Published online Apr 14, 2016. doi: 10.3748/wjg.v22.i14.3758
Peer-review started: September 1, 2015
First decision: September 29, 2015
Revised: November 2, 2015
Accepted: January 17, 2016
Article in press: January 19, 2016
Published online: April 14, 2016
Processing time: 213 Days and 17.1 Hours
AIM: To investigate the biological effects of internal irradiation, and the therapeutic effectiveness was assessed of 131I-labeled anti-epidermal growth factor receptor (EGFR) liposomes, derived from cetuximab, when used as a tumor-targeting carrier in a colorectal cancer mouse model.
METHODS: We described the liposomes and characterized their EGFR-targeted binding and cellular uptake in EGFR-overexpressing LS180 colorectal cancer cells. After intra-tumor injections of 74 MBq (740 MBq/mL) 131I-antiEGFR-BSA-PCL, we investigated the biological effects of internal irradiation and the therapeutic efficacy of 131I-antiEGFR-BSA-PCL on colorectal cancer in a male BALB/c mouse model. Tumor size, body weight, histopathology, and SPECT imaging were monitored for 33 d post-therapy.
RESULTS: The rapid radioiodine uptake of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL reached maximum levels at 4 h after incubation, and the 131I uptake of 131I-antiEGFR-BSA-PCL was higher than that of 131I-BSA-PCL in vitro. The 131I tissue distribution assay revealed that 131I-antiEGFR-BSA-PCL was markedly taken up by the tumor. Furthermore, a tissue distribution assay revealed that 131I-antiEGFR-BSA-PCL was markedly taken up by the tumor and reached its maximal uptake value of 21.0 ± 1.01 %ID/g (%ID/g is the percentage injected dose per gram of tissue) at 72 h following therapy; the drug concentration in the tumor was higher than that in the liver, heart, colon, or spleen. Tumor size measurements showed that tumor development was significantly inhibited by treatments with 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL. The volume of tumor increased, and treatment rate with 131I-antiEGFR-BSA-PCL was 124% ± 7%, lower than that with 131I-BSA-PCL (127% ± 9%), 131I (143% ± 7%), and normal saline (146% ± 10%). The percentage losses in original body weights were 39% ± 3%, 41% ± 4%, 49% ± 5%, and 55% ± 13%, respectively. The best survival and cure rates were obtained in the group treated with 131I-antiEGFR-BSA-PCL. The animals injected with 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL showed more uniform focused liposome distribution within the tumor area.
CONCLUSION: This study demonstrated the potential beneficial application of 131I-antiEGFR-BSA-PCL for treating colorectal cancer. 131I-antiEGFR-BSA-PCL suppressed the development of xenografted colorectal cancer in nude mice, thereby providing a novel candidate for receptor-mediated targeted radiotherapy.
Core tip: This paper describes liposomes that were assessed for EGFR-targeted binding and cellular uptake in EGFR-overexpressing LS180 colorectal cancer cells and a mouse model of colorectal cancer. Anti-EGFR and non-targeted liposomes were labeled with 131I using the chloramine-T method. The time-dependent cellular uptake of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL demonstrated the slow-release effects of nanoparticles. The results of confocal microscopic analysis revealed the significant uptake of antiEGFR-BSA-PCL in LS180 cells. This study also investigated the biological effects of internal irradiation and the therapeutic efficacy of 131I-antiEGFR-BSA-PCL on colorectal cancer in a BALB/c mouse model. To address this issue, tumor size, body weight, histopathology, and SPECT imaging were monitored for 33 d post-therapy. The 131I-antiEGFR-BSA-PCL was demonstrated to be superior in regard to cellular binding and uptake compared with control BSA-PCL in the mouse model.
- Citation: Li W, Ji YH, Li CX, Liu ZY, Li N, Fang L, Chang J, Tan J. Evaluation of therapeutic effectiveness of 131I-antiEGFR-BSA-PCL in a mouse model of colorectal cancer. World J Gastroenterol 2016; 22(14): 3758-3768
- URL: https://www.wjgnet.com/1007-9327/full/v22/i14/3758.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i14.3758
Cancer is a major threat to public health in the 21st century and one of the leading causes of death worldwide. However, the mechanisms of carcinogenesis, cancer invasion, and metastasis remain unclear[1]. Colorectal cancer is the third most common cancer worldwide. Approximately 25% of patients with colorectal cancer present with overt metastatic disease; metastatic disease develops in 40% to 50% of newly diagnosed patients[2]. The clinical need for image-based detection and monitoring for effective therapeutic management to reduce the mortality caused by this disease has not been met to date. Therefore, novel strategies and treatment concepts are needed, particularly because conventional cancer therapies, such as external radiation and chemotherapy, have problems such as poor specificity. These conventional therapies often cause dose-limiting side effects and inhibit the possibility of curative treatments.
Nanomedicine has exploited the possibility of designing tumor-targeted nanocarriers that can deliver radionuclide payloads in a site- or molecule-selective manner based on their magnetic properties, thereby improving the effectiveness and safety of cancer molecular imaging and therapies. Many studies have proven the feasibility of the use of targeted nanoparticle probes. Nanotechnology can lead to fundamental changes and improve the current understanding of biological processes in health and disease as well as enable novel diagnostics and therapeutics for treating cancer; consequently, advances made on the basis of nanotechnology could lead to progress in healthcare[3]. Targeted drug delivery systems, such as passive and active targeting nanoparticles or nanocarriers, have been developed to improve the biodistribution, pharmacological, therapeutic, and toxic properties of chemical agents used in cancer diagnostics and therapeutics[4]. The abundant expression and signal amplification properties of these targets are mandatory for successful receptor-targeted tumor imaging and for their subsequent clinical translation[5]. The epidermal growth factor receptor (EGFR) is a transmembrane receptor belonging to the ErbB family of receptors; the EGFR family includes four transmembrane receptors, namely, EGFR, HER2, HER3, and HER4[6]. EGFR is a cell surface receptor that plays a key role in signaling pathways, and such pathways regulate cell proliferation, angiogenesis, and tumor metastasis[7,8]. Previous studies reported that EGFR is widely overexpressed in several tumor types, including breast cancer, melanoma, brain glioblastoma, non-small cell lung cancer[9], and colorectal cancer[10]; thus, EGFR is an attractive candidate for anti-cancer therapy.
Several radionuclides have been surface-bioconjugated or after-loaded in nanoparticles to improve their effectiveness and reduce the toxicity of cancer imaging and therapies in preclinical and clinical studies. In the broad spectrum of available radionuclides, beta emitters such as 90Y, 131I, and 177Lu are the most commonly used for targeted therapies[11]. 131I provides imaging feasibility and beta-emitting therapeutic effects[12]. The application of nanotechnology for the diagnosis and treatment of colorectal cancer has the potential to improve conventional methods as well as help with the development of novel approaches for detection and therapy[13].
In the present study, 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL were successfully synthesized. These liposomes were characterized for cellular uptake, time-dependent uptake of 131I by LS180 cells in vitro, therapeutic efficacy, histopathology, tissue distribution, and SPECT imaging in an LS180 xenografted nude mouse model.
The following reagents and materials used in this study were obtained: Minimum Essential Medium Eagles with Earle’s Balanced Salts (MEM-EBSS; Gibco), fetal bovine serum (FBS; Gibco), rabbit polyclonal anti-human epidermal growth factor receptor antibody (Abcam, ab2430), 5 mg/mL monoclonal antibody (MERCK Inc., C225; cetuximab), and PV-6000 Polymer Detection System (ZSGB-BIO Co.).
The colorectal cancer cell line LS180 was purchased from the Cell Resource Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences/Peking Union Medical College (Beijing, China). The cells were maintained in MEM-EBSS medium supplemented with 10% FBS and 1% penicillin/streptomycin. The LS180 cells were grown at 37 °C in a humidified incubator with a mixture of 95% air and 5% CO2.
The cetuximab-decorated, BSA-PCL-conjugated antiEGFR-BSA-PCL and BSA-PCL liposomes were synthesized by the researchers from the Department of Polymer Materials Science and Engineering of Tianjin University[14]. The antiEGFR-BSA-PCL and BSA-PCL liposomes were labeled with 131I (Beijing Atomic Hi-tech Co., Ltd.) via the chloramine T method. A detailed description and biological characterization of the compounds are presented by Ickenstein et al[15]. The labeling rate and the radiochemical purity of 131I-labeled nanoparticles were determined by thin layer chromatography.
The cellular binding and uptake of the antiEGFR-BSA-PCL and BSA-PCL liposomes were evaluated by confocal microscopy in LS180 cells. The antiEGFR-BSA-PCL and BSA-PCL liposomes were first labeled by fluorescein isothiocyanate (FITC), then were added at a concentration of 1 mg/106 cells. The cell cultures were then incubated for 4 h at 37 °C. After incubation, cells were washed thrice with PBS, fixed with 4% paraformaldehyde, and analyzed by confocal microscopy (laser scanning confocal microscope, Olympus FV1000; Japan).
To evaluate the time-dependent cellular uptake of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL, approximately 1 × 105 cells per well were seeded in 96-well plates and cultured with 0.37 MBq/mL to 3.7 MBq/mL 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL for 4 h. The medium was completely removed before the cells were washed and lysed twice with ice-cold PBS. Subsequently, 200 μL of DMEM containing 10% FBS was added into each well. The cells were counted every 2 h until 24 h of incubation. Radioactivity was measured with a γ counter (LKB gamma 1261; LKB Instruments, Waverley, Australia) to calculate the counts per minute (CPM) after 24 h. All of these experiments were performed in triplicate.
Experimental subjects were four-week-old BALB/c female nude mice weighing 9 g to 11 g. These mice were purchased from the Beijing Experimental Animal Center of Peking Union Medical, China. Mice were kept under specific pathogen-free conditions, with a constant temperature of 25 °C to 27 °C and a constant humidity of 45% to 50% in the Laboratory Animal Center of the Tianjin Medical University, China. Animal experimentation guidelines were followed according to the regulations of Swiss veterinary law. The LS180 tumor cells, with approximately 1 × 107 cells per 50 μL, were subcutaneously injected into the right flank of the mice. According to the principle of the human thyroid perchlorate discharge test, 0.05 mg/mL sodium perchlorate was added to the drinking water of all mice for 1 d before the experiment (approximately 21 d after tumor inoculation) to reduce the exposure of their thyroids to unwanted radiation and imaging. The growth curve experiment was terminated at 54 d after tumor inoculation, and all the mice were sacrificed. When tumor volume reached an average size of 1.0 cm3, mice were randomly divided into four experimental groups, which were subjected to an intra-tumor injection of 74 MBq (740 MBq/mL) 131I-antiEGFR-BSA-PCL, 131I-BSA-PCL, 131I, or an equivalent volume of normal saline (except in the SPECT imaging experiment).
The mice bearing human colorectal cancer were used for the biodistribution study at day 21 after tumor inoculation. The mice in each group were sacrificed at 4, 24, and 72 h post-injection of drugs, respectively. The heart, spleen, liver, colon, and tumor samples were collected for weighing and for radioactivity measurements with a γ counter (LKB gamma 1261; LKB Instruments). %ID/g represents the percentage injected dose per gram of tissue. %ID/g was determined by the percentage of radioactivity contained within each gram of tissue compared to the total radioactivity injected into the body[16,17]. Meanwhile, the remaining spleen, liver, and tumor tissues were collected to study the histopathology of each group.
Toxicity was monitored by measuring the body weight and tumor volume. During the course of treatment, the animal body weight and in vivo tumor growth were measured at day 21 after tumor cell inoculation. Mice were checked for survival every day; their body weight and tumor volume were measured every 3 d until the end of the study. The growth curve experiment was terminated at 54 d after tumor inoculation, and all mice were then sacrificed. After treatment, the length, width, and height of the tumors were measured with calipers once every 3 d. Tumor volume was calculated as follows: volume = 1/6 ×π× length (cm) × width (cm) × height (cm)[18].
The xenografted colorectal cancer cell line LS180 was characterized for EGFR overexpression by immunohistochemistry. For the immunohistochemical staining of EGFR, the rabbit polyclonal anti-human EGFR antibody (1:500) was used as the primary antibody, followed by PV 6000 via the two-step immunohistochemical detection of cell-associated EGFR. Mice from each group were sacrificed at 4, 24, and 72 h after the injection of different liposomes. The remaining spleen, liver, and tumor tissues were collected to study their histopathology. The organs from the four groups were dissected, fixed in 10% formalin for 48 h, embedded in paraffin, and sectioned. The sections (5-6 μm) were then stained with hematoxylin and eosin. The histopathological changes of the tissue were ultimately examined using light microscopy (Olympus × 40).
For the SPECT imaging (Discovery VH 670; GE, United States), mice from the three treatment groups (except for the normal saline group) received an intra-tumor injection of 74 MBq (740 MBq/mL) of 131I-antiEGFR-BSA-PCL, 131I-BSA-PCL, and 131I, respectively[19]. SPECT imaging was performed at 4, 24, and 72 h after injection.
We ensured that mouse use was conducted in accordance with the highest scientific, humane, and ethical principles and was in compliance with regulations. We also ensured that housing conditions and care were species appropriate and that the environment was safe and secure for both animals and staff.
All in vitro experiments were performed in triplicate unless otherwise indicated. Statistical analyses were conducted with the SPSS software (version 17.0). The results are presented as the mean ± SD. Statistical significance was tested using Student’s t-test or ANOVA, and P-values < 0.05 were considered statistically significant.
The dynamic light scattering measurement showed that the average diameter of 131I-BSA-PCL and 131I-antiEGFR-BSA-PCL was approximately 180 nm, and the polydispersity index was 0.164, in agreement with the TEM result. The mean ζ potential of the obtained protein-based vesicle was also measured and was found to be -38.70 mV. The negative surface charge that resulted from the BSA shell could reduce undesired protein adsorption and provide good protection for the vesicle during the circulation in the body. The specific radioactivity was approximately 370-690 MBq/mg for 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL. The labeling rate of 131I was approximately 50%-85%, and the radiochemical purity was 95%-98%.
Internalization of EGFR-BSA-PCL and BSA-PCL
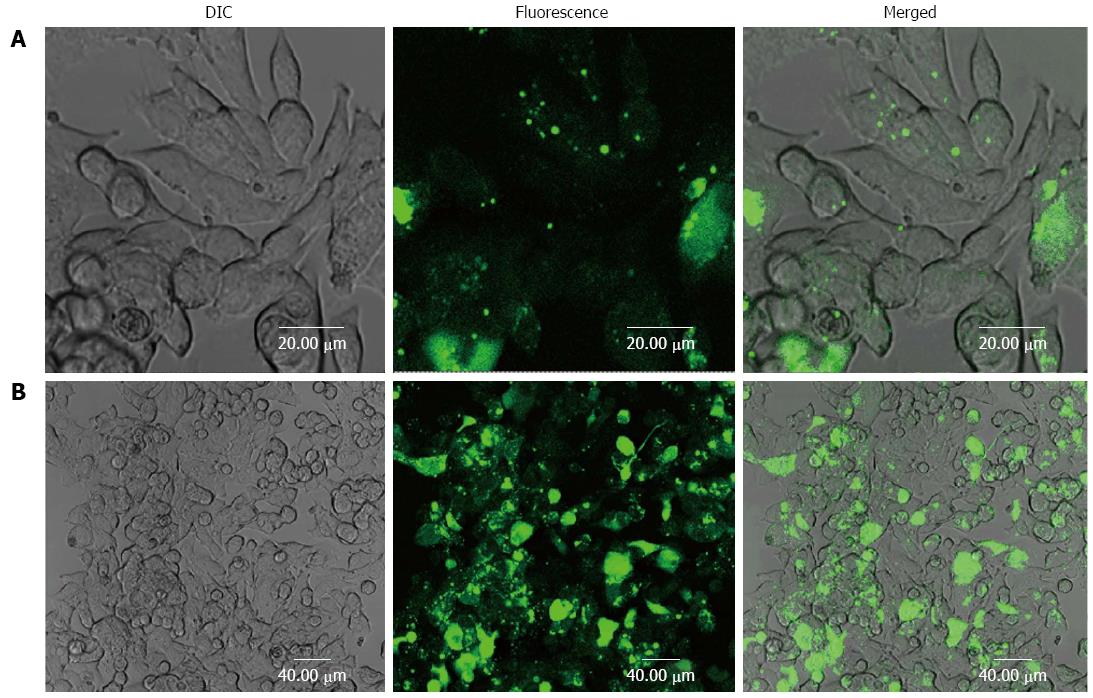
The effective diameter of BSA-PCL and antiEGFR-BSA-PCL was approximately 180 nm[14]. To assess the binding of antiEGFR-BSA-PCL and BSA-PCL to LS180 cells with EGFR overexpression, confocal microscopy was used. The confocal images and the immunofluorescence results of the LS180 cells incubated with antiEGFR-BSA-PCL and BSA-PCL were compared (Figure 1). antiEGFR-BSA-PCL was significantly internalized in the LS180 cells, which was shown by the appearance of strong green fluorescence. Compared with antiEGFR-BSA-PCL, BSA-PCL could similarly bind to cells, but the tumor retention was minimal, and the binding green fluorescence was weaker. This result suggests that the targeting ability of antiEGFR-BSA-PCL to tumor cells was enhanced by EGFR modification.
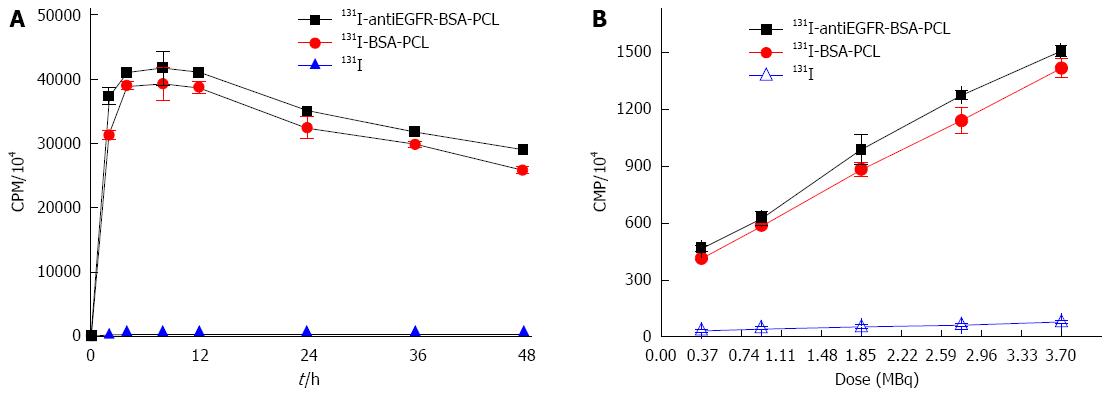
To determine the iodide uptake of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL, time activity measurements were obtained for the LS180 cell line. The radioiodide uptake in this cell line reached its maximum level after 4 h of incubation with 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL. Furthermore, the 131I uptake of 131I-antiEGFR-BSA-PCL was higher than that of 131I-BSA-PCL. Cell-associated 131I activity was still very high after 24 h and reached 79% to 84% in LS 180 cells because the liposomes had slow-release effects (Figure 2A). The CPM in the LS180 cells increased with an increasing dose of 131I-labeled nanoparticles. However, radioactivity was maintained at a low level in the 131I group (Figure 2B).
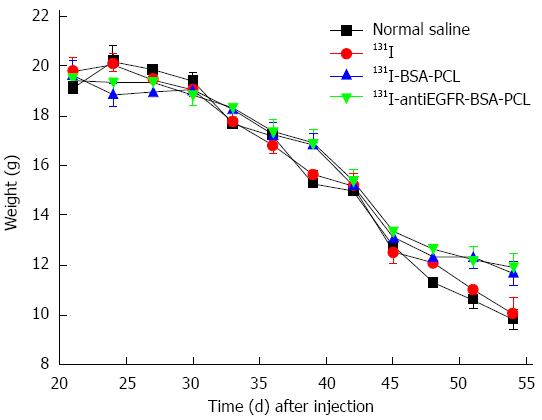
The trend of body weight growth for all study groups is shown in Figure 3. The minimum reduction in body weight was found in the 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL groups. Compared with the original body weight, mice treated with 131I-antiEGFR-BSA-PCL, 131I-BSA-PCL, 131I, and normal saline demonstrated body weight losses of 39% ± 3%, 41% ± 4%, 49% ± 5%, and 55% ± 13%, respectively, on day 54 after tumor inoculation (P < 0.05).
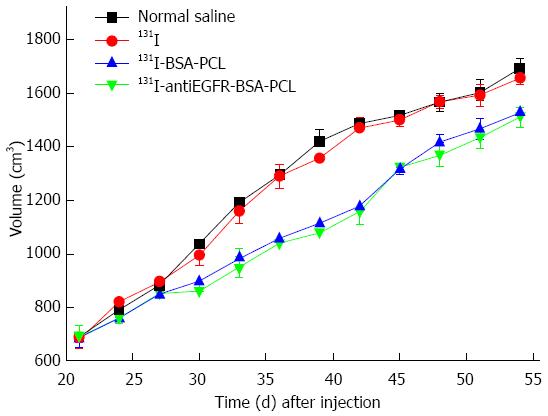
Rapid tumor growth was observed in the xenografted colorectal cancer nude mice treated with saline or 131I. The tumor volume change is presented in Figure 4. By contrast, tumors grew slowly in mice treated with 131I-antiEGFR-BSA-PCL or 131I-BSA-PCL, and the growth curves of these test groups were similar. No significant differences were observed between the other two groups: those treated with 131I or normal saline. The data showed that treatment with 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL significantly inhibited tumor growth. At the end of the study, the volume of tumor increase in the 131I-antiEGFR-BSA-PCL group was 124% ± 7%, which was lower than that of the 131I-BSA-PCL (127% ± 9%), 131I (143% ± 7%), and normal saline (146% ± 10%) groups. The results from the groups treated with 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL were significantly different from those groups treated with 131I and normal saline, suggesting that 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL acted similarly to suppress tumor growth.
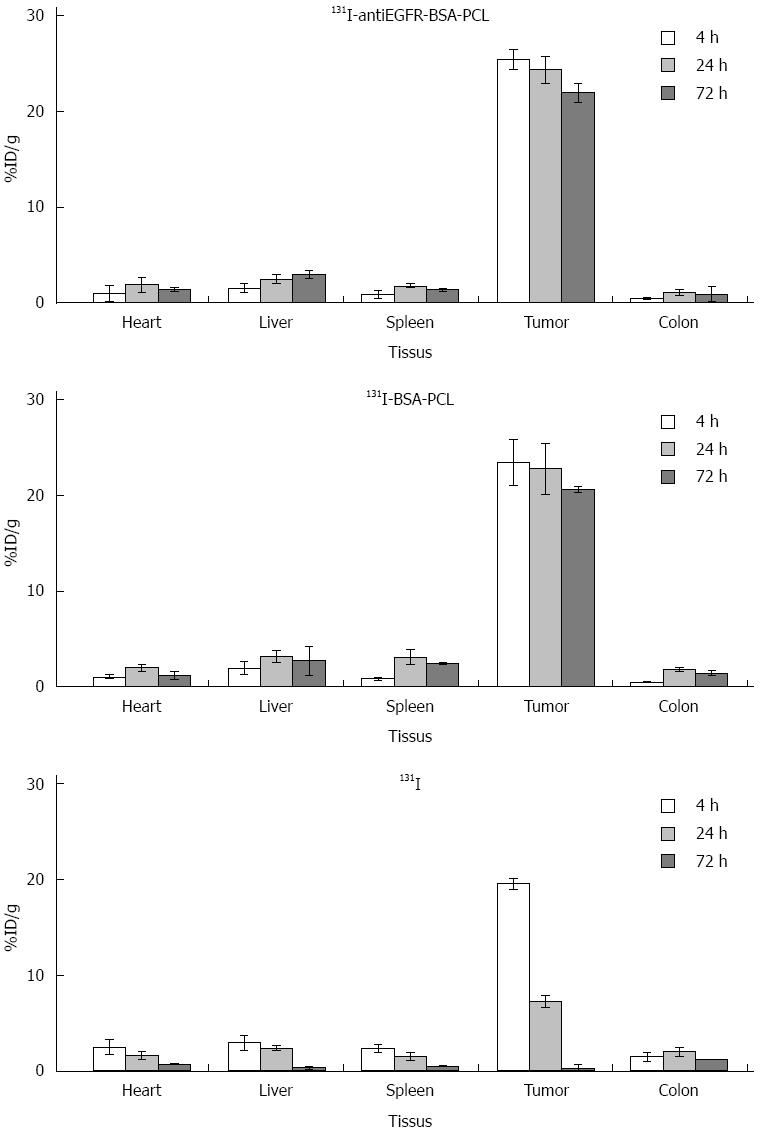
The tissue distribution of 131I-antiEGFR-BSA-PCL in nude mice with human colorectal cancer xenografts was measured by γ photon counts in Figure 5. 131I-antiEGFR-BSA-PCL was markedly taken up by tumor tissues. The tissue distribution assay revealed that 131I-antiEGFR-BSA-PCL was markedly taken up by the tumor and reached its maximal uptake value of 21.0 ± 1.01 %ID/g at 72 h after the therapy. The drug concentration in the tumor was higher than those in the liver, heart, colon, or spleen at 72 h after therapy. Among the non-tumor tissues, 131I-antiEGFR-BSA-PCL mostly accumulated in the liver and spleen. The levels of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL in tumor tissues were significantly higher than those observed in the 131I group at 4, 24, and 72 h after treatment.
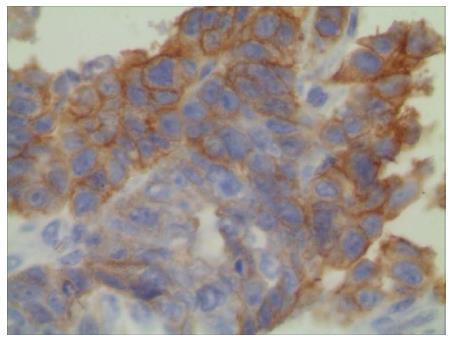
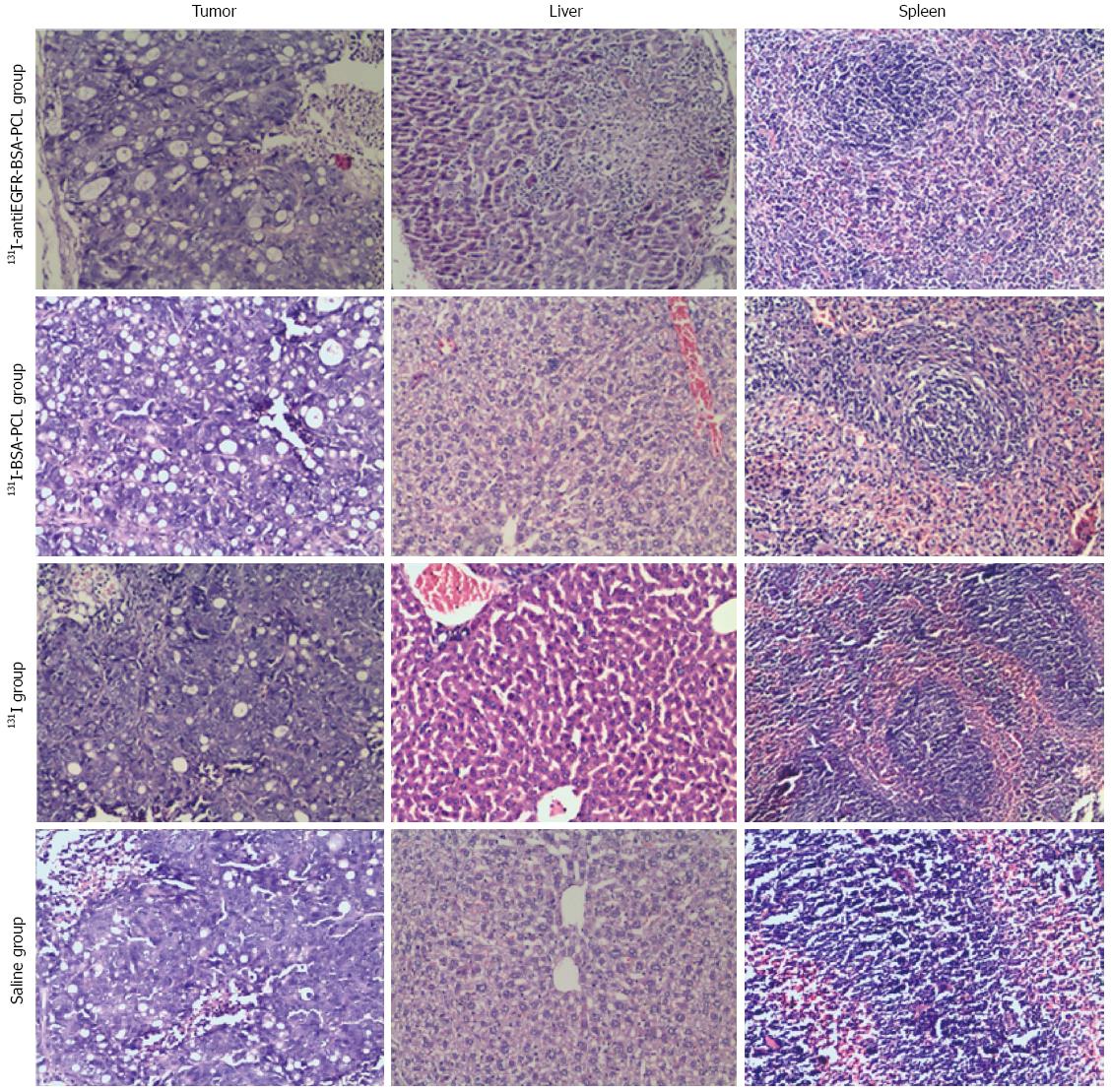
EGFR was overexpressed in the LS180 cell line (Figure 6), and the positive cell membranes were stained brown. The normal saline and 131I-treated groups were characterized by well-developed proliferation of LS180 cells with a compact arrangement and diffusely distributed invading cancer nests; the nuclei were of various sizes and the staining color was variable. Moreover, no apparent necrosis was found in the cancer tissue (Figure 7). After the intratumoral injection of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL, xenografted colorectal cancer tissues were histopathologically examined (Figure 7). Upon comparison, a large field of the tumor tissue appeared necrotic. Evidence of dying tumor cells undergoing vacuolar degeneration of the cytoplasm was observed. Clusters of tumor cells decreased, whereas the intercellular space increased. Cellular arrangements were seen as discrete and not compact, and the cytoplasm was deeply stained. The cancerous nodes were characterized by patchy necrosis, and their tissue structure was smeared.
The SPECT images of LS180 tumor-bearing mice (21 d after tumor inoculation) were acquired at different time points after the intra-tumor injection of 131I-antiEGFR-BSA-PCL, 131I-BSA-PCL, and 131I. The 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL groups both exhibited slow blood clearance and maintained a relatively higher activity (primarily in the tumors, based on biodistribution data). Mice in the 131I group had rapid blood clearance; their activity did not increase after 24 and 72 h compared with the 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL groups. SPECT images showed high uptake and targeting of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL in the tumor. The results were correlated and validated with images of the biodistribution data.
Colorectal cancer is the third most common cancer worldwide[2]. The C26 murine colon carcinoma model has previously been reported to have a high level of metastasis that spreads after the intraperitoneal inoculation of tumor cells in BALB/c mice[20]. EGFR is a transmembrane receptor belonging to the ErbB family of receptors; the EGFR gene is activated or up-regulated in 60% to 80% of colorectal cancer cases[6,10]. Most of the normal tissues express very low levels of EGFR. However, the liver and kidney tissues express moderately high levels of the receptor, which can exceed that of tumors.
Conventional anticancer drugs exhibit a lack of specificity, poor solubility and distribution, unfavorable pharmacokinetics, and high-tissue damage or toxicity[21]. Radiation therapy is typically delivered through high-energy external beam radiation to irradiate the cancerous tissue. Liposomes are spherical vesicles formed by lipid bilayers. These structures have been widely investigated as carriers of tumor-targeting therapy. Radioisotopes can be trapped within the inner space of the liposome, intercalated into the double membrane of the liposome, and connected with the surface of the liposome[22,23]. However, given the close proximity of critical organs to the tumor, the delivery of a therapeutic dose of irradiation without causing serious complications is often impossible, even with technological advancements in radiation dose distribution, such as intensity-modulated radiation therapy[24,25]. Tumor-targeting therapy has overcome the lack of specificity of traditional therapeutic agents. Recent work comparing non-targeted and targeted nanoparticles (lipid-based[26] or polymer-based[27]) has shown that the primary role of the targeting ligands is to enhance the cellular uptake of drugs by cancer cells rather than to increase their accumulation in the tumor. Kao et al[12] demonstrated the enhanced endocytosis and cytotoxicity of 131I-C225-AuNP-PEG against EGFR-overexpressing human A549 lung carcinoma cells as well as its active targeting in an A549 tumor xenograft mouse model[12].
Chen et al[28] and Chang et al[19] reported the biodistribution, pharmacokinetics, and micro-SPECT/CT imaging of the 188Re-liposome in C26 colon carcinoma ascites and solid tumor animal models. Their results demonstrated the potential benefit and advantages of the 188Re-liposome in the C26 animal model. Bao et al[29] reported that the intratumoral injection of liposomal radionuclides had a slower clearance rate. 131I is a radionuclide with a gamma emission of 364 keV (81.7%) and a beta emission of 0.606 MeV (89.9%). This molecule has the advantages of imaging feasibility and beta-emitting therapeutic effects. In the present study, the initial treatment of colorectal cancer with 131I-antiEGFR-BSA-PCL reduced the risk of disease progression compared with 131I-BSA-PCL and 131I. The decreased risk was evidenced by changes in the tumor volume, mouse survival time, and mouse weight. As shown by the data, the rates of increase of the final tumor volumes in the groups treated with 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL were lower than those of tumor-bearing groups treated with 131I and normal saline. However, the tumor volumes of the group treated with normal saline and the group treated with 131I were not significantly different in terms of overall survival. The trend of the body weight growth also had the same characteristics. The 131I-antiEGFR-BSA-PCL group also achieved the maximum suppression of tumor growth, which reflected an enhancement of cancer therapy with the use of 131I-antiEGFR-BSA-PCL. We found that the use of 131I-antiEGFR-BSA-PCL for the in vivo therapy of colorectal cancer was both feasible and reliable, with favorable in vivo characteristics.
Results with mouse models demonstrated the steady extravasation of nanoliposomes into ascites with the gradual release of the drug, followed by drug diffusion into the tumor cellular compartment[30,31]. Wang et al[32] reported that direct intratumoral drug administration in advanced neck and head tumors was clearly appealing and immediately achieved high drug concentrations at the target sites without the associated side effects of external beam radiation or systemic chemotherapy. Their group also proved that direct injection could deliver drugs into areas of the tumor that had high interstitial pressure and large areas of avascular necrosis. This study was relevant to the present study on the pharmacokinetics and effectiveness of 131I-antiEGFR-BSA-PCL administration in the heart, liver, spleen, colon, and tumor cells. The observed iodine uptake demonstrated the potential benefits and advantages of 131I-antiEGFR-BSA-PCL in the LS180 animal model. The accumulation of radioactivity in the tumor was maintained at steady levels for at least 24 h.
SPECT imaging is a non-invasive imaging modality that can monitor the behavior of radiotherapeutics in the same animal at different time points. After the injection of different drugs, the SPECT imaging and iodine uptake showed that the tumor uptake of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL was obvious higher than that of 131I. In addition, the in vitro stability of the passive 131I-antiEGFR-BSA-PCL was found to be higher after 24 h of inoculation. Some mice presented skin ulcerations at around day 13 but were healed by day 19. From the SPECT fusion images, we noticed that the 131I-antiEGFR-BSA-PCL had the tendency to spread into the tumor, liver, spleen, and urinary bladder area. We also observed that the non-tumor tissues of the liver and spleen, which are reticuloendothelial system-rich organs, exhibited significant uptake of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL. The remarkable accumulation of these compounds in the bladder implied that renal clearance is the predominant route of excretion of radioactive metabolites. A glutathione-coated luminescent gold nanoparticle (3 nm diameter)[33] and a gadolinium-loaded dendrimer-entrapped gold nanoparticle (90 nm mean diameter)[34] were previously reported to be excreted via the urinary system. Li et al[16] showed that 131I-labeled rhEGF was markedly absorbed by tumor tissue in xenografted nude mice and reached a maximum uptake rate (16.73 %ID/g) at 120 h after intravenous injection. Their group suggested that 131I-labeled rhEGF could be absorbed by breast cancer tissues and might be a useful clinical candidate. Data from the present study also showed a low immediate clearance for both 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL. The long phase was observed at 72 h after 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL were injected, thereby translating to a high locoregional retention rate when the immediate clearance period had passed. These data supported the notion that the injection of liposomal radionuclides could lead to higher retention rates.
The anti-tumor effect of 131I-antiEGFR-BSA-PCL could also be seen in the histopathological studies. Immunohistochemistry showed that the LS180 cell line overexpressed EGFR. The normal saline and 131I-treated groups were characterized by well-developed proliferation of LS180 cells and diffusely distributed invading cancer nests. No apparent necrosis was observed in the cancer tissue. In the xenografted colorectal cancer tissues of the groups treated with 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL, a large field of the tumor tissue appeared necrotic. Evidence of dying tumor cells undergoing vacuolar degeneration of the cytoplasm was observed. The cancerous nodes were characterized by patchy necrosis, and the tissue structures were smeared. These results demonstrated that treatment with 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL led to histopathological changes in colorectal cancer tissue that were associated with the death of the tumor cells. The above-mentioned reasons made the intraoperative injection of liposomal therapeutic radionuclides a particularly effective treatment option for advanced colorectal cancer.
Although the colorectal cancer xenografted animal model used in this study was not orthotopic, the results are still valuable for interpreting the drug efficiency of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL. Therefore, the protocols used in this study revealed that the designed formulation and treatment trials were novel for a preclinical study.
In conclusion, the results of this study demonstrated that 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL had superior cellular binding and higher cellular uptake compared with 131I in vitro and in vivo. 131I-antiEGFR-BSA-PCL suppressed the development of tumors and exhibited pronounced anti-proliferation ability against the xenografted LS180 cells in a mouse model, as demonstrated by changes in the tumor size, body weight, histopathology, and SPECT imaging. This study revealed that 131I-antiEGFR-BSA-PCL was effective in the killing of tumors, but we did not observe a radiation induced breakdown of the kidney and liver. Therefore, 131I-antiEGFR-BSA-PCL may be a safe drug for radiotherapy in colorectal cancer.
Epidermal growth factor receptor (EGFR) is a cell-surface receptor that plays a key role in signaling pathways. Such pathways regulate cell proliferation, angiogenesis, and tumor metastases. EGFR is widely overexpressed in several tumor types, including breast cancer, melanoma, brain glioblastoma, non-small cell lung cancer, and colorectal cancer. EGFR is an attractive candidate for anti-cancer therapy.
Previous experiments have already proven that EGFR is widely overexpressed in colorectal cancer.
This is the first study evaluating the cellular binding and cellular uptake of 131I-antiEGFR-BSA-PCL and 131I-BSA-PCL in vitro and in vivo. 131I-antiEGFR-BSA-PCL suppressed the development of tumors and exhibited pronounced anti-proliferation ability against the xenografted LS180 cells in a mouse model.
As a novel theranostic agent, 131I-antiEGFR-BSA-PCL exhibited pronounced anti-tumoral effects on colorectal cancer.
This study evaluated the therapeutic effectiveness of 131I-antiEGFR-BSA-PCL in the colorectal cancer mouse model and demonstrated the potential beneficial application of 131I-antiEGFR-BSA-PCL for treating colorectal cancer. The manuscript is well written and properly conducted.
| 1. | Fang M, Peng CW, Pang DW, Li Y. Quantum dots for cancer research: current status, remaining issues, and future perspectives. Cancer Biol Med. 2012;9:151-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13561] [Article Influence: 645.8] [Reference Citation Analysis (3)] |
| 3. | Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1211] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 4. | Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3286] [Cited by in RCA: 3039] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 5. | Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550-6565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 975] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 6. | Messa C, Russo F, Caruso MG, Di Leo A. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol. 1998;37:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1515] [Cited by in RCA: 1586] [Article Influence: 88.1] [Reference Citation Analysis (2)] |
| 8. | Yang J, Lim EK, Lee HJ, Park J, Lee SC, Lee K, Yoon HG, Suh JS, Huh YM, Haam S. Fluorescent magnetic nanohybrids as multimodal imaging agents for human epithelial cancer detection. Biomaterials. 2008;29:2548-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Nurwidya F, Murakami A, Takahashi F, Takahashi K. Molecular mechanisms contributing to resistance to tyrosine kinase-targeted therapy for non-small cell lung cancer. Cancer Biol Med. 2012;9:18-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Porebska I, Harlozińska A, Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol. 2000;21:105-115. [PubMed] |
| 11. | Koppe MJ, Bleichrodt RP, Soede AC, Verhofstad AA, Goldenberg DM, Oyen WJ, Boerman OC. Biodistribution and therapeutic efficacy of (125/131)I-, (186)Re-, (88/90)Y-, or (177)Lu-labeled monoclonal antibody MN-14 to carcinoembryonic antigen in mice with small peritoneal metastases of colorectal origin. J Nucl Med. 2004;45:1224-1232. [PubMed] |
| 12. | Kao HW, Lin YY, Chen CC, Chi KH, Tien DC, Hsia CC, Lin MH, Wang HE. Evaluation of EGFR-targeted radioimmuno-gold-nanoparticles as a theranostic agent in a tumor animal model. Bioorg Med Chem Lett. 2013;23:3180-3185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Su CH, Sheu HS, Lin CY, Huang CC, Lo YW, Pu YC, Weng JC, Shieh DB, Chen JH, Yeh CS. Nanoshell magnetic resonance imaging contrast agents. J Am Chem Soc. 2007;129:2139-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Liu Z, Dong C, Wang X, Wang H, Li W, Tan J, Chang J. Self-assembled biodegradable protein-polymer vesicle as a tumor-targeted nanocarrier. ACS Appl Mater Interfaces. 2014;6:2393-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Ickenstein LM, Edwards K, Sjöberg S, Carlsson J, Gedda L. A novel 125I-labeled daunorubicin derivative for radionuclide-based cancer therapy. Nucl Med Biol. 2006;33:773-783. [PubMed] |
| 16. | Li YC, Xu WY, Tan TZ, He S. 131I-recombinant human EGF has antitumor effects against MCF-7 human breast cancer xenografts with low levels of EGFR. Nucl Med Biol. 2004;31:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Shao X, Zhang H, Rajian JR, Chamberland DL, Sherman PS, Quesada CA, Koch AE, Kotov NA, Wang X. 125I-labeled gold nanorods for targeted imaging of inflammation. ACS Nano. 2011;5:8967-8973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Lee YS, Bullard DE, Zalutsky MR, Coleman RE, Wikstrand CJ, Friedman HS, Colapinto EV, Bigner DD. Therapeutic efficacy of antiglioma mesenchymal extracellular matrix 131I-radiolabeled murine monoclonal antibody in a human glioma xenograft model. Cancer Res. 1988;48:559-566. [PubMed] |
| 19. | Chang YJ, Chang CH, Chang TJ, Yu CY, Chen LC, Jan ML, Luo TY, Lee TW, Ting G. Biodistribution, pharmacokinetics and microSPECT/CT imaging of 188Re-bMEDA-liposome in a C26 murine colon carcinoma solid tumor animal model. Anticancer Res. 2007;27:2217-2225. [PubMed] |
| 20. | Corbett TH, Griswold DP, Roberts BJ, Peckham JC, Schabel FM. Evaluation of single agents and combinations of chemotherapeutic agents in mouse colon carcinomas. Cancer. 1977;40:2660-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Ting G, Chang CH, Wang HE, Lee TW. Nanotargeted radionuclides for cancer nuclear imaging and internal radiotherapy. J Biomed Biotechnol. 2010;2010:pii 953537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Bao A, Goins B, Klipper R, Negrete G, Phillips WT. Direct 99mTc labeling of pegylated liposomal doxorubicin (Doxil) for pharmacokinetic and non-invasive imaging studies. J Pharmacol Exp Ther. 2004;308:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Bao A, Goins B, Klipper R, Negrete G, Phillips WT. 186Re-liposome labeling using 186Re-SNS/S complexes: in vitro stability, imaging, and biodistribution in rats. J Nucl Med. 2003;44:1992-1999. [PubMed] |
| 24. | Lee N, Puri DR, Blanco AI, Chao KS. Intensity-modulated radiation therapy in head and neck cancers: an update. Head Neck. 2007;29:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Xia P, Fu KK, Wong GW, Akazawa C, Verhey LJ. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;48:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 233] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732-6740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 813] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 27. | Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104:15549-15554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 615] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 28. | Chen LC, Chang CH, Yu CY, Chang YJ, Wu YH, Lee WC, Yeh CH, Lee TW, Ting G. Pharmacokinetics, micro-SPECT/CT imaging and therapeutic efficacy of (188)Re-DXR-liposome in C26 colon carcinoma ascites mice model. Nucl Med Biol. 2008;35:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Bao A, Phillips WT, Goins B, Zheng X, Sabour S, Natarajan M, Ross Woolley F, Zavaleta C, Otto RA. Potential use of drug carried-liposomes for cancer therapy via direct intratumoral injection. Int J Pharm. 2006;316:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Bally MB, Masin D, Nayar R, Cullis PR, Mayer LD. Transfer of liposomal drug carriers from the blood to the peritoneal cavity of normal and ascitic tumor-bearing mice. Cancer Chemother Pharmacol. 1994;34:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Gabizon AA. Selective tumor localization and improved therapeutic index of anthracyclines encapsulated in long-circulating liposomes. Cancer Res. 1992;52:891-896. [PubMed] |
| 32. | Wang SX, Bao A, Herrera SJ, Phillips WT, Goins B, Santoyo C, Miller FR, Otto RA. Intraoperative 186Re-liposome radionuclide therapy in a head and neck squamous cell carcinoma xenograft positive surgical margin model. Clin Cancer Res. 2008;14:3975-3983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Zhou C, Hao G, Thomas P, Liu J, Yu M, Sun S, Öz OK, Sun X, Zheng J. Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Angew Chem Int Ed Engl. 2012;51:10118-10122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 34. | Wen S, Li K, Cai H, Chen Q, Shen M, Huang Y, Peng C, Hou W, Zhu M, Zhang G. Multifunctional dendrimer-entrapped gold nanoparticles for dual mode CT/MR imaging applications. Biomaterials. 2013;34:1570-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Meshikhes AW S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma S