Published online Apr 28, 2016. doi: 10.3748/wjg.v22.i16.4149
Peer-review started: January 25, 2016
First decision: February 18, 2016
Revised: February 26, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: April 28, 2016
Processing time: 86 Days and 16.5 Hours
AIM: To elucidate the mechanism of thymoquinone (TQ)-induced apoptosis in human gastric cancer cells in vitro and in vivo.
METHODS: HGC27, BGC823, and SGC7901 cells were cultured in vitro and treated with TQ (0, 10, 25, 50, 75, 100, 125 μmol/L) for 12 h, 24 h, and 36 h, and then the proliferation inhibitory rates were detected by methylthiazole tetrazolium assay. Apoptosis was observed after Hoechst staining. The protein expressions of signal transducer and activator of transcription (STAT)3, p-STAT3, STAT5, p-STAT5, phospho-janus-activated kinase 2 (JAK2), JAK2, p-Src, Src, glyceraldehyde-3-phosphate dehydrogenase, lamin-A, survivin, Cyclin D, Bcl-2, Bax, peroxisome proliferator activated receptor, and caspase-3,7,9 were detected by western blot. Cell cycle and apoptosis were determined with flow cytometry. TQ induced dose-dependent apoptotic cell death in HGC27 cells was measured by Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) analysis and Hoechst 33258.
RESULTS: TQ inhibited the phosphorylation of STAT3 but not STAT5. TQ-induced downregulation of STAT3 activation was associated with a reduction in JAK2 and c-Src activity. TQ also downregulated the expression of STAT3-regulated genes, such as Bcl-2, cyclin D, survivin, and vascular endothelial growth factor, and activated caspase-3,7,9. Consistent with the in vitro results, TQ was significantly effective as an antitumor agent in a xenograft tumor mouse model.
CONCLUSION: This study provides strong evidence that downregulation of the STAT3 signaling pathway mediates TQ-induced apoptosis in gastric cancer.
Core tip: Thymoquinone (TQ) has been demonstrated to exert biological activity in gastric cancer. However, the specific mechanism of TQ in gastric cancer has not been examined. In order to elucidate the mechanism of TQ-induced apoptosis in human gastric cancer cells, we investigated the effects of TQ on signal transducer and activator of transcription (STAT)3 and its related pathway in vitro and in vivo.
- Citation: Zhu WQ, Wang J, Guo XF, Liu Z, Dong WG. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J Gastroenterol 2016; 22(16): 4149-4159
- URL: https://www.wjgnet.com/1007-9327/full/v22/i16/4149.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i16.4149
Based on the Global Burden of Cancer Study (GLOBOCAN) estimates, about 14.1 million new cancer cases and 8.2 million deaths occurred in 2012 worldwide[1]. Among them, gastric cancer ranks fourth and third for incidence rate and mortality rate, respectively, in male patients and ranks fifth in female patients[1]. Chemotherapy is commonly used in the treatment of gastric cancer. Commonly used chemotherapy drugs are 5-fluorouracil (5-FU), cisplatin, and doxorubicin, but they are limited by toxic side effects, the development of resistance, and other defects[2]. Therefore, it is important to search for low toxicity and efficient anti-cancer drugs that inhibit gastric cancer development.
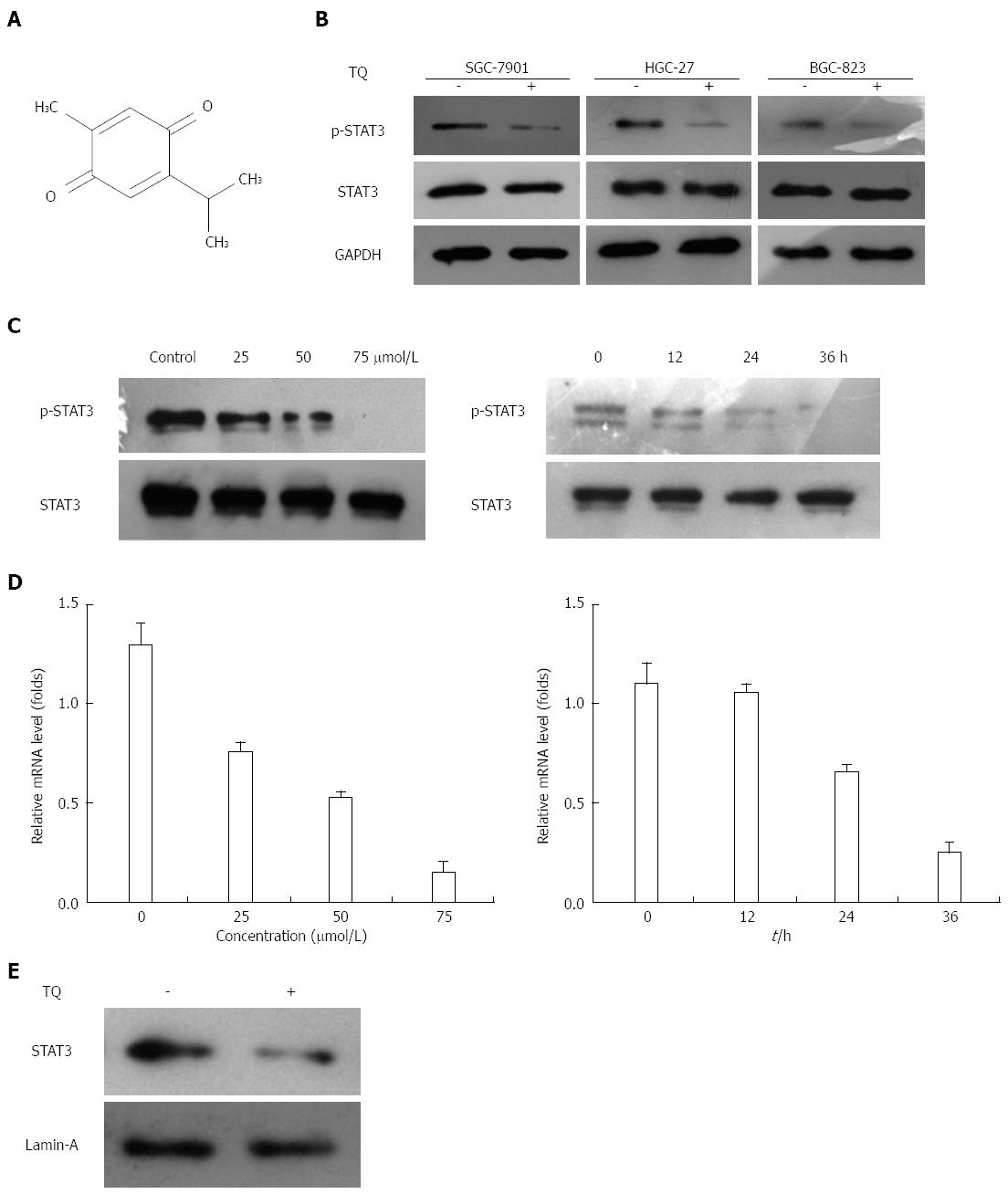
Thymoquinone (TQ, also called 2-isopropyl-5-methyl-1,4-benzo-quinone, C10H12O2) is the active constituent of black cumin (Nigella sativa) seed oil (Figure 1A) and was first extracted by EI-Dakhakhany[3]. TQ has been shown to suppress proliferation and induce apoptosis and drug resistance in various tumor cells, including colorectal carcinoma, pancreatic carcinoma, breast adenocarcinoma, prostate cancer, etc.[4-9]. A phase I study reported that in adult patients with solid tumors or hematological malignancies who were treated with TQ, there were no significant systemic toxicities[10]. It was also reported that the human body could tolerate a dose of 75 to 2600 TQ mg/d. Because of its high efficiency, low toxicity, and natural features in cancer prevention and treatment, TQ has gradually attracted the attention of scholars worldwide, but studies on tumors of the digestive system, especially gastric cancer, are rare.
Signal transducer and activator of transcription (STAT) has been shown to be a fundamental factor in tumor cell survival and proliferation[11]. STAT3, a member of the STAT protein family, is a critical molecule of the janus-activated kinase (JAK)/STAT signaling pathway through many signals, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF). STAT3 can be activated by a variety of ligands related to these signals[12]. It was reported in a variety of human malignancies, such as gastric, colon, breast, and lung, that abnormal expression and constitutive activation of STAT3 are involved[12,13]. Once activated, STAT3 would continue to promote phosphorylation-induced homodimerization, leading to nuclear translocation, DNA binding, and subsequent gene transcription (e.g., Bcl-xL, Bcl-2, survivin, cyclin D, Mcl-1). In addition, STAT3 could also control VEGF expression, which is necessary for angiogenesis and the maintenance of tumor vasculature[14]. STAT3 has been implicated in the inhibition of immune responses to tumor growth by blocking expression of pro-inflammatory factors. A previous study showed that TQ could induce apoptosis and augment 5-FU-induced apoptosis in gastric cancer cells[15]. However, specific mechanisms underlying the effects of TQ in gastric cancer have not been examined.
Based on preliminary studies, we explored the specific mechanisms of TQ-induced regulation of proliferation and apoptosis in gastric cancer cells.
Three human gastric cancer cells (HGC27, BGC823, and SGC7901) were obtained from Key Laboratory of Hubei Province for Digestive System Disease (Wuhan University). The cells were maintained in continuous exponential growth in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 medium supplemented with heat-inactivated 10% fetal bovine serum (FBS) and 1% antibiotics (100 IU penicillin and 100 μg/mL streptomycin) in a humidified incubator at 37 °C and 5% CO2.
Protein expression levels were assessed by western blot analysis. Briefly, cells were collected, washed twice, and lysed with lysis buffer. Cell lysates were kept on ice for 30 min and centrifuged at 4 °C. Samples were then boiled in loading buffer and separated by 10% SDS-PAGE. After electrophoresis, protein was transferred onto a nitrocellulose membrane (Millipore, Bedford, MA, United States), which was incubated with blocking solution [10% non-fat dry milk in Tris-buffered saline with tween (TBST) containing 0.05% Tween-20] for 2 h and immunoblotted with primary antibodies, including STAT3, p-STAT3, STAT5, p-STAT5, p-JAK2, JAK2, p-Src, Src, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Lamin-A, survivin, Cyclin D, Bcl-2, Bax, peroxisome proliferator activated receptor, caspase-3,7,9 (Cell Signaling Technology, Beverly, MA, United States, 1:1000), overnight at 4 °C. After washing with TBST, the membranes were incubated with anti-rabbit (or anti-mouse) immunoglobulin conjugated to horseradish peroxidase secondary antibody (Promega, Madison, WI, United States, 1:500) for 2 h at room temperature, developed using the enhanced chemiluminescence (ECL) western blotting kit (Millipore, Bedford, MA, United States), and then exposed to Kodak X-ray Film. Protein band intensities were determined densitometrically using the video imaging CMIASWIN system (BioRad, Hercules, CA, United States).
Total RNA and complementary DNA (cDNA) were extracted from the cells for real-time reverse transcription polymerase chain reaction (RT-PCR) analysis as described previously[16]. The cDNA was used as the template in an SYBR Green Real-Time PCR Master Mix (Invitrogen, Carlsbad, CA, United States). The PCR reaction was performed with an ABI PRISM 7300 PCR and detection system (Applied Biosystems, Carlsbad, CA, United States). The following primers were used: STAT3 sense 5’- GCCCTTTGGAACGAAGGGTA-3’ and STAT3 antisense 5’- TGGTATTGCTGCAGGTCGTT-3’; GAPDH sense, 5’-TTTGGTATCGTGGAAGGAC-3’, and GAPDH anti-sense, 5’- GTGGAGGAGTGGGTGTCGC-3’. The relative levels of STAT3 mRNA transcripts were normalized to the control GAPDH. For relative quantification, the expression levels of the STAT3 gene were calculated based on the method of 2-ΔΔCt[17].
Cells were seeded into 12-well plates at a density of 5 × 104 cells/well prior to transfection. Cells were transfected with p-STAT3-TA-luc (Clontech, Palo Alto, CA, United States) or control vector using Genefectin transfection reagent (Genetrone Biotech, Seoul, South Korea). After 24 h of transfection, cells were treated with TQ for an additional 24 h, and cell lysis was carried out with 1 × reporter lysis buffer. After mixing the cell lysates with luciferase substrate (Promega), luciferase activity was measured using a luminometer (Tecan Trading AG, Shanghai, China). The β-galactosidase assay was carried out according to the supplier’s instructions (Promega Enzyme Assay System) for normalizing the luciferase activity, and the results are expressed as fold transactivation.
Cell proliferation was assessed with 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) (Sigma St. Louis, MO, United States) colorimetric method. Gastric cancer cells were cultured in a 96-well plate in the presence of 0, 25, 50, 75, or 100 μmol/L TQ for 12, 24, or 36 h. MTT reagent (20 μL) was added into each well and incubated for 4 h at 37 °C. After the purple precipitate was visible, the cells were added with 150 μL dimethylsulfoxide and incubated at room temperature in the dark for 2 h. The absorbance was recorded at 490 nm. For each experiment, the total procedure was repeated three times.
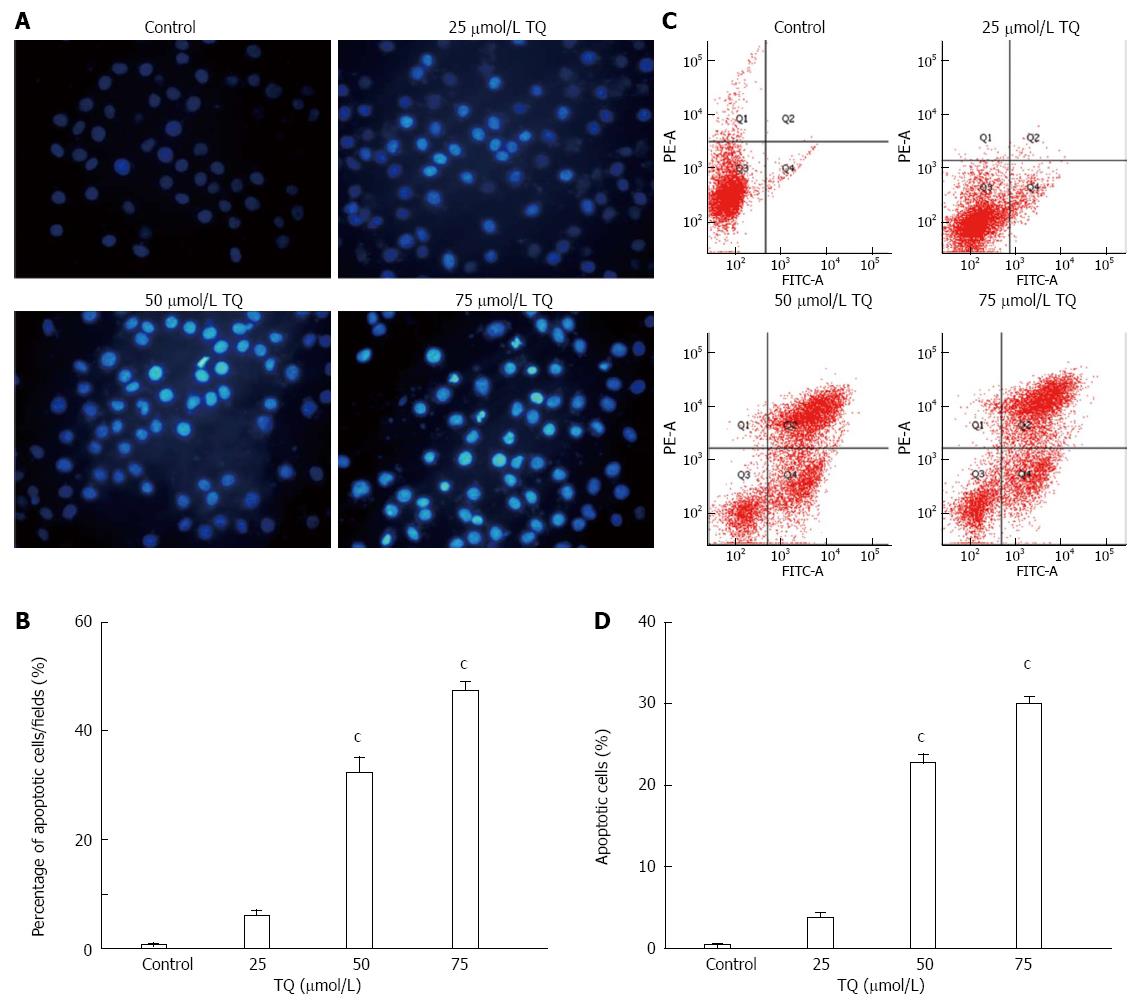
Gastric cancer cells in exponential growth were placed at a final concentration of 5 × 105 cells per well in a six-well plate, which was pretreated with 25 μmol/L, 50 μmol/L, or 75 μmol/L TQ for 24 h. The cells were subsequently fixed, washed three times with phosphate buffered saline (PBS), and stained with Hoechst 33258 (Sigma-Aldrich) according to the manufacturer’s protocol. Apoptotic features were assessed by analyzing chromatin condensation and by staining the fragments under an inverted fluorescent microscope (Olympus, Center Valley, PA, United States).
Gastric cancer cells were cultured in six-well plates at 1 × 106 cells/well in DMEM medium supplemented with 0.5% FBS and different concentrations of TQ as described above. Cells were washed twice with cold PBS and centrifuged, and then incubated with 5 mL of Annexin V-fluorescein isothiocyante (FITC) and 10 mL of propidium iodide (PI) at room temperature for 5 min in the dark. Flow cytometric analysis was performed with a FACSCalibur using the CellQuest software (BDIS, San Jose, CA, United States). For each experiment, the total procedure was repeated three times.
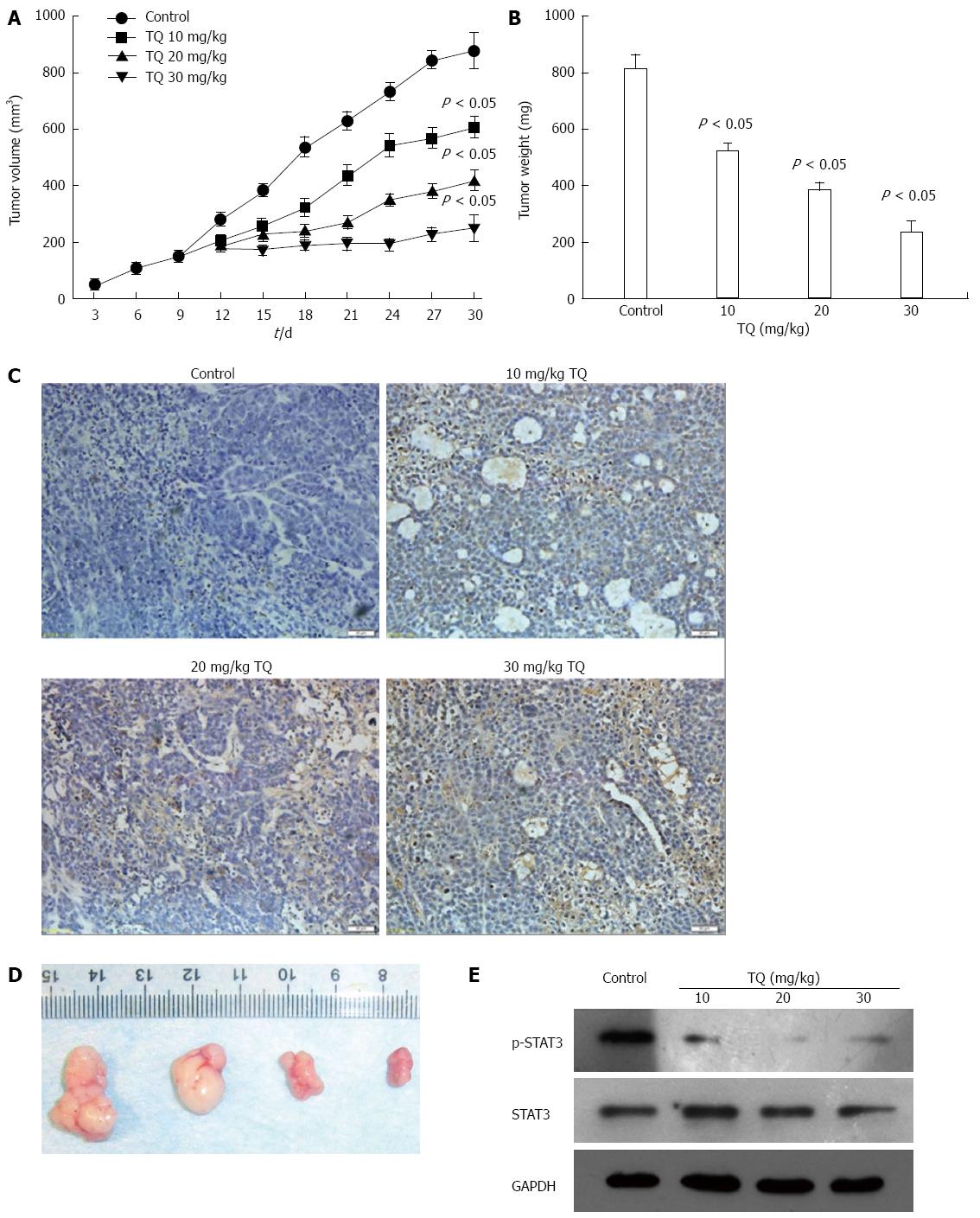
Female BALB/c athymic nude mice (4-wk-old) were obtained from the Center of Experimental Animals of Wuhan University, and all procedures were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Nude mice were divided into four groups, and mice were housed (four animals per cage) in standard mouse plexiglass cages in a light and temperature-controlled environment. Mice were provided with food and water ad libitum. Gastric cancer cells were harvested from subconfluent cultures, washed in serum-free medium, and resuspended in PBS. Cells (5 × 106) resuspended in 0.2 mL PBS were subcutaneously inoculated into the lower right flank of nude mice. When the developing tumors reached 100-150 mm3 in size, mice were randomly assigned to the following treatment groups (n = 5): (1) untreated control, equal volume physiologic saline; (2) 10 mg/kg TQ; (3) 20 mg/kg TQ; and (4) 30 mg/kg TQ. All therapies were administered three times per week via intraperitoneal injection. Next, the mice were weighed, and the size of each tumor and its central necrotic area was monitored using calipers every 3 d. Following the last dose of TQ, all mice were sacrificed on day 30. During the autopsy procedure, the tumor was neatly excised and weighed. One part of the tissue was fixed in formalin, and another part was frozen in liquid nitrogen. Ethical approval was obtained prior to the start of the experiments.
For histological examination, tumor tissues were fixed in 10% buffered formalin and embedded in paraffin, and tissue sections (4 μm) were prepared. The TdT-mediated dUTP-biotin nick end-labeling (TUNEL) assay for apoptosis was conducted using an apoptosis detection kit (Roche Diagnostics, Branchburg, NJ, United States) according to the manufacturer’s instructions. Positive cells were counted as the number of TUNEL-labeled cells per 100 epithelial cancer cells in 10 fields of the most affected tumor areas with 400 × magnification and analyzed using light microscopy (Carl Zeiss, Thornwood, NY, United States).
Results were analyzed by SPSS version 17.0 (SPSS, Inc., Chicago, IL, United States). All data are presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA, multiple comparisons) and t-tests (two groups comparisons) were performed accordingly. P < 0.05 was considered to be statistically significant.
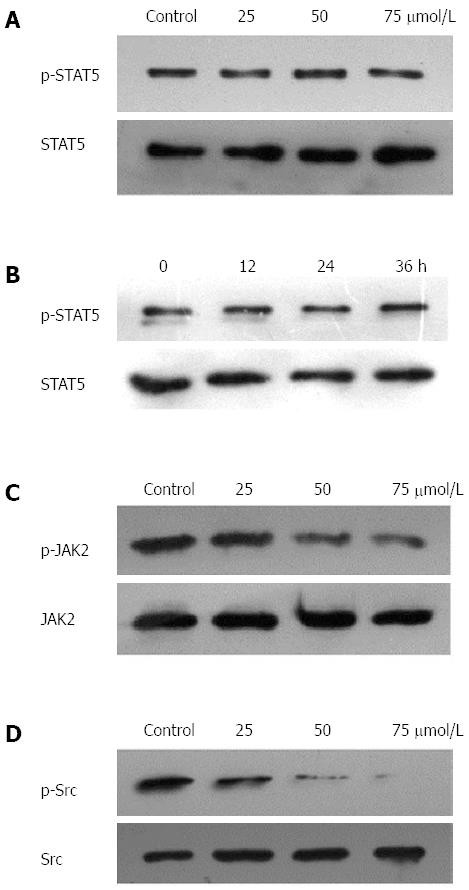
Since HGC27, BGC823, and SGC7901 cells were shown to express constitutive STAT3 activation, we determined whether TQ could inhibit this activation in these cells. Based on western blot results, the protein level of p-STAT3 was significantly reduced in all three types of cancer cells treated with TQ compared to cells not treated with TQ (Figure 1B). These findings suggest that TQ inhibited STAT3 activation in all these cells in a concentration- and time-dependent manner, with maximum inhibition occurring at 75 μmol/L (Figure 1C). Although TQ did not affect protein levels of STAT3 (Figure 1B, lower panel), it did suppress the mRNA expression of STAT3 in a concentration- and time-dependent manner, as demonstrated by real-time RT-PCR (Figure 1D). Because the active dimer of STAT3 is capable of translocating to the nucleus and inducing transcription of specific target genes[18], we determined whether TQ suppresses the nuclear translocation of STAT3. Western blot clearly demonstrated that TQ blocked the translocation of STAT3 into the nucleus in HGC27 cells (Figure 1E). The phosphorylation of STAT5 and the expression of STAT5 were not affected by TQ (Figure 2A and B), indicating that TQ specifically inhibits STAT3 tyrosine phosphorylation.
Since JAK2 and c-Src kinase are two of the main kinases involved in the activation of STAT3[19,20], we examined the effect of TQ on the expression and phosphorylation of these two proteins. As shown in Figure 2C, JAK2 was constitutively active in HGC27 cells, and pretreatment with TQ clearly suppressed its phosphorylation in a time-dependent manner. TQ treatment did not affect total JAK2 levels. In addition, we found that TQ suppressed the constitutive phosphorylation of c-Src kinase in a time-dependent manner (Figure 2D), while the levels of total c-Src kinase remained unchanged under the same conditions (Figure 2D, lower panel).
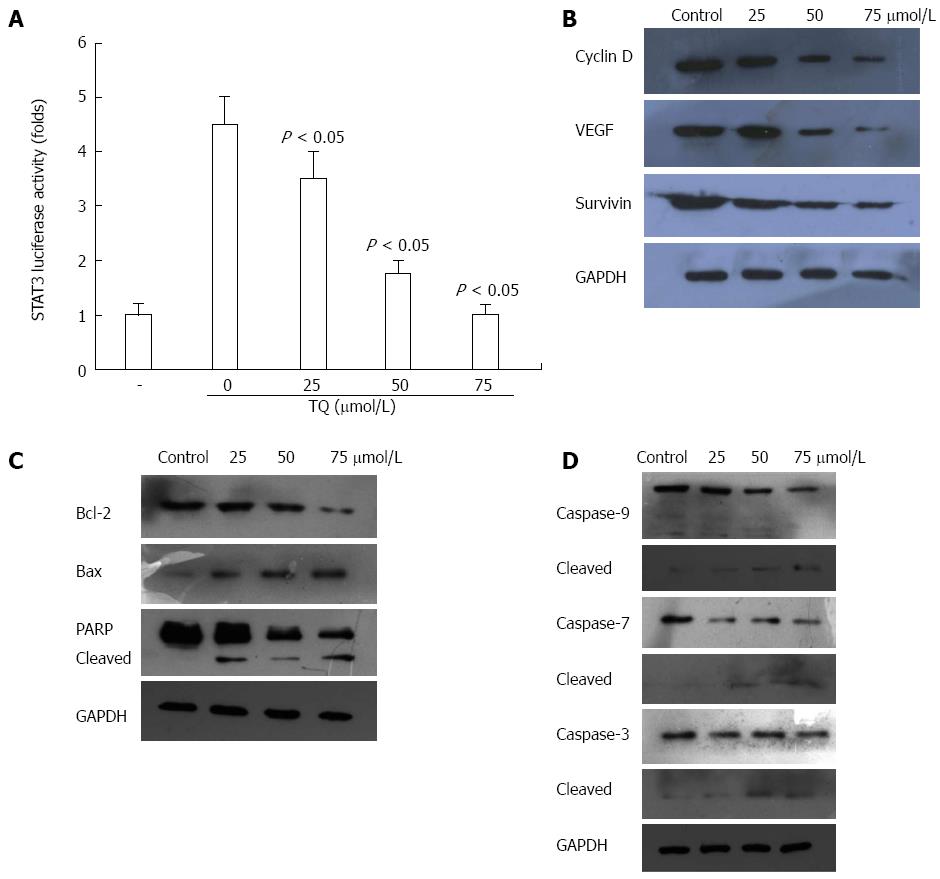
STAT3 is constitutively active in gastric cancer and plays a critical role in cell proliferation through transcriptional activation of pro-survival genes[21]. We found that TQ reduced STAT3 reporter gene activity in HGC27 cells transiently transfected with STAT3-luc vector (Figure 3A). Moreover, TQ attenuated the protein expression of STAT3 target gene products, such as survivin, cyclin-D, VEGF, and Bcl-2 and increased the expression levels of Bax (Figure 3B and C).
Next, we investigated whether suppression of constitutively active STAT3 in HGC27 cells by TQ caused cell apoptosis. In HGC27 cells, TQ activated pro-caspase-3,7,9 in a concentration-dependent manner (Figure 3D). Activation of downstream procaspase-3 led to the cleavage of a 116 kDa poly(ADP-ribose)polymerase (PARP) protein into an 85 kDa fragment (Figure 3C). These results clearly suggested that TQ induces caspase-3-dependent apoptosis in HGC27 cells.
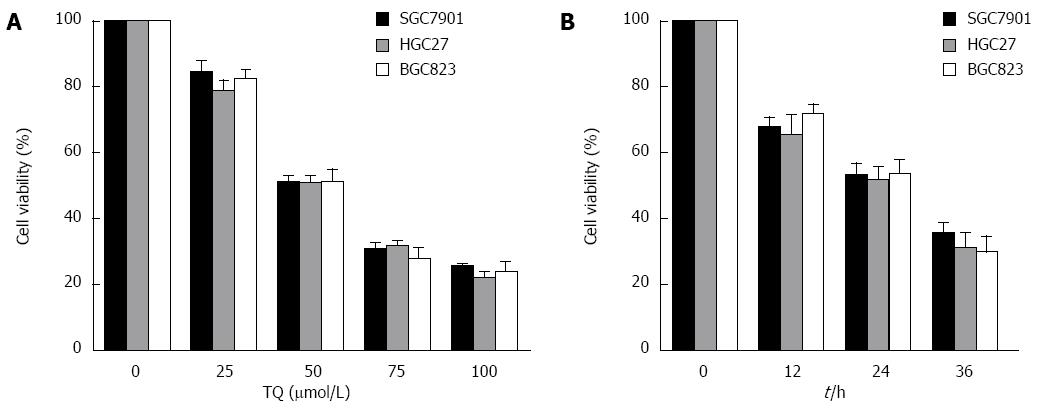
According to the results of the MTT assay, cell viability was reduced in the presence of TQ (25, 50 or 75 μmol/L) in a time- and concentration-dependent manner (Figure 4). Annexin V staining of cells treated with the indicated concentrations of TQ showed that the compound induced apoptosis in a concentration-dependent manner (Figure 5A). In addition, Hoechst 33258 staining was performed in TQ treated cells and showed that apoptotic bodies containing nuclear fragments were generated in apoptotic cells (Figure 5B).
Based on the induction of apoptosis in gastric cancer cell in vitro, the antitumor effect of TQ was dose-dependent. In a xenograft tumor mouse model, the treatments of intraperitoneally administered injections of TQ for 30 d led to significant decreases in tumor weight and size compared to the control group mice (P < 0.05). (Figure 6A and B). Tumor tissues isolated from the xenograft mice of the four groups were processed for TUNEL assay, and the results showed (Figure 6C and D) that the tumors derived from high doses of TQ treated mice exhibited a markedly higher count of apoptotic bodies compared with that from control group mice. In addition, we measured the levels of STAT3 in tumor tissue by western blot and found that TQ inhibited STAT3 phosphorylation in vivo (Figure 6E).
As the second most common cause of cancer-related death in the world, gastric cancer has been reported to have particularly high incidence and mortality rates in eastern Asia. Its survival rate is substantially lower than that of patients with other types of cancers[22].
TQ, the active constituent of black cumin seed oil, was shown to inhibit the proliferation of various types of tumor cells, including lung carcinoma, breast adenocarcinoma, pancreatic cancer, colorectal cancer, acute lymphoblastic leukemia, and prostate cancer[23,24]. It has also been shown to enhance detoxification and inhibit benzo(a)pyrene (BP)-induced fore-stomach tumors in a female Swiss albino mouse model[25]. TQ was also induced apoptosis and augmented 5-FU-induced apoptosis in gastric cancer cells[15]. In this study, we found that TQ inhibited phosphorylation of STAT3 in three gastric cancer cells. TQ also inhibited the expression of the STAT3 gene in a concentration- and time-dependent manner, as demonstrated by real-time RT-PCR.
Phosphorylation of STAT3 is required for its activation and results in dimerization, nuclear translocation, DNA binding, and transcriptional activation of target genes[26]. To investigate the mechanism of TQ-induced STAT3 inhibition in HGC27 cells, we analyzed the activation of upstream protein kinases, such as JAK and Src. We found that TQ could inhibit JAK2 and Src phosphorylation, and presumably the suppression of STAT3 phosphorylation at Tyr705 was due to the inhibition of JAK2 and Src activity. Interestingly, TQ-induced inhibition of tyrosine phosphorylation was only observed for STAT3, not STAT5. These results suggest that TQ specifically inhibited phosphorylation of STAT3 at Tyr705.
We also observed that TQ suppressed the expression of several STAT3-regulated genes, including proliferative (cyclin D1), anti-apoptotic (Bcl-2, survivin), and angiogenic (VEGF) gene products in HGC27 cells. Activation of STAT3 signaling was thought to induce survivin gene expression and confer resistance to apoptosis in human breast cancer cells[27]. It was reported that blocking cell death by Bcl-2 and Bcl-xL could be induced by a variety of chemotherapeutic agents, in parallel with an increase in chemoresistance[28,29]. Thus, we considered that the downregulation of the expression of Bcl-2, Bcl-xL, and survivin was likely to be linked with the ability of TQ to induce apoptosis in HGC27 cells, as evidenced by cleavage of PARP. In addition, we observed that TQ could activate caspase-3,7,9 activities in a concentration-dependent manner. The MTT assay showed that TQ could inhibit cell viability in a concentration- and time-dependent manner. TQ dose-dependently induced apoptotic cell death in HGC27 cells, as estimated by Annexin V-FITC/PI analysis and Hoechst33258.
In addition to these in vitro studies, we further analyzed the effect of TQ on xenograft tumor in vivo. We found that TQ treatment exhibited obvious antitumor effects in a xenograft tumor mouse model. The therapeutic effect was demonstrated by TUNEL analysis, which displayed obvious cell death in the tumor masses via apoptosis. Moreover, STAT3 protein was measured in tumor tissue, and we found that the levels of p-STAT3 in the tumor tissue were significantly decreased after TQ treatment.
In conclusion, the present study demonstrated that TQ inhibited proliferation and induced apoptosis via down-regulation of the STAT3 signaling pathway. TQ may be a promising candidate as a cancer chemopreventive or chemotherapeutic agent.
Thymoquinone (TQ) has been demonstrated to exert biological activity in gastric cancer. However, the specific mechanism by which TQ affects gastric cancer remains to be examined. In order to elucidate the mechanism of TQ-induced apoptosis in human gastric cancer cells, we investigated the effects of TQ on signal transducer and activator of transcription (STAT)3 activation and its relative pathway both in vitro and in vivo.
In 2014, Kundu et al found in human colon cancer HCT116 cells that TQ induced apoptosis through inactivation of STAT3 by blocking janus-activated kinase (JAK)2- and Src-mediated phosphorylation of endothelial growth factor (EGF) receptor tyrosine kinase. This finding is similar with the authors’ study, thus they examined JAK2- and Src as well.
It was previously shown that TQ could induce apoptosis in gastric cancer cells. However, the specific mechanism of TQ in gastric cancer had not been examined. Our study demonstrated in gastric tumor cells that TQ inhibited proliferation and induced apoptosis though downregulation of the STAT3 signaling pathway. These findings suggest that TQ may be a potential chemopreventive or chemotherapeutic candidate for gastric cancer.
Since STAT3 is one of the main signaling pathways involved in TQ-mediated inhibition of gastric cancer, TQ may be used safely clinically. However, it remains to be determined if TQ will be useful in gastric cancer with drug resistance. In the future, this will be examined.
Thymoquinone (TQ, also called 2-isopropyl-5-methyl-1,4-benzo-quinone, C10H12O2) is the active constituent of black cumin (Nigella sativa) seed oil (Figure 1A) and was first extracted by EI-Dakhakhany. STAT refers to signal transducer and activator of transcription. VEGF refers to vascular endothelial growth factor.
In this article, the authors analyzed the mechanism underlying the effect of TQ in the treatment of gastric cancer. This study has practical research significance, providing important information to guide the clinical administration of TQ in the future.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21466] [Article Influence: 1951.5] [Reference Citation Analysis (6)] |
| 2. | Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol. 2008;43:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | El-Dakhakhany M. Studies on the chemical constitution of Egyptian N. Sativa L. seeds. Planta Medica. 1963;11:465-470. [DOI] [Full Text] |
| 4. | Gali-Muhtasib H, Kuester D, Mawrin C, Bajbouj K, Diestel A, Ocker M, Habold C, Foltzer-Jourdainne C, Schoenfeld P, Peters B. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008;68:5609-5618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford). 2009;11:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Connelly L, Barham W, Onishko HM, Sherrill T, Chodosh LA, Blackwell TS, Yull FE. Inhibition of NF-kappa B activity in mammary epithelium increases tumor latency and decreases tumor burden. Oncogene. 2011;30:1402-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M, Dou QP, Reddy GP. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007;67:7782-7788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Das S, Dey KK, Dey G, Pal I, Majumder A, MaitiChoudhury S, kundu SC, Mandal M. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS One. 2012;7:e46641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Attoub S, Sperandio O, Raza H, Arafat K, Al-Salam S, Al Sultan MA, Al Safi M, Takahashi T, Adem A. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam Clin Pharmacol. 2013;27:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Al-Amri AM, Bamosa AO. Phase I safety and clinical activity study of thymoquinone in patients with advanced refractory malignant disease. Shiraz E-Med J. 2009;10:107-111. |
| 11. | Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 540] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 12. | Xiong A, Yang Z, Shen Y, Zhou J, Shen Q. Transcription Factor STAT3 as a Novel Molecular Target for Cancer Prevention. Cancers (Basel). 2014;6:926-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 13. | Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921-4929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 257] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 955] [Article Influence: 39.8] [Reference Citation Analysis (7)] |
| 15. | Lei X, Lv X, Liu M, Yang Z, Ji M, Guo X, Dong W. Thymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem Biophys Res Commun. 2012;417:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Song J, Peng XL, Ji MY, Ai MH, Zhang JX, Dong WG. Hugl-1 induces apoptosis in esophageal carcinoma cells both in vitro and in vivo. World J Gastroenterol. 2013;19:4127-4136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 139175] [Article Influence: 5567.0] [Reference Citation Analysis (3)] |
| 18. | Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 761] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 19. | Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1113] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 20. | Schreiner SJ, Schiavone AP, Smithgall TE. Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J Biol Chem. 2002;277:45680-45687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 566] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 22. | Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535-e547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 23. | Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, Sarkar FH, Mohammad RM. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69:5575-5583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Kundu J, Choi BY, Jeong CH, Kundu JK, Chun KS. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and Src-mediated phosphorylation of EGF receptor tyrosine kinase. Oncol Rep. 2014;32:821-828. [PubMed] |
| 25. | Badary OA, Al-Shabanah OA, Nagi MN, Al-Rikabi AC, Elmazar MM. Inhibition of benzo(a)pyrene-induced forestomach carcinogenesis in mice by thymoquinone. Eur J Cancer Prev. 1999;8:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Groner B, Lucks P, Borghouts C. The function of Stat3 in tumor cells and their microenvironment. Semin Cell Dev Biol. 2008;19:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 447] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 28. | Simonian PL, Grillot DA, Nuñez G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood. 1997;90:1208-1216. [PubMed] |
| 29. | Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S. BCL-X expression in multiple myeloma: possible indicator of chemoresistance. Cancer Res. 1998;58:256-262. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Caboclo JLF, Martin-Villa JM S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang DN