Published online Apr 28, 2016. doi: 10.3748/wjg.v22.i16.4211
Peer-review started: December 12, 2015
First decision: December 30, 2015
Revised: December 31, 2015
Accepted: January 30, 2016
Article in press: January 30, 2016
Published online: April 28, 2016
Processing time: 129 Days and 6.3 Hours
AIM: To test the correlation between lymphocyte-to-monocyte ratio (LMR) and survival after radiofrequency ablation (RFA) for colorectal liver metastasis (CLMs).
METHODS: From July 2003 to Feb 2012, 127 consecutive patients with 193 histologically-proven unresectable CLMs were treated with percutaneous RFA at the University of Foggia. All patients had undergone primary colorectal tumor resection before RFA and received systemic chemotherapy. LMR was calculated by dividing lymphocyte count by monocyte count assessed at baseline. Treatment-related toxicity was defined as any adverse events occurred within 4 wk after the procedure. Overall survival (OS) and time to recurrence (TTR) were estimated from the date of RFA by Kaplan-Meier with plots and median (95%CI). The inferential analysis for time to event data was conducted using the Cox univariate and multivariate regression model to estimate hazard ratios (HR) and 95%CI. Statistically significant variables from the univariate Cox analysis were considered for the multivariate models.
RESULTS: Median age was 66 years (range 38-88) and patients were prevalently male (69.2%). Median LMR was 4.38% (0.79-88) whereas median number of nodules was 2 (1-3) with a median maximum diameter of 27 mm (10-45). Median OS was 38 mo (34-53) and survival rate (SR) was 89.4%, 40.4% and 33.3% at 1, 4 and 5 years respectively in the whole cohort. Running log-rank test analysis found 3.96% as the most significant prognostic cut-off point for LMR and stratifying the study population by this LMR value median OS resulted 55 mo (37-69) in patients with LMR > 3.96% and 34 (26-39) mo in patients with LMR ≤ 3.96% (HR = 0.53, 0.34-0.85, P = 0.007). Nodule size and LMR were the only significant predictors for OS in multivariate analysis. Median TTR was 29 mo (22-35) with a recurrence-free survival (RFS) rate of 72.6%, 32.1% and 21.8% at 1, 4 and 5 years, respectively in the whole study group. Nodule size and LMR were confirmed as significant prognostic factors for TTR in multivariate Cox regression. TTR, when stratified by LMR, was 35 mo (28-57) in the group > 3.96% and 25 mo (18-30) in the group ≤ 3.96% (P = 0.02).
CONCLUSION: Our study provides support for the use of LMR as a novel predictor of outcome for CLM patients.
Core tip: This is a retrospective study to test the correlation between baseline lymphocyte-to-monocyte ratio (LMR) and survival outcomes in colorectal liver metastasis patients treated with radiofrequency ablation. Median overall survival (OS) was 55 mo in patients with LMR > 3.96% and 34 mo in patients with LMR ≤ 3.96% (P = 0.007). Time to recurrence (TTR) was 35 mo in the group > 3.96% and 25 mo in the group ≤ 3.96% (P = 0.02). Nodule size and LMR were the only significant predictors either for OS and for TTR in multivariate analysis. LMR was useful as clinical predictor of survival outcomes.
- Citation: Facciorusso A, Del Prete V, Crucinio N, Serviddio G, Vendemiale G, Muscatiello N. Lymphocyte-to-monocyte ratio predicts survival after radiofrequency ablation for colorectal liver metastases. World J Gastroenterol 2016; 22(16): 4211-4218
- URL: https://www.wjgnet.com/1007-9327/full/v22/i16/4211.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i16.4211
Colorectal cancer (CRC) is the second cause of cancer-related mortality in developed countries and the third most common malignancy worldwide[1]. Liver resection represents a valuable therapeutic option in patients who develop liver metastases, but unfortunately less than 20% of them is suitable for surgery mainly due to high tumor burden or extrahepatic tumoral disease which render systemic chemotherapy the more appropriate treatment in such cases[2,3]. When the surgical option is unfeasible due to patient comorbidities, unwillingness to undergo surgery or tumor location, local ablation may represent a valuable alternative.
Percutaneous radiofrequency ablation (RFA), an ablative technique which determines coagulation necrosis of the tumor by means of radiofrequency-induced heat, has proved effective in prolonging survival in a number of liver malignancies such as hepatocellular carcinoma (HCC)[4-6], liver metastases from CRC (CLMs)[7], breast[8] and ovarian cancer[9]. Cumulative evidence has demonstrated that inflammatory cells infiltrates in the tumor microenvironment have a large influence on the biological behavior of several malignancies, including HCC[10] and CRC[11]. In particular, macrophages constitute the most represented leukocyte lineage in such infiltrates and are well-known to promote tumor proliferation, neo-angiogenesis and metastasis occurrence[12-15]. As a consequence, immunohistochemical studies have validated the association between high monocyte/macrophage density in the tumoral stroma and unfavorable prognosis in a number of malignancies[10,16].
Several inflammatory bio-markers have been tested in CLMs, among them widely available and easy to use are those obtained from peripheral blood cell count such as neutrophil-to-lymphocyte ratio (NLR) and monocyte level (expressed as percentage) but none of them have been definitively and unequivocally validated[17,18]. Since the pre-operative lymphocyte-to-monocyte ratio (LMR) has been recently found to correlate accurately with clinical outcomes in CLM patients undergoing hepatic resection[19], we decided to test whether this marker exerts a prognostic role and therefore can be considered a predictor of overall survival (OS) and time to recurrence (TTR) in CRC patients with liver metastases treated with percutaneous RFA.
From July 2003 to Feb 2012, 127 consecutive patients with 193 histologically-proven CLMs deemed unresectable by consensus of a multidisciplinary team or who refused surgery were treated with percutaneous RFA at the University of Foggia.
All patients had undergone primary colorectal tumor resection before RFA and tumor staging was assessed by multiphasic contrast-enhanced computed tomography (CT) or gadolinium-enhanced magnetic resonance imaging (MRI), according to current guidelines[3].
Inclusion criteria were: (1) confirmed proof of malignancy of CLM; (2) patients not suitable to surgery (due to comorbidities, unfavorable tumor location or those requiring large/difficult surgery) or who refused liver resection; (3) nodule size < 5 cm; (4) no more than three lesions; (5) complete resection of primary neoplasm and no extrahepatic tumors; (6) platelet count > 40000/ mm3 and prothrombin time ratio > 40%; and (7) no pre-treatment hematology disease, infection or hyperpyrexia.
All patients received systemic chemotherapy, mostly according to Douillard regimen (irinotecan, leucovorin, and 5-fluorouracil) for four to six cycles[20], as adjuvant treatment. In cases of tumor progression the FOLFOX (folinic acid, fluorouracil, oxaliplatin) regimen was adopted.
The absolute peripheral blood lymphocyte and monocyte counts were derived from the complete blood cell count before RFA, with LMR calculated by dividing lymphocyte count by monocyte count.
This study was approved by our Institutional Review Board for retrospective evaluation of de-identified patients.
Patients were followed up until July 2015 (median 63 mo, 95%CI: 54-71).
The technical details of the ablative procedures performed in our center have been described elsewhere[4-6]. Briefly, ultrasound-guided RFA was performed under conscious sedation with a 150W generator (Model 1500 L; RITA Medical System, Mountain View, California), connected to a 15-14-gauge probe with a 2.0-cm-long exposed tip able to deploy seven hooks. The needle was then inserted into the centre of the nodul maintaining the temperature of the tip at 80-110 °C for 10-12 min. At the end of the RFA cycle, track ablation was performed in order to prevent tumoral seeding or hemorrhage. In the case of multinodular disease, all nodules were treated in a single session. Aim of the procedure was to achieve complete nodule ablation with a 5 mm safety margin around the target area. All the cycles were performed with no pre-procedural antibiotic or anti-inflammatory drug administration.
Patients were followed-up by means of multiphasic CT scan imaging and adverse events were assessed according to Common Terminology Criteria for Adverse Events 4.0[4-6,21] at 1 mo after the procedure and, in the case of complete response, every 4 mo for the first 3 years and at 5-6 mo thereafter. Treatment-related toxicity was defined as any adverse events occurred within 4 wk after the procedure.
Response rate was defined according to commonly accepted criteria recently proposed by a group of experts and complete response was considered the absence of contrast enhancement in the target nodule[22].
When local tumor progression occurred, RFA was re-planned when technically feasible and on the basis of the likelihood of achieving complete response. For those who developed more extensive metastases or extrahepatic disease, systemic chemotherapy was given whenever possible.
Categorical variables were expressed as frequencies and percentages and continuous variables as medians and ranges.
OS and TTR were analyzed by Kaplan-Meier method and expressed in terms of median (95%CI).
Candidate predictors of survival outcomes were tested with Cox univariate and multivariate regression test and results were described as hazard ratios (HR) and 95%CI. Only those variables which resulted significant in univariate setting were inserted into multivariate model[23].
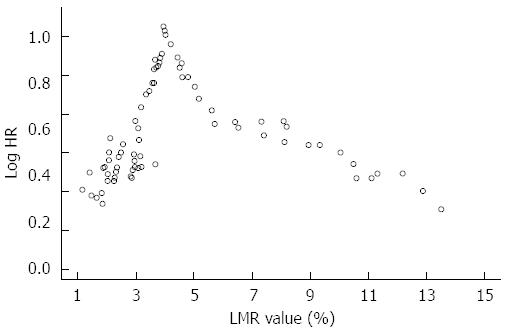
Running log-rank test was performed to identify a reliable LMR prognostic cut-off value[24]. With this method, all the observed LMR values were plotted against survival and log-rank test was performed for each value up to the level that covered 90% of the patients. The LMR value with the highest log-rank statistical value was finally chosen as the optimal cutoff point[24].
Furthermore, in order to assess the independence of LMR from other clinical and tumoral markers, linear and logistic regression models correlating this inflammatory index and the main laboratory and tumoral parameters at baseline were built.
The analysis was performed using R Statistical Software (Foundation for Statistical Computing, Vienna, Austria) and significance threshold was established at the 0.05 level (two-sided).
Clinical and demographic characteristics are summarized in Table 1. Median age was 66 years (range 38-88) and patients were prevalently male (69.2%). Median NLR was 1.74% (0.33-13.09) whereas LMR was 4.38% (0.79-88). Median carcinoembryonic antigen (CEA) level was 34.2 ng/mL (1.5-1198). Median number of nodules was 2 (1-3) with a median maximum diameter of 27 mm (10-45). Most metastatic lesions were synchronous (77.1%) and colon was the most common location of the primary tumor (74.8%).
| Variable | |
| Age (yr) | 66 (38-88) |
| Gender (M/F) | 88 (69.2%)/39 (30.8%) |
| Absolute lymphocyte count (103 cells/μL) | 1.77 (0.45-5.98) |
| Absolute neutrophil count (103 cells/μL) | 3.29 (0.98-12.17) |
| Absolute monocyte count (103 cells/μL) | 0.35 (0.01-2.15) |
| NLR (%) | 1.74 (0.33-13.09) |
| LMR (%) | 4.38 (0.79-88) |
| CEA (ng/mL) | 34.2 (1.5-1198) |
| Number of Nodules | 2 (1-3) |
| Max diameter (mm) | 27 (10-45) |
| Primary tumor (colon/rectum) | 95 (74.8%)/32 (25.2%) |
| Timing of occurrence (synchronous/metachronous) | 98 (77.1%)/29 (22.9%) |
| ECOG performance status (0/1) | 112 (88.1%)/15 (11.9%) |
Regression analysis found no significant correlation between LMR and the other clinical and tumoral markers at baseline. In fact, LMR did not correlate with age (rho = -0.21, P = 0.34), NLR (rho = 0.27, P = 0.10), gender (P = 0.49), CEA (rho = 0.19, P = 0.12), max nodule diameter (rho = 0.12, P = 0.31), timing of metastases occurrence (P = 0.24), and performance status (P = 0.62).
Out of 127 treated patients, 115 reached the complete response (90.5%) after the first RFA and the remaining 12 patients needed a second procedure in order to achieve the complete tumor ablation.
Mean number of RFA sessions needed to achieve the complete ablation was 1.09 ± 0.23 with a median time to response of 3 mo (95%CI: 2-4).
No treatment-related deaths nor severe adverse events were observed.
Running log-rank analysis was performed to find a reliable LMR cut-off value able to predict OS as described in Figure 1.
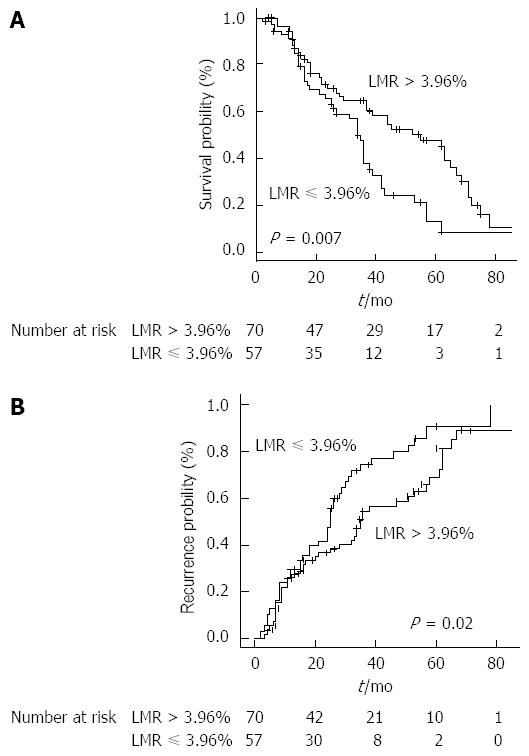
The most significant cut-off value was 3.96%. Stratifying the study population by this cut-off point, median OS resulted 55 mo (37-69) in patients with LMR > 3.96% and 34 (26-39) mo in patients with LMR ≤ 3.96% (HR = 0.53, 0.34-0.85, P = 0.007) (Figure 2A).
As for absolute monocyte count and NLR, the respective cut-off points used in uni/multivariate regression analysis were selected by means of receiver operating characteristic curve (data not shown).
During the study follow-up, 82 patients died.
Median OS was 38 mo (34-53) and survival rate (SR) was 89.4%, 40.4% and 33.3% at 1, 4 and 5 years respectively in the whole cohort.
NLR, LMR, CEA levels, number of nodules and nodule size were found to be predictors of OS in univariate analysis (Table 2). The multivariate Cox analysis restricted the significant predictors of OS to nodule size (P = 0.001) and LMR (P = 0.02) (Table 2).
| Variables | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Age (reference ≤ 65 yr) | 1.16 (0.68-1.95) | 0.39 | ||
| Gender (reference F) | 1.41 (0.58-3.1) | 0.14 | ||
| Monocyte ratio (reference ≤ 5%) | 1.38 (0.46-2.78) | 0.16 | ||
| NLR (reference ≤ 2.1) | 1.62 (0.87-3.64) | 0.03 | 1.48 (0.22- 2.79) | 0.14 |
| LMR (reference ≤ 3.96) | 0.53 (0.34-0.85) | 0.007 | 0.49 (0.29-0.96) | 0.02 |
| CEA (reference ≤ 34 ng/mL) | 1.83 (1.24-4.07) | 0.01 | 1.38 (1.03-2.54) | 0.32 |
| Number of nodules (reference 1) | 1.69 (1.13-4.22) | 0.02 | 1.27 (2.02-6.63) | 0.48 |
| Max diameter (reference ≤ 30 mm) | 2.1 (1.59-5.1) | 0.002 | 2.49 (1.45-5.46) | 0.001 |
| Primary tumor (reference colon) | 1.18 (0.46-1.43) | 0.34 | ||
| Timing (reference synchronous) | 1.29 (0.77-1.84) | 0.21 | ||
| ECOG PS (reference 0) | 1.54 (0.94-2.75) | 0.09 | ||
During the study follow-up, 90 patients experienced tumor recurrence, of which 26 (28.8%) were local recurrences (i.e., in the same liver segment) and 64 (71.2%) new metastases. Median TTR was 29 mo (22-35) with a recurrence-free survival (RFS) rate of 72.6%, 32.1% and 21.8% at 1, 4 and 5 years, respectively.
NLR, LMR, CEA levels, number of nodules and nodule size resulted predictors of TTR in univariate analysis, but only maximum diameter (P = 0.001) and LMR (P = 0.01) were confirmed in multivariate setting (Table 3).
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (reference ≤ 65 yr) | 1.01 (0.57-1.59) | 0.61 | ||
| Gender (reference F) | 1.29 (0.63-1.91) | 0.52 | ||
| Monocyte ratio (reference ≤ 5%) | 1.23 (0.47-2.11) | 0.45 | ||
| NLR (reference ≤ 2.1) | 1.49 (0.88-2.89) | 0.04 | 1.28 (0.21- 4.75) | 0.26 |
| LMR (reference ≤ 3.96) | 0.62 (0.40-0.95) | 0.02 | 0.41 (0.25-0.89) | 0.01 |
| CEA (reference ≤ 38 ng/mL) | 1.69 (1.04-4.21) | 0.02 | 1.38 (1.12-3.48) | 0.22 |
| Number of nodules (reference 1) | 1.41 (1.11-3.64) | 0.05 | 1.53 (1.23-3.49) | 0.10 |
| Max Diameter (reference ≤ 30 mm) | 2.29 (1.58-5.2) | < 0.001 | 3.59 (1.86-6.31) | 0.003 |
| Primary tumor (reference colon) | 1.12 (0.52-1.64) | 0.48 | ||
| Timing (reference synchronous) | 1.21 (0.89-1.75) | 0.41 | ||
| ECOG PS (reference 0) | 1.01 (0.72-1.34) | 0.87 | ||
TTR, when stratified by LMR, was 35 mo (28-57) in the group > 3.96% and 25 mo (18-30) in the group ≤ 3.96% (P = 0.02) (Figure 2B).
RFA represents a valuable therapeutic option for primary and secondary hepatic malignancies in patients unsuitable to surgery or unwilling to undergo major liver resection[3,25].
Because of differences in inclusion criteria, local expertise and use of adjuvant chemotherapy, post-RFA outcomes vary widely between the different published series, with local recurrence rates ranging from 2% to 60%[26,27].
The significant difference in patient outcomes reported throughout the literature has raised an increasing interest on the research and characterization of the main prognostic factors able to influence post-treatment results. Among them, biomarkers of the infiltrating inflammatory microenvironment may represent an important determinant for the clinical outcome in several malignancies such as HCC and CLMs[10,17].
In fact, the immune system plays an important role in cancer as it can destroy cancer cells but also establish the tumor microenvironment that facilitates cancer cell proliferation and metastasis occurrence. Lymphocytes are key immune cells in both humoral and cellular antitumor immune responses while monocytes are recruited into tumors where they alter the tumor microenvironment to promote cancer progression through local immune suppression and angiogenesis[28]. As a consequence high monocyte counts have been reported to be a poor prognostic factor in patients with solid tumors[18,29] and a low LMR, defined as the absolute lymphocyte count divided by the absolute monocyte count, has been proposed as a more reliable predictor of poorer prognosis in a wide range of cancers[30]. Thus a low LMR, which reflects the imbalance in immune response in favor of monocytes/macrophages over lymphocytes, may be responsible of a week antitumor immunity and a favorable microenvironment for tumor growth.
The interesting results of a recent study seem to support the use of pre-operative LMR as prognostic factor in CLMs patients after liver resection[19] but further studies are needed in order to confirm such findings; furthermore, whether this index may represent a reliable predictor of patients survival in other therapeutic fields such as loco-regional treatments is still unknown.
Therefore, aim of our study was to test the correlation between baseline LMR and survival outcomes in our series of CLM patients treated with RFA. To the best of our knowledge our study is the first report on the prognostic role of this novel inflammatory biomarker in metastatic CRC patients.
In order to exclude any theoretical influence of other tumoral and clinical parameters on LMR values, linear and logistic regression analyses were performed which confirmed the independence of lymphocyte-to-monocyte ratio from other baseline features. Afterwards, since LMR is a continuous variable, all values observed in our population were tested with log-rank analysis as predictors of survival in order to identify an accurate cut-off point aimed at stratifying the whole cohort in two different prognostic groups. The higher HR was obtained using as LMR cut-off level 3.96%.
Noteworthy, the cut-off point found in our analysis is consistent with other reports using LMR as predictor of patients survival in several cancers and this aspect further strengthens and puts our results in line with the current literature[19,30].
Patients presenting a higher pre-treatment LMR beyond the aforementioned cut-off showed significantly better survival outcomes with a median OS of 55 mo (vs 34 in patients with LMR ≤ 3.96%, P = 0.007) and median TTR of 35 mo (vs 25 in the group ≤ 3.96%, P = 0.02).
Cox multivariate analysis confirmed LMR, together with tumor size, as a significant predictor of either OS and TTR (P = 0.02 and 0.01; respectively).
Interestingly, LMR resulted superior to both NLR and absolute monocyte count in prognostic accuracy and this represents one of the most important findings in our study. If confirmed in larger prospective series our results could pave the way to the wide use of this useful and commonly available marker in the clinical field.
With regard to toxicity and tumor response, our results are in keeping with most of the published literature and confirm the effectiveness of RFA in CLM patients[3,26,27].
The main strength of the current study is the novelty of our findings that propose a novel, reliable and easily measurable prognostic factor for CLM patients. Second, our series constitutes one of the largest mono-institutional cohort of CLM patients treated with RFA and gives a further proof of the efficacy and safety of such an ablative technique in this oncological setting. Third, the very long recruitment period allowed us to report long-term data up to 10 years from the treatment. To the best of our knowledge only a minority of clinical papers[26,27] provided so complete and long-term data, which are indeed essential for the proper definition of patient prognosis in colorectal cancer.
On the other hand, our paper presents several limitations. First, the findings of the current study could be weakened by its retrospective design. However, completeness of the database and the long follow-up period allowed us to overcome this limitation. Furthermore, the single-center nature of our experience stands for an homogenous approach to CLM patients and exclude any difference in terms of operator expertise and follow-up accuracy. Second, the lack of an external validation cohort requires further studies in order to consider LMR as a reliable prognostic tool. Moreover, the relatively low number of patients with low LMR did not allow to observe a linear trend of log HR for survival. Therefore, LMR cut-off level we propose needs further confirmation in wider series with a larger range of baseline LMR values. Therefore, our study represents a pivotal report aimed at paving the way to well-designed prospective trials.
In conclusion, our study provides support for the use of a novel predictor of outcome for CLM patients. Hence, LMR should be tested in prospective trials in order to verify its accuracy and validate an unequivocal prognostic cut-off point.
Colorectal cancer (CRC) is the second cause of cancer-related mortality in developed countries and the third most common malignancy worldwide. Cumulative evidence has demonstrated that inflammatory cells infiltrates in the tumor microenvironment have a large influence on the biological behavior of several malignancies, including CRC. In particular, macrophages constitute the most represented leukocyte lineage in such infiltrates and are well-known to promote tumor proliferation, neo-angiogenesis and metastasis occurrence. As a consequence, immunohistochemical studies have validated the association between high monocyte/macrophage density in the tumoral stroma and unfavorable prognosis in a number of malignancies. Several inflammatory bio-markers have been tested in CRC liver metastases (CLMs), among them widely available and easy to use are those obtained from peripheral blood cell count such as neutrophil-to-lymphocyte ratio (NLR) and monocyte level (expressed as percentage) but none of them have been definitively and unequivocally validated. Since the pre-operative lymphocyte-to-monocyte ratio (LMR) has been recently found to correlate accurately with clinical outcomes in CLM patients undergoing hepatic resection, we decided to test whether this marker exerts a prognostic role in CRC patients with liver metastases treated with percutaneous RFA.
This study provides support for the use of a novel predictor of outcome for CLM patients. Hence, LMR should be tested in prospective trials in order to verify its accuracy and validate an unequivocal prognostic cut-off point.
The cut-off point found in our analysis (3.96%) is consistent with other reports using LMR as predictor of patients survival in several cancers and this aspect further strengthens and puts the results in line with the current literature. Patients presenting a higher pre-treatment LMR beyond the aforementioned cut-off showed significantly better survival outcomes with a median OS of 55 mo (vs 34 in patients with LMR ≤ 3.96%) and median TTR of 35 mo (vs 25 in the group ≤ 3.96%). Interestingly, LMR resulted superior to both neutrophil-to-lymphocyte ratio and absolute monocyte count in prognostic accuracy and this represents one of the most important findings in our study. With regard to toxicity and tumor response, our results are in keeping with most of the published literature and confirm the effectiveness of RFA in CLM patients.
This study provides support for the use of LMR as a novel predictor of outcome for CLM patients treated with RFA. If a patient has a baseline LMR > 3.96%, it will show better survival outcomes after RFA.
Very good and interesting study focusing on use of biomarkers used to predict efficacy of locoregional treatment. Methodology appears to be correct, the rationale of the study is convincing, and results are useful in clinical practice.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9981] [Article Influence: 907.4] [Reference Citation Analysis (15)] |
| 2. | Kornprat P, Jarnagin WR, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, D’Angelica M. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol. 2007;14:1151-1160. [PubMed] |
| 3. | Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, Chen MH, Choi BI, de Baere T, Dupuy D. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25:3438-3454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 4. | Facciorusso A, Del Prete V, Antonino M, Neve V, Amoruso A, Crucinio N, Di Leo A, Barone M. Conditional survival analysis of hepatocellular carcinoma patients treated with radiofrequency ablation. Hepatol Res. 2015;45:E62-E72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Facciorusso A, Del Prete V, Antonino M, Crucinio N, Neve V, Di Leo A, Carr BI, Barone M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig Liver Dis. 2014;46:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Facciorusso A, Del Prete V, Crucinio N, Muscatiello N, Carr BI, Di Leo A, Barone M. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J Gastroenterol Hepatol. 2015;30:1643-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Lee HY, Ko HK, Kim SH, Lee KS, Ro J, Park IH. Percutaneous radiofrequency ablation for liver metastases in breast cancer patients. Breast J. 2013;19:563-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Gervais DA, Arellano RS, Mueller PR. Percutaneous radiofrequency ablation of ovarian cancer metastasis to the liver: indications, outcomes, and role in patient management. AJR Am J Roentgenol. 2006;187:746-750. [PubMed] |
| 10. | Lin ZX, Ruan DY, Li Y, Wu DH, Ma XK, Chen J, Chen ZH, Li X, Wang TT, Lin Q. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol. 2015;21:10898-10906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Song A, Eo W, Lee S. Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol. 2015;21:12410-12420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 789] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 13. | Facciorusso A, Licinio R, Carr BI, Di Leo A, Barone M. MEK 1/2 inhibitors in the treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2015;9:993-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Facciorusso A, Antonino M, Del Prete V, Neve V, Scavo MP, Barone M. Are hematopoietic stem cells involved in hepatocarcinogenesis? Hepatobiliary Surg Nutr. 2014;3:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 15. | Facciorusso A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: recent findings and new perspectives. Curr Diabetes Rev. 2013;9:382-386. [PubMed] |
| 16. | Liu T, Fang XC, Ding Z, Sun ZG, Sun LM, Wang YL. Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Bio. 2015;5:682-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Chang Z, Zheng J, Ma Y, Zhao J, Wang C, Liu Z. The neutrophil-to-lymphocyte ratio as a predictor for recurrence of colorectal liver metastases following radiofrequency ablation. Med Oncol. 2014;31:855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Haruki K, Shiba H, Fujiwara Y, Furukawa K, Wakiyama S, Ogawa M, Ishida Y, Misawa T, Yanaga K. Perioperative change in peripheral blood monocyte count may predict prognosis in patients with colorectal liver metastasis after hepatic resection. J Surg Oncol. 2012;106:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Neofytou K, Smyth EC, Giakoustidis A, Khan AZ, Williams R, Cunningham D, Mudan S. The Preoperative Lymphocyte-to-Monocyte Ratio is Prognostic of Clinical Outcomes for Patients with Liver-Only Colorectal Metastases in the Neoadjuvant Setting. Ann Surg Oncol. 2015;22:4353-4362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [PubMed] |
| 21. | Facciorusso A, Di Maso M, Antonino M, Del Prete V, Panella C, Barone M, Muscatiello N. Polidocanol injection decreases the bleeding rate after colon polypectomy: a propensity score analysis. Gastrointest Endosc. 2015;82:350-358.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014;25:1691-705.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 23. | Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, Morosi C, Cascella T, Marchianò A, Camerini T, Bhoori S. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Facciorusso A, Del Prete V, Antonino M, Neve V, Crucinio N, Di Leo A, Carr BI, Barone M. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J Gastroenterol Hepatol. 2014;29:1905-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia. 2016;1-6. [PubMed] |
| 26. | Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 27. | Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, Alago W, Durack JC, Maybody M, Brody LA. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes-A 10-year Experience at a Single Center. Radiology. 2016;278:601-611. [PubMed] |
| 28. | Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6:1670-1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1246] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 29. | Lee SD, Kim SH, Kim YK, Lee SA, Park SJ. Prognostic significance of preoperative peripheral blood monocyte ratio in patients with hepatocellular carcinoma. World J Surg. 2014;38:2377-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chen F, Lassandro F S- Editor: Qi Y L- Editor: A E- Editor: Wang CH