Published online Apr 7, 2016. doi: 10.3748/wjg.v22.i13.3663
Peer-review started: November 14, 2015
First decision: December 11, 2015
Revised: December 19, 2015
Accepted: January 11, 2016
Article in press: January 11, 2016
Published online: April 7, 2016
Processing time: 135 Days and 5.9 Hours
AIM: To investigate the prevalence of and risk factors for non-alcoholic fatty liver disease (NAFLD) in a Chinese population.
METHODS: A total of 1948 adults from China was followed for 8 years. A cross-sectional study was performed to investigate the prevalence of NAFLD at baseline, and then the participants were followed for 8 years to investigate risk factors for the development of NAFLD.
RESULTS: A total of 1948 participants were enrolled at baseline, of whom 691 were diagnosed with NAFLD. During the 8-year follow-up, 337 baseline NAFLD-free participants developed NAFLD. They had a greater increase in body mass index (BMI), serum uric acid, fasting plasma glucose, very low-density lipoprotein cholesterol and a considerable decrease in high-density lipoprotein cholesterol. 123 participants who had NAFLD at baseline lost NAFLD during the 8-year follow-up period. They had a greater decrease in BMI, fasting plasma glucose, triglycerides, total cholesterol, low-density lipoprotein cholesterol, alanine aminotransferase, aspartate aminotransferase, and γ-glutamyl transpeptidase.
CONCLUSION: NAFLD is prevalent in Chinese population with a rapidly increasing tendency. It can be reversed when patients lose their weight, control their hyperlipidemia and hyperglycemia, and reduce the liver enzyme levels.
Core tip: This study followed a large sample (n = 1928) for a long term (time = 8 years) to observe the development of non-alcoholic fatty liver disease due to the lifestyle and nutrition changes in a Chinese population.
- Citation: Lu ZY, Shao Z, Li YL, Wulasihan M, Chen XH. Prevalence of and risk factors for non-alcoholic fatty liver disease in a Chinese population: An 8-year follow-up study. World J Gastroenterol 2016; 22(13): 3663-3669
- URL: https://www.wjgnet.com/1007-9327/full/v22/i13/3663.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i13.3663
Non-alcoholic fatty liver disease (NAFLD) refers to hepatic steatosis accounting for over 5% of the total weight of the liver, which is not caused by excessive consumption of alcohol (women ≤ 70 g/wk, men ≤ 140 g/wk)[1,2]. NAFLD is seen worldwide and it is considered the most common chronic liver disease in Western countries, with a prevalence ranging from 17% and 46% in the general population[3,4]. It has also become prevalent in China with the rapid economic development[5-8]. Although NAFLD is not a severe disease, it is among the causes of fatty liver and one of the leading etiologies of chronic liver diseases[9]. NFLD includes a wide clinical and histological spectrum, such as simple steatosis, steatohepatitis, fibrosis, and cirrhosis[10]. Simple steatosis is considered a benign condition, while non-alcoholic steatohepatitis progresses to advanced fibrosis or cirrhosis in nearly 30% of cases, and may lead to end-stage liver disease and hepatocellular carcinoma[9,11-14]. It has been projected that NAFLD will be the most rapidly growing indication for liver transplantation in the next decades[15]. In addition, NAFLD is a well-known risk factor for cardiovascular disease, type 2 diabetes and metabolic syndrome[16-18].
This report consists of two studies of the same population of adult urban subjects. A total of 1948 adults from Zhejiang, China was followed for 8 years. A cross-sectional study was performed to investigate the prevalence and correlative factors of NAFLD and the risk factors for the development of NAFLD after a prospective 8-year follow-up was performed.
This study was performed among adults who underwent routine health examinations at the First Affiliated Hospital, College of Medicine, Zhejiang University between 2006 and 2014. Subjects were excluded if they fulfilled one of the following criteria in 8 years: (1) excess alcohol consumption (men > 140 g/wk, women > 70 g/d); (2) presence of markers of hepatitis B virus infection (hepatitis B surface antigen) and hepatitis C virus infection (anti-hepatitis C virus antibodies); (3) a history of autoimmune hepatitis (e.g., autoimmune hepatitis, primary biliary cirrhosis) or other chronic liver disease with clear causes; and (4) absence of uncontrolled biliary diseases (e.g., bile ductal stone, stenosis, biliary dilatation). A total of 1948 adults above 20 years old were included in the final analysis. The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University.
Body weight and standing height were measured to calculate body mass index (BMI). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded with an automated sphygmomanometer as the subjects sitting calmly. Approximately 10 mL venous blood samples were collected from all subjects following a 12 h overnight-fast. Hemoglobin (Hb), platelet (PLT), white blood cell (WBC), serum uric acid (SUA), fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, very low-density lipoprotein (VLDL) cholesterol, high-density lipoprotein (HDL) cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transpeptidase (γ-GT) were measured with an automatic biochemistry analyzer (Beckman Coulter Inc., CA) using standard methods.
NAFLD was diagnosed based on the ultrasonic criteria suggested by the Chinese Medical Association[2]. The criteria are described as the following items: (1) diffuse enhancement of near field echo in the hepatic region (stronger than in the kidney and spleen region) and gradual attenuation of the far field echo; (2) unclear display of intra-hepatic lacuna structure; (3) mild to moderate hepatomegaly with a round and blunt border; and (4) color Doppler ultrasonography shows a reduction of the blood flow signal in the liver or it is even hard to display, but the distribution of blood flow is normal. NAFLD was diagnosed if item 1 and any one or more of items 2-4 are matched. Hepatic ultrasonic examination was performed and conducted by a trained ultrasonographist in a blind manner.
The population was followed for 8 years, and the end-point examination was repeated in 2014. During this period, patients who took any kind of prescription medicine are excluded. Serological tests were measured with the same automatic analyzer using the same methods. Training course was carried out to make sure that the ultrasonic criteria of NAFLD remain the same and hepatic ultrasonic examination was still performed in a blind manner.
All statistical analyses were conducted with the SPSS software package version 13.0 for Windows (SPSS, Inc., Chicago, IL). Baseline analyses were done using descriptive statistics expressed as mean ± SD, and differences between continuous variables were assessed using Student’s t-test or the Mann-Whitney U-test, depending on the normality of the data. Categorical variables were compared using Pearson’s chi-squared (χ2) test or Fisher’s exact test. Stepwise Binary logistic regression (Forward: Wald; Entry: 0.05, Removal: 0.10) was used to analyse the risk factors associated with the presence of NAFLD. A P value < 0.05 (two-tailed) was considered statistically significant.
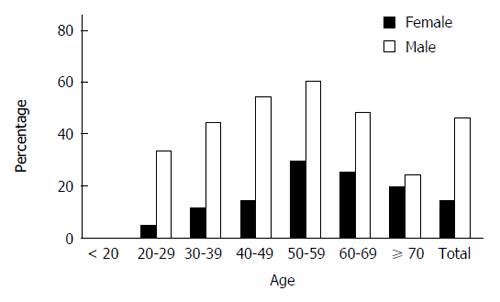
A total of 1948 adult subjects were eventually enrolled in this study, which consisted of 1283 males (65.86%) and 665 females (34.14%). Among 1948 subjects, 691 were diagnosed with NAFLD with the prevalence of 35.47% at baseline (Table 1). Compared with those without NAFLD, the subjects with NAFLD were significantly older, male dominated, and had significantly higher BMI, SBP and DBP, levels of Hb, WBC count, SUA, FPG, TG, TC, LDL cholesterol, VLDL cholesterol, HDL cholesterol, ALT, AST and γ-GT. Meanwhile, NAFLD subjects had significantly lower high-density lipoprotein cholesterol levels. NAFLD also had relatively higher level of PLT count with no significance. Furthermore, the overall prevalence of NAFLD was significantly higher in males than in females (46.61% vs 13.98%, P < 0.001). The gender difference in NAFLD prevalence was noted in groups younger than 60 years but absent in those older than 60 years (Table 2, Figure 1). In addition, the overall prevalence of NAFLD increased with age (in trend analysis, P < 0.001) and reached a peak in the group of 50-60 years (51.74%). This trend was not only in the overall prevalence but also in both males and female’s prevalence (Table 2 and Figure 1).
| Variable | NAFLD | Non-NAFLD | t value | P value |
| Age (yr) | 43.38 (0.42) | 40.14 (0.35) | 5.944 | < 0.001 |
| Gender, male/female (n) | 598/93 | 685/572 | 203.663 | < 0.001 |
| BMI (kg/m2) | 25.69 (0.09) | 21.83 (0.07) | 33.044 | < 0.001 |
| SBP (mmHg) | 128.62 (0.6) | 117.49 (0.43) | 15.253 | < 0.001 |
| DBP (mmHg) | 79.73 (0.36) | 72.71 (0.27) | 15.608 | < 0.001 |
| Hb (g/L) | 151.3 (0.46) | 141.62 (0.41) | 15.679 | < 0.001 |
| PLT (× 109/L) | 209.44 (2.12) | 206.1 (1.5) | 1.299 | 0.194 |
| WBC (× 109/L) | 6.48 (0.06) | 5.91 (0.04) | 8.294 | < 0.001 |
| SUA (μmol/L) | 367.92 (3.45) | 291.89 (2.32) | 18.742 | < 0.001 |
| FPG (mmol/L) | 5.01 (0.05) | 4.59 (0.02) | 8.019 | < 0.001 |
| TG (mmol/L) | 2.47 (0.07) | 1.3 (0.03) | 15.096 | < 0.001 |
| TC (mmol/L) | 4.94 (0.03) | 4.6 (0.02) | 8.128 | < 0.001 |
| LDL (mmol/L) | 2.35 (0.03) | 2.16 (0.03) | 4.487 | < 0.001 |
| VLDL (mmol/L) | 1.41 (0.03) | 1.01 (0.02) | 10.996 | < 0.001 |
| HLDL (mmol/L) | 1.16 (0.01) | 1.44 (0.01) | -20.396 | < 0.001 |
| ALT (U/L) | 36.24 (0.9) | 19.75 (0.41) | 16.649 | < 0.001 |
| AST (U/L) | 27.21 (0.43) | 22.1 (0.25) | 10.200 | < 0.001 |
| γ-GT (U/L) | 49.97 (1.51) | 25.56 (0.99) | 13.512 | < 0.001 |
| Age (yr) | Total | NAFLD | Overall | Males | Females | χ2value | P value |
| < 20 | 3 | 0 | 0.00% | 0.00% | 0.00% | - | - |
| 20-29 | 342 | 69 | 20.18% | 28.07% | 4.39% | 26.471 | < 0.001 |
| 30-39 | 559 | 183 | 32.74% | 44.14% | 10.94% | 63.114 | < 0.001 |
| 40-49 | 608 | 245 | 40.30% | 55.01% | 14.16% | 97.223 | < 0.001 |
| 50-59 | 259 | 134 | 51.74% | 60.00% | 28.99% | 19.499 | < 0.001 |
| 60-69 | 119 | 47 | 39.50% | 48.00% | 25.00% | 6.139 | 0.013 |
| ≥ 70 | 58 | 13 | 22.41% | 25.00% | 19.23% | 0.275 | 0.600 |
| Total | 1948 | 691 | 35.47% | 46.61% | 13.98% | 203.663 | < 0.001 |
Stepwise binary logistic regression model was used to explore the independent risk factors associated with the presence of NAFLD. All the 18 variables in Table 1 were recruited into the original equation, and 10 variables remained in the final equation after removing 8 variables (Table 3). Our results suggest that those 10 variables were significantly associated with the presence of NAFLD including age, BMI, PLT count, SUA, FPG, TG, VLDL cholesterol, HDL cholesterol, ALT and AST.
| Variables | B | S.E. | Wals | Sig. | OR | 95%CI of OR | |
| Age | 0.025 | 0.007 | 14.767 | < 0.001 | 1.025 | 1.012 | 1.038 |
| BMI | 0.450 | 0.036 | 158.411 | < 0.001 | 1.569 | 1.462 | 1.682 |
| PLT | 0.004 | 0.001 | 6.898 | 0.009 | 1.004 | 1.001 | 1.006 |
| SUA | 0.006 | 0.001 | 28.372 | < 0.001 | 1.006 | 1.004 | 1.008 |
| FPG | 0.334 | 0.081 | 16.884 | < 0.001 | 1.397 | 1.191 | 1.638 |
| TG | 0.280 | 0.089 | 9.919 | 0.002 | 1.323 | 1.112 | 1.575 |
| VLDL | 0.336 | 0.118 | 8.148 | 0.004 | 1.400 | 1.111 | 1.764 |
| HDL | -1.053 | 0.298 | 12.469 | < 0.001 | 0.349 | 0.194 | 0.626 |
| ALT | 0.049 | 0.008 | 37.985 | < 0.001 | 1.050 | 1.034 | 1.067 |
| AST | -0.064 | 0.014 | 20.903 | < 0.001 | 0.938 | 0.912 | 0.964 |
After 8-year follow-up, 337 (17.30%) subjects free of NAFLD at baseline developed NAFLD and 123 (6.31%) subjects diagnosed with NAFLD at baseline had not NAFLD any more (Tables 4 and 5). These changes made the prevalence of NAFLD rise by 10.99% to 46.46%. Paired-samples t-test was employed to analyse the changes from baseline to the end point in these two groups, respectively. Subjects who developed NAFLD had a significant change in almost all the parameters during the follow-up. There was an especially greater increase in BMI, SUA, FPG, VLDL cholesterol and a considerable decrease in HDL cholesterol, as their absolute t-values were greater. It is worth noting that these 5 variables were all significantly associated with the presence of NAFLD. Subjects who seceded from NAFLD had a greater decrease in BMI, FPG, TG, TC, LDL cholesterol, ALT, AST, and γ-GT.
| Variables | Difference | t value | P value |
| BMI (kg/m2) | -1.454 | -17.198 | 0.000 |
| SBP (mmHg) | -4.875 | -6.105 | 0.000 |
| DBP (mmHg) | -2.225 | -4.021 | 0.000 |
| Hb (g/L) | -3.537 | -6.972 | 0.000 |
| PLT (× 109/L) | -7.352 | -3.276 | 0.001 |
| WBC (× 109/L) | -0.283 | -3.728 | 0.000 |
| SUA (μmol/L) | -29.304 | -8.814 | 0.000 |
| FPG (mmol/L) | -0.463 | -10.909 | 0.000 |
| TG (mmol/L) | -0.224 | -2.630 | 0.009 |
| TC (mmol/L) | -0.268 | -6.823 | 0.000 |
| LDL (mmol/L) | 0.054 | 1.368 | 0.172 |
| VLDL (mmol/L) | -0.295 | -11.043 | 0.000 |
| HDL (mmol/L) | 0.126 | 10.200 | 0.000 |
| ALT (U/L) | -3.099 | -2.672 | 0.008 |
| AST (U/L) | 0.006 | 0.009 | 0.993 |
| γ-GT (U/L) | -10.43 | -4.912 | 0.000 |
| Variables | Difference | t value | P value |
| BMI (kg/m2) | 0.801 | 4.759 | 0.000 |
| SBP (mmHg) | -2.285 | -1.509 | 0.134 |
| DBP (mmHg) | -0.279 | -0.274 | 0.784 |
| Hb (g/L) | -0.434 | -0.496 | 0.621 |
| PLT (× 109/L) | 0.754 | 0.203 | 0.839 |
| WBC(× 109/L) | -0.040 | -0.274 | 0.785 |
| SUA (μmol/L) | 12.800 | 1.820 | 0.072 |
| FPG (mmol/L) | -0.204 | -2.329 | 0.022 |
| TG (mmol/L) | 0.643 | 6.159 | 0.000 |
| TC (mmol/L) | 0.209 | 2.721 | 0.007 |
| LDL (mmol/L) | 0.357 | 4.532 | 0.000 |
| VLDL (mmol/L) | 0.073 | 1.544 | 0.125 |
| HDL (mmol/L) | -0.005 | -0.194 | 0.846 |
| ALT (U/L) | 8.950 | 5.917 | 0.000 |
| AST (U/L) | 5.084 | 5.997 | 0.000 |
| γ-GT (U/L) | 9.557 | 4.612 | 0.000 |
The prevalence of NAFLD in the Western countries was estimated to be 20% to 30%[19], and varied between 17% and 46% depending on the different population included in the study[4]. With the improvement of economy and westernization of lifestyle in China, the prevalence of NAFLD in the Chinese population had a rapidly increasing tendency, especially in the urban population. Our study showed that the prevalence of NAFLD was 35.47 % in 2006 and went up to 46.46% in 2014 in the same population after follow-up for 8 years. The results also indicated that the prevalence of NAFLD increased with age. The peak of age showed in 50-60 year old subjects. It is important to note that elder people had significantly more known risk factors for NAFLD, such as obesity, diabetes mellitus and hyperlipidaemia[4]. On the other hand, dysfunction of preadipocytes in the elder people impairs the capacity of fat tissue to store lipids, and leads to fat redistribution from subcutaneous to intraabdominal visceral depots including the liver[20,21]. Our results also suggest that there was a significant difference in the prevalence of NAFLD between men and women before the age of 60. This difference might be a consequence of the protective role of estrogens in females. Difference in sex hormone levels would probably correlate to the differences in the amount and distribution of body fat between the sexes[22,23]. As men usually store fat in the abdomen, women store more fat in the subcutaneous tissue. What’s more, logistic regression analyses revealed that gender was not an independent risk factor associated with the prevalence of NAFLD. Different values of serological markers in different genders might directly affect the prevalence of NAFLD.
Our results showed that age, BMI, PLT count, SUA, FPG, TG, VLDL cholesterol, HDL cholesterol, ALT and AST were 10 risk factors independently associated with the prevalence of NAFLD. In the follow-up study, we found not only progressive subjects but also rehabilitative cases. It is the most interesting finding in our study that NAFLD was not always progressing. BMI, SUA, FPG, VLDL cholesterol and HDL cholesterol play the greatest role in the development of NAFLD. They are risk factors for both the prevalence and development of NAFLD. Subjects with NAFLD can be reversed if they lose their weight, control their hyperlipidemia and hyperglycemia, and reduce the liver enzyme levels.
High BMI is without doubt a major risk factor for NAFLD. In this study, the prevalence of NAFLD was 19.94% in non-obese subjects (BMI ≤ 25 kg/m2) and reached 75.05% in obese subjects (BMI > 25 kg/m2). In patients with morbid obesity (BMI > 40 kg/m2) who undergo bariatric surgery, the prevalence of NAFLD may even be in excess of 90%[9]. Previous studies showed that modest weight loss is associated with amelioration of hepatic steatosis and other histological improvements[24,25]. Several recent studies suggested the importance of body weight control, not only in the obese but also in non-obese subjects, for reducing the risk of or preventing NAFLD[26,27].
As universally acknowledged, the proportion of NAFLD is also higher in patients with type 2 diabetes or metabolic syndrome[4]. FPG, TG, VLDL cholesterol and HDL cholesterol were all markers relevant to glucose and lipid metabolism. In this study, we suggest that not only type 2 diabetes and metabolic syndrome but also moderate elevation in parameters mentioned above were responsible for a high prevalence of NAFLD.
Our results demonstrate a close relationship between high SUA and NAFLD. Hyperuricemia is a common finding in recent studies and SUA is independently associated with histological findings of NAFLD regardless of insulin resistance and metabolic syndrome status[28]. As the underlying mechanism is not well studied, further studies are needed to characterize the role of SUA in the development of NAFLD.
In this study, liver enzymes were associated with the prevalence and development of NAFLD. In many previous studies, elevations in the liver enzymes were non-invasive indicators of NAFLD[29,30]. These enzymes indirectly reflect histological changes of livers and severity of NAFLD. It is not surprising that all three enzymes[1-3] significantly decreased in subjects who seceded from NAFLD during the follow-up.
From both clinical experience and research data, the more alcohol people intake, the higher blood TG levels. Patients with NAFLD often have elevated TG. The biopsy also proved that their steatosis correlates directly with alcohol intake. Women may be affected at even lower levels of intake (e.g., half dose). In another word, the alcohol sensitivity of women is different from that of men. Women are more likely to develop NAFLD than men even with lower dose of alcohol intake. That is why we put a lower bar for women (70 g in women vs 140 g in men) in this study according to the Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases[2].
There are several limitations in this study. NAFLD was diagnosed by ultrasonography, which is not sensitive for mild NAFLD and cannot determine the severity of NAFLD. However, ultrasonography as a non-invasive method is widely used in population-based studies with high diagnostic value for detecting NAFLD[31]. Although excluding any medical intervention, during the 8-year follow-up, dietary habits are not fully followed due to the difficult standardization. The role of diet change in NAFLD development and resolution should be also further studied. In addition, admission bias cannot be eliminated because most subjects who participated in health examinations were from population with stable income and high education in urban area.
Our results provide the prevalence of NAFLD and the risk factors for its prevalence and development in a Chinese population. Our findings may make clear the high prevalence in China.
In conclusion, our results showed that NAFLD is prevalent in the Chinese population with a rapidly increasing tendency. NAFLD can be reversed when patients lose their weight, control their hyperlipidemia and hyperglycemia, and reduce the liver enzyme levels.
This study focused on the long-term outcome of non-alcoholic fatty liver disease (NAFLD) in China. The prevalence of NAFLD in China has approximately doubled in the past decade with the increasing pandemic of obesity. Epidemiological data and long-term outcomes of NAFLD in Chinese populations remain unknown and need to be updated, which can be used as a predictor for metabolic disorders and a basis for public health interventions.
Not all individuals with NAFLD develop hepatic steatosis. There also appears to be racial-ethnic variations. So NAFLD racial-ethnic study becomes a hotpot currently. Our study focused on a Chinese population and observed the long-term outcome to provide the initial clues of risk factors for further mechanism study.
The breakthrough of the current study is the finding of natural prognosis of NAFLD by a self-comparison after 8-year follow-up. Among a total 1948 participants, 337 baseline NAFLD-free participants developed NAFLD and 123 participants who had NAFLD at baseline lost NAFLD. Analysis of their clinical characters and laboratory results indicates their metabolic fate without antilipemic drug interventions.
Currently, there is no approved therapy for NAFLD/non-alcoholic steatohepatitis (NASH). Treatment strategies may be grouped into those which address weight loss, reduce lipids, are antioxidants, or target the liver. Our research findings provide the target and biomarkers for the therapies.
NAFLD is a common hepatic disease. Pathologically, it can present as simple steatosis, NASH, and eventually progress to cirrhosis, an end-stage liver disease. The NAFLD is the most common cause of chronic liver disease in the Western world, and it has been increasing in China in the past years. Notably, 1%-5% of patients with simple steatosis can eventually develop actual cirrhosis and even to hepatocellular carcinoma.
Three reviewers have reviewed the manuscript. They all recognized the significance of the study. The only concern is the different criteria for alcohol consumption between males and females. It comes from the sensitivity threshold between different genders. The definition refers to the NAFLD guideline.
| 1. | Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. BMJ. 2014;349:g4596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 2. | Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, Mao YM. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9:108-112. [PubMed] |
| 3. | Weiß J, Rau M, Geier A. Non-alcoholic fatty liver disease: epidemiology, clinical course, investigation, and treatment. Dtsch Arztebl Int. 2014;111:447-452. [PubMed] |
| 4. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2326] [Article Influence: 155.1] [Reference Citation Analysis (1)] |
| 5. | Liao XH, Cao X, Liu J, Xie XH, Sun YH, Zhong BH. Prevalence and features of fatty liver detected by physical examination in Guangzhou. World J Gastroenterol. 2013;19:5334-5339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Wang Z, Xu M, Peng J, Jiang L, Hu Z, Wang H, Zhou S, Zhou R, Hultström M, Lai EY. Prevalence and associated metabolic factors of fatty liver disease in the elderly. Exp Gerontol. 2013;48:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Yan J, Xie W, Ou WN, Zhao H, Wang SY, Wang JH, Wang Q, Yang YY, Feng X, Cheng J. Epidemiological survey and risk factor analysis of fatty liver disease of adult residents, Beijing, China. J Gastroenterol Hepatol. 2013;28:1654-1659. [PubMed] |
| 8. | Xiao SJ, Fu GJ, Lv YL, Zhong XN, Wang RH. Prevalence and risk factors of fatty liver disease in young and middle-aged population: one center study in Southwestern China. J Gastroenterol Hepatol. 2014;29:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1384] [Article Influence: 98.9] [Reference Citation Analysis (5)] |
| 10. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2359] [Article Influence: 87.4] [Reference Citation Analysis (1)] |
| 11. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [PubMed] |
| 12. | Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, Nobili V, Mozzi E, Roviaro G, Vanni E. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 13. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1021] [Article Influence: 42.5] [Reference Citation Analysis (1)] |
| 14. | Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 15. | Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188-2195. [PubMed] |
| 16. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1521] [Article Influence: 95.1] [Reference Citation Analysis (10)] |
| 17. | Sung KC, Kim SH. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Fan JG, Zhu J, Li XJ, Chen L, Lu YS, Li L, Dai F, Li F, Chen SY. Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol. 2005;20:1825-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 919] [Article Influence: 70.7] [Reference Citation Analysis (2)] |
| 20. | Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | DeNino WF, Tchernof A, Dionne IJ, Toth MJ, Ades PA, Sites CK, Poehlman ET. Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care. 2001;24:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Tian GX, Sun Y, Pang CJ, Tan AH, Gao Y, Zhang HY, Yang XB, Li ZX, Mo ZN. Oestradiol is a protective factor for non-alcoholic fatty liver disease in healthy men. Obes Rev. 2012;13:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Zhang H, Liu Y, Wang L, Li Z, Zhang H, Wu J, Rahman N, Guo Y, Li D, Li N. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013;54:345-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Kim HK, Park JY, Lee KU, Lee GE, Jeon SH, Kim JH, Kim CH. Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. Am J Med Sci. 2009;337:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 499] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 26. | Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai Study. Am J Gastroenterol. 2013;108:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Nishioji K, Sumida Y, Kamaguchi M, Mochizuki N, Kobayashi M, Nishimura T, Yamaguchi K, Itoh Y. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011-2012. J Gastroenterol. 2015;50:95-108. [PubMed] |
| 28. | Xie Y, Wang M, Zhang Y, Zhang S, Tan A, Gao Y, Liang Z, Shi D, Huang Z, Zhang H. Serum uric acid and non-alcoholic fatty liver disease in non-diabetic Chinese men. PLoS One. 2013;8:e67152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 408] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 30. | Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 31. | Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 782] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: De Minicis S, Julie NL, Romero MR S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM