Published online Jan 14, 2016. doi: 10.3748/wjg.v22.i2.815
Peer-review started: May 20, 2015
First decision: August 26, 2015
Revised: September 9, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: January 14, 2016
Processing time: 231 Days and 15.2 Hours
Colorectal cancer (CRC) constitutes a major public health problem as the third most commonly diagnosed and third most lethal malignancy worldwide. The prevalence and the physical accessibility to colorectal tumors have made CRC an ideal model for the study of tumor genetics. Early research efforts using patient derived CRC samples led to the discovery of several highly penetrant mutations (e.g., APC, KRAS, MMR genes) in both hereditary and sporadic CRC tumors. This knowledge has enabled researchers to develop genetically engineered and chemically induced tumor models of CRC, both of which have had a substantial impact on our understanding of the molecular basis of CRC. Despite these advances, the morbidity and mortality of CRC remains a cause for concern and highlight the need to uncover novel genetic drivers of CRC. This review focuses on mouse models of CRC with particular emphasis on a newly developed cancer gene discovery tool, the Sleeping Beauty transposon-based mutagenesis model of CRC.
Core tip: Successful implementation of targeted therapy will require a much more sophisticated understanding of colorectal cancer genetics, including the ability to discern “driver” mutations from the more common “passenger” mutations. Interpreting causality from large human genomic datasets will benefit from data produced by animal models and will expedite clinical trials using targeted therapies. This review describes the benefits and limitations of both traditional and new mouse models that are being used to discover and define colorectal cancer driver genes.
- Citation: Clark CR, Starr TK. Mouse models for the discovery of colorectal cancer driver genes. World J Gastroenterol 2016; 22(2): 815-822
- URL: https://www.wjgnet.com/1007-9327/full/v22/i2/815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i2.815
The cause of colorectal cancer (CRC), as with many malignancies, is the accumulation of genetic mutations and other genetic aberrations over an individual’s lifetime. Several decades of genomic studies have revealed that colorectal tumors can be categorized into two general molecular categories known as the “mutator” phenotype and the “chromosome instable” phenotype[1-3].
It is estimated that fifteen percent of patients with CRC have mutations in the DNA mismatch repair system. Because these patients have tumors containing a significantly higher number of somatic mutations vs those with proficient DNA repair systems, their tumors are said to display the mutator phenotype. Sporadic CRC tumors displaying the mutator phenotype typically have a loss of MLH1 expression due to promoter methylation[4]. The MLH1 gene is highly conserved and its gene product functions in repairing DNA replication errors that result in mismatched nucleotide bases. Loss of MLH1 function results in the accumulation of single nucleotide alterations across the genome but are especially apparent in regions of the genome displaying highly repetitive sequence[5]. The result of dysfunctional DNA mismatch repair is illustrated by that fact that MLH1 knockout mice display increased susceptibility to cancer formation[5]. Individuals with Lynch syndrome, also known as Hereditary Non-polyposis Colorectal Cancer (HNPCC), have germline mutations in DNA mismatch repair genes causing a high mutation load and microsatellite instability. Those with Lynch syndrome are at high risk of developing CRC and typically account for 3%-5% of the total number of new CRC diagnoses[6]. For reasons that are not well understood, patients with CRC tumors that are genetically defined as the mutator phenotype generally have a better clinical prognosis vs their non-mutator phenotype counterparts[7,8].
Inactivating mutations in the adenomatous polyposis coli (APC) gene, a tumor suppressor gene, is widely regarded as a key somatic mutation responsible for driving sporadic colorectal tumor formation[9]. This statement is supported by data from large-scale genomic studies that identified mutations in APC in approximately 80% of sporadic CRCs[10,11]. The protein encoded by the APC gene functions as a negative regulator of the Wnt signaling pathway, which is a highly conserved pathway responsible for controlling many cellular processes including cellular proliferation and differentiation[12,13]. Additionally, the Wnt pathway is critical for maintaining organ homeostasis and is particularly important in maintaining homeostasis in the intestinal epithelium. The consequences of deregulated Wnt signaling, usually due to inactivating mutations in APC, are best exemplified in those affected by the relatively uncommon syndrome known as familial adenomatous polyposis (FAP). Individuals with FAP have germline mutations in APC and consequently develop hundreds to thousands of colon polyps by their teenage years. Furthermore, it estimated that nearly all FAP patients are diagnosed with CRC prior to the age of 40[14,15].
The vast majority of sporadic colorectal tumors (about 85%) are molecularly characterized as chromosome instable[2]. Tumors in this category display major chromosomal aberrations including aneuploidy, loss of heterozygosity, gene fusions, and large insertions and deletions[2,16]. The root cause of chromosomal instability in colon cancer is unclear but many mechanisms have been proposed. These mechanisms include, but are not limited to, defects in the system responsible for faithful chromosome segregation during mitosis and inactivation of genes responsible for the repair of DNA damaged by genotoxic stressors (e.g., methylating agents)[2,17]. Regardless of the underlying cause of chromosome instability, it is likely that the accumulation of somatic mutations in tumor suppressor and oncogenes in combination with genome instability leads to the initiation and progression of CRC.
During the 1970’s researchers identified and defined a small number of cancer oncogenes and tumor suppressors based on the study of oncoviruses and recurrent chromosomal abnormalities. In the 1980’s several groups used this knowledge to assay those known cancer genes in CRC and found that RAS mutations were present in 30% to 50% of patients[18,19]. Furthermore, recurrent allelic deletions were identified that eventually implicated TP53, APC and SMAD4 as tumor suppressors in CRC[19-21]. By analyzing tumors at various stages along the continuum of adenoma-adenocarcinoma-metastasis, these researchers were able to hypothesize that certain mutations were early gatekeepers, such as APC and KRAS, while other mutations were only found at later stages, such as SMAD4 and TP53.
In the early 1990s scientists used FAP and Lynch syndrome family cohorts to identify the causative tumor suppressor genes for these two syndromes. The APC gene was identified by positional cloning in FAP cohorts, while linkage analysis implicated mutations at chromosomal regions 2p16 and 3p21 in Lynch syndrome families[22-28]. At the same time it was discovered that a subset of CRC patients had novel microsatellite alleles in their cancers, indicating microsatellite instability. At the same time, microbiologists had recently identified mismatch repair (MMR) genes in yeast. This finding lead to the hypothesis that mutations in human homologs of the yeast MMR genes may be the cause of Lynch Syndrome[29]. This hypothesis was strengthened by the discovery that one of the homologs, MSH2, was in the chromosome 2p region linked to Lynch syndrome[30] Within the next few years mutations in several other MMR genes, including MLH1, PMS1, PMS2, and MSH6, were discovered in Lynch syndrome patients[31-35].
New technologies, such as next generation sequencing, have allowed researchers to comprehensively analyze whole exomes, genomes and transcriptomes of large numbers of CRC patients. These datasets are then mined using various bioinformatic algorithms to identify putative CRC driver genes. In general, these algorithms detect genes that are recurrently mutated, amplified, deleted, or altered by other means and assign a rank or P-value to predict whether or not the gene is a driver of tumorigenesis. For example, a study of the entire exomes of 11 CRC patient tumors identified 140 putative cancer drivers based on frequency of recurrence[36,37]. More recently, 224 CRC patient tumors were assayed for mutations, gene expression, copy number, and methylation status by The Cancer Research Atlas (TCGA) Network. This study produced various lists of genes based on recurrent changes, including 31 genes recurrently mutated and a larger number of genes found in genomic regions that were recurrently amplified or deleted[38]. It is still unknown how many genetic mutations are necessary and sufficient for cancer initiation with estimates ranging from 3 to 14[37,39].
These landmark studies have provided cancer geneticists with a wealth of data regarding the genetic drivers of CRC and were the springboard for the creation of several animal models.
Moser et al[40] developed the first mouse model of FAP in a forward genetic screen using N-Ethyl-N-Nitrosourea (ENU) as a germline mutagen. C57BL/6J (B6) were treated with ENU then crossed to AKR mice to generate progeny harboring ENU-induced germline mutations. The progeny from this breeding scheme exhibited an interesting circling trait that proved to be heritable in the offspring of AKR/B6 (f1) crossed to B6. Adult offspring from this cross were often anemic and frequently passed bloody stool. GI tract adenomas were observed in all anemic mice and tumors eventually progressed to adenocarcinomas in aging mice. Mice displaying these phenotypic features were said to carry the Multiple Intestinal Neoplasia (Min) gene. Further analysis demonstrated the Min mutation was inherited in an autosomal dominant fashion and heterozygous offspring developed hundreds of GI tract tumors resulting in death at approximately 120 d. Su et al[41] later identified the Min locus as the mouse homolog of the human APC gene. A thymine to adenine transversion mutation, creating a premature stop codon, was found at nucleotide 2549 (amino acid 850) of the Apc gene. With similar nonsense mutations often observed in FAP patients, it was determined that the ApcMin mouse was a suitable FAP model.
Several variations of the Apc mutant mouse have since been developed with the main differences being the location of the Apc truncating mutations. The most notable and well characterized of these variations are mice engineered with Apc truncated at amino acids 716 and 1638[42,43]. The development of Cre-lox technology has also enabled researchers to control the location and/or timing of Apc deletions. For example, expressing Cre recombinase from a tissue specific promoter (e.g., Fabpl- and Villin-promoters) results in deletions of Apc specifically in the gastrointestinal (GI) tract epithelial cells[44-48]. Each Apc deletion mutant displays slight phenotypic variations but all develop GI tract adenomas that eventually develop to adenocarcinomas. For more detail on the various Apc mutants please refer to these excellent reviews[49-52]. Because Apc mutant mice rarely develop aggressive metastatic disease these models have been mostly helpful in studying the genetic events driving early CRC tumor formation.
Several attempts to create a mouse model of Lynch syndrome have been made but most are limited in their ability to faithfully recapitulate the early onset of CRC tumors. Early efforts to create a Lynch syndrome mouse model focused on deleting the murine homologs of MSH1 and MSH2, as these genes are mutated in approximately 90% of Lynch syndrome patients[14]. Msh2 knockout mice are viable and develop without abnormalities. Adult Msh2-/- mice have a reduced life span (6-12 mo) due to T-cell malignancies[53,54]. Gastrointestinal tract adenomas and adenocarcinomas are observed in 80% Msh2-/- mice that survive 8-10 mo[55]. The microsatellite instability observed in Lynch syndrome CRC tumors was also found in tumor tissues (T-cell and GI tract) from Msh2-/- mice. Mlh1 knockout mice are phenotypically similar to Msh2-/- mice[56]. It has also been shown that mice deficient in the MMR protein Msh6 develop GI tract tumors but, unlike Msh1-/- and Msh2-/- models, these mice do not display the microsatellite instability characteristic of Lynch syndrome CRC tumors[57].
In order to avoid early death from lymphomas, researchers have bred MMR gene knockout mice to immunocompromised strains (tap1-/-) that lack CD8+ T-cells. Such mice do not die early from lymphomas allowing for the development of CRC tumors resembling those found in Lynch syndrome patients[58]. This approach has been improved upon using the Cre-lox system to restrict MMR gene deletion to the GI tract epithelial cells of immunocompetent mice[59].
Although useful, genetically engineered mice (transgenic or gene, knockout/knockin) are typically designed to harbor only a few mutant alleles and are therefore, inherently limited in their ability to accurately model the complex genetic alterations of sporadic CRC tumors. Another deficiency is the difficulty of simultaneously altering multiple genes, including genes of unknown function. The next section describes models that can overcome these limitations.
To mimic environmentally induced cancers researchers have used carcinogenic chemicals to generate sporadic CRC tumors. Methylazoxymathanol (MAM), 1,2-dimethylhyrdrazine (DMH), and azoxymethane (AOM) are examples of popular compounds used to generated CRC tumors in mice. Although the number of tumors varies depending on the strain, mice treated with these compounds quickly develop CRC tumors in the distal colon that moderately resemble human CRC tumors (e.g., KRAS mutations). Although these models reliably produce sporadic CRC tumors, it is challenging to determine their mutational landscape. To do so, one must target previously identify cancer drivers for DNA sequencing or use a more unbiased method of analysis (e.g., whole exome, RNA-seq, etc.), which can be cost prohibitive. For a comprehensive review of the many carcinogen-induced CRC models refer to these reviews[60-63].
The advancement CRC therapeutics, specifically the development of molecularly targeted therapies, is critically dependent on the identification of novel CRC driver mutations. Insertional mutagenesis forward genetic screens are an excellent method to identify novel cancer genes. Retroviruses (e.g., MMTV and MuLV) have long been used as insertional mutagens and their use has led to the discovery of several major cancer genes[64-67]. There are two flavors of retroviruses, the acute transforming and the slow transforming virus. Slow transforming retroviruses are often used as insertional mutagens because they do not carry viral oncogenes within their genomes. Instead, slow transforming viruses promote tumor formation by proviral insertional into oncogenes and tumor suppressors. Upon insertion next to, or within, a gene, elements within the viral genome can act in cis to alter the expression of cellular genes[68]. The identification of the virally disrupted genes is possible using PCR to amplify the viral-host genome DNA junction and subsequent sequencing. Due to tissue tropisms, the use of retroviruses has mainly been used to model mammary and blood cancers.
Class II transposable elements represents a novel insertional mutagen used for the discovery of novel CRC driver genes. Class II transposons are DNA elements that rely on the enzymatic activity of a transposase to be “cut” from one genomic location and “pasted” to another. Transposons have been used for decades to study gene function in a wide array of organisms but the use of transposons in vertebrates is a relatively new advancement[69,70]. Ivics et al[71] (1997) were the first to construct a synthetic transposon, Sleeping Beauty (SB), and transposase that showed activity in vertebrate cells. Quickly after its introduction, the SB transposon system was engineered into the mouse genome and transposition was shown to occur in vivo[72,73]. Enhancements that increased the transposition frequency of the SB transposon lead to the development of the oncogenic transposon known as T2/Onc[74,75]. The T2/Onc transposon carries multiple mutagenic elements that make it an ideal tool for the discovery of candidate cancer genes. To mimic gain of function mutations, the T2/Onc transposon carries a strong viral promoter and a splice donor sequence. In the event of T2/Onc integration upstream of a gene and in the same transcriptional orientation, the strong viral promoter carried by the transposon will drive expression of the cellular gene. Similarly, if the transposon lands in an intron it can also produce an active truncated protein. T2/Onc also carries splice acceptor sites on both DNA strands and a bidirectional poly(A) signal. If T2/Onc integrates within a gene, in either orientation, the splice acceptors and poly(A) signal will terminate transcription effectively mimicking loss of function mutations (Figure 1). In separate publications Collier et al[76] and Dupuy et al[77] used the T2/Onc transposon and SB transposase to demonstrate the mutagenic potential of the T2/Onc transposon in somatic cells.
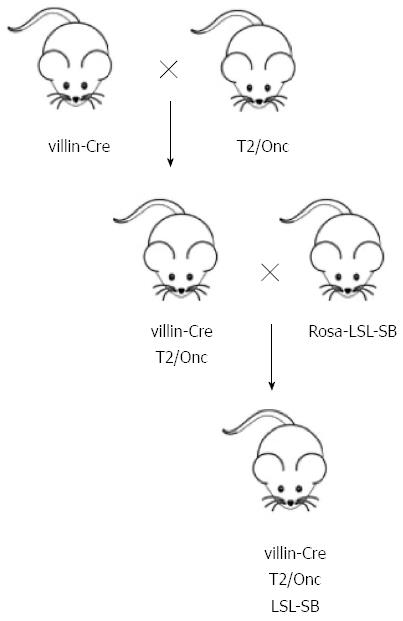
Soon after the debut of the T2/Onc transposon, Starr et al[78] harnessed its mutagenic potential for the discovery of novel CRC driver genes. In this forward genetic screen, the authors created a triple transgenic mouse by crossing mice harboring a Cre-responsive SB transposase allele with double transgenic mice engineered to express the Cre recombinase only in the gut as well as carry a concatemer of T2/Onc transposons (Figure 2). In this model the T2/Onc transposon is mobilized by the SB transposase only after Cre recombinase (villin-Cre) removes the LoxP-STOP-LoxP cassette located upstream of the SB transposase. The results from this study revealed that triple transgenic mice become moribund at a faster rate than control mice (double transgenics) and that the vast majority of these triple transgenic mice developed intestinal lesions. Histopathology revealed the resulting GI tract growths to be intraepithelial neoplasias, adenomas, and adenocarcinomas. To identify the disrupted host genes, DNA was harvested from 135 tumors for linker-mediated PCR (LM-PCR) amplification of the transposon-host genome junction. Sequencing of the LM-PCR products revealed each tumor contained, on average, 124 T2/Onc insertions. Using statistical approaches to identify common insertion site (CIS) loci the authors were able to hone in on a list of 77 candidate CRC genes. The ability of this tool to model sporadic CRC was confirmed by the finding that APC, PTEN, and SMAD4, which are commonly disrupted in human CRC tumors, were also identified as T2/Onc CISs in mouse tumors. Of the 77 CIS genes, 17 were identified as novel CRC driver genes. One of these genes, RSPO2, has recently been identified as a tumor suppressor gene in human CRC tumors[79].
Similar screens have been carried out using the T2/Onc transposon as an insertional mutagen in Apc mutant mice. This approach was used by Starr et al[80] (2011) to identify novel CRC genes that cooperate with Apc mutations. T2/Onc transposition in ApcMin mice increased the polyp count from 112 (controls) to an average of 360 polyps per mouse. DNA analysis of 96 polyps from 12 mice revealed the presence of greater than 30 thousand transposon insertions. CIS analysis identified 37 genes in this set of transposon insertions. The remaining wild-type Apc allele of the ApcMin mouse was the most commonly mutated gene in this study, a result that further demonstrates the importance of APC mutations in CRC development. To validate some of the 37 CIS genes the authors used RNAi to decrease the expression of nine CIS genes (CNOT1, PDE4DIP, PDCD6IP, ATF2, SF11, FNBP1L, MYO5B, SNX24, and STAG1) in vitro. It was determined that knockdown of CNOT, PDE4DIP, PDCD6IP, ATF2, and SF11 significantly decreased the proliferation of the SW480 CRC cell line[80]. Using a similar approach March et al[81] also determined that SB transposition increased the morbidity and tumor burden of Apc mutant mice. CIS analysis of DNA from 446 transposon-induced tumors identified hundreds of CIS genes. Using pathway analysis the authors determined that genes from their CIS list were involved in 38 cancer related genetic networks (e.g., K-Ras signaling pathway) and as many as 183 CIS genes were identified as Wnt pathway targets. A more recent publication by Takeda et al[82] expanded upon this approach to include transposon based insertional mutagenesis on KRAS, SMAD4, and TP53 mutant strains of mice. In human CRC tumors the loss of APC is a tumor initiating mutation followed by activating mutations in KRAS in early to intermediate adenomas and loss of function mutations in SMAD4 and TP53 occuring in later stages. By generating mouse models with initiating mutations in these three genes (KRAS, SMAD4, and TP53) the authors attempted to identify mutations that cooperate with these three genes. Using this strategy the authors discovered different sets of CIS genes that were unique to each genetic background suggesting that the initiating mutation influences which genes are mutated during CRC tumor development. One of their findings was that the gatekeeper Apc mutation was common in all backgrounds, while activating mutations in the Wnt pathway members Rspo1 and Rspo2 were only found in the SMAD4 mutant mice.
The ultimate goal of cancer gene discovery is to translate new knowledge into more effective therapies for treating CRC. Identification of recurrently altered genes is informative, but does not directly result in reduced mortality rates for CRC patients. The limited success of targeted therapies is likely attributed to tumor heterogeneity and the action of unidentified CRC driver genes. Mouse models continue to be an essential tool used to unveil novel CRC driver genes with therapeutic potential. One promising example is the identification of ion channel genes as candidate drivers of CRC. Both KCNQ1 and CFTR were identified in multiple Sleeping Beauty insertional mutagenesis screens[80-82]. Based on these findings, Than et al[83] reported that KCNQ1 is a tumor suppressor in human CRC, while recent findings from cystic fibrosis patients support the hypothesis that CFTR is also a human tumor suppressor[84]. These findings suggest that ion channel modulators may represent a new class of drugs for treating a subset of CRC patients.
Eventually, clinical trials using approved and experimental therapies are required. Once the panoply of driver genes has been defined, we will need to develop clinical lab diagnostics capable of determining the exact drivers of each individual patient’s cancer based on tumor biopsies. Such information will allow physicians to select drugs and therapies specifically designed to target those drivers.
| 1. | Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816-819. [PubMed] |
| 2. | Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138:2059-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 625] [Article Influence: 39.1] [Reference Citation Analysis (1)] |
| 3. | Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8187] [Cited by in RCA: 11714] [Article Influence: 901.1] [Reference Citation Analysis (0)] |
| 4. | Bertagnolli MM, Redston M, Compton CC, Niedzwiecki D, Mayer RJ, Goldberg RM, Colacchio TA, Saltz LB, Warren RS. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803. J Clin Oncol. 2011;29:3153-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Ellison AR, Lofing J, Bitter GA. Human MutL homolog (MLH1) function in DNA mismatch repair: a prospective screen for missense mutations in the ATPase domain. Nucleic Acids Res. 2004;32:5321-5338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Bonadona V, Bonaïti B, Olschwang S, Grandjouan S, Huiart L, Longy M, Guimbaud R, Buecher B, Bignon YJ, Caron O. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 816] [Article Influence: 54.4] [Reference Citation Analysis (1)] |
| 7. | Merok MA, Ahlquist T, Røyrvik EC, Tufteland KF, Hektoen M, Sjo OH, Mala T, Svindland A, Lothe RA, Nesbakken A. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013;24:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Hveem TS, Merok MA, Pretorius ME, Novelli M, Bævre MS, Sjo OH, Clinch N, Liestøl K, Svindland A, Lothe RA. Prognostic impact of genomic instability in colorectal cancer. Br J Cancer. 2014;110:2159-2164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1312] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 10. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] |
| 11. | Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1300] [Article Influence: 86.7] [Reference Citation Analysis (2)] |
| 12. | Jin YR, Yoon JK. The R-spondin family of proteins: emerging regulators of WNT signaling. Int J Biochem Cell Biol. 2012;44:2278-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | de Sousa EM, Vermeulen L, Richel D, Medema JP. Targeting Wnt signaling in colon cancer stem cells. Clin Cancer Res. 2011;17:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 945] [Cited by in RCA: 886] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 15. | Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19-27. [PubMed] |
| 16. | Duval A, Hamelin R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res. 2002;62:2447-2454. [PubMed] |
| 17. | Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr Biol. 2010;20:R285-R295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 459] [Article Influence: 28.7] [Reference Citation Analysis (2)] |
| 18. | Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1250] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 19. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4493] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 20. | Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, vanTuinen P, Ledbetter DH, Barker DF, Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989;244:217-221. [PubMed] |
| 21. | Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49-56. [PubMed] |
| 22. | Joslyn G, Carlson M, Thliveris A, Albertsen H, Gelbert L, Samowitz W, Groden J, Stevens J, Spirio L, Robertson M. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66:601-613. [PubMed] |
| 23. | Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589-600. [PubMed] |
| 24. | Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661-665. [PubMed] |
| 25. | Kinzler KW, Nilbert MC, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hamilton SR, Hedge P, Markham A. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991;251:1366-1370. [PubMed] |
| 26. | Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665-669. [PubMed] |
| 27. | Lindblom A, Tannergård P, Werelius B, Nordenskjöld M. Genetic mapping of a second locus predisposing to hereditary non-polyposis colon cancer. Nat Genet. 1993;5:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 270] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Peltomäki P, Aaltonen LA, Sistonen P, Pylkkänen L, Mecklin JP, Järvinen H, Green JS, Jass JR, Weber JL, Leach FS. Genetic mapping of a locus predisposing to human colorectal cancer. Science. 1993;260:810-812. [PubMed] |
| 29. | Strand M, Prolla TA, Liskay RM, Petes TD. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993;365:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 737] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 30. | Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1994;77:1 p following 166. [PubMed] |
| 31. | Nicolaides NC, Papadopoulos N, Liu B, Wei YF, Carter KC, Ruben SM, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994;371:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1021] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 32. | Papadopoulos N, Nicolaides NC, Wei YF, Ruben SM, Carter KC, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, Adams MD. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994;263:1625-1629. [PubMed] |
| 33. | Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, Jass JR, Dunlop M, Wyllie A, Peltomäki P. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996;2:169-174. [PubMed] |
| 34. | Wijnen J, van der Klift H, Vasen H, Khan PM, Menko F, Tops C, Meijers Heijboer H, Lindhout D, Møller P, Fodde R. MSH2 genomic deletions are a frequent cause of HNPCC. Nat Genet. 1998;20:326-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 154] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Montazer Haghighi M, Radpour R, Aghajani K, Zali N, Molaei M, Zali MR. Four novel germline mutations in the MLH1 and PMS2 mismatch repair genes in patients with hereditary nonpolyposis colorectal cancer. Int J Colorectal Dis. 2009;24:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2562] [Cited by in RCA: 2556] [Article Influence: 127.8] [Reference Citation Analysis (0)] |
| 37. | Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2283] [Cited by in RCA: 2274] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 38. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6856] [Article Influence: 489.7] [Reference Citation Analysis (10)] |
| 39. | Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1310] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 40. | Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322-324. [PubMed] |
| 41. | Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668-670. [PubMed] |
| 42. | Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, Breukel C, Alt E, Lipkin M, Khan PM. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA. 1994;91:8969-8973. [PubMed] |
| 43. | Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci USA. 1995;92:4482-4486. [PubMed] |
| 44. | Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, Matsumoto H, Takano H, Akiyama T, Toyoshima K. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120-123. [PubMed] |
| 45. | Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem. 1999;274:38071-38082. [PubMed] |
| 46. | Pinto D, Robine S, Jaisser F, El Marjou FE, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999;274:6476-6482. [PubMed] |
| 47. | Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275-33283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 679] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 48. | el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 887] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 49. | Heyer J, Yang K, Lipkin M, Edelmann W, Kucherlapati R. Mouse models for colorectal cancer. Oncogene. 1999;18:5325-5333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | McCart AE, Vickaryous NK, Silver A. Apc mice: models, modifiers and mutants. Pathol Res Pract. 2008;204:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Nandan MO, Yang VW. Genetic and Chemical Models of Colorectal Cancer in Mice. Curr Colorectal Cancer Rep. 2010;6:51-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Karim BO, Huso DL. Mouse models for colorectal cancer. Am J Cancer Res. 2013;3:240-250. [PubMed] |
| 53. | de Wind N, Dekker M, Berns A, Radman M, te Riele H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995;82:321-330. [PubMed] |
| 54. | Reitmair AH, Schmits R, Ewel A, Bapat B, Redston M, Mitri A, Waterhouse P, Mittrücker HW, Wakeham A, Liu B. MSH2 deficient mice are viable and susceptible to lymphoid tumours. Nat Genet. 1995;11:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 283] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 55. | Reitmair AH, Cai JC, Bjerknes M, Redston M, Cheng H, Pind MT, Hay K, Mitri A, Bapat BV, Mak TW. MSH2 deficiency contributes to accelerated APC-mediated intestinal tumorigenesis. Cancer Res. 1996;56:2922-2926. [PubMed] |
| 56. | Edelmann W, Yang K, Kuraguchi M, Heyer J, Lia M, Kneitz B, Fan K, Brown AM, Lipkin M, Kucherlapati R. Tumorigenesis in Mlh1 and Mlh1/Apc1638N mutant mice. Cancer Res. 1999;59:1301-1307. [PubMed] |
| 57. | Edelmann W, Yang K, Umar A, Heyer J, Lau K, Fan K, Liedtke W, Cohen PE, Kane MF, Lipford JR. Mutation in the mismatch repair gene Msh6 causes cancer susceptibility. Cell. 1997;91:467-477. [PubMed] |
| 58. | de Wind N, Dekker M, van Rossum A, van der Valk M, te Riele H. Mouse models for hereditary nonpolyposis colorectal cancer. Cancer Res. 1998;58:248-255. [PubMed] |
| 59. | Kucherlapati MH, Lee K, Nguyen AA, Clark AB, Hou H, Rosulek A, Li H, Yang K, Fan K, Lipkin M. An Msh2 conditional knockout mouse for studying intestinal cancer and testing anticancer agents. Gastroenterology. 2010;138:993-1002.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 568] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 61. | De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 428] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 62. | Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30:183-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 63. | Tong Y, Yang W, Koeffler HP. Mouse models of colorectal cancer. Chin J Cancer. 2011;30:450-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99-109. [PubMed] |
| 65. | Selten G, Cuypers HT, Zijlstra M, Melief C, Berns A. Involvement of c-myc in MuLV-induced T cell lymphomas in mice: frequency and mechanisms of activation. EMBO J. 1984;3:3215-3222. [PubMed] |
| 66. | Johansson FK, Brodd J, Eklöf C, Ferletta M, Hesselager G, Tiger CF, Uhrbom L, Westermark B. Identification of candidate cancer-causing genes in mouse brain tumors by retroviral tagging. Proc Natl Acad Sci USA. 2004;101:11334-11337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Mikkers H, Allen J, Knipscheer P, Romeijn L, Hart A, Vink E, Berns A. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat Genet. 2002;32:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 302] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 68. | Uren AG, Kool J, Berns A, van Lohuizen M. Retroviral insertional mutagenesis: past, present and future. Oncogene. 2005;24:7656-7672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 69. | Osborne BI, Baker B. Movers and shakers: maize transposons as tools for analyzing other plant genomes. Curr Opin Cell Biol. 1995;7:406-413. [PubMed] |
| 70. | Spradling AC, Stern DM, Kiss I, Roote J, Laverty T, Rubin GM. Gene disruptions using P transposable elements: an integral component of the Drosophila genome project. Proc Natl Acad Sci USA. 1995;92:10824-10830. [PubMed] |
| 71. | Ivics Z, Hackett PB, Plasterk RH, Izsvák Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501-510. [PubMed] |
| 72. | Dupuy AJ, Fritz S, Largaespada DA. Transposition and gene disruption in the male germline of the mouse. Genesis. 2001;30:82-88. [PubMed] |
| 73. | Fischer SE, Wienholds E, Plasterk RH. Regulated transposition of a fish transposon in the mouse germ line. Proc Natl Acad Sci USA. 2001;98:6759-6764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 74. | Zayed H, Izsvák Z, Walisko O, Ivics Z. Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol Ther. 2004;9:292-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 75. | Geurts AM, Yang Y, Clark KJ, Liu G, Cui Z, Dupuy AJ, Bell JB, Largaespada DA, Hackett PB. Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol Ther. 2003;8:108-117. [PubMed] |
| 76. | Collier LS, Carlson CM, Ravimohan S, Dupuy AJ, Largaespada DA. Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature. 2005;436:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 330] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 77. | Dupuy AJ, Akagi K, Largaespada DA, Copeland NG, Jenkins NA. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature. 2005;436:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 403] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 78. | Starr TK, Allaei R, Silverstein KA, Staggs RA, Sarver AL, Bergemann TL, Gupta M, O’Sullivan MG, Matise I, Dupuy AJ. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science. 2009;323:1747-1750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 292] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 79. | Wu C, Qiu S, Lu L, Zou J, Li WF, Wang O, Zhao H, Wang H, Tang J, Chen L. RSPO2-LGR5 signaling has tumour-suppressive activity in colorectal cancer. Nat Commun. 2014;5:3149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 80. | Starr TK, Scott PM, Marsh BM, Zhao L, Than BL, O’Sullivan MG, Sarver AL, Dupuy AJ, Largaespada DA, Cormier RT. A Sleeping Beauty transposon-mediated screen identifies murine susceptibility genes for adenomatous polyposis coli (Apc)-dependent intestinal tumorigenesis. Proc Natl Acad Sci USA. 2011;108:5765-5770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 81. | March HN, Rust AG, Wright NA, ten Hoeve J, de Ridder J, Eldridge M, van der Weyden L, Berns A, Gadiot J, Uren A. Insertional mutagenesis identifies multiple networks of cooperating genes driving intestinal tumorigenesis. Nat Genet. 2011;43:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 82. | Takeda H, Wei Z, Koso H, Rust AG, Yew CC, Mann MB, Ward JM, Adams DJ, Copeland NG, Jenkins NA. Transposon mutagenesis identifies genes and evolutionary forces driving gastrointestinal tract tumor progression. Nat Genet. 2015;47:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 83. | Than BL, Goos JA, Sarver AL, O’Sullivan MG, Rod A, Starr TK, Fijneman RJ, Meijer GA, Zhao L, Zhang Y. The role of KCNQ1 in mouse and human gastrointestinal cancers. Oncogene. 2014;33:3861-3868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 84. | Billings JL, Dunitz JM, McAllister S, Herzog T, Bobr A, Khoruts A. Early colon screening of adult patients with cystic fibrosis reveals high incidence of adenomatous colon polyps. J Clin Gastroenterol. 2014;48:e85-e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Cao GW, Sam MR S- Editor: Ma YJ L- Editor: A E- Editor: Ma S