Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3496
Peer-review started: April 16, 2015
First decision: June 19, 2015
Revised: July 16, 2015
Accepted: September 13, 2015
Article in press: September 14, 2015
Published online: March 28, 2016
Processing time: 344 Days and 16.1 Hours
Mycosis fungoides (MF) is a cutaneous T-cell lymphoma that can undergo local progression with possible systemic dissemination. We report a case of a patient affected by MF with a pancreatic mass that was a diagnostic challenge between primitive tumor and pancreatic metastasis from MF. Clinical setting findings and imaging studies raised the suspicion of a pancreatic primary neoplasm. A diagnostic clue was provided by the combined histomorphologic/immunohistochemical study of pancreatic and cutaneous biopsies, which revealed a pancreatic localization of MF. Considering the rarity of metastatic localization of MF to the pancreas, we next investigated whether chemokine-chemokine receptor interactions could be involved in the phenomenon to provide new insight into the possible mechanisms underlying metastatic localization of MF to the pancreas. Histological analyses of archival pancreatic tissue demonstrated that glucagon-secreting cells of the pancreatic islets expressed the CCL27 chemokine, which may have attracted in our case metastatic MF cells expressing the complementary receptor CCR10.
Core tip: We believe that this clinical case is very interesting both for its rarity (mycosis fungoides metastasized to the pancreas) and the complexity of the differential diagnosis. In addition, the investigations performed provide new insight into the possible mechanisms underlying metastatic localization of mycosis fungoides to the pancreas. Indeed, histological analyses of archival pancreatic tissue demonstrated for the first time that glucagon-secreting cells of the pancreatic islets express the CCL27 chemokine, which may have attracted, in our case, metastatic mycosis fungoides cells expressing the complementary receptor CCR10.
- Citation: Ceriolo P, Fausti V, Cinotti E, Bonadio S, Raffaghello L, Bianchi G, Orcioni GF, Fiocca R, Rongioletti F, Pistoia V, Borgonovo G. Pancreatic metastasis from mycosis fungoides mimicking primary pancreatic tumor. World J Gastroenterol 2016; 22(12): 3496-3501
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3496.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3496
Cutaneous T-cell lymphoma (CTCL) includes a group of mature (peripheral) T-cell neoplasms characterized by clonal expansion of a mature CD4-positive T-cell, putatively belonging to a skin homing subset of memory T-cells[1]. According to World Health Organization (WHO) criteria, mycosis fungoides (MF) is the most common form of CTCL and accounts for almost 50% of all primary cutaneous lymphomas[2].
MF is an indolent disease initially limited to the skin that undergoes variable cutaneous progression with possible systemic dissemination in late stages. Although spread of MF to the gastrointestinal tract has been described previously, there have been only two reports on symptomatic pancreatic involvement[3,4].
Herein, we report a case of MF in anaplastic transformation with pancreatic involvement, mimicking primary pancreatic tumor.
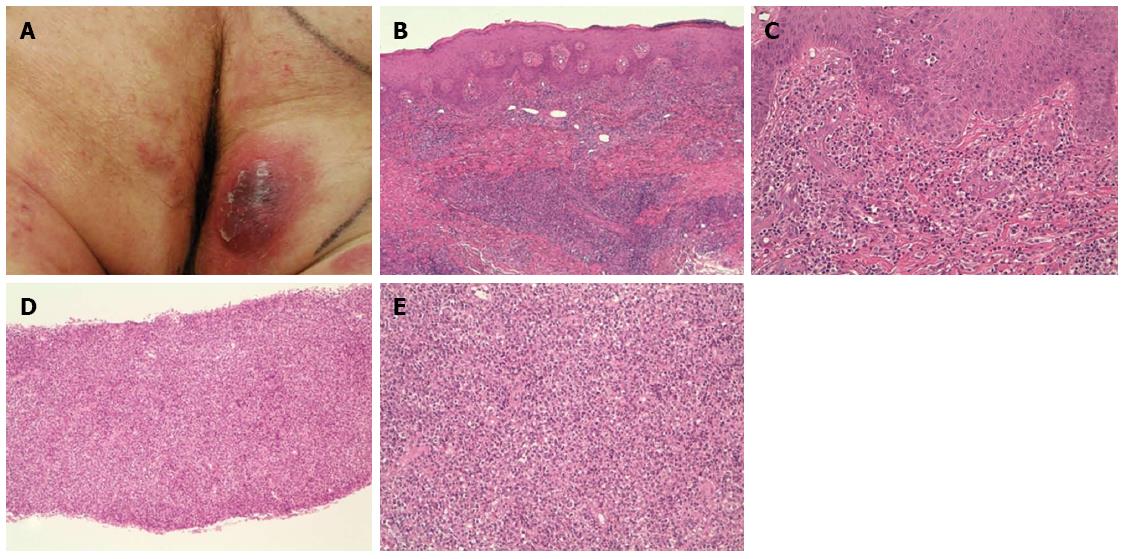
A 64-year-old man presented with erythematous and nodular lesions on the trunk and extremities (erythematous patches on the trunk, red-brown sharply demarcated plaques on the extremities, and violaceous nodules and exophytic tumor lesions on the occipital region, right forearm, and gluteus) (Figure 1A). Erythematous skin lesions manifested 6 years before admission and followed an indolent course until 1 year ago when the full aggressive clinical picture described above became apparent.
Since the lesions were increasing in number and unresponsive to the conventional topical therapy, a nodule on the right arm of the patient was biopsied. The histopathological evaluation led to the diagnosis of CTCL, MF-type. The patient was studied with abdominal computed tomography (CT) that did not show any visceral or lymph node involvement.
Initially, the patient was treated with narrow-band ultraviolet B therapy (three sessions a week on nonconsecutive days, initial dose 0.1 J/cm2, increment of the dose 0.1 J/cm2 per session) followed 2 mo later by Interferon α2b, 3 million units subcutaneously three times a week. Interferon α2b therapy was temporarily stopped because of side effects (nausea, gastric pyrosis, diarrhea, neutropenia, and anemia) and restarted 3 mo later with the addition of oral bexarotene (75 mg twice a day for 10 d, subsequently three times a day for another 10 d and then four times a day for 3 mo) and local radiotherapy of the occipital region (36 Gy five times a week for 2 mo). The patient responded with partial resolution of the skin lesions, especially the nodular ones. Five months later, the patient complained of persistent abdominal pain, but blood chemistry and abdominal ultrasonography did not reveal any abnormality.
Eight months later, the patient presented with jaundice, asthenia, and worsening of abdominal pain.
Blood exams and gastrointestinal ultrasonography were initially performed; thereafter, a percutaneous ultrasound guided pancreatic core biopsy and a cutaneous biopsy were performed for a combined histomorphologic and immunohistochemical study. In particular, immunohistochemical staining for CD3, CD4, CD30, CD45, glucagon, somatostatin, pancreatic polypeptide, CCL27, and CCR10 was carried out.
Blood chemistry disclosed abnormal levels of amylase (625 U/L; n.v. 5-53 U/L), lipase (9228 U/L; n.v. 114-286 U/L), aspartate aminotransferase: 105 U/L; n.v. 0-40 U/L), alanine aminotransferase (171 U/L; n.v. 0-40 U/L), alkaline phosphatase (1539 U/L; n.v. 98-280 U/L), gamma-glutamyltransferase ( 675 U/L; n.v. 11-50), total bilirubin (1.36 mg/dL; n.v. 0.2-1.2 mg/dL), and direct bilirubin (1 mg/dL; n.v. 0.0-0.4 mg/dL). These results were indicative of biliary stasis.
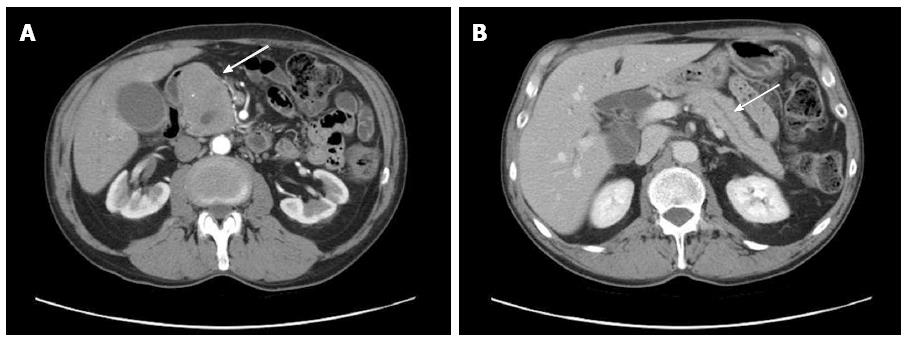
Thereafter, gastrointestinal ultrasonography was performed showing a mass measuring 70 mm × 48 mm × 68 mm and involving the head of pancreas with peripheric vascularization pattern and Wirsung duct dilation. An abdominal CT with contrast agent showed an irregular pancreatic mass involving the head of the pancreas (Figure 2A, arrow), with dilation of the intrahepatic and the common bile ducts (Figure 2B, arrow) in the absence of further pancreatic lesions. No involvement of spleen, liver, kidney, or lymph node was detected. Imaging studies suggested the presence of a pancreatic primary neoplasm. A diagnosis of pancreatic adenocarcinoma was unlikely because of the homogeneous contrast enhancement and the regular margins of the mass. Other potential diagnoses included papillary neoplasm, neuroendocrine tumors, or lymphoma. Genetic analysis of K-ras, P16, and p53, which are highly mutated in pancreatic cancer, was not performed. The patient was subjected to percutaneous ultrasound guided core biopsy of the pancreatic mass, which suggested a metastatic localization of MF but did not allow drawing definitive conclusions due to the paucity of the material.
Considering also the extreme rarity of this occurrence, the pancreatic needle biopsy was repeated in parallel with a cutaneous biopsy to compare the histological features of the two specimens. A lymphomatous infiltrate consistent with MF was detected in both bioptic specimens.
The cutaneous specimen (Figure 1B and C) revealed a dermal nodular infiltrate mainly composed of small-medium size cells with focal evidence of epidermotropism. Neoplastic lymphocytes were positive for CD45 and CD4 and showed a heterogeneous expression of CD3. A minority (< 25%) of CD30+ larger elements and an eosinophilic component were also observed.
The pancreatic parenchyma was completely replaced by a neoplastic infiltrate (Figure 1D and E) showing features overlapping with those detected in the cutaneous specimen.
These findings led us to diagnose peripheral T cell lymphoma, MF type. The medium-large CD30+ component suggested a tendency toward histological transformation[2].
Extracutaneous localizations of MF are rare and occur in long-standing MF with lymph node involvement. The literature reports involvement of lungs, spleen, liver, kidney, thyroid gland, bone marrow, oral cavity, larynx, heart[5], breast[6], central nervous system[7], esophagus[8,9], stomach, small intestine[10] and pancreas[3,4].
The diagnosis ante mortem of an extracutaneous manifestation of MF is rare and limited to few case reports. This is in contrast with autopsy findings, showing a high rate of extracutaneous involvement in lungs (75%), spleen (60%), liver (53), kidney (44%), and pancreas (41%)[11].
To date, only two patients with clinically detectable pancreatic metastasis from MF have been reported[3,4]. In these cases, intrapancreatic metastases displayed an infiltrative pattern without any gross tumor, a feature associated with most neoplasms that metastasize to the pancreas[4].
Pancreatic involvement is rare not only in MF but also in other T-cell cutaneous lymphomas. Only two such cases have been reported in association with disseminated pagetoid reticulosis[12] and adult T-cell leukaemia/lymphoma[13].
Chemokines and their receptors have been associated with tumor metastasis, invasion of lymphatic vessels, and trafficking of lymphoma cells[1]. Due to the extraordinary rarity of metastatic localization of MF cells to the pancreas, we investigated whether chemokine-chemokine receptor interactions could be involved in the phenomenon. We focused on the CCL27-CCR10 axis that has a primary role in T cell-mediated skin inflammation[14] and cutaneous T cell malignancy[15]. Thus, CCR10+ CD4+ T lymphocytes are attracted to the skin where the CCR10 ligand CCL27 is secreted by keratinocytes of the basal layers of the epidermis in normal skin, immobilized on extracellular matrix, and displayed on the surface of endothelial cells[14,15].
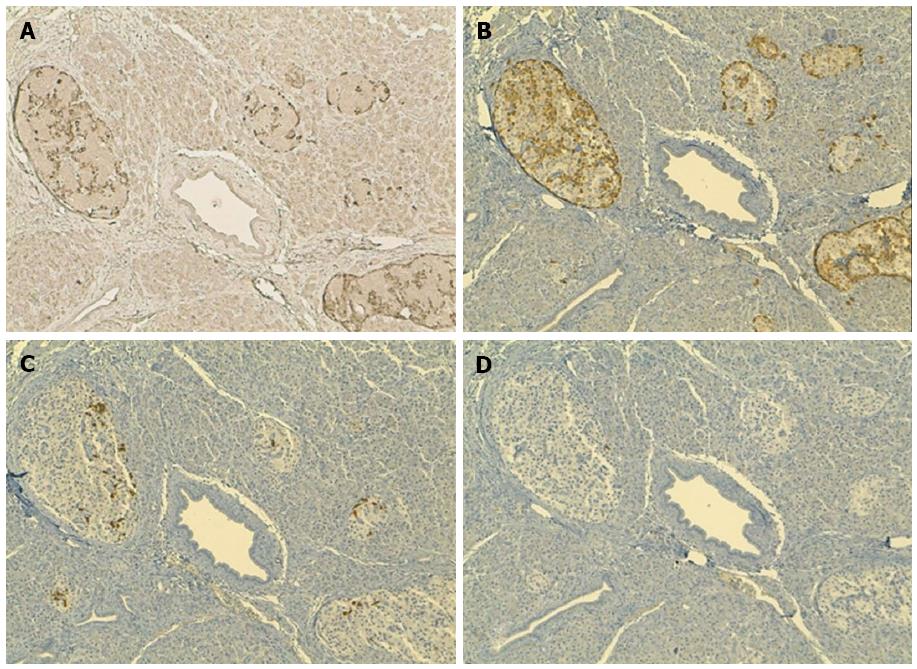
By immunohistochemical analysis, primary MF cells from our patient expressed CCR10, consistent with previous reports[1,15]. We hypothesized that a subset of MF cells circulating in the peripheral blood had upregulated CCR10 and was attracted by CCL27 expressed in the pancreas. However, CCR10 expression in metastatic MF cells and CCL27 expression in infiltrated pancreas could not be investigated due to the minute amount of pancreatic tissue obtained from the biopsy. We found no evidence in the literature for CCL27 expression in normal pancreas. Nonetheless, upon staining of five different archival pancreatic tissue samples from three patients with pancreatic ductal adenocarcinoma and two patients with papillary adenocarcinoma of ampulla (one with a concomitant well differentiated neuroendocrine tumor), we discovered that the islets of the pancreas, but not the exocrine portion, tested positive for CCL27 expression. Immunohistochemical staining on serial sections demonstrated that glucagon-secreting, but not somatostatin - or pancreatic polypeptide (PP) - secreting, pancreatic endocrine cells expressed CCL27 (Figure 3). These results point to the attraction of CCR10+ malignant lymphocytes by glucagon-secreting insulae expressing CCL27 as a potential mechanism driving localization of MF cells to the pancreas in our patient. To the best of our knowledge, this is the first demonstration of CCL27 expression in the pancreas. It is important to note, however, that these results on CCL27 expression were derived from archival pancreatic tissue samples from patients with primary malignancies, and the possibility that CCL27 may be abnormally upregulated in these conditions cannot be excluded.
The CCL27/CCR10 axis is not the only one that has been implicated in MF pathogenesis. Recent studies have highlighted the pivotal role of the CCL20/CCR6 axis in cutaneous T cell lymphoma invasiveness and metastasis[16]. Thus, our findings may have disclosed only one of the possible mechanisms promoting MF cell metastasis to the pancreas.
In conclusion, our case posed a clinical diagnostic challenge between pancreatic primitive tumor and a pancreatic metastasis from MF. In the clinical setting, the patient had: (1) jaundice and blood exams indicative of bile stasis; and (2) imaging studies raising the suspicion of a pancreatic primary neoplasm. A diagnostic clue came from the combined histomorphologic and immunohistochemical study of pancreatic and cutaneous biopsies, which revealed a pancreatic localization of MF. Although the prognosis of MF patients that develop lymph node or visceral metastasis (stage IV disease) is poor[17], our patient is still alive after a 3 year follow up.
A 64-year-old man with a previous history of erythematous skins lesions and a diagnosis of cutaneous T cell lymphoma, mycosis fungoides (MF) type, presented with jaundice, asthenia, and severe abdominal pain.
Persistent abdominal pain combined with jaundice suggested a disorder of bile ducts or pancreas.
Pancreatic adenocarcinoma, papillary neoplasm, neuroendocrine tumor, lymphoma.
Abnormally increased levels of amylase, lipase, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyltransferase, total bilirubin, and direct bilirubin.
Gastrointestinal ultrasonography showed a mass involving the head of the pancreas and Wirsung duct dilation. An abdominal computed tomography (CT) with contrast agent showed an irregular pancreatic mass involving the head of the pancreas, with dilation of the intrahepatic and the common bile ducts.
Percutaneous biopsy of the pancreatic mass showed a lymphomatous infiltrate consistent with metastatic localization of MF.
The patient was subjected to palliative endoscopic retrograde cholangiopancreatography.
Extracutaneous localizations of MF are rare and occur in long-standing MF with lymph node involvement. The literature reports involvement of lungs, spleen, liver, kidney, thyroid gland, bone marrow, oral cavity, larynx, heart, breast, central nervous system, esophagus, stomach, small intestine, as well as pancreas (two cases).
MF is the most common form of cutaneous T-cell lymphoma and accounts for almost 50% of all primary cutaneous lymphomas. Primary pancreatic tumors may involve the exocrine or the endocrine portion.
This case posed a clinical diagnostic challenge between pancreatic primitive tumor and a pancreatic metastasis from MF. A diagnostic clue came from the combined histomorphologic and immunohistochemical study of pancreatic and cutaneous biopsies, which revealed a pancreatic localization of MF.
This manuscript described a rare case with the diagnosis of pancreatic metastasis from MF. It further provides interesting study on the mechanism.
| 1. | Notohamiprodjo M, Segerer S, Huss R, Hildebrandt B, Soler D, Djafarzadeh R, Buck W, Nelson PJ, von Luettichau I. CCR10 is expressed in cutaneous T-cell lymphoma. Int J Cancer. 2005;115:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Ralfkiaer E, Cerroni L, Sander CA, Smoller BR, Willemze R. Mycosis fungoides. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC 2008; 296-298. |
| 3. | Kaplanski G, Koeppel MC, Deharo C, Durand JM, Andrac L, Sayag J. Secondary pancreatic involvement of mycosis fungoides detected by a clinically palpable mass. Dermatology. 1994;189:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Gottlieb K, Anders K, Kaya H. Obstructive jaundice in a patient with mycosis fungoides metastatic to the pancreas. EUS findings. JOP. 2008;9:719-724. [PubMed] |
| 5. | Maleki Z, Azmi F. Mycosis fungoides of the true vocal cord: a case report and review of the literature. Arch Iran Med. 2010;13:429-431. [PubMed] |
| 6. | Jaspars LH, Bonnet P, Willemze R, Meijer CJ. Mycosis fungoides with extracutaneous localization in the breast. Br J Dermatol. 1996;134:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Li N, Kim JH, Glusac EJ. Brainstem involvement by mycosis fungoides in a patient with large-cell transformation: a case report and review of literature. J Cutan Pathol. 2003;30:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Kim OD, Cantave I, Schlesinger PK. Esophageal involvement by cutaneous T-cell lymphoma, mycosis fungoides type: diagnosis by endoscopic biopsy. J Clin Gastroenterol. 1990;12:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Redleaf MI, Moran WJ, Gruber B. Mycosis fungoides involving the cervical esophagus. Arch Otolaryngol Head Neck Surg. 1993;119:690-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Slater DN, Bleehen SS, Beck S. Gastrointestinal complications of mycosis fungoides. J R Soc Med. 1984;77:114-119. [PubMed] |
| 11. | Rappaport H, Thomas LB. Mycosis fungoides: the pathology of extracutaneous involvement. Cancer. 1974;34:1198-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Shiozawa E, Shiokawa A, Shibata M, Nakada T, Yamochi-Onizuka T, Saito B, Takaba E, Iijima M, Takimoto M, Ota H. Autopsy case of CD4/CD8 cutaneous T-cell lymphoma presenting disseminated pagetoid reticulosis with aggressive granulomatous invasion to the lungs and pancreas. Pathol Int. 2005;55:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Mori A, Kikuchi Y, Motoori S, Watanabe J, Shinozaki M, Eguchi M. Acute pancreatitis induced by diffuse pancreatic invasion of adult T-cell leukemia/lymphoma cells. Dig Dis Sci. 2003;48:1979-1983. [PubMed] |
| 14. | Homey B, Alenius H, Müller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bünemann E. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 601] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Fujita Y, Abe R, Sasaki M, Honda A, Furuichi M, Asano Y, Norisugi O, Shimizu T, Shimizu H. Presence of circulating CCR10+ T cells and elevated serum CTACK/CCL27 in the early stage of mycosis fungoides. Clin Cancer Res. 2006;12:2670-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Ito M, Teshima K, Ikeda S, Kitadate A, Watanabe A, Nara M, Yamashita J, Ohshima K, Sawada K, Tagawa H. MicroRNA-150 inhibits tumor invasion and metastasis by targeting the chemokine receptor CCR6, in advanced cutaneous T-cell lymphoma. Blood. 2014;123:1499-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 447] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Lu Z S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang DN