Published online Apr 7, 2016. doi: 10.3748/wjg.v22.i13.3687
Peer-review started: November 4, 2015
First decision: December 15, 2015
Revised: December 17, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: April 7, 2016
Processing time: 146 Days and 22.4 Hours
Ampullary adenoma is a common indication for endoscopic papillectomy. Ampullary neuroendocrine tumor (NET) is a rare disease for which complete surgical resection is the treatment of choice. However, because of the morbidity and mortality associated with surgical resection, endoscopic papillectomy is increasingly used in selected cases of low grade, with no metastasis and no invasion of the pancreatic or bile duct. Also, confirmed and complete endoscopic resection of ampullary NET accompanied by adenoma has not been reported to date. We report herein a rare case of an ampullary NET accompanied with adenoma, which was successfully and completely resected via endoscopic papillectomy. Prior to papillectomy, this case was diagnosed as an ampullary adenoma.
Core tip: In selected cases without metastasis or invasion of the pancreatic and bile duct, endoscopic papillectomy can be a treatment of choice for ampullary neuroendocrine tumor (NET). To the best of our knowledge, the complete cure case of successful endoscopic papillectomy for ampullary NET accompanied with ampullary adenoma has not been reported in the English-language literature. This unusual ampullary NET accompanied with adenoma was successfully treated by endoscopic papillectomy.
- Citation: Lee SH, Lee TH, Jang SH, Choi CY, Lee WM, Min JH, Cho HD, Park SH. Ampullary neuroendocrine tumor diagnosed by endoscopic papillectomy in previously confirmed ampullary adenoma. World J Gastroenterol 2016; 22(13): 3687-3692
- URL: https://www.wjgnet.com/1007-9327/full/v22/i13/3687.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i13.3687
The incidence of gastrointestinal neuroendocrine tumor (NET) has increased in recent years due to the increased frequency of healthcare examinations and improvements in diagnostic techniques. However, NET of the ampulla of Vater is extremely rare[1-3]. Pancreaticoduodenectomy is generally considered the procedure of choice for NET of the ampulla of Vater larger than 2 cm and for cases of neuroendocrine carcinoma. Endoscopic local resection and surgical ampullectomy have been considered to be safe for small NET of the ampulla of Vater (less than 2-cm diameter) or in patients with severe comorbidities[2-6]. Endoscopic papillectomy is now commonly indicated for adenomas of the ampulla of Vater[7,8].
In the case presented herein, we first diagnosed ampullary adenoma by routine endoscopic examination. However, subsequent endoscopic papillectomy confirmed combined ampullary NET. Ampullary NET accompanied by adenoma of the ampulla of Vater was resected completely by endoscopic papillectomy, without local recurrence during 2 years.
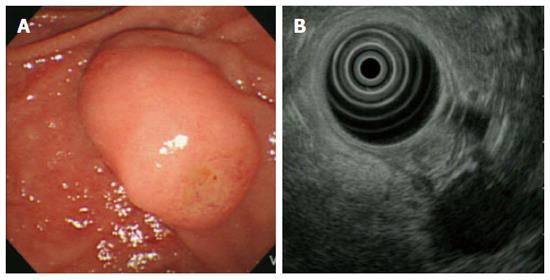
A 53-year-old female patient visited our hospital for dyspepsia of 3-mo duration. She had no specific medical or surgical history. Her vital signs at admission were blood pressure 110/70 mmHg, pulse rate 82/min, respiratory rate 20/min, and body temperature 36.3 °C. Physical examination revealed no marked features. The laboratory data also showed no abnormalities, including tumour markers (CEA 1.0 ng/mL, CA 19-9 2.0 U/mL). Screening upper gastrointestinal endoscopy showed protruding major papilla and subsequent endoscopic biopsy of the ampulla of Vater revealed low-grade ampullary adenoma. A duodenoscopic image showed an enlarged major papilla with central umbilication and fine nodularity. Endoscopic ultrasonography (EUS) at the major ampulla revealed a 1.1 × 0.9-cm, slightly hypoechoic round ampullary mass confined to the submucosa without definite wall disruption or adjacent invasion (Figure 1). Abdomen computed tomography (CT) did not show an abnormally dilated pancreatic or biliary duct, ductal invasion, or enlarged lymph nodes.
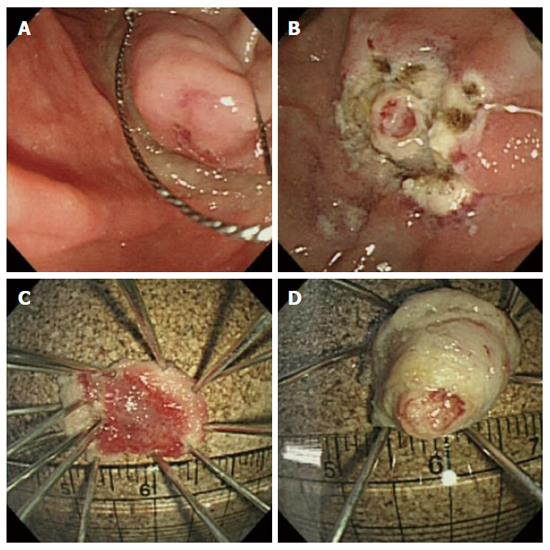
Based on pathology and an imaging study, we planned endoscopic papillectomy for the removal of unexposed ampullary adenoma. Following submucosal injection of 1:10000 diluted epinephrine, snaring papillectomy was performed. However, primary complete resection of major papilla was not achieved. Following the first papillectomy, a remnant, whitish, round mass-like lesion was seen to protrude, and was difficult to differentiate from remnant tumor or a second combined tumor. Therefore, subsequently a second resection was performed successfully in the same manner as the first. An endoscopic image acquired immediately following the two-step papillectomy showed complete resection without complications. Resected tissues showed a papillary roof lesion and a whitish, round, mass-like lesion (Figure 2). Insertion of a prophylactic pancreatic stent failed due to technical difficulties with selective pancreatic duct cannulation. Due to the risk of post-procedure pancreatitis, the number of attempts to cannulate the pancreatic duct was not permitted to exceed five. Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis did not occur.
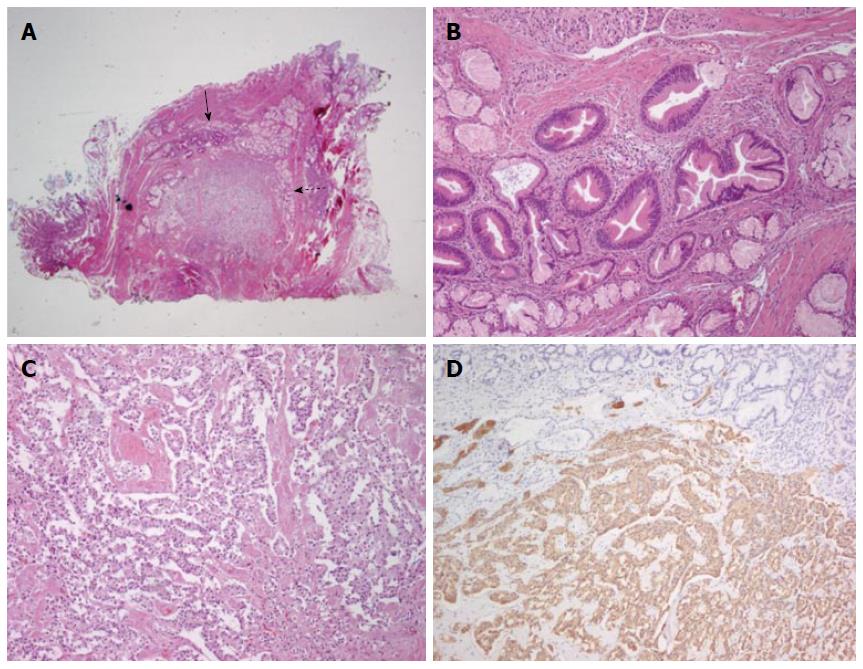
Microscopic findings of the resected specimens were as follows. There was no evidence of NET and tubular adenoma in the first primary papillectomy specimen. But, the second resection specimen showed a collision tumor composed of tubular adenoma (closed arrow) and NET [open arrow, hematoxylin-eosin (HE) staining, magnification × 4] and it measured about 1.0 cm. The tubular adenoma component was mainly found in intra-ampullary portion of the ampulla of Vater and exhibited round-to-oval enlarged glands with stratified epithelial cells (HE staining, magnification × 100). The NET component was also found in the second resection specimen, abutting the tubular adenoma and showed cord-like arrangement of monotonous tumor cells (HE staining, magnification × 100). Immunohistochemistry showed that the tumour cells were positive for synaptophysin (magnification × 100) (Figure 3).
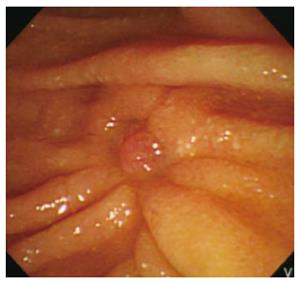
Follow-up endoscopic biopsy of the papillectomy site performed one and three month later did not show remnant tumor except reepithelization (Figure 4). No local recurrence or metastasis of NET or adenoma was detected during 24 mo of follow-up.
NET of the ampulla of Vater, formerly known as carcinoid tumors, is extremely rare. It accounts for only about 0.3%-1% of all gastrointestinal NETs, and less than 2% of all periampullary cancers. The natural history of this disease entity has not been well established[2,9-11]. Well-differentiated (low and intermediate grade) NETs have been variously termed carcinoid tumor (typical and atypical), neuroendocrine tumor (grade 1 and 2), or neuroendocrine carcinoma (low and intermediate grade). The previously used term, carcinoids of the ampulla of Vater, comprises a broad spectrum of morphologically and biologically diverse tumors. In the latest World Health Organization classification, published in 2010, it is recommended to distinguish between (1) neuroendocrine neoplasm, grade 1 (low grade); (2) neuroendocrine neoplasm, grade 2 (intermediate grade); and (3) neuroendocrine carcinoma, grade 3 (high grade)[6].
The diagnosis of ampullary NET is challenging till now. This tumor frequently originates from the deep mucosa or submucosa, so that cannot easily be detected in biopsy specimens. Most reports describe such lesions as a round or oval mass, with intact overlying duodenal mucosa, with negative biopsies. Also, early lymphatic metastasis is possible despite the small size of the lesions, and an accurate diagnosis is difficult[4,12]. Accuracy rates of biopsy for the preoperative diagnosis of NET range from to 14% to 66%[3,4,13]. Duodenoscopy in combination with ERCP is the diagnostic method of choice for deeper biopsies with the aim of identifying intrapapillary lesions. EUS, CT or magnetic resonance imaging (MRI) are used for staging and differential diagnosis, together with detection of invasion of the bile or pancreatic duct and metastasis to lymph nodes or other organs[4].
Clinically, NET can cause carcinoid syndrome, which presents as diarrhea or facial flushing, due to increased secretion of serotonin. However, carcinoid syndrome in ampullary NET is rare and the clinical and laboratory findings typical of carcinoid syndrome are frequently absent[14]. Anatomically, ampullary NET develops at the conjugation of the pancreatic and biliary ducts. Jaundice is the predominant symptom (53%) at the time of admission to hospital, followed by pain (24.6%), acute pancreatitis (6%) and weight loss (3.7%)[4,15]. The case presented herein exhibited no specific symptoms due to the tumour, with the exception of mild dyspepsia.
The treatment of choice for NET is complete resection. Metastasis is rare, particularly in ampullary NETs. Therefore, complete resection is required as the primary therapy[1,4,16]. In terms of prognosis, the 5-year survival rate of completely resected NET exceeds 95%[1]. The classical partial pancreaticoduodenectomy (Kausch-Whipple operation) or pylorus-preserving pancreaticoduodenectomy (PPPD) are considered the treatments of choice for ampullary NETs > 2.0 cm in diameter. The mortality and morbidity rates for the two approaches are less than 5% and 15%, respectively[4,17]. Alternatively, in patients with multiple comorbidities or elderly individuals, conservative treatment or minimally invasive endoscopic papillectomy should be considered[5,7]. Local excision may be an option for the treatment of ampullary NET, if the tumor is small and there is no evidence of regional lymph node or distant metastasis. Compared to local surgical excision, endoscopic papillectomy may be less harmful to the patient and reduce the hospital stay duration, and complete resection is possible in selected patients. Similar to the management of an ampullary adenoma, endoscopic papillectomy may be a reasonable alternative to surgical resection[7]. When endoscopic papillectomy is decided upon, the differentiation of NET, tumor size, and lymph node metastasis should be considered. Endoscopic papillectomy may be a good alternative in highly differentiated tumours that do not infiltrate the muscularis, tumors < 2 cm in size, and with no distant metastasis. The prognosis is reported to be excellent, with an overall 5-year survival rate of 90%[15].
This presented case was diagnosed initially as an ampullary adenoma (low grade) by endoscopic biopsy during screening endoscopy. Following endoscopic papillectomy for removal of adenoma, the lesion was diagnosed as NET, grade 1 (< 2 mitoses/10 HPF and < 3% Ki67 index) accompanied by low-grade adenoma. Pathologically deep resection margin was not clear for the tumor; however, no evidence of local or distant metastasis was detected by repeated biopsies and radiologic examination during 24 mo of follow-up. Surgical resection was not performed due to successful complete endoscopic resection and imaging studies did not show lymph node or distant metastasis.
To the best of our knowledge, this is the first reported case of endoscopic papillectomy for ampullary NET accompanied by adenoma in the English-language literature. Some cases of ampullary small cell neuroendocrine carcinoma associated with or mixed with adenoma, adenocarcinoma and squamous cell carcinoma have been reported[18-20]. However, pancreaticoduodenectoy were performed in all of the above mentioned cases due to a carcinomatous change. Until now, there is a hypothesis that two different types of tumors arise from common progenitor cell and differentiate different[21,22]. In the case of low grade neuroendocrine tumor with adenoma, the low grade neuroendocrine tumor had good prognosis and no death and no lymph node metastasis were found[23]. However, since the study is with regard to lesion of duodenum and colorectum, further study in necessary to examine a prognosis of adenoma and low grade neuroendocrine tumor confined to ampulla of Vater.
In conclusion, low-grade ampullary adenoma is a common indication for endoscopic papillectomy. Indeed, well-differentiated ampullary NET may be also a good candidate for complete endoscopic resection in selected indications. Even though combined case such as a presented case, may be completely resected by endoscopic papillectomy. The clinical or pathological correlation between NET and adenoma is unclear. Further clinical follow-up is needed to confirm the long term clinical outcome or these combined cases.
A 53-year-old female patient visited hospital for dyspepsia of 3-mo duration.
Based on endoscopic finding and given pathology, clinically the authors diagnosed an unexposed ampullary adenoma.
Unexposed adenocarcinoma arising from the ampulla of Vater.
All labs were within normal limits.
A duodenoscopic image showed an enlarged major papilla with central umbilication and fine nodularity. Endoscopic ultrasonography (EUS) at the major ampulla revealed a 1.1 × 0.9-cm, slightly hypoechoic round ampullary mass confined to the submucosa.
Low grade neuroendocrine tumor combined with adenoma.
Complete endoscopic papillectomy of tumors.
Some cases of ampullary small cell neuroendocrine carcinoma associated with or mixed with adenoma, adenocarcinoma and squamous cell carcinoma have been reported, however these cases were managed by surgical resection.
Neuroendocrine tumor (NET) of the ampulla of Vater is formerly known as carcinoid tumors. NET is distinguish between neuroendocrine neoplasm, grade 1 (low grade); neuroendocrine neoplasm, grade 2 (intermediate grade); and neuroendocrine carcinoma, grade 3 (high grade).
Well-differentiated ampullary NET may be a good candidate for complete endoscopic papillectomy in selected indications even though combined case such as an adenoma.
This case report is worthy because of the rarity of the NET in ampulla of Vater accompanied by tubular adenoma treated with endoscopic papillectomy.
| 1. | Strosberg JR, Weber JM, Feldman M, Coppola D, Meredith K, Kvols LK. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol. 2013;31:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Arnold R. Endocrine tumours of the gastrointestinal tract. Introduction: definition, historical aspects, classification, staging, prognosis and therapeutic options. Best Pract Res Clin Gastroenterol. 2005;19:491-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Neuroendocrine tumors of the ampulla of Vater: biological behavior and surgical management. Arch Surg. 2009;144:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Hartel M, Wente MN, Sido B, Friess H, Büchler MW. Carcinoid of the ampulla of Vater. J Gastroenterol Hepatol. 2005;20:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Howe JR, Karnell LH, Menck HR, Scott-Conner C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer. 1999;86:2693-2706. [PubMed] |
| 6. | Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | De Palma GD. Endoscopic papillectomy: indications, techniques, and results. World J Gastroenterol. 2014;20:1537-1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 8. | Will U, Müller AK, Fueldner F, Wanzar I, Meyer F. Endoscopic papillectomy: data of a prospective observational study. World J Gastroenterol. 2013;19:4316-4324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Yakaitis RW, Thomas JD, Mahaffey JE. Influence of pH and hypoxia on the success of defibrillation. Crit Care Med. 2010;3:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Jayant M, Punia R, Kaushik R, Sharma R, Sachdev A, Nadkarni NK, Attri A. Neuroendocrine tumors of the ampulla of vater: presentation, pathology and prognosis. JOP. 2012;13:263-267. [PubMed] |
| 11. | Mavroudis N, Rafailidis S, Symeonidis N, Aimoniotou E, Antonopoulos V, Evgenidis N, Venizelos L, Sakadamis A. Carcinoid of the ampulla of Vater--report of two cases. Acta Chir Belg. 2005;105:213-216. [PubMed] |
| 12. | Clements WM, Martin SP, Stemmerman G, Lowy AM. Ampullary carcinoid tumors: rationale for an aggressive surgical approach. J Gastrointest Surg. 2003;7:773-776. [PubMed] |
| 13. | Hwang S, Lee SG, Lee YJ, Han DJ, Kim SC, Kwon SH, Ryu JH, Park JI, Lee HJ, Choi GW. Radical surgical resection for carcinoid tumors of the ampulla. J Gastrointest Surg. 2008;12:713-717. [PubMed] |
| 14. | Onaitis MW, Kirshbom PM, Hayward TZ, Quayle FJ, Feldman JM, Seigler HF, Tyler DS. Gastrointestinal carcinoids: characterization by site of origin and hormone production. Ann Surg. 2000;232:549-556. [PubMed] |
| 15. | Hatzitheoklitos E, Büchler MW, Friess H, Poch B, Ebert M, Mohr W, Imaizumi T, Beger HG. Carcinoid of the ampulla of Vater. Clinical characteristics and morphologic features. Cancer. 1994;73:1580-1588. [PubMed] |
| 16. | Dumitrascu T, Dima S, Herlea V, Tomulescu V, Ionescu M, Popescu I. Neuroendocrine tumours of the ampulla of Vater: clinico-pathological features, surgical approach and assessment of prognosis. Langenbecks Arch Surg. 2012;397:933-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Roder JD, Stein HJ, Hüttl W, Siewert JR. Pylorus-preserving versus standard pancreatico-duodenectomy: an analysis of 110 pancreatic and periampullary carcinomas. Br J Surg. 1992;79:152-155. [PubMed] |
| 18. | Sugawara G, Yamaguchi A, Isogai M, Watanabe Y, Kaneoka Y, Suzuki M. Small cell neuroendocrine carcinoma of the ampulla of Vater with foci of squamous differentiation: a case report. J Hepatobiliary Pancreat Surg. 2004;11:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Nassar H, Albores-Saavedra J, Klimstra DS. High-grade neuroendocrine carcinoma of the ampulla of vater: a clinicopathologic and immunohistochemical analysis of 14 cases. Am J Surg Pathol. 2005;29:588-594. [PubMed] |
| 20. | Sun JH, Chao M, Zhang SZ, Zhang GQ, Li B, Wu JJ. Coexistence of small cell neuroendocrine carcinoma and villous adenoma in the ampulla of Vater. World J Gastroenterol. 2008;14:4709-4712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Cox WF, Pierce GB. The endodermal origin of the endocrine cells of an adenocarcinoma of the colon of the rat. Cancer. 1982;50:1530-1538. [PubMed] |
| 22. | Vortmeyer AO, Lubensky IA, Merino MJ, Wang CY, Pham T, Furth EE, Zhuang Z. Concordance of genetic alterations in poorly differentiated colorectal neuroendocrine carcinomas and associated adenocarcinomas. J Natl Cancer Inst. 1997;89:1448-1453. [PubMed] |
| 23. | Estrella JS, Taggart MW, Rashid A, Abraham SC. Low-grade neuroendocrine tumors arising in intestinal adenomas: evidence for alterations in the adenomatous polyposis coli/β-catenin pathway. Hum Pathol. 2014;45:2051-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Aiura K, Boy C, Kim HS, Zhao HT S- Editor: Yu J L- Editor: A E- Editor: Wang CH