopic polyp excision, duodenectomy, and pancreaticoduodenectomy[1]. The morbidity and mortality of these procedures are dependent on patient co-morbidities, together with operator experience and case volume[1]. These techniques are usually reserved for malignant lesions. In this review, we will discuss the clinicopathological characteristics and management of sporadic and FAP-related nonampullary duodenal adenomas.
PREVALENCE AND NATURAL HISTORY OF NONAMPULLARY DUODENAL ADENOMAS
Sporadic duodenal adenomas
In a large retrospective endoscopic series conducted in Germany, 378 duodenal polyps were identified in the course of more than 25000 esophagogastroduodenoscopy (EGD) procedures, corresponding to an incidence of 1.5%[6]. That study showed that 6.9% of 378 patients had duodenal adenomas. A prospective study from Denmark reported that the prevalence of duodenal polyps was 4.6% (27/584) in patients referred for diagnostic EGD, and two of the polyps were adenomas (0.4%)[7]. Our group demonstrated that the prevalence of duodenal polyps was 1.02% (510/50114), and 14 of the polyps were adenomas (0.03%)[8]. With the increasingly widespread use of endoscopy, these tumors are being diagnosed more frequently. These lesions are of particular interest because of the acceptance of the adenoma to carcinoma progression sequence of colorectal tumors, which has also been postulated to be associated with those of the small bowel[9]. Because 30%-85% of duodenal adenomas undergo malignant transformation, their surgical or endoscopic excision is mandatory[10-14].
Okada et al[15] evaluated the risk of adenocarcinoma posed by nonampullary SDAs with an initial diagnosis of low-grade dysplasia (LGD) or high-grade dysplasia (HGD), based on the results of subsequent endoscopic observations and histological assessments. In this study, 46 SDAs (43 LGD lesions, 3 HGD lesions) were followed up for ≥ 6 mo without treatment. Among 43 LGD lesions, 34 (79.1%) showed no histopathological changes during follow-up, whereas the remaining 9 (20.9%) showed progression to HGD, including 2 (4.7%) that eventually progressed to noninvasive carcinoma. All three HGD lesions remained unchanged histologically, based on biopsy, although one of the three lesions resected endoscopically revealed evidence of noninvasive carcinoma. Among 11 lesions initially diagnosed as HGD from biopsy samples, nine were resected immediately or during follow-up. A high percentage of the cancers (54.5%, 6 of 11) were diagnosed from resected specimens. A multivariate analysis identified HGD diagnosed at first biopsy and a lesion diameter of ≥ 20 mm as being significantly predictive of progression to adenocarcinoma. The authors concluded that LGD lesions show a low risk of progression to adenocarcinoma, but some risk of progression to HGD, which warrants careful follow-up biopsy. However, HGD lesions and large nonampullary SDAs ≥ 20 mm in diameter show a high risk of progression to adenocarcinoma and therefore, they should be treated immediately.
Duodenal adenomas in FAP
Duodenal cancer is a leading cause of death in FAP patients with previous colectomy[16,17]. It develops from pre-existing adenomas with a cumulative risk of almost 100% in patients with FAP[16,18-20]. The cumulative risk of duodenal cancer is 3%-10%[19,21]. Duodenal adenomas usually are multiple, sessile, and predominantly located in the mucosal folds of the descending duodenum[16]. In 1989, Spigelman et al[17] developed a staging system for evaluation of the severity of duodenal adenomatosis. Using this system, classification is determined by a five-grade scale (stage 0-IV) based on adenoma number (1-4, 5-20, or > 20), size (< 5, 5-10, or > 10 mm), histologic type (tubular, tubulovillous, or villous) and severity of dysplasia (mild, moderate, or severe). This system allowed evaluation of the duodenal adenoma burden in patients with FAP and estimation of the risk of developing duodenal cancer, which was confirmed in subsequent studies[20,22]. The risk of duodenal cancer increases with age and with progressive adenoma stage[16,22]. It is lowest at 0.7% in stage 0-III disease and greatest in stage IV disease, with rates of 7%-36% over follow-up periods of 7.6-10 years[21,22]. The risk of developing stage IV duodenal polyposis is estimated to be 20%-50% at age 70 years[19-21].
MOLECULAR FEATURES OF NONAMPULLARY DUODENAL ADENOMAS
Duodenal adenomas are thought to progress to duodenal adenocarcinoma in a stepwise manner, with the accumulation of genetic mutations, including those in APC, KRAS, and p53[23]. Despite epidemiologic and histologic evidence suggesting an adenoma-carcinoma sequence analogous to that proposed for most colorectal adenomas, the pathogenesis of small intestinal adenomas and adenocarcinomas is poorly characterized[24]. Nonampullary SDAs show a tubular architecture in all cases[24,25]. Wagner et al[24] evaluated the molecular characteristics of a series of nonampullary SDAs (n = 22) that developed distal to the ampulla, and compared them with the features of sporadic ampullary adenoma (n = 9) and FAP-related polyps (n = 12). Regardless of their anatomic location and whether they were sporadic or FAP-related, approximately 75% of the duodenal adenomas showed Wnt signaling pathway abnormalities, which probably reflect underlying APC, rather than β-catenin, mutations. KRAS mutations were infrequent in nonampullary SDAs (18%), and FAP-related adenomas (9%), moderatley frequent in ampullary adenomas (44%). None of the cases harbored BRAF mutations. In addition, p53 alterations and DNA mismatch repair were rare. These results indicate that duodenal adenomas share morphologic and molecular features with colorectal adenomas, suggesting that they develop via similar mechanisms. Rubio[26] reviewed a cohort of 306 FAP-related or sporadic duodenal adenomas and found gastric duodenal metaplasia (GMD) covering portion of the adenomas in 31.7% (66/208) of the duodenal FAP adenomas and in 59.5% (58/98) of the duodenal sporadic adenomas (P < 0.05). This result suggests that a subset of GMD of unknown cause might be present in the duodenal mucosa before adenomatous transformation, with neoplastic proclivity similar to that of other metaplasias of the gastrointestinal tract (intestinal metaplasia of the esophagus and of the stomach and metaplastic-hyperplastic polyposis of the colon). Adenomatous neoplastic transformation in these patients may be due to the carcinogenic effect of the high concentrations of bile acids and pancreatic juices in the duodenum. Similar to colonic tumors, small intestinal adenocarcinomas are usually preceded by noninvasive precursor lesions such as adenomas, as evidenced by the occurrence of invasive adenocarcinoma within small bowel adenomas, and the presence of residual adenomatous tissue adjacent to, or within, most carcinomas[9,10]. Sun et al[27] evaluated the CpG island methylator phenotype (CIMP) in ampullary and nonampullary SDAs and determined a correlation between CIMP and MLH1 and p16 methylation, as well as KRAS and BRAF mutations. They showed that CIMP+ (more than two markers methylated) was found in 33.3% of duodenal adenomas; 61% of these CIMP+ adenomas were CIMP-high (more than three markers methylated). In addition, CIMP+ status significantly correlated with older patient age, larger size and villous type of tumor, coexistent dysplasia and periampullary location, MLH1 methylation and KRAS mutation in duodenal adenomas. These results suggest that patients with CIMP+ duodenal adenomas have a higher risk of developing malignancy and may require more aggressive management and surveillance.
ENDOSCOPIC DIAGNOSIS
Certain ampullary neoplasms may present early with obstructive jaundice or pancreatitis, as small lesions can cause obstruction of the ampullary orifice, whereas almost all nonampullary neoplasms are incidentally discovered during routine endoscopy. Kiesslich et al[28] demonstrated that conventional chromoendoscopy with indigocarmine detected a significantly greater number of lesions in the duodenum than did standard high-resolution white-light endoscopy (98 vs 28, P = 0.0042). However, the dye spray technique is considered cumbersome, and, no study on adenoma differentiation has been published to date[29]. In 2006, Uchiyama et al[30] reported narrow band imaging (NBI) findings of ampullary polyps classified as type I, oval-shaped villi, type II, pinecone/leaf-shaped villi, or type III, irregular/non-structured. They showed a perfect correlation (100%) of type II and/or type III surface structures with histological findings of adenoma and adenocarcinoma. However, there were a few positive lesions in their study. A recent study involving a large number of patients applied NBI endoscopy in 65 gastric and duodenal polyps using the same criteria, and revealed that NBI had 80% accuracy for the detection of adenoma[31]. In a recent study, probe-based confocal laser endomicroscopy (pCLE) was used together with NBI (GIF H-180; Olympus) for duodenal adenoma diagnosis. It was concluded that pCLE provided greater sensitivity than NBI (92% vs 83%, P = 0.8)[31]. Of note, the criteria for duodenal adenoma diagnosis of pCLE and NBI in this study were adopted from those for Barrett’s esophagus. More recently, a Japanese researcher developed a novel diagnostic algorithm for magnifying endoscopy with NBI (ME-NBI) for nonampullary superficial duodenal epithelial tumors[32]. Lesions displaying a single surface pattern were classified as monotype, and those displaying multiple surface patterns as mixed type. Surface pattern was classified as preserved, micrified, or absent. In addition, vascular pattern was classified as absent, network, intrastructural vascular (ISV), or unclassified. The results showed that all mixed-type lesions (23/23) were category 4 (mucosal high-grade neoplasia) or category 5 (submucosal invasion by carcinoma) tumors according to the revised Vienna classification[33]. Approximately 50% (10/23) of monotype lesions were category 3 (mucosal low grade neoplasia) tumors. Among the monotype lesions, the probability of category 4/5 tumor was 100% (2/2) in lesions with an unclassified vascular pattern, 64.3% (9/14) in lesions with an ISV pattern, 33.3% (1/3) in lesions with an absent pattern, and 25.5% (1/4) in lesions with a network pattern. Taken together, these findings suggest that detailed observation of both the surface and vascular pattern is necessary for monotype lesions, and ME-NBI classification can be used to perform histological diagnosis of nonampullary superficial duodenal epithelial tumors. However, further study is needed to accumulate a greater number of cases and to conduct careful comparisons with pathological findings. A Japanese multicenter study including 364 patients with 396 nonampullary superficial duodenal epithelial tumors showed that a significantly greater number of high-grade dysplasia or superficial adenocarcinomas compared with low-grade dysplasia was found in tumors with a diameter > 5 mm as well as solely or predominantly red coloration[34].
MANAGEMENT OF NONAMPULARRY DUODENAL ADENOMAS
As mentioned previously, endoscopic or surgical resection is recommended for nonampullary SDAs because of malignant potential of this lesion[15,35]. The method of resection depends on the size, location morphology, and pathology of the adenoma. Surgical resection is considered in cases of adenoma ≥ 2 cm, with severe dysplasia, suspicious carcinomatous infiltration, or recurrence after complete endoscopic resection[1].
Endoscopic treatment
Endoscopic resection for the treatment of nonampullary SDAs has the advantages of being less invasiveness. However, the endoscopic techniques are not standardized and most studies regarding endoscopic resection have been of retrospective design or a case series. Several endoscopic resection techniques-including snare polypectomy (Figure 1), EMR (Figure 2), ESD (Figure 3), and APC ablative methods-for management of nonampullary SDAs are available. Endoscopic resection is feasibe as a treatment method for nonampullary SDAs, but it also has limitations regarding complications and recurrence. The extensive second-order arterial blood supply and thin wall of the duodenum contribute to the occurrence of immediate/delayed bleeding and transmural thermal injury causing perforation.
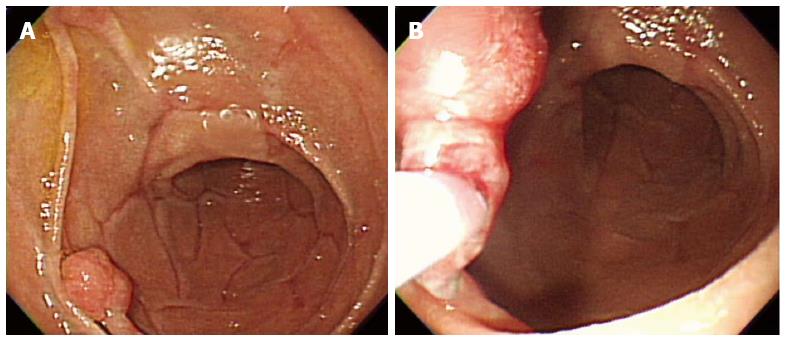
Figure 1 Snare polypectomy.
A: A approximately 10 mm peduculated adenoma in the second portion of the duodenum; B: Snare polypectomy procedure.
Figure 2 Endoscopic mucosal resection.
A: An approximately 20 mm polypoid mass in the second portion of the duodenum; B: Injection of submucosal saline solution with indigocarmine; C: Endoscopic mucosal resection (EMR) procedure; D: A clear, post-EMR ulcer.
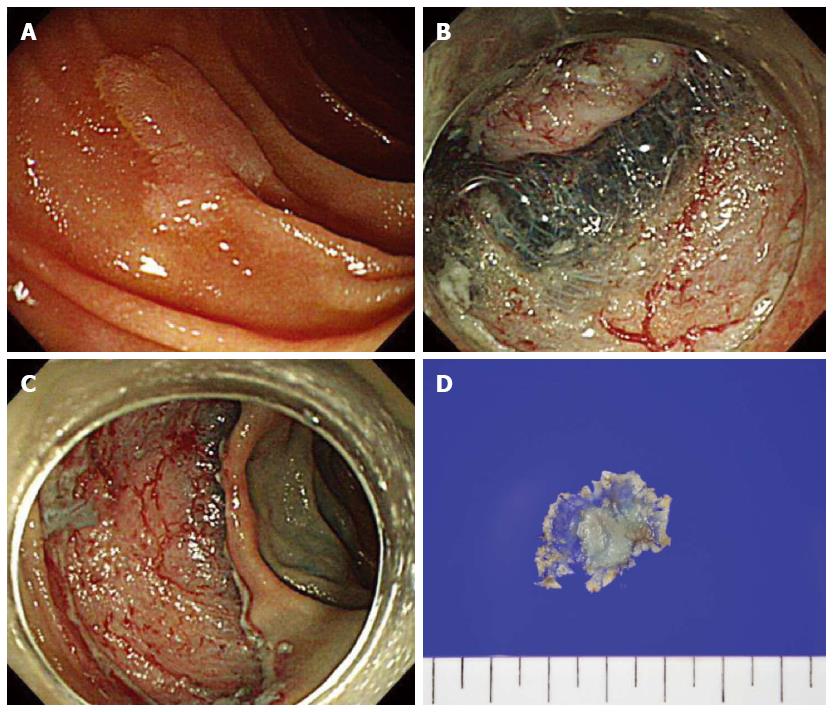
Figure 3 Endoscopic submucosal dissection.
A: A 12 mm sized superficial elevated type (IIa) lesion in the second portion of the duodenum; B: Circumferential mucosal incision and submucosal dissedtion; C: The lesion successfully removed en bloc without complications; D: A 27 mm resected specimen with adenoma.
An endoscope that enables optimal enface access to the lesion should be used. A side-viewing endoscope is optimal for lesions on the anterior or medial wall, particularly within 5 cm of the major papilla, and a pediatric colonoscope is preferable for lesions on the posterior or lateral wall[3]. Double balloon endoscopy was reported to enable stable manipulation and successful resection, even in the deep portion of the duodenum[36,37]. Snare polypectomy was reported to be effective, with an 85% eradication rate, in 20 duodenal adenoma cases, but it required repetitive intervention, combined or additional APC ablation, and follow-up due to incomplete resection or tumor recurrence[38]. EMR has been used in the majority of studies on endoscopic adenoma treatment of nonampullary SDAs. A flat or sessile morphology is most common in duodenal adenomas, and submucosal injection of lifting solution raises the lesion on a cushion of submucosal fluid, separating the mucosal and muscle layers. EMR has shown complete resection rates ranging 59% to 100%[5,39-50]. Other modified EMR methods, such as cap-assisted EMR[51], underwater EMR[3], and the band-and-slough technique[52], were reported to be effective endoscopic treatments for adenoma. The extent of the luminal circumference involved by the tumor was reported as the strongest predictor of successful eradication of nonampullary SDAs by endoscopic treatment[42]. The en bloc resection rate of EMR was reported to be 38.1% to 100%, and multiple resections or APC ablation are frequently required due to piecemeal resection[40,42-48,50]. The adenoma recurrence rate after EMR has been reported to be 0%-37%[5,39-41,43-50]. The majority of recurrent adenomas after EMR have been treated by endoscopic resection or ablation techniques[40,41,47,48]. Adenomas of > 2 cm are associated with a higher incidence of recurrence[41]. Adenomas with a villous nature were also reported to have a higher rate of recurrence[48]. The following complications after EMR have been reported: an intraprocedural bleeding rate of 0%-29.2%, delayed bleeding rate of 0%-16.7% and perforation rate of 0%-4.3%[37,39,43,44,46,49,51,53]. There have been fewer reports on ESD for the treatment of nonampullary SDAs, involving a limited number of cases, compared with EMR. ESD has been reported to be an effective method, with a complete resection rate of 80%-100% for nonampullary SDAs; moreover, no recurrence was reported[5,36,37,54-56]. However, duodenal ESD has been reported to have high rates of perforation (6%-50%) and bleeding (0%-7%). Duodenal ESD should be performed with caution in selected patients to avoid serious complications. Endoscopic APC ablation has been applied as both primary and adjunctive treatment for nonampullary SDAs. Entire pathologic evaluation is impossible in APC ablation. Two studies on APC ablation as primary treatment involving limited numbers of patients have been performed, with reported recurrence rates of 10% and 39%[46,57]. APC ablation has been used mainly as an adjunct modality with EMR for removal of remnant or locally recurrent nonampullary SDAs[38-42,46,48,50-52].
Following successful endoscopic resection of nonampullary SDA, follow-up endoscopy at 3-6 mo is recommended to check for recurrence. If recurrence is not detected, endoscopic surveillance at 6-12 mo is required[38,58]. Annual follow-up endoscopy should be performed for at least 2 years after complete resection[41].
Surgical treatment
Surgery remains as the standard treatment for large and complex nonampullary SDAs which are technically impossible to remove using endoscopic techniques. The optimal surgical treatment of nonampullary SDAs has not been established. Four major surgical procedures exist for the removal of duodenal tumors[2,12,14,59-61]; namely, transduodenal excision (transduodenal submucosal excision)[62], local full-thickness resection (wedge resection), pancreas-sparing segmental duodenectomy[63], and pancreaticoduodenectomy. The first three are the so-called “limited resections” and are usually indicated for selected tumors not amenable to endoscopic resection that have no or negligible risk of nodal metastasis[59-61]. The local recurrence rates for these surgical managements of nonampullary SDAs are reportedly low[2,14]. The endoscopy-assissted laparoscopic technique for nonampullary SDAs has been reported to be a safe and minimally invasive treatment option[64,65].
Surveillance and management of duodenal adenomas in patients with FAP
Considering the high incidence of duodenal adenomas with severe dysplasia in FAP and the increased risk of developing duodenal cancer, upper gastrointestinal (GI) endoscopic surveillance is highly recommended. To provide adequate visualization of the entire duodenal mucosa, a forward- and side-viewing endoscope should be used[53]. Although recommendations concerning patient age for initiation of upper GI surveillance are not uniform, performance of the first endoscopy in patients with FAP is recommended at 25-30 years of age[16,66]. Continued endoscopic surveillance after baseline endoscopy is usually performed according to the Spigelman stage. In general, patients with stage 0 receive repeat surveillance endoscopy every 4 years, those with stage I and II every 2-3 years, those with stage III every 6-12 mo with consideration for surgery, and those with stage IV should be referred to a pancreato-biliary surgeon for consideration of surgery[22,66]. Despite routine endoscopic surveillance, a considerable percentage of advanced ampullary neoplasias may not be detected[19]. In addition, several authors report that random biopsy samples from the ampullary region are needed because 12%-54% of normal-appearing papilla may harbor adenoma[16,19-22,67]. The use of chromoendoscopy leads to a marked increase in the number and size of duodenal adenomas detected in patients with FAP and an upgrade of the Spigelman stage[68,69]. A recent study showed that the use of NBI resulted in detection of a greater number of duodenal adenomas, resulting in upgrade of the Spigelman stage in 4.4% of patients[70]. However, NBI did not improve the detection of gastric polyps in comparison with high-resolution endoscopy.
The management of duodenal adenoma in patients with FAP remains a major challenge. It is at present clear whether medical or endoscopic therapies significantly alter long-term cancer risk or improve survival or quality of life in patients with a significant duodenal burden[53,66]. Nonsteroidal anti-inflammatory drugs (NSAIDs) can lead to regression of colorectal adenomas in patients with FAP. One study compared the effect of sulindac with placebo on the number of duodenal polyps[71]. Treatment with sulindac resulted in regressed small (≤ 2 mm) duodenal polyps, whereas larger (≥ 3 mm) duodenal polyps were unaffected. However, most studies regarding the effects of NASIDs and other compounds on prevention or regression of duodenal adenomas in FAP have reported disappointing results[66]. Current endoscopic treatment options for duodenal polyps in patients with FAP include snare polypectomy, EMR, thermal ablation, APC ablation, and photodynamic therapy[53,66]. Most studies indicate the complete resection of non-polypoid flat lesions by snare polypectomy is difficult. EMR may facilitate removal of large flat duodenal polyps in FAP. Endoscopic treatment has been proposed for patients with stage II or III disease[22]. Although current clinical practice recommends endoscopic resection of all large duodenal adenomas unless there is suspicion of advanced histology and submucosal invasion, endoscopic treatment is usually insufficient to guarantee a polyp-free duodenum and should be individualized based on the patient’s overall polyp burden, size and location, and comorbidities[53,66]. Surgical management includes local surgical treatment (duodenotomy with polypectomy), pancreas- or pylorus-preserving duodenectomy and pancreaticoduodenectomy[2,66]. Duodenotomy and polypectomy have proven ineffective in FAP patients with severe duodenal adenomatosis, as evidenced by high recurrence rates[72]. Definitive resection in the form of pancreaticoduodenectomy (standard or pylorus preserving) or pancreas-preserving duodenectomy has been indicated for patients with severe polyposis (stage IV) or polyps that are not amenable to endoscopic resection[2,66]. Recurrence rates are low after these operations, but the risks of morbidity and mortality are relatively high[66].
CONCLUSIONS AND FUTURE PERSPECTIVES
Nonampullary duodenal adenomas are uncommon but are associated with a risk of developing duodenal cancer. The development of endoscopic techniques for early detection and differentiation between adenoma and cancerous lesions, as well as between mucosal and submucosal cancer, is important for the management of nonampullary duodenal adenomas. Although remarkable progress in advanced endoscopic resection throughout the gastrointestinal tract was made during the last decade, endoscopic techniques to remove nonampullary duodenal adenomas have not yet been standardized. Compared with EMR, ESD has a superior complete resection rate with no recurrence. However, duodenal ESD remains challenging due to the high risk of immediate or delayed perforation and bleeding. The use of classical surgery for nonampullary duodenal adenomas that are not amenable to endoscopic resection has decreased with the development of minimally invasive surgery. Current treatment options for duodenal adenomatosis in patients with FAP include frequent endoscopic surveillance and targeted endoscopic treatment or surgical management according to the severity of duodenal lesions. Further studies should be focus on developing new endoscopic techniques to guide diagnostic and therapeutic decisions for future management of nonampullary duodenal adenomas.