Published online Jan 14, 2016. doi: 10.3748/wjg.v22.i2.681
Peer-review started: June 1, 2015
First decision: July 14, 2015
Revised: October 5, 2015
Accepted: November 30, 2015
Article in press: December 1, 2015
Published online: January 14, 2016
Processing time: 222 Days and 19.2 Hours
Obesity plays relevant pathophysiological role in the development of health problems, arising as result of complex interaction of genetic, nutritional, and metabolic factors. Due to the role of adipose tissue in lipid and glucose metabolism, and low grade inflammation, it is necessary to classify obesity on the basis of body fat composition and distribution, rather than the simply increase of body weight, and the Body Mass Index. The new term of adiposopathy (‘‘sick fat’’) clearly defines the pathogenic role of adipose tissue. Four phenotypes of obese individuals have been described: (1) normal weight obese (NWO); (2) metabolically obese normal weight; (3) metabolically healthy obese; and (4) metabolically unhealthy obese or “at risk” obese. Moreover, sarcopenic obesity has been related to all the phenotypes. The category of normal weight lean, represented by metabolically healthy normal weight has been classified to distinguish from NWO. It is crucial to recommend a bariatric surgery taking into account adiposopathy and sick fat that occurs with the expansion of fat mass, changing the inflammatory and metabolic profile of the patient. Body fat percentage and genetic polymorphism have to be evaluated to personalize the best bariatric surgery intervention.
Core tip: Obesity is a global public health problem due to its association with several diseases and reduced lifespan, as result of complex interaction of genetic, nutritional, and metabolic factors. The term of adiposopathy clearly defines the pathogenic role of adipose tissue. Four phenotypes of obesity have been described, based on body fat composition and distribution: (1) normal weight obese; (2) metabolically obese normal weight; (3) metabolically healthy obese; and (4) metabolically unhealthy obese. Sarcopenic obesity has been characterized, related to all the described phenotypes. Body fat percentage and genetic polymorphism have to be evaluated to personalize the best bariatric surgery intervention.
- Citation: De Lorenzo A, Soldati L, Sarlo F, Calvani M, Di Lorenzo N, Di Renzo L. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol 2016; 22(2): 681-703
- URL: https://www.wjgnet.com/1007-9327/full/v22/i2/681.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i2.681
The American Medical Association’s Council on Science and Public Health Report 4, has identified the following common criteria to define a disease as: (1) an impairment of the normal functioning of some aspect of the body; (2) characteristic signs or symptoms; and (3) harm or morbidity[1].
The World Health Organization (WHO) defines obesity as “a condition in which percentage body fat (PBF) is increased to an extent in which health and well-being are impaired, and, due to the alarming prevalence increase, declared it as a “global epidemic”[2].
The high prevalence of obesity is a global public health problem due to its association with several diseases[3,4], and reduced lifespan[5]. It arise as a result of complex interaction of genetic, life style, dietary habitus, energy expenditure, nutritional and metabolic factors, as the adipocyte metabolism[6,7].
The shared definition that the adipose tissue and skeletal muscle are an energy storage has been replaced by the notion that these tissues have a role in lipid and glucose metabolism due to the large number of bioactive proteins, termed adipokines and myokines produced[8], that are related to some cardiovascular risk factors influences of obesity[9].
Moreover, obesity and glucose metabolism are intimately related to low grade systemic inflammation, involving a number of pro-inflammatory cytokines produced by many cell types that also appear to be major regulators of adipose tissue metabolism[10].
Therefore, the greatest limitation of any measure that relegates the diagnosis of obesity to the mere quantity of weight and circumference gain, without taking into account the body composition, in terms of body fat increase and body lean decrease, is the failure to consider the impact of adiposity on physiological and metabolic processes that result in increased morbidity and mortality[11].
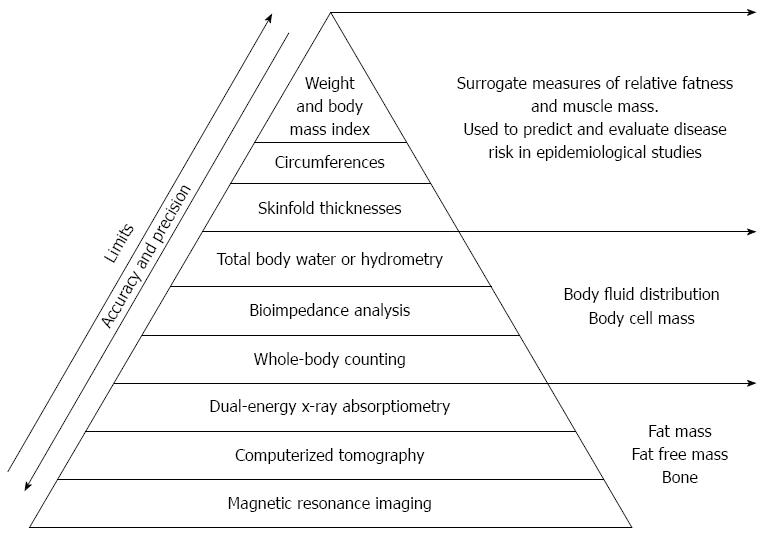
Due to the endocrine and inflammatory role of the adipose tissue it is necessary to classify obesity condition on the basis of body fat composition and distribution, rather than simply on the increase of body weight (Figure 1). Therefore, the body mass index (BMI), a ratio between weight to the squared height (kg/m2) of a subject, used to easily approximate body fat percentage and stratify people into categories, leads to a large error and misclassification.
According to BMI, general population is classified in five categories: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5-24.9 kg/m2), class I obesity - overweight (BMI 25.0-29.9 kg/m2), class II obesity - obesity (BMI 30.0-39.9 kg/m2), class III obesity - extreme obesity (BMI > 40 kg/m2).
The currently used BMI cut-off values for pre-obesity and obesity are based on morbidity and mortality studies in relation to Caucasian population[8-10].
However, there is a controversy in the literature, termed the “obesity paradox”, which associates better survival and fewer cardiovascular events in patients with mildly elevated BMI afflicted with chronic diseases[11].
In fact, the outdated BMI formula developed nearly 200 years ago by Quetelet, is not a measurement of adiposity, but merely an imprecise mathematical estimate[12-14].
Its popularity stems in part from its convenience, safety, and minimal cost, and its use is widespread, despite the fact, BMI ignores several important factors affecting adiposity. Moreover, the error in the diagnosis of obesity generates important effects on health care costs.
BMI formula is only an arithmetic approximation for the relative quantity of adiposity and is used to predict and evaluate disease risk in epidemiological studies, thereby acting only as a population-level indicator of obesity.
Because BMI does not measure PBF directly and poorly distinguishes between total body fat and total body lean, or bone mass, the use of BMI as an index of PBF for a person may be inaccurate and not useful as a cardiovascular risk factor[15-17].
However, according to a WHO expert committee, “there is no agreement about cut-off points for the PBF that constitutes obesity”[10].
Current research suggests that the obesity cut-off points of PBF are in the 23%-25% range in men and 30%-35% range in women[18].
The clinical use of WHO BMI cut-off values when applied to the Italian population cause misclassifications, and a considerable number of subjects, both males and females, will not be classified as obese based on their BMI alone[19].
The disagreement became impressive in the classification of obese women: in the class of age 30-40 the proportion of obese women according to BMI is 30% reaching about 82% if the classification is based on PBF. Notably, among women that were classified as normal according to their observed PBF, the median BMI was 20.1, ranging from 15.6 to 26.7.
For the Italian population, the percentage of obese women according to PBF classification increases as age increases, ranging from 63.17% in women younger than 20 years to 87.39% in women older than 60[20].
Moreover, values corresponding to normal weight, overweight, and various subgroups of obesity are confounded by body frame and muscularity, fluid retention, sarcopenia in aging or disease, spinal deformities, physical disabilities, and transcultural differences. A person with the same BMI, may have a large proportion of total body fat mass and be obese, or may have a considerable muscle mass and be a weight-lifter. Moreover, PBF at a given BMI will tend to vary across gender, age, and race-ethnicity[21-23].
Moreover, it is recommended to measure waist circumference (WC) in adults with BMIs below 35 kg/m2, to further assess disease risk[24].
Anyway, obese individuals differ not only in the amount of excess fat mass, but also in the regional distribution of the fat within the body. The fat distribution affects the risk associated with obesity. It is useful therefore, to be able to distinguish between those at increased risk as a result of abnormal fat distribution or android obesity from those with the less serious gynoid fat distribution, in which fat is more evenly and peripherally distributed around the body[2].
On the hand even if there are some obese people are prone to develop alterations in fat distribution and metabolic disease, others are protected from the adverse metabolic effects of weight gain and increased adiposity[25].
Some studies suggested that the main issue to explain the metabolic abnormalities in normal weight individuals was fat distribution.
Certain attributes of visceral fat, the adipose tissue surrounding abdominal organs, make its accumulation more worrisome than the accumulation of subcutaneous fat, which resides below the skin[26-28].
Other markers for excess body fat evaluation have to be used in clinical practice and investigation (e.g., WC, skin fold thickness, waist-to-hip ratio, waist-to-height ratio).
WC or waist-to-hip ratio has been used as a proxy measure for body fat distribution when investigating the health risk increased with an increasing ratio. Some studies have suggested that WC, either singly or in combination with BMI, may have a stronger relation to some health outcomes than BMI alone[29]. Moreover, progressively higher values of BMI and WC are associated with a progressive elevation in metabolic markers of cardiovascular disease (CVD) risk such as total serum cholesterol, triglycerides, blood glucose and a progressive reduction of HDL-cholesterol, with a clear-cut increase in the incidence of all-cause and cardiovascular deaths as well as of cardiovascular morbid and fatal events[30]. In post-menopausal women, it was reported that both BMI and WC were associated with mortality, but WC may be more important than BMI[31], as it reflects abdominal fat levels. In the Nurses’ Health Study, waist-to-hip ratio and WC were also independently strongly associated with increased risk of coronary heart disease among women with a BMI of < 25 kg/m2[32]. WC reflects abdominal or intra-abdominal fat, and hip circumference reflects different aspects of body composition in the gluteo-femoral region, i.e., muscle mass, bone, and fat mass. The importance of waist and hip measurements, and the waist to hip ratio, lies in the apparently different physical and metabolic characteristics of these two regions, and therefore the diverse clinical outcomes in subjects with a gynoid (low waist to hip ratio, lower body obesity) or android (high waist to hip ratio, upper body obesity) body conformation. This may be due to the tendency for abdominal adipocytes to enlarge (hypertrophy) whereas subcutaneous femoral adipocytes increase in number (hyperplasia), perhaps due to increased levels of the adipogenic transcription factors CCAAT/ enhancer-binding protein α (C/EBPα; GeneBank accession No: NC_000019) and peroxisome proliferator-activated receptor-γ2 (PPARγ2; GeneBank accession No: NC_000003) in hypertrophic adipocytes[33]. Hypertrophic adipocytes tend to be associated with dyslipidemia and insulin resistance[34]. It has been suggested that the composition of gluteal fat deposits correspond more closely with that of visceral deposits rather than femoral deposits[35].
In conclusion, diagnosis, therapy and follow up of all subtypes of obesity must not be based on “body weight” parameter, but body composition parameters and energetic expenditure are required.
To overcome misclassifications, direct measurements of PBF, by magnetic resonance imaging (MRI), computerized tomography (CT), dual energy X-ray absorptiometry (DXA), bioimpedence analysis, total body water or hydrometry, and skinfold thickness would be a better tool for diagnosing the obese phenotypes (Figure 1).
Bays et al[36] have coined a new term that well defines the concept of “sick fat”, the adiposopathy, in order to highlight the pathogenic role of adipose tissue. The adiposopathy is caused by positive caloric balance, that occurred in hypercaloric diet and sedentary lifestyle in genetically predisposed and environmentally sensitive individual[36]. Impaired adipocyte proliferation or differentiation (adipogenesis), visceral adiposity, growth of adipose tissue beyond adequate vascular supply and ectopic fat deposition are anatomical manifestations of adiposopathy that are associated with adverse endocrine and immune responses leading to metabolic disease[37,38].
In order to understand the importance of the role of adipose tissue in the onset of a pathological condition and distinguish the degree and type of disease, we agree with the definition proposed by Bays of adiposopathy, as “sick fat disease”, and obesity as “fat mass disease”[38]. Different adverse physical and metabolic health consequences could occur as directed or undirected consequences of adipose tissue mass spread and dysfunction[39], such as metabolic syndrome (MS), respiratory disorders, joint pain, diabetic retinopathy, low self-esteem, cardiovascular, neurologic, pulmonary, musculoskeletal, dermatologic, gastrointestinal, genitourinary, renal and psychological diseases and cancer[3,4,40-42]. Moreover, also a slight rise of body weight may represent a risk factor for the onset of metabolic abnormalities that lead to development metabolic disease[43].
According to Patel and Abate[44], the subcutaneous adipose tissue (SAT) can be a major contributor of systemic free fatty acid flux, more than visceral or retroperitoneal fat, able to determine the insulin resistance development.
Apovian et al[45] explained the insulin resistance and vascular endothelial dysfunction in obese subjects with the phenomenon of adipose tissue macrophage (ATM) infiltration, in the form of crown-like structures, associated with and elevated plasma high sensitivity C-reactive protein (hs-CRP) levels.
Because of the complexity of the obesity condition, it is clear that we need a new and more adequate model, possibly based on findings at the physiopathological level and a new method to correctly identify all the affected subjects for an efficient and successful treatment.
If Bays et al[39] affirmed that to deny the adiposopathy, also in mildly overweight patients, represents denying the opportunity of cure to this individuals, and perhaps to entire populations, we added the importance to evaluate PBF in normal weight individuals to predict in advance the risk of adiposopathy, and to verify the real state of health.
The association between visceral fat and metabolic and cardiovascular disorders is also related to accumulation of ectopic fat that accompanies visceral adiposity[46]. Moreover, the hepatic fat observed in patients with insulin resistance could be independent from the visceral fat content[47].
Healthy expansion of subcutaneous fat in response to obesity is accompanied by enlargement of fat mass through enhanced recruitment of preadipocytes along with adequate vascularization of expanding adipose tissue and minimal fibrosis and minimal infiltration of inflammatory ATMs. In contrast, pathologic expansion is associated with rapid growth of fat tissue through the enlargement of existing adipocytes and inadequate vascularization leading to fibrosis and inflammatory ATM infiltration that secrete high levels of inflammatory cytokines[48].
This pathologic expansion is associated with adipose tissue accumulation in ectopic locations such as liver, skeletal muscle, and pancreas as well as visceral adiposity[49].
The existence of “metabolically healthy obese (MHO)”[50,51] further supports this concept as these individuals have less ectopic fat despite high amount of subcutaneous fat in gluteal depot and are insulin sensitive[51].
Therefore, adipose tissue distribution is a more significant predictor of metabolic and cardiovascular risk than overall adiposity. Inability to expand subcutaneous depots in response to positive energy balance[52] results in metabolic and cardiovascular complications.
Adipose tissue, found in several locations throughout the body and long thought to be primarily a repository for triglycerides, is also important for regulating metabolism and body’s physiologic. Fat is an endocrine tissue and, indeed, may constitute the largest endocrine organ in the body[53,54].
If in the past, adipose tissue was considered to be a metabolically inactive fat depot, the current view of adipose tissue is that of an active secretory organ, sending out and responding to signals that modulate appetite, energy expenditure, insulin sensitivity, endocrine and reproductive systems, bone metabolism, and inflammation and immunity[55].
Expansion of the adipose tissue is accompanied by an increased infiltration of immune cells, in particular macrophages and T-cells[56], with a pro-inflammatory phenotype. The “cross-talk” between the infiltrating cells and the tissue-resident adipocytes leads to secretion of adipokines, cytokines, chemokines, and lipids with a predominant proinflammatory character[57].
Several indirect lines of evidence suggest that fatty acids can modulate the immune response. One of these is that levels of several fatty acids are associated with levels of inflammatory markers in healthy individuals[58].
More directly, the type of fatty acids contained in the diet has been suggested to influence the risk of development of inflammatory diseases in which the immune system plays an important role.
This cross-talk has an also been shown to affect the function of adipocytes, such as lipolysis, which will most likely result in altered concentration of circulating free fatty acids.
Some of obesity phenotypes are associated with a high risk of developing diabetes type 2 (T2DM) however for a given adiposity, there is a large heterogeneity in the metabolic risk mainly linked to the location of excessive adipose tissue.
The cause of glucose, lipid, or atherogenic disorders can be found in the visceral adipose tissue (VAT), representing a predictive factor for those disorders[59].
The role of visceral fat in the development of peripheral insulin resistance in T2DM is related to the insulin-mediated glucose uptake[60], that can be directly affected by inflammatory cytokines, like tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), and interleukin 6 (IL-6) secreted by visceral fat depot.
Moreover, it has been demonstrated that both hepatic fat accumulation than hepatic insulin resistance are related to visceral fat depots, representing approximately 20% of total body fat mass in men and 6% in women[61], as approximately 80% of hepatic blood supply is derived from the portal vein[62].
Due to the interactions within adipose tissue and muscle, involved in the endocrine bi-directional “cross-talk” between these tissues[63], it was observed a loss of muscle strength, that depends on both decrease in muscle mass and accumulation of inter-muscular adipose tissue, contributing to the decline of muscle quality[64]. Therefore, the evaluation of body lean mass, together with body fat mass analysis and body force assessment, could contribute to predict sarcopenic obesity risk.
The obese state is a low-grade systemic inflammation, characterized by abnormal adipokine production, and activation of some pro-inflammatory signalling pathways, resulting in the induction of several biological markers of inflammation. In fact, inflammatory markers, such as C-reactive protein (CRP), IL-6, IL-1 family [IL-1α, IL-1β and IL-1 receptor antagonist (IL-1Ra)] and TNF-α are increased in obese individuals compared with lean subjects, although not to the same extent observed in classic inflammatory conditions[65].
Though all the functional consequences of the increase in inflammatory processes in obese are not yet known, is an established fact that cytokines have metabolic activities, as many “adipokines” can modulate of both the metabolic and vascular homeostasis[66], acting not only as autocrine/paracrine regulators, but also reaching, from the perivascular fat depot, the surrounding target organs, through the systemic circulation (“outside-to-inside” cellular cross-talk)[67-69].
This inflammatory mechanism is the basis of the increased risk of development of T2DM, acute heart attacks and Alzheimer’s disease[70,71] in obesity.
Polymorphisms and allele variants of cytokines genes are presumed to be involved in obesity and related chronic-degenerative diseases, therefore an understanding of their food association could be useful for nutritional pre-emption.
The practical application of predictive and preventive medicine requires early identification of the individuals who are on a path toward earlier development of disease, followed by the introduction of a targeted intervention. Ideally, one could use the identification of specific polymorphisms, an early marker of disease susceptibility, to prevent complications of disease by the use of nutritional agents that modulate the biology resulting from the genetic variation.
A high degree of sequence variation recently has been shown to exist in cytokine genes. These gene polymorphisms are relatively common in regulatory regions of the cytokine genes and therefore may be functionally significant in defining inter-individual differences in transcription or post-transcriptional processes. These genetic variations therefore provide a potential mechanism by which individuals may have different degrees of response to the same stimulus.
Among the first genes activated with any injurious challenge are the genes for IL-6, IL-1 and TNF-α. These molecules activate each other and both are critical components of the inflammatory process.
Besides regulating the immune system, IL-6 also plays a role in the regulation of body fat and energy expenditure. Recent studies have shown that IL-6 deficient mice develop mature onset obesity with high leptin levels in the circulation[28].
Moreover, intracerebroventricular IL-6 treatment decreases body fat and increases energy expenditure in rodents[72].
In humans, high cytokine levels (including IL-6) and cytokine brain synthesis were found to increase resting energy expenditure and induce cachexia. Additionally, a subcutaneous injection of IL-6 increased resting metabolic rate and hypothalamic-pituitary-adrenal axis activity in a dose-dependent fashion, suggesting that hypothalamic corticotrophin-releasing hormone may mediate both of these actions in humans. A second possibility by which IL-6 may affect energy expenditure is enhanced adrenergic stimulation. In the other hand, high circulating IL-6 concentrations have been found to predict the development of type 2 diabetes[73,74]. IL-6 has a central role in the mechanism of pro-inflammation pathway and there are some evidences suggesting the implication of a network of cytokines for the development of metabolic disorders, as type 2 diabetes, CVD, and sarcopenia.
Polymorphisms that affect the gene transcription and production of IL-6, like the gene promoter -174 G/C polymorphism, are examples of genetically determined-changes in metabolism and energy homeostasis. In fact, IL-6 gene promoter polymorphism -174 G/C is shown to influence IL-6 transcription as well as overweight and insulin sensitivity.
Data suggest that endogenous IL-6 has effects on energy expenditure but upper endogenous IL-6 production, e.g., due to the presence of allele C of -174G/C polymorphism, contributes to obesity in humans and related pathologies and is a disadvantage for longevity.
Macrophages, whose adipose tissue is composed, are the major source of TNF-α produced by the white adipose tissue and contribute approximately 50% of white adipose tissue-derived IL-6[75].
TNF-α participates to inflammatory events but it’s also an important autocrine/paracrine of fat cell function which limits adipose tissue and skeletal muscle expansion, by inducing lipolysis[76], insulin resistance[77], and muscle apoptosis[78]. Its presence also results in an increase in circulating leptin concentrations[79] and, finally, its overexpression, by exceeding fat, aimed at stopping the growth of tissues[80].
Several population-based studies have suggested an association between TNF-α polymorphisms and obesity-related phenotypes[81].
TNF-α has a direct (possibly paracrine) function in adipose tissue where it limits its mass by stimulating lipolysis and decreasing lipoprotein lipase expression and activity, that means it’s involved in the adipostatic function[76].
TNF-α can induce hyperlipidemia and insulin resistance, especially in presence of single nucleotide polymorphisms (SNPs) on the promoter (-308 G/A and -238 G/A)[82].
In fact, TNF-α is an antagonist of insulin receptor and an overproduction could impair insulin signalling as well as down-regulate adipose lipase producing hyperlipidemia. Levels of TNF-α are high in an obese population and moreover experiments in vitro show that allele A alters binding of nuclear factors to the -308 region causing a general increase in transcriptional[83]. Interestingly, cases with the -308 A allele of the TNF-α gene have significantly higher hip and WC, BMI and body fat mass values than obese individuals carrying the -308 G allele, but not the waist-to-hip ratio[84].
An higher fasting insulin levels, higher Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), higher systolic blood pressure and lower HDL cholesterol were highlighted among -308 A allele TNF-α obese carriers than -308 G obese carriers, and overweight subjects with impaired glucose tolerance showing a genotype have higher risk of developing T2DM than subjects without this genotype[85].
This observation is consistent with previous findings, suggesting that leptin secretion and insulin resistance are affected by TNF-α expression. Disturbances in circulating levels of leptin may be accompanied by a lower control of appetite and thermogenesis or may occur as a consequence of leptin resistance.
Other intriguing molecules produced by adipose tissue and involved in metabolism are that belonging to the class of IL-1. IL-1 biological activity involves two agonists, IL-1α and IL-1β, specific receptors, and a naturally occurring antagonist, IL-1Ra. Some of the most relevant properties of IL-1 biological activity are the ability to initiate cyclooxygenase type 2 and inducible nitric oxide, leading to substantial expression of prostaglandin E2 and nitric oxide by cells exposed to IL-1β.
Moreover, IL-1β is able to determine lipolysis, glucose transport, and adipocyte maturation in adipose tissue, to inhibit lipogenesis, via the IL-1 receptor type 1 and the IL-1 receptor accessory protein, like TNF-α.
In obese individuals the serum level of IL-1Ra is up-regulated for a feedback down-regulation, resulting in an acquired resistance to leptin, rising the high values as in inflammatory autoimmune diseases and sepsis[86,87].
Polymorphisms affecting IL-1 production, like the SNP at IL-1β (+3954), the SNP at IL-1α (-889) and the SNP at IL-1α (+4845) are associated with increased levels of inflammatory mediators, and also with increased severity of several chronic diseases, including Alzheimer’s disease[88], periodontal disease and others. This proinflammatory IL-1 genotype pattern recently has been associated with cardiovascular acute coronary events. For example, despite having total cholesterol levels below 200 mg/dL, individuals who carried two copies of IL-1α (+4845) allele 2 were four times (P < 0.01) more likely to have a coronary heart disease event during an 11-y monitoring period than were individuals with the same level of cholesterol but who did not carry this IL-1 genotype (unpublished data, Interleukin Genetics, Waltham, MA, United States).
The IL-1Ra antagonizes the effects of IL-1α and -1β[89], and the anti-inflammatory cytokine interferon-beta and leptin can induce IL-1Ra production without increasing IL-1[86,90]. In subjects with hyperleptinemic obese individuals the serum sIL-1Ra levels are higher as compared to non obese subjects[91].
Furthermore, in subjects that underwent to intestinal bypass surgery, sIL-1Ra levels dropped after weight loss, correlating with BMI and the degree of insulin resistance better than with serum leptin levels, according to metabolic control unmediated by leptin.
The ability of the pancreatic alpha and beta cells to increase the uptake of glucose can be compromised by constant hyperglycemia, even mild. This mechanism, compromising the glucose transport mediated by insulin, prevents the possibility of self correction, and start a vicious cycle mechanism that leads to deterioration of the metabolic process[92].
Continuous overstimulation of the beta-cell by glucose could eventually lead to depletion of insulin stores, worsening of hyperglycemia, and finally deterioration of beta-cell function.
The mechanisms responsible for changes of insulin sensitivity vs insulin resistance clearly vary and involve changes in both beta -cell function and beta -cell mass, although in most instances it appears that functional changes predominate. Moreover, the beta-cell must also alter its activity when this critical modulator changes for more prolonged periods[60].
Several mechanisms might explain the glucotoxicity due to prolonged hyperglycemia[93,94], such as beta -cell exhaustion, oxidative stress induced by free radical oxygen species, endoplasmic reticulum (ER) stress, inflammation caused by proinflammatory cytokines and chemokines, loss of neogenesis, proliferation of beta -cells, gradual loss of insulin gene expression and other beta-cell specific genes, changes in mitochondrial number, morphology and function, disruption in calcium homeostasis[95].
In the presence of hyperglycemia, prolonged exposure to increased free fatty acids result in accumulation of toxic metabolites in the cells (“lipotoxicity”), finally causing decreased insulin gene expression and impairment of insulin secretion.
Chronic exposure to abnormally high blood glucose has detrimental effects on insulin synthesis/secretion, cell survival and insulin sensitivity through multiple mechanisms (“glucotoxicity”), which in turn lead to hyperglycemia and finally to the vicious circle of continuous deterioration of beta cell function[96].
Beta cells exposed to an increased insulin secretory request place a high demand on ER for the synthesis of proinsulin, leading to cellular stress[97].
Due to chronic ER stress and strong unfolded protein response, beta cell death through apoptosis (mediated by stress kinases and transcription factors) may be initiated. In addition to glucose, free fatty acids (FFA) and islet amyloid polypeptide are triggers of beta cell ER stress[98]. Moreover, reactive oxygen species (ROS), free radicals that are intermediate metabolites derived from oxygen metabolism in mitochondria, primarily due to hyperglycemia, causes oxidative stress in various tissues, playing an important role in both physiology and pathology in beta-cells. ROS are continuously produced by the mitochondrial electron transport system as a byproduct of the oxidative phosphorylation pathway; however, normal cells have antioxidant defenses to rapidly neutralize ROS and maintain an optimal redox potential for appropriate biological cell function[99]. This optimal redox balance is impaired because of increased ROS production and insufficient endogenous anti-oxidant defenses of the β-cells.
Chronic hyperglycemia leads to decreased number of mitochondria and changes of their morphology[99] in the β-cells. This is associated with impaired oxidative phosphorylation, decreased mitochondrial Ca2+ capacity and decline in ATP generation[100]. In addition, disruption in Ca2+ homeostasis (e.g., changes in glucose-induced Ca2+ influx, ER Ca2+ depletion) negatively impacts on beta cell growth/function and on the insulin secretion pathway[101].
When adipose tissue cannot meet the demand of storing excessive energy, triglyceride is accumulated in non-adipose tissues as ectopic fat, which may lead to insulin resistance in the liver and skeletal muscle and insufficient insulin secretion in the pancreas[48,102,103].
High plasma FFA and triglyceride levels lead to increased import of FFAs into non-adipose tissues (i.e., liver, muscle, pancreas), contributing to intracellular lipid accumulation. This phenomenon, known as lipotoxicity, may play an important role in the pathogenesis of diabetes and heart failure in humans[104].
Adverse consequences of lipid overload have been observed in many organ systems, mainly in the heart, skeletal muscle, pancreas, liver, and kidney[104].
In addition to primary hyperlipidemias, serum triglycerides[105,106] and FFAs[107] are elevated in type 1 and type 2 diabetics and plasma FFA levels are elevated in obese individuals[108].
The cross-talk has been shown to affect the function of adipocytes, such as lipolysis, which will most likely result in an altered concentration of circulating free fatty acids. Indeed, obese persons have higher levels of free fatty acids in plasma compared to lean subjects[109-111].
Several indirect lines of evidence suggest that fatty acids, contained in the diet, can modulate the immune response. One of these is that levels of several fatty acids are associated with levels of inflammatory markers in healthy individuals[58].
The overall data suggest that low concentrations of fatty acids can influence the proliferation of T-cells, whereas higher concentrations induce apoptosis in a dose- dependent manner, through the induction of several pathways[112-117].
In addition, the concentration at which apoptosis occurs is also determined by the degree of saturation and the length of the fatty acid. While short-chain fatty acids are not toxic even at a concentration of 800 mmol/L or higher[113], longer, and more unsaturated fatty acids can already be toxic in low concentrations (e.g., linoleic acids) is toxic at 100 mmol/L[114,115]. In contrast to the apoptotic effects, the modulatory effects of low, non-toxic concentrations of fatty acids on proliferation do not seem to correlate with length of the fatty acids tested.
de Jong et al[59] conclude that, free fatty acids induce proliferation of resting T-cells in low concentrations, while higher concentrations induce apoptosis.
Etiological contributing factors to VAT depot and distribution are represented by age, gender, genetics, and ethnicity[118].
Substantial anatomical differences between males, that accumulate adipose tissue in the upper body (trunk, abdomen), and females, with a usual accumulation of adipose tissue in the lower body (hips, thighs), are practically unique in human[119-121].
Vague[122] identified two different body shapes, proposing the two terms “android obesity”, to refer to adipose tissue accumulated preferentially in the trunk/upper body area closely related to hypertension, CVD, gout, and diabetes, and the term “gynoid obesity” to refer to adipose tissue accumulation in the hips and thighs, with less associated complications[123,124].
Visceral fat depot, evaluated by MRI and computed tomography (CT), was found to be specifically associated with the metabolic alterations related to obesity, both in men and women[125-127]; subcutaneous fat in general define a positive effect on insulin sensitivity[128].
Genetic predisposition could explain the fact that females, although high BMI, are protected against insulin resistance more than males[118]. Furthermore, the capacity to increase the fat depots depends on the ease with which local preadipocytes can be readily activated toward differentiation process[129].
Although ethnicity is a factor that determines the different adipose tissue distribution and responses to the cardiometabolic risks[118,130,131], as highlighted by Deurenberg et al[132], that observed ethnic differences in BMI at similar levels of PBF, in Chinese, Indonesian, and Thai populations respect to Caucasians (American, Australian, and European Whites analyzed as one group). Moreover, Asian Indians have more abdominal fat than Caucasians[133,134], and Caucasians have more VAT than African Americans[135-137]. On the other hand, in Asians and Indian Asians the accumulation of fat is mainly in the visceral area[131,138,139].
Genetic and epigenetic programming is one of the most credible explanations for the observed differences in the storage of lipid depot[118,139,140].
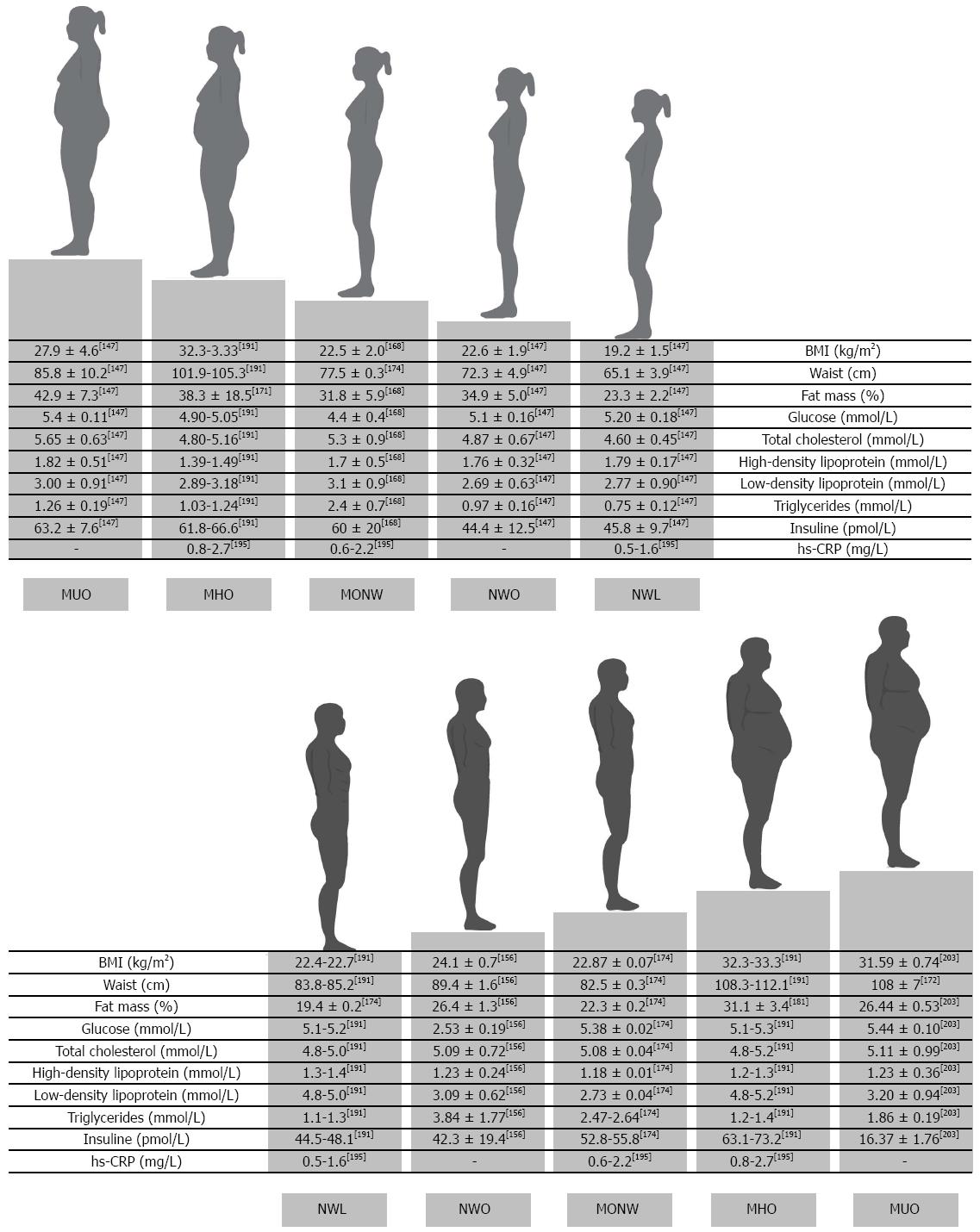
Nowadays four phenotypes of obese individuals have been widely described (Table 1): (1) normal weight obese (NWO)[141]; (2) metabolically obese normal weight (MONW)[142,143]; (3) MHO; and (4) metabolically unhealthy obese (MUO) or “at risk” obese with MS[144].
| Publication | Year | Definition |
| Normal weight obesity - NWO | ||
| De Lorenzo et al[141] | 2005 | Normal weight (by BMI) + PBF > 30 (dual X-ray absorptiometry) + ↓ lean body composition of the left leg. Do not have metabolic syndrome |
| Di Renzo et al[162] | 2006 | NWO syndrome is characterized by wild type homozygotes genotypes regarding IL-15 R-α and MTHFR 677C/T polymorphism |
| De Lorenzo et al[147] | 2007 | ↑ concentrations of pro-inflammatory cytokines IL-1α, IL-1β, IL-6, IL-8, TNF-α in NWO respect to non obese group |
| Di Renzo et al[160] | 2007 | The allele 2 (A2) of IL-1 receptor antagonist (Ra) in NWO subjects was associated with ↑ of IL-1β plasma amount |
| Marques-Vidal et al[151] | 2008 | Normal weight (by BMI) + ↑PBF (Bioelectrical impedance) or fat mass index ≥ 8.3 kg/m2 (men) or ≥ 11.8 kg/m2 (women) |
| Di Renzo et al[161] | 2008 | Genotyping of -175 G/C IL-6 polymorphism: in G/G the serum IL-6 level of NWO (10.70 ± 2.52 pg/mL) and obese (10.67 ± 1.09 pg/mL) was significantly higher compared with NWL women (5.54 ± 1.51 pg/mL). Positive correlation between PBF and plasma IL-6 and between HOMA-IR and plasma IL-6 only in NWO e obese G/G carrier |
| Marques-Vidal et al[149] | 2010 | ↑ blood pressure, ↑ lipid levels and ↑ prevalence of dyslipidaemia [OR = 1.90 (1.34-2.68)] and fasting hyperglycaemia [OR = 1.63 (1.10-2.42)] respect to lean women, whereas no differences were found between NWO and overweight women. |
| Di Renzo et al[159] | 2010 | ↓ glutathione and nitric oxide metabolites were significantly lower in pre-obese-obese and NWO compared to normal weight individuals. Lipid peroxide levels negatively correlated to FFM% and positively correlated to PBF, IL-15, TNF-α, insulin, total cholesterol, LDL, and triglycerides |
| Romero-Corral et al[157] | 2010 | NWO not manifest the metabolic syndrome, despite a cluster of metabolic and genetic features such as the higher prevalence of dyslipidemia, hypertension (men), CVD (women), and a 2.2-fold increased risk of CVD mortality (women) compared with those with low PBF |
| Kim et al[156] | 2013 | Normal weight (by BMI) + ↑ PBF |
| Madeira et al[155] | 2013 | Normal weight (by BMI < 25) + Sum of triceps and subscapular skinfolds > 90th percentiles |
| Di Renzo et al[164] | 2013 | G/A -308 TNF-α polymorphism contributes to sarcopenic obesity susceptibility in NWO |
| Di Renzo et al[165] | 2014 | TP53 codon 72 in exon 4 polymorphism was associated to the reduction of appendicular skeletal muscle mass index in NWO, leading to increase of sarcopenia risk |
| Oliveros et al[146] | 2014 | Normal BMI, ↑ PBF content and at increased risk for metabolic dysregulation, systemic inflammation and mortality |
| Jean et al[158] | 2014 | Highlight the importance of PBF correct assessment and body fat distribution in the clinical setting to identify NWO phenotype |
| Metabolically healty obese - MHO | ||
| Bonora et al[178] | 1998 | Subgroup of obese individuals with a normal metabolic response |
| Sims[179] | 2001 | The MHO subset include family members with uncomplicated obesity, early onset of the obesity, fasting plasma insulin within normal range, and normal distribution of the excess fat |
| Karelis et al[190] | 2004 | ↑ Fat mass + Normal Metabolic profile + ↑ insulin sensitivity |
| Karelis et al[143] | 2005 | Favorable inflammation profile: ↓ hsCRP, ↓α-1 antitrypsin levels compared with insulin-resistant women, suggesting that lower inflammation state could play a role in the protection of this phenotype |
| Succurro et al[171] | 2008 | Respect to MONW, MHO have a healthier metabolic risk profile and ↑ disposition index (insulin sensitivity x insulin secretion) |
| Wildman et al[50] | 2008 | In NHANES sample, it was found a prevalence of 32% among obese adults over the age of 20 |
| Arnlöv et al[192] | 2010 | MHO individuals were at an increased risk of major CVD events as compared to MHNW individuals in follow-up periods (> 15 yr) |
| Eshtiaghi et al[194] | 2014 | MHO phenotype over a 10 yr period progressed to frank metabolic syndrome |
| Achilike et al[196] | 2015 | Among subjects classified as MHO at baseline, almost half (47.6%) of them progressed to metabolically unhealthy obese (MUO) within the 7.8-yr follow-up period |
| Shaharyar et al[195] | 2015 | Both MHO individuals and MONW phenotypes were associated with ↑ high hsCRP, and hepatic steatosis |
| Metabolically obese but normal weight - MONW | ||
| Ruderman et al[166] | 1981 | Subtle increase in adiposity and/or hyperinsulinism creating obese associated diseases in normal weight (by standard weight tables) |
| Ruderman et al[167] | 1998 | CHD, T2DM and other disorders associated with obesity + normal weight (< 115% of ideal body weight or BMI < 28 kg/m2) |
| Dvorak et al[168] | 1999 | Impaired insulin sensitivity, BMI < 26.3 kg/m2↑ total fat mass (+20%), ↑ PBF (+16%) (dual x-ray absorptiometry), ↑ subcutaneous fat (+33%), ↑ visceral fat (+26%) |
| Esposito et al[176] | 2004 | ↑ inflammation biomarkers, TNF-α and IL-6, in as a result of the larger visceral fat areas in this group |
| Conus et al[169] | 2004 | Insulin sensitivity determined by HOMA > 1.69 with normal weight (by BMI < 25 kg/m2), ↑PBF (dualX-ray absorptiometry), ↓ FFM, ↓ physical activity energy expenditure, ↓ peak oxygen uptake |
| Meigs et al[170] | 2006 | BMI < 25 kg/m2 + Metabolic Syndrome criteria/insulin resistance |
| Succurro et al[171] | 2008 | Normal weight (by BMI) + Impaired insulin sensitivity, ↑ visceral adiposity, ↓ HDL, ↑ fasting glucose, ↑ triglycerides, hypertension |
| Thomas et al[175] | 2012 | According magnetic resonance imaging, they refined MONW phenotype and renaming this sub-phenotype as “thin-on-the-outside fat-on-the-inside” (TOFI), with a higher ratio of visceral:subcutaneous abdominal adipose tissue |
| Eckel et al[172] | 2015 | ↑ waist circumference (women: 75.5 cm vs 73.1 cm; men: 88.0 cm vs 85.1 cm), ↑ HbA1c (6.1% vs 5.3%), ↑ triglycerides (1.47 mmol/L vs 1.11 mmol/L), and ↑ hsCRP (0.81 mg/L vs 0.51 mg/L) , ↓ HDL (1.28 mmol/L vs 1.49 mmol/L) and ↓ adiponectin (6.32 μg/L vs 8.25 μg/mL) |
| Du et al[173] | 2015 | Lipid accumulation product and visceral adiposity index, two markers of visceral obesity, identify the MONW phenotype |
| Metabolically unhealthy obese - MUO | ||
| Alberti et al[201] | 2005 | MUO subjects are characterized by a BMI ≥ 30 kg/m2, a PBF > 30% and high visceral fat mass, closely linked to the development of the metabolic syndrome, T2DM, and atherosclerotic cardiovascular disease |
| Fabbrini et al[47] | 2009 | In MAO subjects, but not MNO subjects, moderate weight exacerbated several metabolic risk factors for CVD: ↑ blood pressure, ↑ plasma triglyceride,↑ in intra hepatic triglyceride, ↑ VLDL apoB100 and ↓ plasma adiponectin concentrations and insulin sensitivity in the liver, skeletal muscle, and adipose tissues, ↓ adipose tissue expression of genes involved in glucose uptake and lipogenesis |
| O'Connell et al[210] | 2010 | MUO and obese with T2DM subjects had a omental adipocyte size greater than MHO, moreover MUO group had an intermediate degree of steatosis (43%) respect to MHO (3%) and obese with T2DM (74%). |
| Di Daniele et al[202] | 2013 | 6 mo of dietary intervention based on Italian Mediterranean Diet in “at risk” obese subjects, determined a reduction (-52%) in the prevalence of the metabolic syndrome and a reduction in terms of waist circumference, BMI and total body weight. |
| Calanna et al[211] | 2013 | At-risk obese individuals showed ↑ plasma glucose dependent insulinotropic polypeptide, ↓ post-glucose load glucagone-like-peptide-1, and ↑ levels at baseline and after glucose load, indicating inappropriate glucagone suppression |
The characteristic of the four obese phenotypes are depicted in Figure 2.
Moreover, the sarcopenic obesity has been characterized, and related to all the described phenotypes[145]. Moreover the category of normal weight lean (NWL), represented by metabolically healthy normal weight (MHNW) has been classified to distinguish from NWO.
According to the causality and anatomic, pathophysiologic, and clinical manifestations of obese phenotypes, we can define NWO and MHO belonging to the category of “fat mass disease”, and MONW and at risk obesity to “sick fat disease”. Moreover, NWO women are quite different from MHO women and may also be distinguished from MONW women, according to fat distribution, inflammatory and metabolic parameters, and genetic variants.
The new concept of NWO had been proposed[141,146]. NWO subjects have normal BMI (18.5-24.9 kg/m2), and highest body fat percentage (BF%) (men, ≥ 23.5%, women, ≥ 29.2%), associated at a higher degree of subclinical vascular inflammation and risk for cardiometabolic disease, of which body fat is a major contributing factor[147-149].
Worldwide prevalence of NWO is near to 10%, and the prevalence is higher in women than in men[150-152]. In women, prevalence of NWO increased considerably with age, and virtually all women aged over 55 with a BMI < 25 kg/m2 were actually considered as NWO. Even if the proportion of PBF changes with age and sex[23,153], using sex and age-specific thresholds for PBF led to a much lower prevalence of NWO in women, whereas little differences were found for men[15,20].
As demonstrated by Kang et al[154], NWO subjects highlighted a significantly higher blood pressure, fasting glucose level, and worse lipid profile compared to normal weight subjects, independently associated with elevated TBRmax values, that may determine and justify the metabolic and CVD occurring risks.
Due to the peculiarity of the relationship between lean mass distribution (lean of the right part of the body trunk and of the left leg), and CVD risk indexes, NWO could be considered an additional metabolic subset of obesity[141,155,156].
They also do not manifest the MS, despite a cluster of metabolic and genetic features such as the higher prevalence of dyslipidemia, hypertension (men), CVD (women), and a 2.2-fold increased risk of CVD mortality (women) compared with those with low total body fat mass[157].
Jean et al[158] have provided three potential explanations for why normal-weight central obesity is linked to increased mortality include the following: (1) the accumulation of abdominal visceral fat as manifested by an increased girth: there is a very strong association between WC and visceral adiposity and the excess adipose tissue in the abdominal cavity organs, which are a source of inflammation and insulin resistance; (2) the reduced amounts of subcutaneous fat in the legs, hips, and buttocks, known to be protective for CVD and with a favorable metabolic function; and (3) the limited muscle mass, because like in NWO, people with normal-weight central obesity may also have sarcopenic obesity since, in order to have normal weight in the setting of large amounts of visceral fat, they likely have limited amounts of muscle mass.
De Lorenzo et al[141] firstly described the NWO syndrome characterized by higher oxidative stress level[159], early inflammatory status[147] and few metabolic abnormalities.
The presence of SNPs of triad inflammatory genes, characterizes the NWO syndrome.
In NWO syndrome, the IL-1Ra allele 2 increases the risk of ovarian, pancreatic, cervical and gastric cancer, probably due to increased IL-1 production and inhibition of gastric acid secretion[160].
According to the -174G/C promoter polymorphism of the IL-6 gene, G/G NWO showed a strong correlation between HOMA-IR and PBF[161].
Moreover, NWO are characterized by a wild type homozygotes genotypes regarding IL-15 receptor-alpha and methylenetetrahydrofolate reductase (MTHFR) enzyme[162], suggesting a “conversation” between adipose tissue and skeletal muscle[163].
The importance of the TNF-α gene polymorphism to total body lean mass variation in NWO syndrome, demonstrating that G/A -308 TNF-α polymorphism contributes to sarcopenic obesity susceptibility[164].
TP53 codon 72 in exon 4 polymorphism was associated to the reduction of appendicular skeletal muscle mass index (ASMMI) in NWO, leading to increase of sarcopenia risk[165].
This finding gives much more support to the idea that a genetic approach could be predictive for finding a vulnerable category of people[160-162,164].
MONW subjects, first described and revisited by Ruderman et al[166,167], represents a subset of persons who have a normal weight and normal BMI but have a cluster of metabolic characteristics that may increase the possibility of developing the MS.
MONW women, also defined as “metabolically unhealthy normal weight” (MUNW) have metabolic disturbances typical of obese persons and are characterized by having a high amount of visceral fat, a low BMI, a high fat mass, a low lean body mass, low insulin sensitivity, and high triacylglycerol concentrations, premature chronic degenerative disease and liver fat[168-171].
In a recent paper Eckel et al[172] found that, MUNW individuals were characterized by known diabetes risk factors, e.g., they were significantly more likely to be male, former smokers, hypertensive, and less physically active compared to normal weight individuals without incident diabetes. Higher WC (women: 75.5 cm vs 73.1 cm; men: 88.0 cm vs 85.1 cm), higher HbA1c (6.1% vs 5.3%), higher triglycerides (1.47 mmol/L vs 1.11 mmol/L), and higher levels of high sensitive C-reactive protein (0.81 mg/L vs 0.51 mg/L) as well as lower levels of HDL-cholesterol (1.28 mmol/L vs 1.49 mmol/L) and adiponectin (6.32 μg/mL vs 8.25 μg/mL) characterized this phenotype.
Moreover, lipid accumulation product (LAP) and visceral adiposity index, two markers of visceral obesity, identify the MONW phenotype[173].
Lee et al[174] recently found that in MONW individuals, the triglycerides index [(fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2)] was higher respect to normal weight population, managing to clearly discriminate individuals at metabolic risk from NWL subjects.
Thomas et al[175] refined MONW phenotype using MRI, showing a disproportionate deposition of VAT respect to overweight or obese subjects; they called this sub-phenotype, “thin-on-the-outside fat-on-the-inside” (TOFI).
TOFI subjects showed a higher ratio of visceral:subcutaneous abdominal adipose tissue, and it was been observed in both male and female subjects and it was accompanied by increased levels of both liver and muscle fat[175].
Higher concentrations of inflammation biomarkers, TNF-α and IL-6, in the MONW group could be the result of the larger visceral fat areas in this group, supporting the correlation between visceral fat and both insulin resistance and CVD[176].
However, in different population, CRP levels were significantly higher in MONW women than in the control group, and serum IL-6, IL-18 levels in males and females did not differ in both groups[177].
MHO is a new concept in which an individual may exhibit an obese phenotype in the absence of any metabolic abnormalities[178,179].
MHO persons, despite having excess of body fat, have a metabolic profile characterized by high insulin sensitivity, a favorable lipid profile, and no sign of hypertension[144].
However, there is no consensus as to how metabolic normality should be defined, so the reported prevalence of MHO ranges from 2% to 50%, depending on the specific criteria used and the population studied[50,180-189].
MHO subjects when compared with obese insulin resistant adults have a healthier metabolic risk profile and higher disposition index (insulin sensitivity X insulin secretion)[171].
According to Karelis et al[190], the selection criteria for MHO individuals were partially based on the National Cholesterol Education Program’s Adult Treatment Panel III report for lipid profiles [triglycerides: < 1.7 mmol/L, total cholesterol: < 5.2 mmol/L, HDL-cholesterol: > 1.3 mmol/L and low-density lipoprotein (LDL)-cholesterol: < 2.6 mmol/L] and from the study of Brochu et al[187] for insulin sensitivity (HOMA < 1.95); when 4 out of 5 criteria are met, the diagnosis of the MHO individual could be made. Intrahepatic triglyceride (IHTG) content and VAT volume were much higher in metabolically abnormal obese subjects than in MHO subjects.
In 2005 Karelis et al[143] indicated that postmenopausal women displaying the MHO phenotype also have a favorable inflammation profile, as shown by lower CRP and α-1 antitrypsin levels compared with insulin-resistant women, suggesting that lower inflammation state could play a role in the protection of this phenotype.
Manu et al[191] found that MHO was similar to NWL group regarding terms of age, fasting glucose and triglyceride levels, but with higher insulin resistance, C-reactive protein levels, LDL cholesterol levels levels and systolic blood pressure, and lower intake of dietary fiber and levels of physical activity.
However, studies with longer follow-up periods (> 15 years) have demonstrated that MHO individuals were at an increased risk of major CVD events as compared to MHNW individuals[192,193].
Examining the natural history of the MHO phenotype it has been showed that over half of subjects progressed to frank MS over a 10 years period[194].
Moreover, Shaharyar et al[195] demonstrated that both MHO individuals and MONW phenotypes were associated with elevated hs-CRP, and hepatic steatosis.
In a recent paper, Achilike et al[196] showed that among subjects classified as MHO at baseline, almost half (47.6%) of them progressed to MUO within the 7.8-year follow-up period. MHO individuals who developed MUO were older and had more adiposity, higher 10-year CHD risk and lower HDL cholesterol than those who remained as MHO or become non-obese. They conclude that, MHO may not be a stable condition, because it confers markedly increased risk of developing multiple metabolic abnormalities in the future.
According to the editorial comment of Puri[197], it must be simply accepted that obesity is a disease, and no level of obesity can be considered healthy. In fact, the studies by Chang et al[198], demonstrated that, on the basis of the coronary artery calcium scores, MHO had a significantly greater prevalence of coronary atherosclerosis than the metabolically-healthy but normal weight subjects. Moreover, Kramer et al[199] observed that compared with NWL subjects, MHO were at significantly greater risk for death and cardiovascular events.
All these data raised serious doubts on the concept of obese people maintaining a benign prognosis and highlight the fact that obesity per se is a genuine disease[197].
Therefore, both MHO and MONW phenotypes, in the same manner of “at risk obese” phenotype, may not be benign and physicians should strive to treat individuals in these subgroups to reverse these conditions.
The MUO, also defined as “at risk” obese subjects are characterized by a BMI ≥ 30 kg/m2, a PBF > 30% and high visceral fat mass, closely linked to the development of the MS, T2DM, and atherosclerotic CVD[200-203].
Although a unifying definition of the MS does not exist[204-206], there is a worldwide agreement about the role of insulin resistance and abdominal obesity as the main pathophysiological mechanisms for the development of metabolic disorders characterizing the MS[207,208].
Hormonal differences after an oral glucose tolerance test may explain the propensity for impaired glucose homeostasis in then “at-risk” obese phenotype[209].
O’Connell et al[210] divided the obese subjects in three class: MHO, MUO and obese with DM. They found that MUO and T2DM obese subjects had an omental adipocyte size greater than MHO, while subcutaneous adipocyte size, was related to metabolic health, and possibly progression from hepatic steatosis to fibrosis. Moreover, they showed that MUO group had an intermediate degree of steatosis (43%) respect to MHO (3%) and obese with T2DM (74%). Finally, they suggested that the size of the individual’s adipocytes is more important than the size of the individual.
MUO individuals showed higher plasma glucose dependent insulinotropic polypeptide, lower post-glucose load glucagone-like-peptide-1, and higher levels at baseline and after glucose load, indicating inappropriate glucagone suppression[211].
Many studies have demonstrated that increased IHTG content (i.e., NAFLD) is a robust marker of metabolic dysfunction in obese people[47,212,213], and the amount of IHTG is directly correlated with the degree of insulin resistance in the liver, skeletal muscle, and adipose tissue[214].
However, not all obese persons develop NAFLD, insulin resistance, and cardiometabolic disease. A subgroup of obese people are those have increased IHTG content are the “Metabolic Abnormal Obese” (MAO)[47].
Roberts et al[215] and others[216-219] have found that, compared with Metabolic Normal Obese (MNO) subjects, MAO have decreased adipose tissue expression of genes involved in glucose uptake and lipogenesis[47].
Moreover, Fabbrini et al[47] demonstrated distinct differences in the response to weight gain in MNO and MAO subjects. In MAO subjects, but not MNO subjects, moderate weight gain exacerbated several metabolic risk factors for CVD, including increased blood pressure, plasma triglyceride levels, VLDL apoB100 concentrations, and VLDL apoB100 secretion rates, and decreased plasma adiponectin concentrations and insulin sensitivity in the liver, skeletal muscle, and adipose tissues. Weight gain also caused a greater absolute, but not relative, increase in intra hepatic triglyceride content in MAO subjects compared with that seen in MNO subjects. Together, these data suggest that increased adipose tissue capacity for lipogenesis helps protect against the adverse metabolic effects of weight gain.
In general, metabolic disorders are associated more strongly with visceral adiposity, rather than with subcutaneous adiposity; also, the anatomic location of VAT means that fatty acids are released directly into the portal circulation and fat accumulation in the liver has been shown to be an important feature of the MS[220,221].
Sarcopenia is defined as a loss of muscle mass leading to muscle weakness, limited mobility, and increased susceptibility to injury; an understanding of the underlying causes of muscle loss is critical for the development of strategies and therapies to preserve muscle mass and function[222].
It is well known that muscle is a type of endocrine organ, and excess fat mass exerts harmful effects on vascular inflammation BMI can miscategorize a significant proportion of subjects who have lower muscle mass content and higher body fat levels as those having a same cardiovascular risk as healthy, non-obese subjects[17].
Moreover, according to PBF cut-off classification, Di Renzo et al[165] found that 14.68% of individuals were affected by sarcopenic obesity (81.25% NWO and 18.75% MHO/at risk obese, respectively), and, according to the population attributable risk (PAR), the sarcopenia incidence could be reduced at least of 20%, by appropriate detection and treatment of obesity.
The combination of sarcopenia and obesity[223,224], defined as sarcopenic obesity, is an important public health associated with functional limitations and increased mortality[225,226].
The concept of sarcopenic obesity was firstly proposed by Roubenoff who suggested how the inflammatory cytokines, produced by adipose tissue, especially visceral fat, can accelerate muscle catabolism and thus contribute to the vicious cycle that initiates and sustains sarcopenic obesity[145].
These cytokines have effects on the brain, liver, and pancreas that drive appetite, carbohydrate and fat metabolism, and energy balance. Thus, an increase in fat mass causes higher cytokines levels, which can affect protein metabolism both directly, via its effect on muscle amino acid balance, and indirectly, via insulin sensitivity.
Schrager et al[227], showed that components of sarcopenic obesity were associated with elevated levels of IL-6, C-reactive protein, IL-1 receptor antagonist, and soluble IL-6 receptor. They suggest that global obesity and, to a greater extent, central obesity directly affect inflammation, which in turn negatively affects muscle strength, contributing to the development and progression of sarcopenic obesity. These results suggest that proinflammatory cytokines may be critical in both the development and progression of sarcopenic obesity.
The key problem for this disease is the diagnosis. For this reason, in order to avoid the risk of sarcopenic obesity, the diagnosis of obesity requires the utilization of various methods, including body composition evaluation, metabolic, functional and a genetic approach[17,228,229].
The assessment of the physical status in association with genotype represents a very important information to evaluate both the health status and quality of life. Moreover, while a number of risk factors and diagnostic methodologies are available, it would be very useful to be able to develop additional predictive tools and risk indexes for this pathology.
Among various methods using to assess body composition, DXA-derived total body fat mass, total body lean mass and ASMMI measures can reflect both the PBF, than muscle mass and muscle strength, providing a reliable measure for assessment of sarcopenia and obesity[15,19,230].
More significant deviations from normal healthy body composition trajectories configure the development of abnormal phenotypes such as high adiposity[41], low muscle mass, or a combination of the two[231].
For this reason, as highlighted by Prado et al[232], there is a need to detect the high adiposity with low muscle mass phenotypes at younger ages in order to carry out timely personalized treatment interventions, in which weigh loss strategies are aimed to increase or preserve muscle mass and reduce fat mass.
For this reason measurement of body composition are fundamental for individual risk stratification.
The success rates of suggested obesity prevention and treatment strategies including lifestyle modification, behavioral therapy, and pharmacotherapy are dissatisfactory and lack efficacy in the management of morbid obesity[233].
Surgery for the treatment of severe obesity is gaining increasing favor, an surgical interventions are currently the most effective evidence-based approach towards clinically significant and sustainable weight loss along with reduction in mortality and obesity-related comorbidities[234,235].
According to the different classification of obesity subtypes, it is important to choose as the selection criteria of the subjects that may be underwent to bariatric surgery both the PBF, the distribution of adipose tissue and the metabolic variables.
Bariatric surgery, included Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric band (LB), duodenal switch, and sleeve gastrectomy[236-238], has proven to be a treatment of choice for morbid obesity[234,239], and it is recommended for patients with BMI above 40 kg/m2 or higher than 35 kg/m2 when associated with comorbidities which include the different components of MS and type 2 diabetes[240].
As previously described, the expansion of adipose tissue lead to inflammation, hypoxia, insulin resistance, limitations on energy storage, net increase in circulating free fatty acids, lipotoxicity, adverse endocrine and immune responses.
For example, in order to reduce serious sleep apnea, immobility, CVD and other medical disorders, obese patients with pathological obesity may undergo surgical treatments interventions, to reduce the adipose tissue[241].
Thus, a reduction in fat cell size and reduction in adipose tissue growth beyond its vascular supply are favorable effects that at least partially explain the observed reduction in inflammatory markers with a reduction in adiposity, occurs with bariatric surgery.
Reversion of the low-grade inflammation and of risk factors seem to occur when a reduction in BMI is achieved and loss of adipose tissue is observed in obese individuals, after LAGB[241,242].
As previously described the different adipose tissue depots may contribute differently to obesity related comorbidities, most studies have focused on VAT and SAT findings from a single abdominal slice, as an appropriate surrogate measure of total VAT and total SAT.
However, Weiss et al[243] reported that the ratio between VAT and SAT remains fairly constant 6 mo following bariatric. In fact, they observed a weight reduction of 47% in male and 42.6% in female subjects, with a reduction of VAT and total SAT of 35% and 32%, respectively, in both sexes.
Weight loss after bariatric surgery is usually associated with an improvement in insulin resistance[244].
Most studies have demonstrated a strong association between VAT and insulin resistance[245]. In one study, reduction in VAT was associated with improvement in oral glucose tolerance even after adjusting for overall weight loss[246], even if it is unclear whether greater VAT is a marker of insulin resistance or plays a causal role[247].
Moreover, insulin sensitivity correlated with generalized and regional adiposity, however, the magnitude of improvement in insulin sensitivity was predicted by the percent decrease in VAT, but not by other changes in body composition[248].
Data from Carroll et al[249] indicated that 6 mo after LGBS there were significant improvements in many cardiovascular and metabolic risk markers, with a major decreasing in insulin resistance associated to VAT reduction. The calculated total VAT volume (by CT) from eight abdominal slices decreased by 22% at 6 mo post-LB surgery, and the reduction in VAT was significantly correlated with reductions in insulin, HOMA, and glucose.
Korner et al[250], using whole-body MRI in postsurgery weight-stable female patients (at 19-25 mo post-LB and RYGB surgery), found that in women post-surgery, VAT was 44% less than in control subjects, and the difference remained significant after adjustment for total adipose tissue (TAT) and menopausal status. After adjustment for TAT, SAT in women post-surgery was significantly greater than matched controls, and there was a significant negative correlation of VAT and the degree of weight loss in women but this relationship was not significant in men.
In fact, VAT was nearly identical in men post-surgery compared with matched controls even though the degree of weight reduction was not significantly different from women (27.4% vs 32.6%).
Interestingly Toro-Ramos et al[251] showed that bariatric surgery caused substantial and robust loss in total and regional adipose tissue at 12 and 24 mo postbariatric surgery, with continued losses between 12 and 24 mo in women, when body weight change was not significant. These results indicate that bariatric surgery has an important effect on reducing adipose tissue depots even after body weight has begun to stabilize. They found a remarkable 77% reduction in VAT at 12 mo postsurgery in both men and women.
Weight loss obtained after bariatric surgery is associated with a highly significant reduction in cardiovascular risk factors[252,253]. Moreover, systematic review and meta-analysis reported complete resolution of T2DM in high percentage of the cases[239], and the rank order of increasing efficacy of the most common surgical procedures progresses from the purely restrictive to the mostly restrictive to the mostly malabsorptive.
Surgical treatment of massive obesity is being extended to adolescents, seemingly with similar success and risk rates as in adults[254].
Pontiroli et al[237] have previous observed that, although LAGB induces a durable weight loss in morbidly obese patients, a significant proportion of these patients (25%) had a relatively modest weight loss.
It is not clear demonstrated whether genetic factors that play an important role in body weight homeostasis may account for the differences in the therapeutic effects of bariatric surgery. However, in literature there are some evidences that reported a relationship between -174G>C IL-6 polymorphism with diabetes, insulin resistance, MS, longevity and cardiovascular risk[255-257].
Di Renzo et al[258] evaluated the efficacy of LAGB surgery and the effects on anthropometry, body composition, fluid distribution, some cardiometabolic parameters and IL-6 plasma levels of selected patients after 6 mo follow-up, according to 174G>C IL-6 polymorphism. The authors have shown that in obesity bariatric treatment, LAGB seemed to determine a weight loss, sparing muscle mass and causing only mild body fluid alterations. The loss of fat mass was significant, despite a slight proportional loss of lean mass. Moreover, for the first time, this study has provided evidence that, the promoter polymorphism of IL-6 (-174G>C) gene is associated both with body composition and fluid distribution, in obese subjects, at baseline and at 6-mo follow-up after LAGB. C (-) obese carrier showed a lower capability to lose weight and body fat mass after LAGB, and a higher LAGB induced detrimental effect on bone density. This implies that LAGB was less effective if the subjects were carrying risk genotypes (C-carriers) for obesity[258].
In conclusion, it is possible suggest that an accurate and complete body composition evaluation together with genetic variations analysis would be a important tools for the selection of the type of the bariatric surgery, and for screening in order to predict therapeutic response of obese subjects, in terms of fat loss. Further studies of the court must be made to understand what is the best type of bariatric surgery, depending on the pathology.
Remains the undoubted value of the assessment of body composition in obese patient whose treatment of bariatric surgery is recommended, in the control of quality and effectiveness of the intervention, ensuring patient safety.
It is crucial, therefore, to recommend a bariatric surgery independently from BMI and body size, but taking into account adiposopathy and sick fat that occurs with the expansion of body fat mass, changing the inflammatory and metabolic profile of the patient.
Interactions between genetic and environmental factors such as diet and lifestyle, particularly over-nutrition and sedentary behavior, promote the progression and pathogenesis of polygenic diet-related diseases.
In the different obese phenotype, it is of primary importance to highlight a potential connection between body composition (as weight, body lean mass, body fat mass), and genetic variants, to identify individuals, who were at increased risk of reduction of skeletal muscle mass, that could lead to sarcopenic obesity.
The highlighted findings underline that it is critical to assess body composition evaluation, looking to body fat mass and lean mass, and to identify a useful biomarker for selecting the population at risk for adipose tissue-associated inflammation, for preventive medicine purposes.
In this context, the mounting influx of global quantitative data from body composition, blood biomarkers, and genetic led to change the healthcare system, transforming of the concept of medicine, intended not only as a curative intervention but as a proactive P4 medicine, that is predictive, preventive, personalized, and participatory[259].
| 1. | American Medical Association. Council on Science and Public Health Report, 2013. . |
| 2. | Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253. [PubMed] |
| 3. | Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 426] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 4. | Recognition of Obesity as a Disease. Available from: http://www.npr.org/documents/2013/jun/ama-resolution-besity.pdf. |
| 5. | Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109-117. [PubMed] |
| 6. | Shuldiner AR. Obesity genes and gene-environment-behavior interactions: recommendations for a way forward. Obesity (Silver Spring). 2008;16 Suppl 3:S79-S81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Sesti G, Perego L, Cardellini M, Andreozzi F, Ricasoli C, Vedani P, Guzzi V, Marchi M, Paganelli M, Ferla G. Impact of common polymorphisms in candidate genes for insulin resistance and obesity on weight loss of morbidly obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. J Clin Endocrinol Metab. 2005;90:5064-5069. [PubMed] |
| 8. | Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1011] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 9. | Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J Parenter Enteral Nutr. 2008;32:638-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452. [PubMed] |
| 11. | Poirier P. Adiposity and cardiovascular disease: are we using the right definition of obesity? Eur Heart J. 2007;28:2047-2048. [PubMed] |
| 12. | Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Millán D, Vila N, Ibañez P, Gil MJ, Valentí V. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond). 2012;36:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 439] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 13. | Flegal KM. Commentary: the quest for weight standards. Int J Epidemiol. 2010;39:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Sun Q, van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol. 2010;172:1442-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | De Lorenzo A, Bianchi A, Maroni P, Iannarelli A, Di Daniele N, Iacopino L, Di Renzo L. Adiposity rather than BMI determines metabolic risk. Int J Cardiol. 2013;166:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Franzosi MG. Should we continue to use BMI as a cardiovascular risk factor? Lancet. 2006;368:624-625. [PubMed] |
| 17. | Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond). 2008;32:959-966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1089] [Cited by in RCA: 1046] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 18. | Snitker S. Use of body fatness cutoff points. Mayo Clin Proc. 2010;85:1057; author reply 1057-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 19. | De Lorenzo A, Deurenberg P, Pietrantuono M, Di Daniele N, Cervelli V, Andreoli A. How fat is obese? Acta Diabetol. 2003;40 Suppl 1:S254-S257. [PubMed] |
| 20. | De Lorenzo A, Nardi A, Iacopino L, Domino E, Murdolo G, Gavrila C, Minella D, Scapagnini G, Di Renzo L. A new predictive equation for evaluating women body fat percentage and obesity-related cardiovascular disease risk. J Endocrinol Invest. 2014;37:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 573] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 22. | Li C, Ford ES, Zhao G, Balluz LS, Giles WH. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr. 2009;90:1457-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694-701. [PubMed] |
| 24. | Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S-209S. [PubMed] |
| 25. | Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, Fraterrigo G, Okunade AL, Patterson BW, Klein S. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426-430. [PubMed] |
| 27. | Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45-62. [PubMed] |
| 28. | Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75-79. [PubMed] |
| 29. | Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379-384. [PubMed] |
| 30. | Bombelli M, Facchetti R, Fodri D, Brambilla G, Sega R, Grassi G, Mancia G. Impact of body mass index and waist circumference on the cardiovascular risk and all-cause death in a general population: data from the PAMELA study. Nutr Metab Cardiovasc Dis. 2013;23:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Kanaya AM, Vittinghoff E, Shlipak MG, Resnick HE, Visser M, Grady D, Barrett-Connor E. Association of total and central obesity with mortality in postmenopausal women with coronary heart disease. Am J Epidemiol. 2003;158:1161-1170. [PubMed] |
| 32. | Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843-1848. [PubMed] |
| 33. | Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA. 2010;107:18226-18231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 34. | Veilleux A, Caron-Jobin M, Noël S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60:1504-1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr. 2008;87:56-63. [PubMed] |
| 36. | Bays H, Abate N, Chandalia M. Adiposopathy: sick fat causes high blood sugar, high blood pressure and dyslipidemia. Future Cardiol. 2005;1:39-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Bays H, Ballantyne C. Adiposopathy: why do adiposity and obesity cause metabolic disease? Future Lipidol. 2006;1:389-420. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Bays H. Adiposopathy, “sick fat,” Ockham’s razor, and resolution of the obesity paradox. Curr Atheroscler Rep. 2014;16:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Bays HE, González-Campoy JM, Henry RR, Bergman DA, Kitabchi AE, Schorr AB, Rodbard HW. Is adiposopathy (sick fat) an endocrine disease? Int J Clin Pract. 2008;62:1474-1483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Bays HE, González-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 41. | Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583-2589. [PubMed] |
| 42. | Kushner RF, Roth JL. Assessment of the obese patient. Endocrinol Metab Clin North Am. 2003;32:915-933. [PubMed] |
| 43. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [PubMed] |
| 44. | Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013;5:2019-2027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 45. | Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 46. | Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1063] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 47. | Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430-15435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 763] [Article Influence: 44.9] [Reference Citation Analysis (6)] |
| 48. | Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1425] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 49. | Sam S, Mazzone T. Adipose tissue changes in obesity and the impact on metabolic function. Transl Res. 2014;164:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008;168:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1178] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 51. | Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 791] [Article Influence: 43.9] [Reference Citation Analysis (1)] |
| 52. | Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 53. | Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145-1158. [PubMed] |
| 54. | Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548-2556. [PubMed] |
| 55. | Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911-99; quiz 920. [PubMed] |
| 56. | Anderson EK, Gutierrez DA, Hasty AH. Adipose tissue recruitment of leukocytes. Curr Opin Lipidol. 2010;21:172-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (3)] |
| 58. | Perreault M, Roke K, Badawi A, Nielsen DE, Abdelmagid SA, El-Sohemy A, Ma DW, Mutch DM. Plasma levels of 14: 0, 16: 0, 16: 1n-7, and 20: 3n-6 are positively associated, but 18: 0 and 18: 2n-6 are inversely associated with markers of inflammation in young healthy adults. Lipids. 2014;49:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | de Jong AJ, Kloppenburg M, Toes RE, Ioan-Facsinay A. Fatty acids, lipid mediators, and T-cell function. Front Immunol. 2014;5:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Carrera Boada CA, Martínez-Moreno JM. Pathophysiology of diabetes mellitus type 2: beyond the duo “insulin resistance-secretion deficit”. Nutr Hosp. 2013;28 Suppl 2:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 61. | Ross R, Shaw KD, Martel Y, de Guise J, Avruch L. Adipose tissue distribution measured by magnetic resonance imaging in obese women. Am J Clin Nutr. 1993;57:470-475. [PubMed] |
| 62. | Campra JL, Reynolds TB. The hepatic circulation. The liver: biology and pathobiology. New York: Raven Press 1982; 627-645. |
| 63. | Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7:e33308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 380] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 64. | Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res. 2012;2012:629637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 65. | Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29-35. [PubMed] |
| 66. | Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2276-2283. [PubMed] |
| 67. | Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594-2599. [PubMed] |
| 68. | Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460-2466. [PubMed] |
| 69. | Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817-1820. [PubMed] |
| 70. | Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes. 2004;53:2735-2740. [PubMed] |
| 72. | Li G, Klein RL, Matheny M, King MA, Meyer EM, Scarpace PJ. Induction of uncoupling protein 1 by central interleukin-6 gene delivery is dependent on sympathetic innervation of brown adipose tissue and underlies one mechanism of body weight reduction in rats. Neuroscience. 2002;115:879-889. [PubMed] |
| 73. | Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506-512. [PubMed] |
| 74. | Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084-2089. [PubMed] |
| 75. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [PubMed] |
| 76. | Hauner H, Petruschke T, Russ M, Röhrig K, Eckel J. Effects of tumour necrosis factor alpha (TNF alpha) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture. Diabetologia. 1995;38:764-771. [PubMed] |
| 77. | Dalziel B, Gosby AK, Richman RM, Bryson JM, Caterson ID. Association of the TNF-alpha -308 G/A promoter polymorphism with insulin resistance in obesity. Obes Res. 2002;10:401-407. [PubMed] |
| 78. | Meadows KA, Holly JM, Stewart CE. Tumor necrosis factor-alpha-induced apoptosis is associated with suppression of insulin-like growth factor binding protein-5 secretion in differentiating murine skeletal myoblasts. J Cell Physiol. 2000;183:330-337. [PubMed] |
| 79. | Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152-2157. [PubMed] |
| 80. | Argilés JM, López-Soriano J, Almendro V, Busquets S, López-Soriano FJ. Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med Res Rev. 2005;25:49-65. [PubMed] |
| 81. | Dahlman I, Arner P. Obesity and polymorphisms in genes regulating human adipose tissue. Int J Obes (Lond). 2007;31:1629-1641. [PubMed] |
| 82. | Fontaine-Bisson B, Wolever TM, Chiasson JL, Rabasa-Lhoret R, Maheux P, Josse RG, Leiter LA, Rodger NW, Ryan EA, El-Sohemy A. Tumor necrosis factor alpha -238G& gt; A genotype alters postprandial plasma levels of free fatty acids in obese individuals with type 2 diabetes mellitus. Metabolism. 2007;56:649-655. [PubMed] |
| 83. | Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391-399. [PubMed] |
| 84. | Brand E, Schorr U, Kunz I, Kertmen E, Ringel J, Distler A, Sharma AM. Tumor necrosis factor-alpha--308 G/A polymorphism in obese Caucasians. Int J Obes Relat Metab Disord. 2001;25:581-585. [PubMed] |
| 85. | Kubaszek A, Pihlajamäki J, Komarovski V, Lindi V, Lindström J, Eriksson J, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S. Promoter polymorphisms of the TNF-alpha (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes. 2003;52:1872-1876. [PubMed] |
| 86. | Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA. Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab. 2001;86:783-791. [PubMed] |
| 87. | Um JY, Lee KM, Kim HM. Polymorphism of interleukin-1 receptor antagonist gene and obesity. Clin Chim Acta. 2004;340:173-177. [PubMed] |
| 88. | Griffin WS, Nicoll JA, Grimaldi LM, Sheng JG, Mrak RE. The pervasiveness of interleukin-1 in alzheimer pathogenesis: a role for specific polymorphisms in disease risk. Exp Gerontol. 2000;35:481-487. [PubMed] |
| 89. | Juge-Aubry CE, Somm E, Giusti V, Pernin A, Chicheportiche R, Verdumo C, Rohner-Jeanrenaud F, Burger D, Dayer JM, Meier CA. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes. 2003;52:1104-1110. [PubMed] |
| 90. | Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303-312. [PubMed] |
| 91. | Jungo F, Dayer JM, Modoux C, Hyka N, Burger D. IFN-beta inhibits the ability of T lymphocytes to induce TNF-alpha and IL-1beta production in monocytes upon direct cell-cell contact. Cytokine. 2001;14:272-282. [PubMed] |
| 92. | Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985;28:119-121. [PubMed] |
| 93. | Bensellam M, Laybutt DR, Jonas JC. The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol Cell Endocrinol. 2012;364:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 94. | Cernea S, Dobreanu M. Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochem Med (Zagreb). 2013;23:266-280. [PubMed] |
| 95. | Chae H, Gilon P. Can Tea Extracts Exert a Protective Effect Against Diabetes by Reducing Oxidative Stress and Decreasing Glucotoxicity in Pancreatic β-Cells? Diabetes Metab J. 2015;39:27-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 96. | Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53 Suppl 1:S119-S124. [PubMed] |
| 97. | Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802-1812. [PubMed] |
| 98. | Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42-61. [PubMed] |
| 99. | Roma LP, Duprez J, Takahashi HK, Gilon P, Wiederkehr A, Jonas JC. Dynamic measurements of mitochondrial hydrogen peroxide concentration and glutathione redox state in rat pancreatic β-cells using ratiometric fluorescent proteins: confounding effects of pH with HyPer but not roGFP1. Biochem J. 2012;441:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Ma Z, Wirström T, Borg LA, Larsson-Nyrén G, Hals I, Bondo-Hansen J, Grill V, Björklund A. Diabetes reduces β-cell mitochondria and induces distinct morphological abnormalities, which are reproducible by high glucose in vitro with attendant dysfunction. Islets. 2012;4:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Herchuelz A, Nguidjoe E, Jiang L, Pachera N. β-Cell preservation and regeneration in diabetes by modulation of β-cell Ca²⁺ homeostasis. Diabetes Obes Metab. 2012;14 Suppl 3:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 2172] [Article Influence: 135.8] [Reference Citation Analysis (1)] |
| 103. | Suganami T, Tanaka M, Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr J. 2012;59:849-857. [PubMed] |
| 104. | Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281-287. [PubMed] |
| 105. | Hallgren B, Stenhagen S, Svanborg A, Svennerholm L. Gas chromatographic analysis of the fatty acid composition of the plasma lipids in normal and diabetic subjects. J Clin Invest. 1960;39:1424-1434. [PubMed] |
| 106. | Laakso M, Voutilainen E, Sarlund H, Aro A, Pyörälä K, Penttilä I. Serum lipids and lipoproteins in middle-aged non-insulin-dependent diabetics. Atherosclerosis. 1985;56:271-281. [PubMed] |
| 107. | Zuniga-Guajardo S, Zinman B. The metabolic response to the euglycemic insulin clamp in type I diabetes and normal humans. Metabolism. 1985;34:926-930. [PubMed] |
| 108. | Campbell PJ, Carlson MG, Nurjhan N. Fat metabolism in human obesity. Am J Physiol. 1994;266:E600-E605. [PubMed] |
| 109. | Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37:635-46, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 653] [Cited by in RCA: 646] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 110. | Björntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover rate in obesity. Acta Med Scand. 1969;185:351-356. [PubMed] |
| 111. | OPIE LH, WALFISH PG. Plasma free fatty acid concentrations in obesity. N Engl J Med. 1963;268:757-760. [PubMed] |
| 112. | Zurier RB, Rossetti RG, Seiler CM, Laposata M. Human peripheral blood T lymphocyte proliferation after activation of the T cell receptor: effects of unsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 1999;60:371-375. [PubMed] |
| 113. | Lima TM, Kanunfre CC, Pompéia C, Verlengia R, Curi R. Ranking the toxicity of fatty acids on Jurkat and Raji cells by flow cytometric analysis. Toxicol In Vitro. 2002;16:741-747. [PubMed] |
| 114. | Cury-Boaventura MF, Pompéia C, Curi R. Comparative toxicity of oleic acid and linoleic acid on Jurkat cells. Clin Nutr. 2004;23:721-732. [PubMed] |
| 115. | Cury-Boaventura MF, Gorjão R, de Lima TM, Newsholme P, Curi R. Comparative toxicity of oleic and linoleic acid on human lymphocytes. Life Sci. 2006;78:1448-1456. [PubMed] |
| 116. | Gorjão R, Cury-Boaventura MF, de Lima TM, Curi R. Regulation of human lymphocyte proliferation by fatty acids. Cell Biochem Funct. 2007;25:305-315. [PubMed] |
| 117. | RE: Complications of whole bladder dihematoporphyrin ether photodynamic therapy. J Urol. 1990;144:750-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1791] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 119. | Pond CM. An evolutionary and functional view of mammalian adipose tissue. Proc Nutr Soc. 1992;51:367-377. [PubMed] |
| 120. | Krotkiewski M, Björntorp P, Sjöström L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150-1162. [PubMed] |
| 121. | Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351-1361. [PubMed] |
| 122. | Vague J. La différenciation sexuelle; facteur déterminant des formes de l’obésité. Presse Med. 1947;55:339. [PubMed] |
| 123. | Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20-34. [PubMed] |
| 124. | Kissebah AH, Peiris AN. Biology of regional body fat distribution: relationship to non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1989;5:83-109. [PubMed] |
| 125. | Després JP, Lemieux S, Lamarche B, Prud’homme D, Moorjani S, Brun LD, Gagné C, Lupien PJ. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord. 1995;19 Suppl 1:S76-S86. [PubMed] |
| 126. | Banerji MA, Chaiken RL, Gordon D, Kral JG, Lebovitz HE. Does intra-abdominal adipose tissue in black men determine whether NIDDM is insulin-resistant or insulin-sensitive? Diabetes. 1995;44:141-146. [PubMed] |
| 127. | Albu JB, Kovera AJ, Johnson JA. Fat distribution and health in obesity. Ann N Y Acad Sci. 2000;904:491-501. [PubMed] |
| 128. | Grundy SM, Adams-Huet B, Vega GL. Variable contributions of fat content and distribution to metabolic syndrome risk factors. Metab Syndr Relat Disord. 2008;6:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 129. | Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010;21:345-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 130. | Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Ravussin E, Ryan DH, Bouchard C. Ethnic-specific BMI and waist circumference thresholds. Obesity (Silver Spring). 2011;19:1272-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 131. | Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr. 2007;86:353-359. [PubMed] |
| 132. | Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164-1171. [PubMed] |
| 133. | McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382-386. [PubMed] |
| 134. | Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141-146. [PubMed] |
| 135. | Després JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20:1932-1938. [PubMed] |
| 136. | Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Ravussin E, Ryan DH, Smith SR, Bouchard C. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 137. | Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119-1124. [PubMed] |
| 138. | Kadowaki T, Sekikawa A, Murata K, Maegawa H, Takamiya T, Okamura T, El-Saed A, Miyamatsu N, Edmundowicz D, Kita Y. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond). 2006;30:1163-1165. [PubMed] |
| 139. | Misra A, Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metab Syndr Relat Disord. 2009;7:497-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 140. | Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220-225. [PubMed] |
| 141. | De Lorenzo A, Martinoli R, Vaia F, Di Renzo L. Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. Nutr Metab Cardiovasc Dis. 2006;16:513-523. [PubMed] |
| 142. | Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569-2575. [PubMed] |
| 143. | Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud’homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145-4150. [PubMed] |
| 144. | Seo MH, Rhee EJ. Metabolic and cardiovascular implications of a metabolically healthy obesity phenotype. Endocrinol Metab (Seoul). 2014;29:427-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 145. | Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553-557. [PubMed] |
| 146. | Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 147. | De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: early inflammation? Am J Clin Nutr. 2007;85:40-45. [PubMed] |
| 148. | Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ Cardiovasc Imaging. 2012;5:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 149. | Marques-Vidal P, Pécoud A, Hayoz D, Paccaud F, Mooser V, Waeber G, Vollenweider P. Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis. 2010;20:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 150. | Di Renzo L, Del Gobbo V, Bigioni M, Premrov MG, Cianci R, De Lorenzo A. Body composition analyses in normal weight obese women. Eur Rev Med Pharmacol Sci. 2006;10:191-196. [PubMed] |
| 151. | Marques-Vidal P, Pécoud A, Hayoz D, Paccaud F, Mooser V, Waeber G, Vollenweider P. Prevalence of normal weight obesity in Switzerland: effect of various definitions. Eur J Nutr. 2008;47:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 152. | Marques-Vidal P, Chiolero A. Paccaud F. Large differences in the prevalence of normal weight obesity using various cut-offs for excess body fat. E Spen Eur E J Clin Nutr Metab. 2008;3:e159-e162. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Kyle UG, Genton L, Slosman DO, Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition. 2001;17:534-541. [PubMed] |
| 154. | Kang S, Kyung C, Park JS, Kim S, Lee SP, Kim MK, Kim HK, Kim KR, Jeon TJ, Ahn CW. Subclinical vascular inflammation in subjects with normal weight obesity and its association with body fat: an 18 F-FDG-PET/CT study. Cardiovasc Diabetol. 2014;13:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 155. | Madeira FB, Silva AA, Veloso HF, Goldani MZ, Kac G, Cardoso VC, Bettiol H, Barbieri MA. Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PLoS One. 2013;8:e60673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 156. | Kim JY, Han SH, Yang BM. Implication of high-body-fat percentage on cardiometabolic risk in middle-aged, healthy, normal-weight adults. Obesity (Silver Spring). 2013;21:1571-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 157. | Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 463] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 158. | Jean N, Somers VK, Sochor O, Medina-Inojosa J, Llano EM, Lopez-Jimenez F. Normal-weight obesity: implications for cardiovascular health. Curr Atheroscler Rep. 2014;16:464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 159. | Di Renzo L, Galvano F, Orlandi C, Bianchi A, Di Giacomo C, La Fauci L, Acquaviva R, De Lorenzo A. Oxidative stress in normal-weight obese syndrome. Obesity (Silver Spring). 2010;18:2125-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 160. | Di Renzo L, Bigioni M, Del Gobbo V, Premrov MG, Barbini U, Di Lorenzo N, De Lorenzo A. Interleukin-1 (IL-1) receptor antagonist gene polymorphism in normal weight obese syndrome: relationship to body composition and IL-1 alpha and beta plasma levels. Pharmacol Res. 2007;55:131-138. [PubMed] |
| 161. | Di Renzo L, Bertoli A, Bigioni M, Del Gobbo V, Premrov MG, Calabrese V, Di Daniele N, De Lorenzo A. Body composition and -174G/C interleukin-6 promoter gene polymorphism: association with progression of insulin resistance in normal weight obese syndrome. Curr Pharm Des. 2008;14:2699-2706. [PubMed] |
| 162. | Di Renzo L, Bigioni M, Bottini FG, Del Gobbo V, Premrov MG, Cianci R, De Lorenzo A. Normal Weight Obese syndrome: role of single nucleotide polymorphism of IL-1 5Ralpha and MTHFR 677C--& gt; T genes in the relationship between body composition and resting metabolic rate. Eur Rev Med Pharmacol Sci. 2006;10:235-245. [PubMed] |
| 163. | Di Renzo L, Gloria-Bottini F, Saccucci P, Bigioni M, Abenavoli L, Gasbarrini G, De Lorenzo A. Role of interleukin-15 receptor alpha polymorphisms in normal weight obese syndrome. Int J Immunopathol Pharmacol. 2009;22:105-113. [PubMed] |
| 164. | Di Renzo L, Sarlo F, Petramala L, Iacopino L, Monteleone G, Colica C, De Lorenzo A. Association between -308 G/A TNF-α polymorphism and appendicular skeletal muscle mass index as a marker of sarcopenia in normal weight obese syndrome. Dis Markers. 2013;35:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 165. | Di Renzo L, Gratteri S, Sarlo F, Cabibbo A, Colica C, De Lorenzo A. Individually tailored screening of susceptibility to sarcopenia using p53 codon 72 polymorphism, phenotypes, and conventional risk factors. Dis Markers. 2014;2014:743634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 166. | Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutr. 1981;34:1617-1621. [PubMed] |
| 167. | Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699-713. [PubMed] |
| 168. | Dvorak RV, DeNino WF, Ades PA, Poehlman ET. Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes. 1999;48:2210-2214. [PubMed] |
| 169. | Conus F, Allison DB, Rabasa-Lhoret R, St-Onge M, St-Pierre DH, Tremblay-Lebeau A, Poehlman ET. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. J Clin Endocrinol Metab. 2004;89:5013-5020. [PubMed] |
| 170. | Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D’Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906-2912. [PubMed] |
| 171. | Succurro E, Marini MA, Frontoni S, Hribal ML, Andreozzi F, Lauro R, Perticone F, Sesti G. Insulin secretion in metabolically obese, but normal weight, and in metabolically healthy but obese individuals. Obesity (Silver Spring). 2008;16:1881-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 172. | Eckel N, Mühlenbruch K, Meidtner K, Boeing H, Stefan N, Schulze MB. Characterization of metabolically unhealthy normal-weight individuals: Risk factors and their associations with type 2 diabetes. Metabolism. 2015;64:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 173. | Du T, Yu X, Zhang J, Sun X. Lipid accumulation product and visceral adiposity index are effective markers for identifying the metabolically obese normal-weight phenotype. Acta Diabetol. 2015;52:855-863. [PubMed] |
| 174. | Lee SH, Han K, Yang HK, Kim MK, Yoon KH, Kwon HS, Park YM. Identifying subgroups of obesity using the product of triglycerides and glucose: the Korea National Health and Nutrition Examination Survey, 2008-2010. Clin Endocrinol (Oxf). 2015;82:213-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 175. | Thomas EL, Frost G, Taylor-Robinson SD, Bell JD. Excess body fat in obese and normal-weight subjects. Nutr Res Rev. 2012;25:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 176. | Esposito K, Giugliano D. The metabolic syndrome and inflammation: association or causation? Nutr Metab Cardiovasc Dis. 2004;14:228-232. [PubMed] |
| 177. | Bednarek-Tupikowska G, Zdrojowy-Wełna A, Stachowska B, Kuliczkowska-Płaksej J, Matczak-Giemza M, Kubicka E, Tworowska-Bardzińska U, Milewicz A, Bolanowski M. Accumulation of abdominal fat in relation to selected proinflammatory cytokines concentrations in non-obese Wrocław inhabitants. Endokrynol Pol. 2014;65:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 178. | Bonora E, Willeit J, Kiechl S, Oberhollenzer F, Egger G, Bonadonna R, Muggeo M. U-shaped and J-shaped relationships between serum insulin and coronary heart disease in the general population. The Bruneck Study. Diabetes Care. 1998;21:221-230. [PubMed] |
| 179. | Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499-1504. [PubMed] |
| 180. | van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, Gaye A, Gögele M, Heier M, Hiekkalinna T. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 414] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 181. | Yoo HK, Choi EY, Park EW, Cheong YS, Bae RA. Comparison of Metabolic Characteristics of Metabolically Healthy but Obese (MHO) Middle-Aged Men According to Different Criteria. Korean J Fam Med. 2013;34:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 182. | Singh-Manoux A, Czernichow S, Elbaz A, Dugravot A, Sabia S, Hagger-Johnson G, Kaffashian S, Zins M, Brunner EJ, Nabi H. Obesity phenotypes in midlife and cognition in early old age: the Whitehall II cohort study. Neurology. 2012;79:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 183. | Pajunen P, Kotronen A, Korpi-Hyövälti E, Keinänen-Kiukaanniemi S, Oksa H, Niskanen L, Saaristo T, Saltevo JT, Sundvall J, Vanhala M. Metabolically healthy and unhealthy obesity phenotypes in the general population: the FIN-D2D Survey. BMC Public Health. 2011;11:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 184. | Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, Mannino S, Crosignani P, Bosi E, Luzi L. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34:210-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 185. | Lopez-Garcia E, Guallar-Castillon P, Leon-Muñoz L, Rodriguez-Artalejo F. Prevalence and determinants of metabolically healthy obesity in Spain. Atherosclerosis. 2013;231:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 186. | Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97:2482-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 431] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 187. | Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, Poehlman ET. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020-1025. [PubMed] |
| 188. | Durward CM, Hartman TJ, Nickols-Richardson SM. All-cause mortality risk of metabolically healthy obese individuals in NHANES III. J Obes. 2012;2012:460321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 189. | Shea JL, Randell EW, Sun G. The prevalence of metabolically healthy obese subjects defined by BMI and dual-energy X-ray absorptiometry. Obesity (Silver Spring). 2011;19:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 190. | Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30:569-572. [PubMed] |
| 191. | Manu P, Ionescu-Tirgoviste C, Tsang J, Napolitano BA, Lesser ML, Correll CU. Dysmetabolic signals in “metabolically healthy” obesity. Obes Res Clin Pract. 2012;6:e1-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 192. | Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 450] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 193. | Flint AJ, Hu FB, Glynn RJ, Caspard H, Manson JE, Willett WC, Rimm EB. Excess weight and the risk of incident coronary heart disease among men and women. Obesity (Silver Spring). 2010;18:377-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 194. | Eshtiaghi R, Keihani S, Hosseinpanah F, Barzin M, Azizi F. Natural course of metabolically healthy abdominal obese adults after 10 years of follow-up: the Tehran Lipid and Glucose Study. Int J Obes (Lond). 2015;39:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 195. | Shaharyar S, Roberson LL, Jamal O, Younus A, Blaha MJ, Ali SS, Zide K, Agatston AA, Blumenthal RS, Conceição RD. Obesity and metabolic phenotypes (metabolically healthy and unhealthy variants) are significantly associated with prevalenceof elevated C-reactive protein and hepatic steatosis in a large healthy brazilian population. J Obes. 2015;2015:178526. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 196. | Achilike I, Hazuda HP, Fowler SP, Aung K, Lorenzo C. Predicting the development of the metabolically healthy obese phenotype. Int J Obes (Lond). 2015;39:228-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 197. | Puri R. Is it finally time to dispel the concept of metabolically-healthy obesity? J Am Coll Cardiol. 2014;63:2687-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 198. | Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung HS, Choi Y, Ahn J. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 199. | Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159:758-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 721] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 200. | Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 1020] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 201. | Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059-1062. [PubMed] |
| 202. | Di Daniele N, Petramala L, Di Renzo L, Sarlo F, Della Rocca DG, Rizzo M, Fondacaro V, Iacopino L, Pepine CJ, De Lorenzo A. Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean Diet in obese patients with metabolic syndrome. Acta Diabetol. 2013;50:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 203. | Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 1001] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 204. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [PubMed] |
| 205. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [PubMed] |
| 206. | Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [PubMed] |
| 207. | Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 1986;124:903-915. [PubMed] |
| 208. | Wycherley TP, Brinkworth GD, Clifton PM, Noakes M. Comparison of the effects of 52 weeks weight loss with either a high-protein or high-carbohydrate diet on body composition and cardiometabolic risk factors in overweight and obese males. Nutr Diabetes. 2012;2:e40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 209. | Teixeira TF, Alves RD, Moreira AP, Peluzio Mdo C. Main characteristics of metabolically obese normal weight and metabolically healthy obese phenotypes. Nutr Rev. 2015;73:175-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 210. | O’Connell J, Lynch L, Cawood TJ, Kwasnik A, Nolan N, Geoghegan J, McCormick A, O’Farrelly C, O’Shea D. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS One. 2010;5:e9997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 211. | Calanna S, Piro S, Di Pino A, Maria Zagami R, Urbano F, Purrello F, Maria Rabuazzo A. Beta and alpha cell function in metabolically healthy but obese subjects: relationship with entero-insular axis. Obesity (Silver Spring). 2013;21:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 212. | Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 444] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 213. | Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183-1192. [PubMed] |
| 214. | Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 465] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 215. | Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, Micklem KJ, Frayn KN. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 216. | Herman MA, Peroni OD, Villoria J, Schön MR, Abumrad NA, Blüher M, Klein S, Kahn BB. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 486] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 217. | Hoffstedt J, Förster D, Löfgren P. Impaired subcutaneous adipocyte lipogenesis is associated with systemic insulin resistance and increased apolipoprotein B/AI ratio in men and women. J Intern Med. 2007;262:131-139. [PubMed] |
| 218. | Kursawe R, Eszlinger M, Narayan D, Liu T, Bazuine M, Cali AM, D’Adamo E, Shaw M, Pierpont B, Shulman GI. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes. 2010;59:2288-2296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 219. | Graham TE, Kahn BB. Tissue-specific alterations of glucose transport and molecular mechanisms of intertissue communication in obesity and type 2 diabetes. Horm Metab Res. 2007;39:717-721. [PubMed] |
| 220. | Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3490-3497. [PubMed] |
| 221. | Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75:721-728. [PubMed] |
| 222. | Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 481] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 223. | Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013;2013:204164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 224. | Frühbeck G. The Sir David Cuthbertson Medal Lecture. Hunting for new pieces to the complex puzzle of obesity. Proc Nutr Soc. 2006;65:329-347. [PubMed] |
| 225. | Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Pérusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring). 2006;14:529-644. [PubMed] |
| 226. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8782] [Article Influence: 548.9] [Reference Citation Analysis (4)] |
| 227. | Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985). 2007;102:919-925. [PubMed] |
| 228. | Silva AO, Karnikowski MG, Funghetto SS, Stival MM, Lima RM, de Souza JC, Navalta JW, Prestes J. Association of body composition with sarcopenic obesity in elderly women. Int J Gen Med. 2013;6:25-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 229. | De Lorenzo A, Di Renzo L, Puja A, Saccucci P, Gloria-Bottini F, Bottini E. A study of acid phosphatase locus 1 in women with high fat content and normal body mass index. Metabolism. 2009;58:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 230. | Hsu FC, Lenchik L, Nicklas BJ, Lohman K, Register TC, Mychaleckyj J, Langefeld CD, Freedman BI, Bowden DW, Carr JJ. Heritability of body composition measured by DXA in the diabetes heart study. Obes Res. 2005;13:312-319. [PubMed] |
| 231. | Prado CM, Siervo M, Mire E, Heymsfield SB, Stephan BC, Broyles S, Smith SR, Wells JC, Katzmarzyk PT. A population-based approach to define body-composition phenotypes. Am J Clin Nutr. 2014;99:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 232. | Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38:940-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 428] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 233. | Kral JG, Kava RA, Catalano PM, Moore BJ. Severe obesity: the neglected epidemic. Obes Facts. 2012;5:254-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 234. | Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-752. [PubMed] |
| 235. | Mitka M. Bariatric surgery continues to show benefits for patients with diabetes. JAMA. 2012;307:1901-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 236. | Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132:2253-2271. [PubMed] |
| 237. | Pontiroli AE, Pizzocri P, Librenti MC, Vedani P, Marchi M, Cucchi E, Orena C, Paganelli M, Giacomelli M, Ferla G. Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three-year study. J Clin Endocrinol Metab. 2002;87:3555-3561. [PubMed] |
| 238. | Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3-60 month follow-up. Obes Surg. 2000;10:233-239. [PubMed] |
| 239. | Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753-761. [PubMed] |
| 240. | Dixon JB, Zimmet P, Alberti KG, Rubino F. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med. 2011;28:628-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 241. | Ramalho R, Guimarães C. [The role of adipose tissue and macrophages in chronic inflammation associated with obesity: clinical implications]. Acta Med Port. 2008;21:489-496. [PubMed] |
| 242. | Ramalho R, Guimarães C, Gil C, Neves C, Guimarães JT, Delgado L. Morbid obesity and inflammation: a prospective study after adjustable gastric banding surgery. Obes Surg. 2009;19:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 243. | Weiss R, Appelbaum L, Schweiger C, Matot I, Constantini N, Idan A, Shussman N, Sosna J, Keidar A. Short-term dynamics and metabolic impact of abdominal fat depots after bariatric surgery. Diabetes Care. 2009;32:1910-1915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 244. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [PubMed] |
| 245. | Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab. 2004;89:4206-4210. [PubMed] |
| 246. | Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54-59. [PubMed] |
| 247. | Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005;28:2322-2325. [PubMed] |
| 248. | Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839-847. [PubMed] |
| 249. | Carroll JF, Franks SF, Smith AB, Phelps DR. Visceral adipose tissue loss and insulin resistance 6 months after laparoscopic gastric banding surgery: a preliminary study. Obes Surg. 2009;19:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 250. | Korner J, Punyanitya M, Taveras C, McMahon DJ, Kim HJ, Inabnet W, Bessler M, Gallagher D. Sex differences in visceral adipose tissue post-bariatric surgery compared to matched non-surgical controls. Int J Body Compos Res. 2008;6:93-99. [PubMed] |
| 251. | Toro-Ramos T, Goodpaster BH, Janumala I, Lin S, Strain GW, Thornton JC, Kang P, Courcoulas AP, Pomp A, Gallagher D. Continued loss in visceral and intermuscular adipose tissue in weight-stable women following bariatric surgery. Obesity (Silver Spring). 2015;23:62-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 252. | Benaiges D, Goday A, Ramon JM, Hernandez E, Pera M, Cano JF; Obemar Group. Laparoscopic sleeve gastrectomy and laparoscopic gastric bypass are equally effective for reduction of cardiovascular risk in severely obese patients at one year of follow-up. Surg Obes Relat Dis. 2011;7:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 253. | Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223-4231. [PubMed] |
| 254. | Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, Wolfe LG. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102-107; discussion 107-108. [PubMed] |
| 255. | Vozarova B, Fernández-Real JM, Knowler WC, Gallart L, Hanson RL, Gruber JD, Ricart W, Vendrell J, Richart C, Tataranni PA. The interleukin-6 (-174) G/C promoter polymorphism is associated with type-2 diabetes mellitus in Native Americans and Caucasians. Hum Genet. 2003;112:409-413. [PubMed] |
| 256. | Moleres A, Rendo-Urteaga T, Azcona C, Martínez JA, Gómez-Martínez S, Ruiz JR, Moreno LA, Marcos A, Marti A. Il6 gene promoter polymorphism (-174G/C) influences the association between fat mass and cardiovascular risk factors. J Physiol Biochem. 2009;65:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 257. | Möhlig M, Boeing H, Spranger J, Osterhoff M, Kroke A, Fisher E, Bergmann MM, Ristow M, Hoffmann K, Pfeiffer AF. Body mass index and C-174G interleukin-6 promoter polymorphism interact in predicting type 2 diabetes. J Clin Endocrinol Metab. 2004;89:1885-1890. [PubMed] |
| 258. | Di Renzo L, Carbonelli MG, Bianchi A, Iacopino L, Fiorito R, Di Daniele N, De Lorenzo A. Body composition changes after laparoscopic adjustable gastric banding: what is the role of -174G& gt; C interleukin-6 promoter gene polymorphism in the therapeutic strategy? Int J Obes (Lond). 2012;36:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 259. | Tian Q, Price ND, Hood L. Systems cancer medicine: towards realization of predictive, preventive, personalized and participatory (P4) medicine. J Intern Med. 2012;271:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Boileve JB S- Editor: Yu J L- Editor: A E- Editor: Ma S