Published online Jan 14, 2016. doi: 10.3748/wjg.v22.i2.490
Peer-review started: April 27, 2015
First decision: September 11, 2015
Revised: September 25, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: January 14, 2016
Processing time: 255 Days and 23.3 Hours
Colorectal cancer (CRC) is the second most common cause of cancer-related death in western countries. Approximately one-quarter of newly diagnosed patients for CRC have metastases, and a further 40%-50% experience disease recurrence or develop metastases after all standard therapies. Therefore, understanding the molecular mechanisms involved in the progression of CRC and subsequently developing novel therapeutic targets is crucial to improve management of CRC and patients’ long-term survival. Several tyrosine kinase receptors have been implicated in CRC development, progression and metastasis, including epidermal growth factor receptor (EGFR) and vascular EGFR. Recently, tropomyosin-related kinase B (TrkB), a tyrosine kinase receptor, has been reported in CRC and found to clearly exert several biological and clinical features, such as tumor cell growth and survival in vitro and in vivo, metastasis formation and poor prognosis. Here we review the significance of TrkB and its ligand brain derived-neurotrophic factor in CRC. We focus on their expression in CRC tumor samples, and their functional roles in CRC cell lines and in in vivo models. Finally we discuss therapeutic approaches that can lead to the development of novel therapeutic agents for treating TrkB-expressing CRC tumors.
Core tip: Recently, the tropomyosin-related kinase B (TrkB)/brain derived-neurotrophic factor (BDNF) signaling pathway has emerged as a key player in the pathogenesis and prognosis of several non-neural cancers. Indeed, the TrkB tyrosine kinase receptor has been recently found to play an important role in the biological and clinical behavior of colorectal cancer (CRC). Here, we review the implications of TrkB and its ligand BDNF in CRC. Additionally, we discuss possible therapeutic strategies targeting this pathway.
- Citation: Akil H, Perraud A, Jauberteau MO, Mathonnet M. Tropomyosin-related kinase B/brain derived-neurotrophic factor signaling pathway as a potential therapeutic target for colorectal cancer. World J Gastroenterol 2016; 22(2): 490-500
- URL: https://www.wjgnet.com/1007-9327/full/v22/i2/490.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i2.490
Colorectal cancer (CRC) is the second most common cause of cancer-related death in the western world and the third most common malignancy diagnosed worldwide[1]. With present-day therapies, curative (R0) surgical resection is used in approximately 70%-80% of newly diagnosed patients with localized CRC[2]. However, the overall prognosis remains poor for CRC patients, with a median survival time of approximately 20-24 mo[3-5]. In fact, the remaining 20%-30% of newly diagnosed patients present advanced unresectable metastatic disease, and an additional 40%-50% of patients with initially localized CRC experience disease recurrence despite surgical resection and adjuvant therapy, or develop hepatic and/or lung metastases[5,6].
In recent decades, the development of early detection methods for CRC, together with the evolution of systemic chemotherapy for patients with CRC, has led to a decrease in CRC incidence and mortality worldwide[1]. 5-Fluorouracil (5-FU), discovered in 1957 by Heidelberger et al[7], was for many years, in combination with leucovorin the only treatment for patients with metastatic CRC (mCRC)[8-11]. In the early 2000s, the use of the topoisomerase I inhibitor irinotecan, and subsequently the platinum-containing agent oxaliplatin, were key developments in CRC treatment[5]. These agents were found to significantly prolong the median overall survival (OS) of patients with mCRC from 12.6 mo (5-FU/leucovorin alone as first-line therapy) to 14.8 mo (bolus 5-FU/leucovorin with irinotecan)[12], then from 15 mo (5-FU/leucovorin with irinotecan) to 19.5 mo (FOLFOX; 5-FU/leucovorin with oxaliplatin)[13]. In 2004, Hurwitz et al[14] reported that treatment of CRC patients with bevacizumab (humanized monoclonal antibody against vascular endothelial growth factor) led to a significant increase in OS. Next, in several key trials, monoclonal antibodies to the anti-epidermal growth factor receptor (EGFR), namely cetuximab and panitumumab, were proposed as a novel agents for mCRC treatment. These anti-tyrosine kinase receptor antibodies were the first therapeutic targeted agents in patients with the KRAS wild-type metastatic CRC (mCRC)[5,15,16]. Recently, Regorafenib, the first small-molecule tyrosine kinase inhibitor, was approved as a potential new line of therapy for patients who have progressed after all standard therapies[17].
Expression of the tyrosine kinase receptor TrkB is increasingly reported in CRC, and there is a growing body of evidence that the TrkB/BDNF pathway is an important player in CRC tumor growth and progression. Although mainly recognized for its role in the nervous system, the TrkB/BDNF pathway has been implicated in the pathogenesis and prognosis of several tumors outside of the brain, such as pancreatic[18-20], prostatic[21,22], pulmonary[23,24], ovarian[25], bladder[26] and head and neck cancers[27,28], in addition to choriocarcinoma[29], myeloma[30] and B-lymphocytic leukemia[31,32]. This review will focus on the role of TrkB and its ligand BDNF in promoting CRC progression and survival. In addition, we outline potential therapeutic approaches targeting the TrkB/BDNF pathway.
BDNF is a member of the neurotrophin (NT) family, which is a group of closely related growth factors originally known for their involvement in the differentiation, proliferation, and survival of neural cells in both the central and peripheral nervous systems[33].
The NT family includes NGF (nerve growth factor), BDNF (brain-derived neurotrophic factor), NT-3 (neurotrophin 3) and NT-4/5 (neurotrophin 4/5). In 1982, Barde et al[34] first isolated BDNF from pig brain as a survival factor for cultured embryonic chick sensory neurons. NTs are synthesized via the same process of maturation. Indeed, NTs are generated from single protein precursors of approximately 27 kDa called pre-pro-NTs. The latter is composed of a pre-pro-domain, a pro-domain and a mature domain that are sequentially cleaved to obtain mature NTs of approximately 13 kDa. Immature NTs (pre-pro-NTs) are cleaved in the endoplasmic reticulum to generate pro-NTs[35,36]. Pro-NTs are then proteolytically cleaved to mature NTs. Cleavage can occur either intracellularly, by the action of furin or proconvertase, or extracellularly, by the action of plasmin and matrix metalloproteinases (MMPs) such as MMP-3, MMP-7 or MMP-9[36,37]. Accordingly, pro-NTs can be released as soluble forms or processed as mature NTs, each with specific receptor binding properties and physiological consequences. Generally, immature NTs or pro-NTs bind to p75NTR and sortilin (a member of the VPS10p-domain receptor family) to induce apoptosis via the JNK kinase pathway[38]. In their mature forms, NTs mediate their effects through binding to two structurally unrelated cell membrane receptors, the Trk tyrosine kinase receptors and the common NT receptor p75NTR[39,40]. BDNF effectively signals through the low affinity receptor (p75NTR) that belongs to the tumor necrosis factor (TNF) receptor superfamily and through the high-affinity TrkB tyrosine kinase receptor.
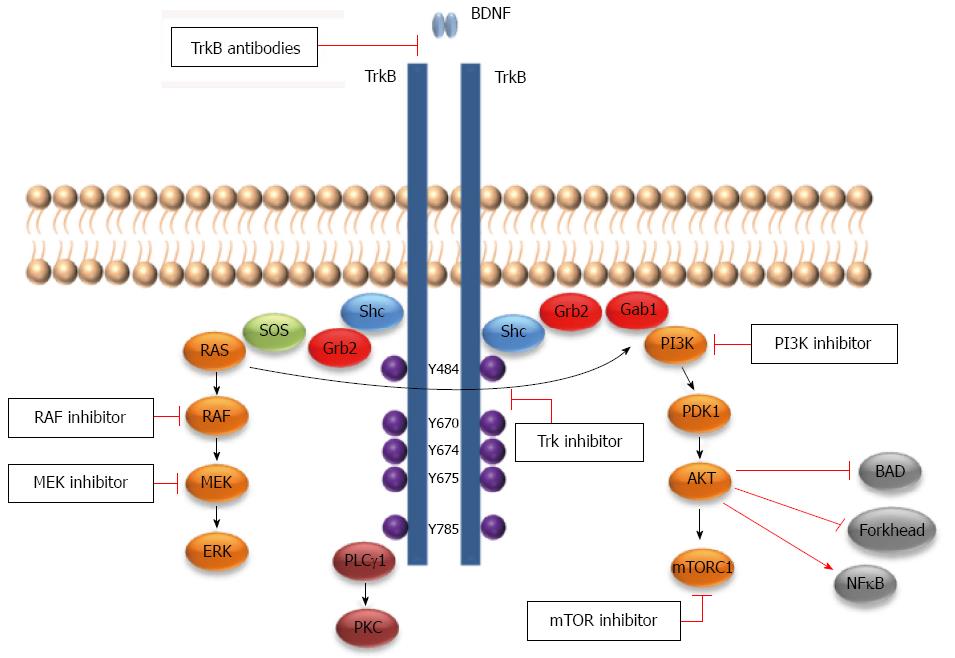
TrkB belongs to the Trk family of tyrosine kinase receptors, which consists of an extracellular ligand-binding domain, a transmembrane domain and a cytoplasmic tyrosine kinase domain[41,42]. The extracellular domain of these receptors consists of a leucine-rich domain surrounded by two cysteine rich regions that are thought to be essential for NTs high affinity binding. Near those regions and close to the cell membrane there are two immunoglobulin-like (IG-like) domains that are followed by the transmembrane domain. The IG-like domain directs ligand-binding specificity. The cytoplasmic domain consists of a Shc binding site, a tyrosine kinase domain and a tail region that includes a phospholipase C-gamma binding site (PLC-γ)[43]. Trk was first described in 1986 by Martin-Zanca et al[44] as an oncogene in colonic carcinoma. The Trk family includes three members, TrkA, TrkB and TrkC, each of which is activated by one or more of the four NTs. NGF preferentially binds to TrkA and BDNF and NT-4/5 preferentially bind to TrkB. NT-3 preferentially binds to TrkC but also to TrkA and TrkB to a lesser extent and only in certain cellular contexts[45-48]. The TrkB gene (NTRK2 about 590 kb) is located on chromosome 9q22 and contains 24 exons[43,49]. The TrkB gene can create more than 100 RNA transcripts (due to alternative splicing) and at least 36 of these transcripts are translated, among which there are three major protein isoforms[43,50]. In addition to the full-length TrkB (TrkB-FL, 145 kDa), there are two alternatively spliced TrkB isoforms (95 kDa) that lack the tyrosine kinase domain, TrkB-T1 and TrkB-Shc, which are slightly longer and include the Shc binding site[50]. BDNF binding to the TrkB-FL (full-length) receptor results in its dimerization and subsequent autophosphorylation of tyrosine (Y) Y484 and Y785. Generally, TrkB-FL induces survival and differentiation through the three principle growth factor signaling pathways: Ras/MAPK, PI3Kinase (PI3K)/Akt and PLC-γ/PKC pathways[42,50] (Figure 1). For an expanded review on Trk signaling, see[45,46].
TrkB-T1 is abundantly expressed in adult neural cells but also in non-neural cells, yet its biological role in BDNF-mediated signaling remains poorly understood[51]. For many years, both truncated TrkB receptor isoforms were though to act as dominant-negative inhibitors of TrkB-FL and did not signal directly[52-54]. For example, TrkB-T1 or TrkB-Shc can bind to and internalize BDNF or dimerize with TrkB-FL to prevent its autophosphorylation and subsequently inhibit intracellular signaling pathways[50,55]. However, recent studies have demonstrated that TrkB-T1 sequesters and translocate BDNF[56], induces filopodia and neurite outgrowth[57-59], regulates Rho GTPase signaling[60] and intracellular signaling cascades[30,61,62], and mediates cell proliferation[63,64]. Rose et al[65] have demonstrated that astrocytes predominantly express TrkB-T1 and that BDNF-mediated activation of the truncated TrkB-T1 controls calcium release from intracellular stores through an inositol-1,45-phosphate (IP3)-dependent pathway. In addition, Li et al[63] have shown that TrkB-T1 enhanced cell proliferation, promoted formation of colonies in soft agar, stimulated tumor cell invasion and induced liver metastasis in an orthotopic xenograft mouse model of pancreatic cancer. For a recent and extensive review on truncated TrkB, refer to Fenner[55].
Overexpression of the TrkB receptor was first described in human neuroblastoma, which is a childhood malignant tumor derived from primitive cells of the developing sympathetic nervous system[66]. TrkB and BDNF are highly expressed in biologically unfavorable, MYCN-amplified, aggressive neuroblastomas[67]. Indeed, patients with neuroblastoma, whose tumors express elevated levels of TrkB and BDNF, have a poor prognosis[67,68]. It has also been shown that in neuroblastoma tumor cells the TrkB/BDNF pathway is involved in epithelial-mesenchymal-transition-like transformation (EMT), metastasis[69,70], anoikis[71], chemotherapeutic resistance[72,73], and hypoxia via activation of angiogenesis[74]. Thus, BDNF/TrkB signaling in neuroblastoma contributes to tumor growth and an unfavorable outcome in an autocrine/paracrine manner[75].
Activity of the BDNF/TrkB pathway is mainly recognized in the field of neurobiology. However there is now increasing recognition that the TrkB/BDNF pathway regulates important processes and may contribute to the pathogenesis of numerous non-neural carcinomas whose cells express the TrkB receptor, including pancreas[18,19], prostate[21,22], ovarian[76,77], Wilms’ tumors, head and neck[27,28], lung[23,78], breast[79,80], gastric[81,82], and hepatocellular carcinomas[83,84], as well as myelomas[30,85,86] and lymphoid cancers[31,32,87]. Indeed, overexpression of TrkB was found in the highly metastatic pancreatic cancer cells. Moreover, TrkB expression was correlated with perineural invasion, positive retroperitoneal margin, and earlier liver metastasis development in patient samples[19]. In prostate cancer, TrkB and BDNF may be considered as possible diagnostic markers[21]. Furthermore, TrkB therapeutic effects were shown in an in vivo xenograft model using Trk-specific inhibitors, such as CEP-751 or CEP-701. In these studies, results have shown that targeting of the TrkB receptor induces cell death in a cell cycle-independent manner and inhibits tumor growth and metastasis development in prostate cancer[88,89]. In Wilms’ tumors, TrkB overexpression correlated with a higher risk of mortality[90]. The role of the TrkB/BDNF pathway in head and neck squamous cell carcinoma (HNSCC) was also investigated by Kupferman et al[27] who demonstrated that these proteins are expressed in greater than 50% of human HNSCC tumors, but not in the normal upper aerodigestive tract. In vitro and in vivo studies have revealed that TrkB is directly implicated in EMT, tumor growth and invasive behavior of HNSCC. Furthermore, BDNF stimulation of HNSCC cells enhances cell migration and invasion, drug-resistance and anti-apoptotic protein expression[27,28]. BDNF was also described as an autocrine factor in human multiple myeloma cells contributing in tumor progression and survival by activating signaling in the mitogen-activated protein kinase (MAPK) pathway and the phosphatidylinositol 3-kinase-Akt (PI3K/Akt) pathway. In addition, BDNF was able to protect human multiple myeloma (MM) cell lines from apoptosis induced by dexamethasone or bortezomib[30]. Finally, TrkB and BDNF were also reported to be associated with a significant increase in the survival and proliferation of non-Hodgkin lymphoma cells[91] and malignant B cell lines[31,32]. For example, Fauchais et al[31] have shown that endogenous BDNF released under stress culture conditions exerts antiapoptotic effects in human B cell lines. Such autocrine production was linked to the presence of sortilin, an endogenous protein that is able to transport and release BDNF in culture media. In diffuse large B cell lymphoma, in vitro studies have shown that the Trk-inhibitor K252a enhances apoptosis and exhibits additive apoptotic effects with rituximab, a chimeric anti-CD20 monoclonal antibody[32].
There is now accumulating evidence that the TrkB/BDNF pathway is involved in CRC tumor growth, progression and metastasis. The first investigation on the effect of TrkB in CRC was published in 2010 by Yu et al[92] who demonstrated that TrkB was up-regulated in tumor tissues compared with the non-tumorous counterparts, and this overexpression correlates with lymphatic vessel density and metastasis. Next, the expression of TrkB was confirmed in samples from CRC patients at both the mRNA and protein levels, with a higher expression in tumor tissues compared to adjacent non-cancerous tissues[93-96]. Thereafter, TrkB expression has been correlated with clinicopathological parameters in CRC[95-99]. These studies have shown that TrkB mRNA expression levels are positively correlated with lymph node metastasis, distant metastasis and CRC tumor stage progression[97]. Moreover, high TrkB mRNA expression was correlated with poor prognosis[97,98]. However, TrkB mRNA expression was not correlated with histological grade of CRC[97]. Interestingly, immunohistochemistry studies have also shown that TrkB was overexpressed in CRC tissues compared with adjacent non-cancerous tissues[95,96]. Additionally, TrkB expression was correlated with local progression, clinical stage, nodal metastasis, distant metastasis, and poor prognosis[95,96,99]. Interestingly, Dawson et al[99] further showed that TrkB is overexpressed in tumor budding cells, and this overexpression was positively correlated with KRAS-mutated tumors. More importantly, they found that among patients with membranous/cytoplasmic TrkB-positive buds, high tumor membranous/cytoplasmic TrkB expression is a significant independent adverse prognostic factor in CRC. These data clearly suggest that the TrkB receptor may indeed be a useful diagnostic and prognostic factor for CRC.
BDNF has also been studied in CRC patient tissues. Like its receptor TrkB, BDNF mRNA expression levels are significantly higher in CRC patient samples compared with control samples[93-95,100]. Further, this overexpression is positively correlated with differentiated CRC tumors, T-staging and poor prognosis[100]. In line with this finding, a recent report showed significant association between BDNF mRNA expression level and poor overall survival, liver metastasis and peritoneal metastasis. Nonetheless, no significant associations were found between BDNF mRNA and any other clinicopathological parameters in CRC tissues[98]. On the other hand, serum levels of BDNF were significantly decreased in CRC patients compared with control patients, and there were no significant associations between serum BDNF levels and Dukes’ stages[101].
In vitro studies have shown that TrkB and its ligand, BDNF, are expressed in several CRC cell lines (corresponding to different CRC tumor stages) at both mRNA and protein levels[92-98]. In addition, under stress culture conditions these cells were able to produce and secrete the BDNF[93,94], together with a relocation of its receptor TrkB to the cell membrane[94]. Thus, the expression of both BDNF and TrkB within the same cell lines has raised the question of an autocrine effect in CRC. Indeed, it was found that exogenous BDNF enhanced cell survival, proliferation, migration, invasion, and inhibited anoikis through the TrkB receptor in CRC cell lines[93,94,98,102]. BDNF was also found to trigger Akt phosphorylation in CRC cell lines through the TrkB receptor, as assessed by K252a, a Trk tyrosine kinase inhibitor that competes with Trks’ ATP binding site. K252a suppressed both BDNF-induced TrkB and Akt activation, in addition to cell survival, proliferation, migration, invasion and anoikis resistance[93,94,98]. Furthermore, gene knockdown of TrkB decreased the expression of PI3K (PIK3CA) and AKT1, their downstream effector mTOR, and the MAPK p38[95]. Recent studies have shown that cell survival, anoikis and chemotherapeutic agent resistance in tumor cells are mediated in part by TrkB receptor through activation of the PI3K/Akt pathway[30,71,83,103]. Thus, it is possible that BDNF/TrkB-induced CRC cell survival and anoikis resistance may be mediated through the PI3K/Akt pathway. TrkB silencing by siRNA also enhanced apoptosis and anoikis, while proliferation, migration and invasion were inhibited in TrkB siRNA-transfected cells[92,95-97]. Conversely, Fan et al[96] have shown by using regulated-TrkB expression models that TrkB overexpression protects CRC cell lines from anoikis. They also showed that Akt signaling pathway was functionally linked to TrkB-induced anoikis resistance in CRC cells. Furthermore, Akt phosphorylation levels were related with the expression pattern of TrkB in CRC cell lines[96].
In vivo, TrkB inhibition has been suggested to sensitize CRC cells to anoikis, which consequently protects against metastases formation in xenograft mouse models[96,98]. In these studies, TrkB blockade using the Trk pharmacological inhibitor K252a[98] or short hairpin (sh) RNA[96] significantly reduces the size and number of peritoneal and lung metastatic nodules in treated mice. Furthermore, a lower risk of metastasis-induced death has been found in mice injected with CRC-shTrkB cells compared with control[96].
Though TrkB-T1 has been found in CRC cells, in vitro and in vivo data suggest that the effects of the autocrine TrkB/BDNF loop are likely mediated by TrkB-FL, as assessed by K252a-induced inhibition of TrkB activation. Moreover, it is important to note that PCR primers used in some of the aforementioned studies were not able to clearly distinguish between TrkB-FL and TrkB-T1 in patient samples or in CRC cell lines. Further assessment will be necessary to clarify the functional role of TrkB-T1 in CRC.
EMT is now thought to play a fundamental role in tumor progression and metastasis formation. In fact, EMT enhances tumor dissemination by inducing individual cell delamination from its primary tumor site, which allows tumor cells to migrate through stroma and further invade adjacent tissues[104]. Additionally, the loss of E-cadherin expression is considered as a fundamental step in EMT[104]. In colorectal cancer, EMT takes place at the invasive front, allowing tumor cells that lost E-cadherin expression to migrate and gain the ability to disseminate to the metastatic sites[105]. This phenomenon was also found in other solid tumors such as papillary thyroid carcinoma, breast carcinoma and cervical carcinoma, whose cells show increased vimentin expression and loss of E-cadherin[104]. The relationship between TrkB and EMT has been clearly described in neuroblastoma tumors, yet there are now recent reports indicating that TrkB may induce EMT in cells derived from endometrial carcinoma[106], lung[107] and oral cancer cells[108], and head and neck[27] and CRC tumor cells[97,99]. Indeed, Fujikawa et al[97] have shown that TrkB was inversely correlated with E-cadherin at both the mRNA and protein levels in CRC patient tissues. In vitro, TrkB silencing enhances E-cadherin and reduces vimentin expressions in CRC cell lines. In line with these findings, Dawson et al[99] found that membranous/cytoplasmic TrkB expression was inversely correlated with both Ki-67 and caspase-3 in CRC tumor buds, and cytoplasmic TrkB overexpression was correlated with tumors presenting high-grade tumor budding at the invasive front, in agreement with the EMT-inducing function of TrkB. Thus, these results strongly suggest that TrkB receptor may be a promoter of EMT, anoikis resistance, and consequently metastasis formation in CRC.
Because the TrkB/BDNF pathway plays an important role in the biological and clinical behavior of CRC, the TrkB tyrosine kinase receptor as a potential therapeutic target is beginning to draw increasing attention. Thus, inhibiting the tyrosine kinase activity of TrkB may be an important addition to CRC therapy. Indeed, Lestaurtinib (CEP-701) a Trk-selective tyrosine kinase inhibitor derived from the prototypical K252a that blocks NT-induced Trk activation, has shown efficacy in treating solid cancers whose cells overexpress TrkB receptor in preclinical xenograft models, including models of neuroblastoma[109,110], prostate[88], pancreatic[111,112], and breast cancer[113]. The use of Lestaurtinib was also found to be feasible, as this agent does not show toxicity against normal tissue. Furthermore, Lestaurtinib (CEP-701) and the prodrug CEP-2563 and CEP-751 are currently in phase I and phase II clinical trials for treatment of neuroblastoma and other non-neural solid and blood-derived cancers[114-116]. Likewise, other selective TrK kinase inhibitor compounds have been recently developed and tested for cancer treatment, such as the pan-Trk inhibitor ZD6918 and AZ-23 (TrkA/TrkB selective kinase inhibitor; orally bioavailable)[117]. Based on these preclinical and clinical data, investigating the therapeutic potential of the TrkB receptor would be of great importance in CRC treatment. Thus, inhibiting the TrkB receptor may become an important adjunct target to improve clinical outcome in patients with CRC. It is worth mentioning that both TrkA and TrkC, two other members of the Trk family, were not found in CRC cell lines, as described in our previous work[94]. In line with this finding, the TrkC receptor is highly downregulated in CRC tumor samples in comparison with adjacent normal tissues. Furthermore, reestablishment of TrkC expression in CRC cell lines induces apoptosis and inhibits tumor growth in vitro and in vivo, supporting the idea that TrkC is a CRC tumor suppressor[118]. However, the TrkC receptor was recently found in CRC cell lines and tumor samples, and its expression was positively correlated with liver metastasis[95]. Presumably, targeting TrkB receptor will likely render CRC tumor cells more sensitive to chemotherapy-induced apoptosis, as shown in neuroblastoma and other solid cancers[119].
It has also been shown that TrkB has an anti-apoptotic effect in CRC. Therefore, combining Trk-targeted therapy with recombinant TRAIL (TNF related apoptosis induced ligand) or Fas ligand may also be useful in treatment of CRC. Indeed, normal colonic cells are resistant to TRAIL-induced apoptosis, whereas colonic tumor cells are sensitive to the TRAIL recombinant. Additionally, both 5-FU and oxaliplatin, two of the main chemotherapeutic agents used in the treatment of CRC, act by inducing apoptosis through the Fas (CD95) receptor[120]. Accordingly, inhibition of the TrkB receptor in CRC may potentiate the anti-tumor effects of apoptogenic agents used in treatment of advanced CRC.
Crosstalk between TrkB and the EGFR has been described in numerous solid tumors, such as ovarian cancer[25]. Indeed, BDNF-induced activation of TrkB was found to activate EGFR in ovarian cancer, and subsequently induce cell proliferation and migration through the PI3K/Akt pathway. Additionally, EGFR and TrkB kinase inhibitors blocked EGF- and BDNF-induced TrkB and EGFR activation, respectively, and blocked Akt phosphorylation. In line with this finding, De farias et al[102] showed in an HT-29 CRC cell line that the TrkB/BDNF pathway protects CRC cells from the inhibitory effects of cetuximab (anti-EGFR monoclonal antibody). Furthermore, TrkB blockade was found to potentiate the inhibitory effects of cetuximab on CRC cell proliferation and survival[102]. Such results suggest a potential therapeutic benefit from the crosstalk between the TrkB/BDNF pathway and EGFR in CRC cells. It is possible that EGFR inhibition could be more effective in combination with TrkB inhibition in the treatment of advanced CRC. In addition to the crosstalk with EGFR, TrkB has also been found to activate angiogenesis in neuroblastoma and ovarian cancer. Although functional evidence supporting this possibility is lacking at present in CRC, Sasahira et al[95], showed that the TrkB receptor positively regulates VEGF-A and VEGF-C gene expression.
Targeting the TrkB/BDNF pathway, points toward a promising addition to the strategies for CRC therapy.
Currently, among conventional therapies, advanced CRC remains a major therapeutic challenge with poor overall survival. Therefore, identifying novel molecular mechanisms involved in driving CRC development and progression is crucial for improving clinical management of CRC and patient outcome. TrkB has been shown to associate with CRC local progression, clinical stage, nodal metastasis, distant metastasis, and poor prognosis. Additionally, emerging data suggest that the TrkB/BDNF pathway enhances several biological processes in CRC, including proliferation, invasion, migration, EMT, apoptosis resistance and anoikis resistance. Hence, inhibition of the TrkB/BDNF pathway alone or in combination with other conventional and targeted agents in CRC may hold promise as an important approach to improve patients’ long-term survival.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20722] [Article Influence: 1883.8] [Reference Citation Analysis (23)] |
| 2. | Lombardi L, Morelli F, Cinieri S, Santini D, Silvestris N, Fazio N, Orlando L, Tonini G, Colucci G, Maiello E. Adjuvant colon cancer chemotherapy: where we are and where we’ll go. Cancer Treat Rev. 2010;36 Suppl 3:S34-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1371] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 4. | Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 502] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 5. | Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, Tveit KM, Gibson F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 6. | Lucas AS, O’Neil BH, Goldberg RM. A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer. 2011;10:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179:663-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1040] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 8. | Park JG, Collins JM, Gazdar AF, Allegra CJ, Steinberg SM, Greene RF, Kramer BS. Enhancement of fluorinated pyrimidine-induced cytotoxicity by leucovorin in human colorectal carcinoma cell lines. J Natl Cancer Inst. 1988;80:1560-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Poon MA, O’Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, Krook JE, Mailliard JA, Laurie JA, Tschetter LK. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7:1407-1418. [PubMed] |
| 10. | Machover D, Schwarzenberg L, Goldschmidt E, Tourani JM, Michalski B, Hayat M, Dorval T, Misset JL, Jasmin C, Maral R. Treatment of advanced colorectal and gastric adenocarcinomas with 5-FU combined with high-dose folinic acid: a pilot study. Cancer Treat Rep. 1982;66:1803-1807. [PubMed] |
| 11. | Madajewicz S, Petrelli N, Rustum YM, Campbell J, Herrera L, Mittelman A, Perry A, Creaven PJ. Phase I-II trial of high-dose calcium leucovorin and 5-fluorouracil in advanced colorectal cancer. Cancer Res. 1984;44:4667-4669. [PubMed] |
| 12. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2273] [Cited by in RCA: 2228] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 13. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1729] [Article Influence: 78.6] [Reference Citation Analysis (2)] |
| 14. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7794] [Article Influence: 354.3] [Reference Citation Analysis (8)] |
| 15. | Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992-3995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1669] [Cited by in RCA: 1717] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 16. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3160] [Article Influence: 185.9] [Reference Citation Analysis (6)] |
| 17. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2213] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 18. | Ketterer K, Rao S, Friess H, Weiss J, Büchler MW, Korc M. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res. 2003;9:5127-5136. [PubMed] |
| 19. | Sclabas GM, Fujioka S, Schmidt C, Li Z, Frederick WA, Yang W, Yokoi K, Evans DB, Abbruzzese JL, Hess KR. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res. 2005;11:440-449. [PubMed] |
| 20. | Wang W, Zhao H, Zhang S, Kang E, Chen Y, Ni C, Zhang S, Zhu M. Patterns of expression and function of the p75(NGFR) protein in pancreatic cancer cells and tumours. Eur J Surg Oncol. 2009;35:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Bronzetti E, Artico M, Forte F, Pagliarella G, Felici LM, D’Ambrosio A, Vespasiani G, Bronzetti B. A possible role of BDNF in prostate cancer detection. Oncol Rep. 2008;19:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Montano X, Djamgoz MB. Epidermal growth factor, neurotrophins and the metastatic cascade in prostate cancer. FEBS Lett. 2004;571:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Ricci A, Greco S, Mariotta S, Felici L, Bronzetti E, Cavazzana A, Cardillo G, Amenta F, Bisetti A, Barbolini G. Neurotrophins and neurotrophin receptors in human lung cancer. Am J Respir Cell Mol Biol. 2001;25:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Wilson CM, Naves T, Vincent F, Melloni B, Bonnaud F, Lalloué F, Jauberteau MO. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J Cell Sci. 2014;127:3983-3997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Qiu L, Zhou C, Sun Y, Di W, Scheffler E, Healey S, Kouttab N, Chu W, Wan Y. Crosstalk between EGFR and TrkB enhances ovarian cancer cell migration and proliferation. Int J Oncol. 2006;29:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Lai PC, Chiu TH, Huang YT. Overexpression of BDNF and TrkB in human bladder cancer specimens. Oncol Rep. 2010;24:1265-1270. [PubMed] |
| 27. | Kupferman ME, Jiffar T, El-Naggar A, Yilmaz T, Zhou G, Xie T, Feng L, Wang J, Holsinger FC, Yu D. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene. 2010;29:2047-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Lee J, Jiffar T, Kupferman ME. A novel role for BDNF-TrkB in the regulation of chemotherapy resistance in head and neck squamous cell carcinoma. PLoS One. 2012;7:e30246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Kawamura N, Kawamura K, Manabe M, Tanaka T. Inhibition of brain-derived neurotrophic factor/tyrosine kinase B signaling suppresses choriocarcinoma cell growth. Endocrinology. 2010;151:3006-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005;105:4429-4436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Fauchais AL, Lalloué F, Lise MC, Boumediene A, Preud’homme JL, Vidal E, Jauberteau MO. Role of endogenous brain-derived neurotrophic factor and sortilin in B cell survival. J Immunol. 2008;181:3027-3038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Bellanger C, Dubanet L, Lise MC, Fauchais AL, Bordessoule D, Jauberteau MO, Troutaud D. Endogenous neurotrophins and Trk signaling in diffuse large B cell lymphoma cell lines are involved in sensitivity to rituximab-induced apoptosis. PLoS One. 2011;6:e27213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 2012;846:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 34. | Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549-553. [PubMed] |
| 35. | Suter U, Heymach JV, Shooter EM. Two conserved domains in the NGF propeptide are necessary and sufficient for the biosynthesis of correctly processed and biologically active NGF. EMBO J. 1991;10:2395-2400. [PubMed] |
| 36. | Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247-250. [PubMed] |
| 37. | Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: Recent advances and challenges. Dev Neurobiol. 2010;70:350-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1258] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 39. | Schecterson LC, Bothwell M. Neurotrophin receptors: Old friends with new partners. Dev Neurobiol. 2010;70:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Chen Y, Zeng J, Cen L, Chen Y, Wang X, Yao G, Wang W, Qi W, Kong K. Multiple roles of the p75 neurotrophin receptor in the nervous system. J Int Med Res. 2009;37:281-288. [PubMed] |
| 41. | Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272-280. [PubMed] |
| 42. | Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148-155. [PubMed] |
| 43. | Stoilov P, Castren E, Stamm S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem Biophys Res Commun. 2002;290:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Martin-Zanca D, Mitra G, Long LK, Barbacid M. Molecular characterization of the human trk oncogene. Cold Spring Harb Symp Quant Biol. 1986;51 Pt 2:983-992. [PubMed] |
| 45. | Teng KK, Hempstead BL. Neurotrophins and their receptors: signaling trios in complex biological systems. Cell Mol Life Sci. 2004;61:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1697] [Cited by in RCA: 1698] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 47. | Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65:895-903. [PubMed] |
| 48. | Rydén M, Ibáñez CF. Binding of neurotrophin-3 to p75LNGFR, TrkA, and TrkB mediated by a single functional epitope distinct from that recognized by trkC. J Biol Chem. 1996;271:5623-5627. [PubMed] |
| 49. | Nakagawara A, Liu XG, Ikegaki N, White PS, Yamashiro DJ, Nycum LM, Biegel JA, Brodeur GM. Cloning and chromosomal localization of the human TRK-B tyrosine kinase receptor gene (NTRK2). Genomics. 1995;25:538-546. [PubMed] |
| 50. | Thiele CJ, Li Z, McKee AE. On Trk--the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res. 2009;15:5962-5967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 51. | Luberg K, Wong J, Weickert CS, Timmusk T. Human TrkB gene: novel alternative transcripts, protein isoforms and expression pattern in the prefrontal cerebral cortex during postnatal development. J Neurochem. 2010;113:952-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Brodeur GM, Minturn JE, Ho R, Simpson AM, Iyer R, Varela CR, Light JE, Kolla V, Evans AE. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15:3244-3250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 53. | Ohira K, Hayashi M. Expression of TrkB subtypes in the adult monkey cerebellar cortex. J Chem Neuroanat. 2003;25:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123-3129. [PubMed] |
| 55. | Fenner BM. Truncated TrkB: beyond a dominant negative receptor. Cytokine Growth Factor Rev. 2012;23:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 56. | Alderson RF, Curtis R, Alterman AL, Lindsay RM, DiStefano PS. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin-4/5 by rat astrocytes and schwann cells in vitro. Brain Res. 2000;871:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 222] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 58. | Haapasalo A, Saarelainen T, Moshnyakov M, Arumäe U, Kiema TR, Saarma M, Wong G, Castrén E. Expression of the naturally occurring truncated trkB neurotrophin receptor induces outgrowth of filopodia and processes in neuroblastoma cells. Oncogene. 1999;18:1285-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Hartmann M, Brigadski T, Erdmann KS, Holtmann B, Sendtner M, Narz F, Lessmann V. Truncated TrkB receptor-induced outgrowth of dendritic filopodia involves the p75 neurotrophin receptor. J Cell Sci. 2004;117:5803-5814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Ohira K, Homma KJ, Hirai H, Nakamura S, Hayashi M. TrkB-T1 regulates the RhoA signaling and actin cytoskeleton in glioma cells. Biochem Biophys Res Commun. 2006;342:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Ohira K, Funatsu N, Homma KJ, Sahara Y, Hayashi M, Kaneko T, Nakamura S. Truncated TrkB-T1 regulates the morphology of neocortical layer I astrocytes in adult rat brain slices. Eur J Neurosci. 2007;25:406-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Hu Y, Wang YD, Guo T, Wei WN, Sun CY, Zhang L, Huang J. Identification of brain-derived neurotrophic factor as a novel angiogenic protein in multiple myeloma. Cancer Genet Cytogenet. 2007;178:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Li Z, Chang Z, Chiao LJ, Kang Y, Xia Q, Zhu C, Fleming JB, Evans DB, Chiao PJ. TrkBT1 induces liver metastasis of pancreatic cancer cells by sequestering Rho GDP dissociation inhibitor and promoting RhoA activation. Cancer Res. 2009;69:7851-7859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Tervonen TA, Ajamian F, De Wit J, Verhaagen J, Castrén E, Castrén M. Overexpression of a truncated TrkB isoform increases the proliferation of neural progenitors. Eur J Neurosci. 2006;24:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Rose CR, Blum R, Pichler B, Lepier A, Kafitz KW, Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 286] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 66. | Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1600] [Cited by in RCA: 1650] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 67. | Aoyama M, Asai K, Shishikura T, Kawamoto T, Miyachi T, Yokoi T, Togari H, Wada Y, Kato T, Nakagawara A. Human neuroblastomas with unfavorable biologies express high levels of brain-derived neurotrophic factor mRNA and a variety of its variants. Cancer Lett. 2001;164:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol. 1994;14:759-767. [PubMed] |
| 69. | Smit MA, Geiger TR, Song JY, Gitelman I, Peeper DS. A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol. 2009;29:3722-3737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 70. | Smit MA, Peeper DS. Zeb1 is required for TrkB-induced epithelial-mesenchymal transition, anoikis resistance and metastasis. Oncogene. 2011;30:3735-3744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 454] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 72. | Middlemas DS, Kihl BK, Moody NM. Brain derived neurotrophic factor protects human neuroblastoma cells from DNA damaging agents. J Neurooncol. 1999;45:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Middlemas DS, Kihl BK, Zhou J, Zhu X. Brain-derived neurotrophic factor promotes survival and chemoprotection of human neuroblastoma cells. J Biol Chem. 1999;274:16451-16460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249-4255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 75. | Schramm A, Schulte JH, Astrahantseff K, Apostolov O, Limpt Vv, Sieverts H, Kuhfittig-Kulle S, Pfeiffer P, Versteeg R, Eggert A. Biological effects of TrkA and TrkB receptor signaling in neuroblastoma. Cancer Lett. 2005;228:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Au CW, Siu MK, Liao X, Wong ES, Ngan HY, Tam KF, Chan DC, Chan QK, Cheung AN. Tyrosine kinase B receptor and BDNF expression in ovarian cancers - Effect on cell migration, angiogenesis and clinical outcome. Cancer Lett. 2009;281:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 77. | Yu X, Liu L, Cai B, He Y, Wan X. Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Sci. 2008;99:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Okamura K, Harada T, Wang S, Ijichi K, Furuyama K, Koga T, Okamoto T, Takayama K, Yano T, Nakanishi Y. Expression of TrkB and BDNF is associated with poor prognosis in non-small cell lung cancer. Lung Cancer. 2012;78:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 79. | Vanhecke E, Adriaenssens E, Verbeke S, Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X, Hondermarck H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin Cancer Res. 2011;17:1741-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 80. | Patani N, Jiang WG, Mokbel K. Brain-derived neurotrophic factor expression predicts adverse pathological & amp; clinical outcomes in human breast cancer. Cancer Cell Int. 2011;11:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Zhang Y, Fujiwara Y, Doki Y, Takiguchi S, Yasuda T, Miyata H, Yamazaki M, Ngan CY, Yamamoto H, Ma Q. Overexpression of tyrosine kinase B protein as a predictor for distant metastases and prognosis in gastric carcinoma. Oncology. 2008;75:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Okugawa Y, Tanaka K, Inoue Y, Kawamura M, Kawamoto A, Hiro J, Saigusa S, Toiyama Y, Ohi M, Uchida K. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br J Cancer. 2013;108:121-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Zhang Z, Han L, Liu Y, Liang X, Sun W. Up-regulation of Tropomyosin related kinase B contributes to resistance to detachment-induced apoptosis in hepatoma multicellular aggregations. Mol Biol Rep. 2009;36:1211-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST, Poon RT. Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: implication in hepatocellular carcinoma. Clin Cancer Res. 2011;17:3123-3133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Hu Y, Sun CY, Wang HF, Guo T, Wei WN, Wang YD, He WJ, Wu T, Tan H, Wu TC. Brain-derived neurotrophic factor promotes growth and migration of multiple myeloma cells. Cancer Genet Cytogenet. 2006;169:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Zhang L, Hu Y, Sun CY, Li J, Guo T, Huang J, Chu ZB. Lentiviral shRNA silencing of BDNF inhibits in vivo multiple myeloma growth and angiogenesis via down-regulated stroma-derived VEGF expression in the bone marrow milieu. Cancer Sci. 2010;101:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | D’Onofrio M, de Grazia U, Morrone S, Cuomo L, Spinsanti P, Frati L, Gulino A, Ragona G. Expression of neurotrophin receptors in normal and malignant B lymphocytes. Eur Cytokine Netw. 2000;11:283-291. [PubMed] |
| 88. | George DJ, Dionne CA, Jani J, Angeles T, Murakata C, Lamb J, Isaacs JT. Sustained in vivo regression of Dunning H rat prostate cancers treated with combinations of androgen ablation and Trk tyrosine kinase inhibitors, CEP-751 (KT-6587) or CEP-701 (KT-5555). Cancer Res. 1999;59:2395-2401. [PubMed] |
| 89. | Dionne CA, Camoratto AM, Jani JP, Emerson E, Neff N, Vaught JL, Murakata C, Djakiew D, Lamb J, Bova S. Cell cycle-independent death of prostate adenocarcinoma is induced by the trk tyrosine kinase inhibitor CEP-751 (KT6587). Clin Cancer Res. 1998;4:1887-1898. [PubMed] |
| 90. | Eggert A, Grotzer MA, Ikegaki N, Zhao H, Cnaan A, Brodeur GM, Evans AE. Expression of the neurotrophin receptor TrkB is associated with unfavorable outcome in Wilms’ tumor. J Clin Oncol. 2001;19:689-696. [PubMed] |
| 91. | Sniderhan LF, Garcia-Bates TM, Burgart M, Bernstein SH, Phipps RP, Maggirwar SB. Neurotrophin signaling through tropomyosin receptor kinases contributes to survival and proliferation of non-Hodgkin lymphoma. Exp Hematol. 2009;37:1295-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Yu Y, Zhang S, Wang X, Yang Z, Ou G. Overexpression of TrkB promotes the progression of colon cancer. APMIS. 2010;118:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Brunetto de Farias C, Rosemberg DB, Heinen TE, Koehler-Santos P, Abujamra AL, Kapczinski F, Brunetto AL, Ashton-Prolla P, Meurer L, Reis Bogo M. BDNF/TrkB content and interaction with gastrin-releasing peptide receptor blockade in colorectal cancer. Oncology. 2010;79:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Akil H, Perraud A, Mélin C, Jauberteau MO, Mathonnet M. Fine-tuning roles of endogenous brain-derived neurotrophic factor, TrkB and sortilin in colorectal cancer cell survival. PLoS One. 2011;6:e25097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 95. | Sasahira T, Ueda N, Kurihara M, Matsushima S, Ohmori H, Fujii K, Bhawal UK, Yamamoto K, Kirita T, Kuniyasu H. Tropomyosin receptor kinases B and C are tumor progressive and metastatic marker in colorectal carcinoma. Hum Pathol. 2013;44:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Fan M, Sun J, Wang W, Fan J, Wang L, Zhang X, Yang A, Wang W, Zhang R, Li J. Tropomyosin-related kinase B promotes distant metastasis of colorectal cancer through protein kinase B-mediated anoikis suppression and correlates with poor prognosis. Apoptosis. 2014;19:860-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Fujikawa H, Tanaka K, Toiyama Y, Saigusa S, Inoue Y, Uchida K, Kusunoki M. High TrkB expression levels are associated with poor prognosis and EMT induction in colorectal cancer cells. J Gastroenterol. 2012;47:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 98. | Tanaka K, Okugawa Y, Toiyama Y, Inoue Y, Saigusa S, Kawamura M, Araki T, Uchida K, Mohri Y, Kusunoki M. Brain-derived neurotrophic factor (BDNF)-induced tropomyosin-related kinase B (Trk B) signaling is a potential therapeutic target for peritoneal carcinomatosis arising from colorectal cancer. PLoS One. 2014;9:e96410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 99. | Dawson H, Grundmann S, Koelzer VH, Galván JA, Kirsch R, Karamitopoulou E, Lugli A, Inderbitzin D, Zlobec I. Tyrosine kinase receptor B (TrkB) expression in colorectal cancers highlights anoikis resistance as a survival mechanism of tumour budding cells. Histopathology. 2015;66:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Yang X, Martin TA, Jiang WG. Biological influence of brain-derived neurotrophic factor (BDNF) on colon cancer cells. Exp Ther Med. 2013;6:1475-1481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Brierley GV, Priebe IK, Purins L, Fung KY, Tabor B, Lockett T, Nice E, Gibbs P, Tie J, McMurrick P. Serum concentrations of brain-derived neurotrophic factor (BDNF) are decreased in colorectal cancer patients. Cancer Biomark. 2013;13:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | de Farias CB, Heinen TE, dos Santos RP, Abujamra AL, Schwartsmann G, Roesler R. BDNF/TrkB signaling protects HT-29 human colon cancer cells from EGFR inhibition. Biochem Biophys Res Commun. 2012;425:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 103. | Jaboin J, Kim CJ, Kaplan DR, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3’-kinase pathway. Cancer Res. 2002;62:6756-6763. [PubMed] |
| 104. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7905] [Article Influence: 465.0] [Reference Citation Analysis (1)] |
| 105. | Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356-10361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 866] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 106. | Bao W, Qiu H, Yang T, Luo X, Zhang H, Wan X. Upregulation of TrkB promotes epithelial-mesenchymal transition and anoikis resistance in endometrial carcinoma. PLoS One. 2013;8:e70616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 107. | Ricci A, De Vitis C, Noto A, Fattore L, Mariotta S, Cherubini E, Roscilli G, Liguori G, Scognamiglio G, Rocco G. TrkB is responsible for EMT transition in malignant pleural effusions derived cultures from adenocarcinoma of the lung. Cell Cycle. 2013;12:1696-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 108. | Dudás J, Bitsche M, Schartinger V, Falkeis C, Sprinzl GM, Riechelmann H. Fibroblasts produce brain-derived neurotrophic factor and induce mesenchymal transition of oral tumor cells. Oral Oncol. 2011;47:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Evans AE, Kisselbach KD, Liu X, Eggert A, Ikegaki N, Camoratto AM, Dionne C, Brodeur GM. Effect of CEP-751 (KT-6587) on neuroblastoma xenografts expressing TrkB. Med Pediatr Oncol. 2001;36:181-184. [PubMed] |
| 110. | Evans AE, Kisselbach KD, Yamashiro DJ, Ikegaki N, Camoratto AM, Dionne CA, Brodeur GM. Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clin Cancer Res. 1999;5:3594-3602. [PubMed] |
| 111. | Miknyoczki SJ, Chang H, Klein-Szanto A, Dionne CA, Ruggeri BA. The Trk tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits significant antitumor efficacy in preclinical xenograft models of human pancreatic ductal adenocarcinoma. Clin Cancer Res. 1999;5:2205-2212. [PubMed] |
| 112. | Miknyoczki SJ, Dionne CA, Klein-Szanto AJ, Ruggeri BA. The novel Trk receptor tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits antitumor efficacy against human pancreatic carcinoma (Panc1) xenograft growth and in vivo invasiveness. Ann N Y Acad Sci. 1999;880:252-262. [PubMed] |
| 113. | Vazquez-Ortiz G, Chisholm C, Xu X, Lahusen TJ, Li C, Sakamuru S, Huang R, Thomas CJ, Xia M, Deng C. Drug repurposing screen identifies lestaurtinib amplifies the ability of the poly (ADP-ribose) polymerase 1 inhibitor AG14361 to kill breast cancer associated gene-1 mutant and wild type breast cancer cells. Breast Cancer Res. 2014;16:R67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 114. | Marshall JL, Kindler H, Deeken J, Bhargava P, Vogelzang NJ, Rizvi N, Luhtala T, Boylan S, Dordal M, Robertson P. Phase I trial of orally administered CEP-701, a novel neurotrophin receptor-linked tyrosine kinase inhibitor. Invest New Drugs. 2005;23:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 115. | Chan E, Mulkerin D, Rothenberg M, Holen KD, Lockhart AC, Thomas J, Berlin J. A phase I trial of CEP-701 + gemcitabine in patients with advanced adenocarcinoma of the pancreas. Invest New Drugs. 2008;26:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 116. | Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, Clark R, Levis MJ, Small D. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 320] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 117. | Thress K, Macintyre T, Wang H, Whitston D, Liu ZY, Hoffmann E, Wang T, Brown JL, Webster K, Omer C. Identification and preclinical characterization of AZ-23, a novel, selective, and orally bioavailable inhibitor of the Trk kinase pathway. Mol Cancer Ther. 2009;8:1818-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | Genevois AL, Ichim G, Coissieux MM, Lambert MP, Lavial F, Goldschneider D, Jarrosson-Wuilleme L, Lepinasse F, Gouysse G, Herceg Z. Dependence receptor TrkC is a putative colon cancer tumor suppressor. Proc Natl Acad Sci USA. 2013;110:3017-3022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 119. | Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, Camoratto AM, Evans AE, Brodeur GM. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462-6466. [PubMed] |
| 120. | Prabhudesai SG, Rekhraj S, Roberts G, Darzi AW, Ziprin P. Apoptosis and chemo-resistance in colorectal cancer. J Surg Oncol. 2007;96:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ananiev J S- Editor: Qi Y L- Editor: A E- Editor: Wang CH