Published online Mar 14, 2016. doi: 10.3748/wjg.v22.i10.2971
Peer-review started: September 17, 2015
First decision: November 5, 2015
Revised: November 22, 2015
Accepted: December 19, 2015
Article in press: December 21, 2015
Published online: March 14, 2016
Processing time: 176 Days and 8 Hours
AIM: To explore the synergistic effect of docosahexaenoic acid (DHA)/5-fluorouracil (5-FU) on the human gastric cancer cell line AGS and examine the underlying mechanism.
METHODS: AGS cells were cultured and treated with a series of concentrations of DHA and 5-FU alone or in combination for 24 and 48 h. To investigate the synergistic effect of DHA and 5-FU on AGS cells, the inhibition of cell proliferation was determined by MTT assay and cell morphology. Flow cytometric analysis was also used to assess cell cycle distribution, and the expression of mitochondrial electron transfer chain complexes (METCs) I, II and V in AGS cells was further determined by Western blot analysis.
RESULTS: DHA and 5-FU alone or in combination could markedly suppress the proliferation of AGS cells in a significant time and dose-dependent manner. DHA markedly strengthened the antiproliferative effect of 5-FU, decreasing the IC50 by 3.56-2.15-fold in an apparent synergy. The morphological changes of the cells were characterized by shrinkage, cell membrane blebbing and decreased adherence. Cell cycle analysis showed a shift of cells into the G0/G1 phase from the S phase following treatment with DHA or 5-FU (G0/G1 phase: 30.04% ± 1.54% vs 49.05% ± 6.41% and 63.39% ± 6.83%, respectively, P < 0.05; S phase: 56.76% ± 3.14% vs 34.75% ± 2.35% and 25.63% ± 2.21%, respectively, P < 0.05). Combination treatment of DHA and 5-FU resulted in a significantly larger shift toward the G0/G1 phase and subsequent reduction in S phase (G0/G1 phase: 69.06% ± 2.63% vs 49.05% ± 6.41% and 63.39% ± 6.83%, respectively, P < 0.05; S phase: 19.80% ± 4.30% vs 34.75% ± 2.35% and 25.63% ± 2.21%, respectively, P < 0.05). This synergy was also reflected in the significant downregulation of the expression of METCs in AGS cells.
CONCLUSION: Synergistic anticancer properties of DHA and 5-FU may involve interference with energy production of AGS cells via downregulation of METCs and cell cycle arrest.
Core tip: We explored the synergistic anticancer properties of docosahexaenoic acid (DHA) and 5-fluorouracil (5-FU) against gastric cancer cells and the underlying mechanism. DHA and 5-FU alone or in combination could markedly suppress the proliferation of AGS cells in a significant time and dose-dependent manner. Co-administration of DHA with 5-FU resulted in a significantly larger shift toward the G0/G1 phase and a significant downregulation of the expression of mitochondrial electron transfer chain complexes in AGS cells. The associated mechanism may involve interference with energy production of AGS cells via downregulation of mitochondrial electron transfer chain complexes and cell cycle arrest in G0/G1 phase.
- Citation: Gao K, Liang Q, Zhao ZH, Li YF, Wang SF. Synergistic anticancer properties of docosahexaenoic acid and 5-fluorouracil through interference with energy metabolism and cell cycle arrest in human gastric cancer cell line AGS cells. World J Gastroenterol 2016; 22(10): 2971-2980
- URL: https://www.wjgnet.com/1007-9327/full/v22/i10/2971.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i10.2971
Gastric cancer is the fourth most frequently occurring malignancy worldwide[1] and the second leading cause of cancer-related deaths[2]. In particular East Asia, including Japan, South Korea and China, reports the highest mortality rates. East Asia accounts for approximately 60% of the global prevalence of gastric cancer and 41% in China alone[1]. Surgical intervention remains the only therapeutic modality with a potentially curative effect[3] with increased success rates following postoperative adjuvant chemotherapy[4]. 5-fluorouracil (5-FU) is the first-line chemotherapeutic agent recommended for gastric cancer; however, its therapeutic effect is often hampered by lower response rate and considerable adverse effects. The degree of these side effects often limits the dosage to a sub-effective range compromising the quality of life of patients[5]. Therefore, it is imperative to find a better solution to improve the efficacy of current anticancer drugs. Several studies have observed that docosahexaenoic acid (DHA) has the potential to augment the efficacy of chemotherapeutics. This subsequently allowed lower dosages of 5-FU to be administered in combination with DHA in the human colorectal cancer cell lines and colon adenocarcinoma model[6,7]. The studies in cancer cell lines and cancer-bearing animals showed that DHA supplementation had a powerful adjuvant activity and has then emerged as an innovative approach to chemosensitize cancer cells[8]. Although many studies have been performed at present to investigate the underlying mechanisms of this synergy, there is still no commonly accepted answer.
DHA is one of the most important members of the omega-3 polyunsaturated fatty acids (ω-3 PUFAs) which are essential fatty acids that can not be synthesized by the body and thus must be obtained from dietary sources. Omega-3 PUFAs play many physiological roles in the body including acting as sources of cellular energy, constructing the phospholipids required for cell membranes and providing membrane fluidity[9]. It was not until recently that evidence from both in vitro and in vivo studies began to show DHA possesses anticancer properties against several cancers such as liver cancer[10], colon cancer[11], bladder cancer[12], breast cancer[13] and lung cancer[14]. In this regard, DHA not only suppresses carcinogenesis but also inhibits disease progression. But when it comes to gastric cancer, there are few studies and little evidence reviewing the effects of DHA. Meta-analyses examining an association between DHA consumption and the risk of gastric cancer are inconclusive[15,16], but high-dose DHA has been shown to induce apoptosis through activator protein-1 (AP-1) activation in gastric cancer cells AGS[17]. The studies further demonstrated that the mechanisms by which DHA in combination with 5-FU exerts an apoptotic effect are believed to be the regulation of apoptosis-associated gene expression in gastric cancer cells SGC7901 and MGC803[18,19]. As a unique cellular organelle, mitochondria play a major part in apoptosis process and cellular energy metabolism. Thus, the effect of co-administration of DHA with 5-FU on mitochondria of human gastric cancer cells needs to be further investigated.
The energy metabolism of cancer cells is a heated topic. The Warburg effect indicates that cancer cells have faults in mitochondrial oxidative phosphorylation and therefore rely on chiefly anaerobic glycolysis in cytosol, even in the presence of plentiful oxygen, as the major source of ATP to support cellular proliferation[20]. However, many researchers have showed that mitochondria have distinct functions in most cancer cells and are the primary contributors to ATP production[21-24]. Omega-3 PUFAs are involved in a variety of mitochondrial processes including mitochondrial calcium homeostasis, respiratory function and mitochondrial apoptosis. Thus, competing influences over mitochondria could be the foundation for the disruptive effects of DHA on cancer cells, and further examination of its interaction with the components of the mitochondrial electron transfer chain complexes (METCs) is needed.
The present study aimed to investigate a synergistic effect of DHA together with the chemotherapeutic agent 5-FU most commonly administrated in stomach cancer on human gastric cancer cell line AGS and to identify the probable underlying mechanism.
DHA and 5-FU were bought from Sigma Chemical Company (St. Louis, MO, United States). DHA was dissolved in ethanol at a concentration of 5 mg/mL. RPMI-1640 medium was bought from GIBCO (Grand Island, NY, United States). METCs I, II and V monoclonal antibodies were purchased from Invitrogen (Carlsbad, United States). MTT [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] was purchased from Beijing Cellchip Biotechnology Corporation (Beijing, China).
The human gastric adenocarcinoma cell line AGS was kindly provided by Prof. You-Fen Li (Xi’an Jiaotong University, Xi’an, China). AGS cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) (Hangzhou Biotechnology Co. Ltd), 100 U/mL penicillin and 0.1 mg/mL streptomycin in a humidified incubator at 37 °C with 5% CO2. The cells growing in logarithmic phase were seeded in 96-well plates at 1.0 × 104 cells per well and allowed to adhere overnight, after which a series of concentrations of DHA (7.5, 15, 22.5, 30, 37.5, and 45 μg/mL) and/or 5-FU (1.5625, 3.125, 6.25, 12.5, 25, and 50 μg/mL) were added to the well for 24 or 48 h. Control experiments were carried out by adding ethanol without DHA to AGS cells. After 24 or 48 h of incubation, 20 μL MTT (5 mg/mL) was added to each well and the cells were re-incubated for another 4 h at 37 °C. After removal of the supernatant gently, 200 μL of dimethyl sulfoxide (DMSO) was added to each well to solubilize the purple formazan crystals completely. Absorbance values at 570 nm were measured with a microplate reader and are reported as a percentage of growth with respect to the control. Inhibition rate of cell growth was measured using the formula: inhibition rate (%) = [1 - OD570 (experiment group)/OD570 (control group)] × 100. All experiments were repeated in triplicate. The drug concentration that produced 50% inhibition of cell proliferation (IC50) was calculated and analyzed for 5-FU, DHA and the combination. Based on the IC50 values obtained for DHA and 5-FU from the MTT assay, the optimal treatment concentrations for DHA and 5-FU were determined to be 30.00 μg/mL and 12.50 μg/mL, respectively.
Cell cycle distribution was analysed by flow cytometry. Briefly, AGS cells were seeded in 6-well plates with growth medium (control group), DHA in medium (30.00 μg/mL), 5-FU in medium (12.50 μg/mL), or DHA plus 5-FU in medium (30.00 μg/mL + 12.50 μg/mL). After incubation for 48 h, the cells were harvested by trypsinization, washed twice with ice-cold phosphate buffered saline (PBS) and pelleted in a centrifuge. Cells were fixed in 70% ethanol at -20 °C overnight. The ethanol was decanted following centrifugation and the cells were washed once with cold PBS. The cells were then treated with 1.0 μg/mL RNase for 30 min and stained with propidium iodide. The cell cycle distribution of the stained cells was then analyzed by flow cytometry (FACSCalibur; Becton-Dickinson, San Diego, CA, United States). The data were analyzed with CellQuest Pro (Becton-Dickinson) and ModFit LT software (Verity Software House, Inc., Topsham, ME, United States) to determine the cell distribution in G0/G1 (stationary phase), S (DNA synthesis phase), and G2/M.
AGS cells were incubated with medium (control group), DHA in medium (30.00 μg/mL), 5-FU in medium (12.50 μg/mL) or DHA plus 5-FU in medium (30.00 μg/mL + 12.5 μg/mL). Following incubation for 24 or 48 h, the cells from the different treatment groups were harvested, washed with PBS twice and lysed with 500 μL of Mito-Cyto (mitochondrial cytosol) isolation buffer. The BSA assay was used to determine the concentration of protein samples. Equal amounts of protein samples were loaded onto a 12% sodium dodecyl sulfate-polyacrylamide gel for electrophoresis. After electrophoresis the proteins were transferred into PVDF membranes. The membranes were blocked with 5% skim milk in PBST (phosphate-buffered saline with 0.1% tween 20) for 2 h and incubated with specific monoclonal antibodies against METCs I, II and V at 4 °C for 2 h. After three washes with PBST buffer, the membranes were incubated with an anti-mouse secondary antibody (Jackson, United States) conjugated with peroxidase for 2 h at room temperature. The bands of proteins were visualized using the enhanced chemiluminescence detection system. Quantification of the bands of proteins was evaluated by densitometric scanning.
The results are expressed as the mean ± SE. Statistical analyses were performed using Student’s t test and one-way ANOVA. Post hoc testing was performed for inter-group comparisons using the least significance difference (LSD). Significance was defined as P < 0.05. The combination index (CI) was calculated using the formula CI = %AB/%A × %B, where %A and %B are the inhibition rate of DHA and 5-FU alone on AGS cell growth, and %AB is the inhibition rate of DHA and 5-FU in combination on AGS cell growth[25]. When combination index is 1, the effect between DHA and 5-FU is considered additive; when combination index is significantly greater than or less than 1, the effect is considered subadditive or supraadditive, respectively.
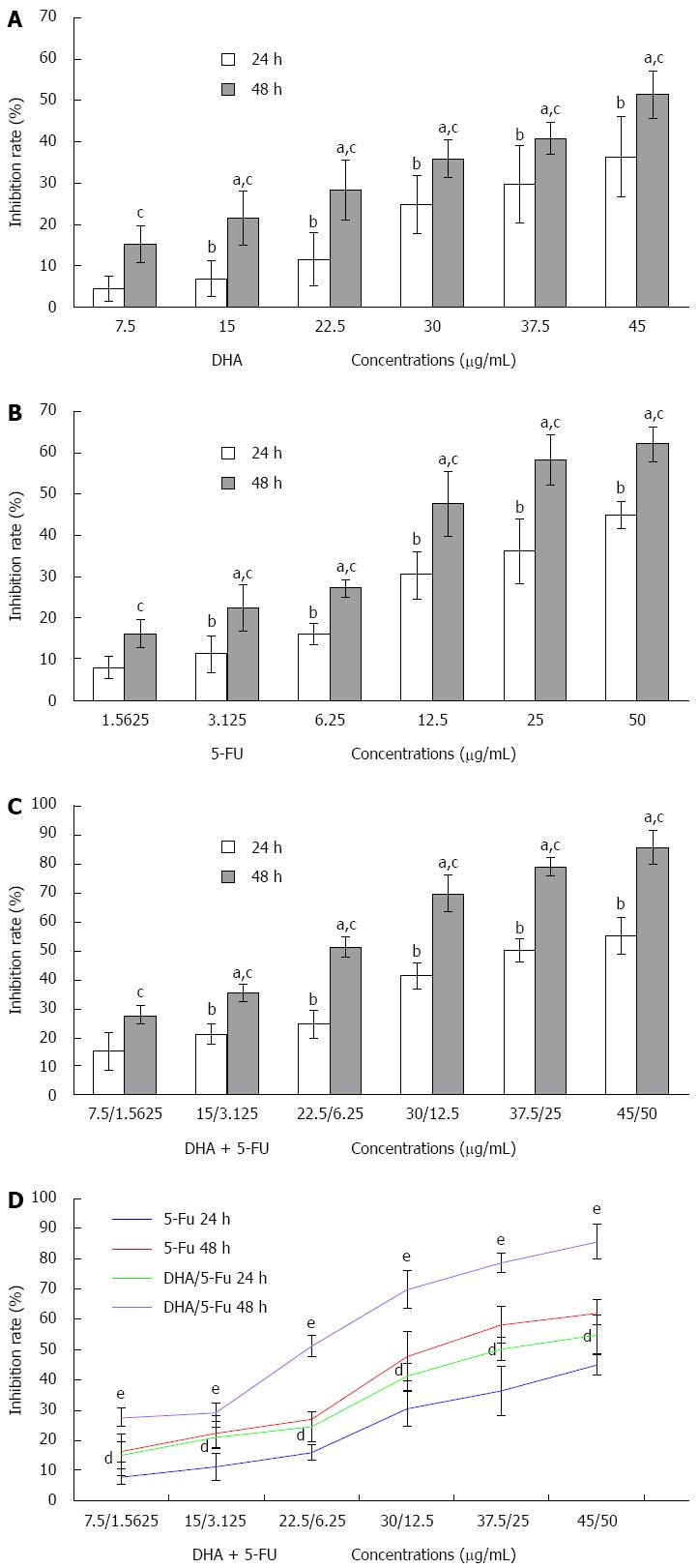
To investigate the synergistic effect of DHA and 5-FU on AGS cells, the inhibition of cell proliferation was determined by a MTT assay. The growth inhibition of AGS cells treated with the two compounds individually was first measured. The results demonstrated that DHA and 5-FU could markedly inhibit the proliferation of AGS cells in a significant time and dose-dependent manner (Figure 1). Furthermore, the values of IC50 for DHA or 5-FU administered for 24 and 48 h were 51.60 μg/mL (DHA: 24 h), 34.82 μg/mL (DHA: 48 h), 45.90 μg/mL (5-FU: 24 h), and 16.86 μg/mL (5-FU: 48 h). When the two compounds were used in combination, the IC50 values for DHA reduced to 34.17 μg/mL and 23.06 μg/mL for 24 and 48 h incubations, respectively. DHA could notably strengthen the inhibitory effect of 5-FU on the growth of AGS (Figure 1). The IC50 values for 5-FU in the presence of DHA sharply dropped to 12.90 μg/mL and 7.84 μg/mL at 24 and 48 h, respectively. The evaluation of the interaction between DHA and 5-FU on AGS cell growth (Table 1) yielded a combination index < 1 for different time points (24 h and 48 h), suggesting a synergistic effect between the two drugs.
| Time (h) | Combination index | Pavalue |
| 24 | 0.161 ± 0.065 | < 0.05 |
| 48 | 0.057 ± 0.013 | < 0.05 |
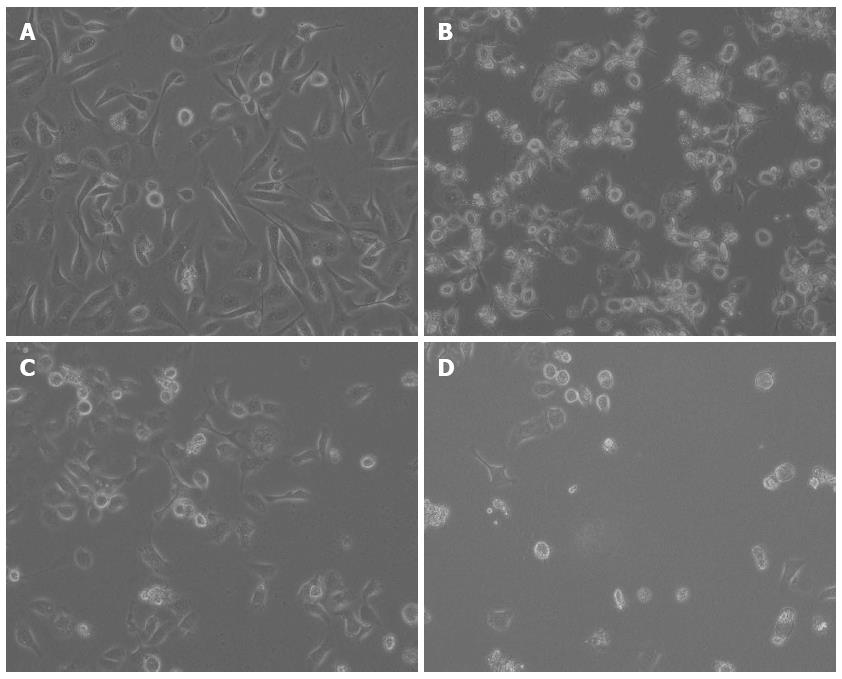
The AGS cell morphology following exposure to the test compounds was evaluated successively under an inverted-phase microscope. Cells grown in medium alone appeared spindle-shaped with dense intercellular gaps and an active proliferative capacity. Cells treated with DHA or 5-FU exhibited a ring-shaped appearance, had a lower viability and fewer adhered cells. Cells exposed to the combination treatment were characterized by shrinkage, cell membrane blebbing and the lower level of cell adherence (Figure 2).
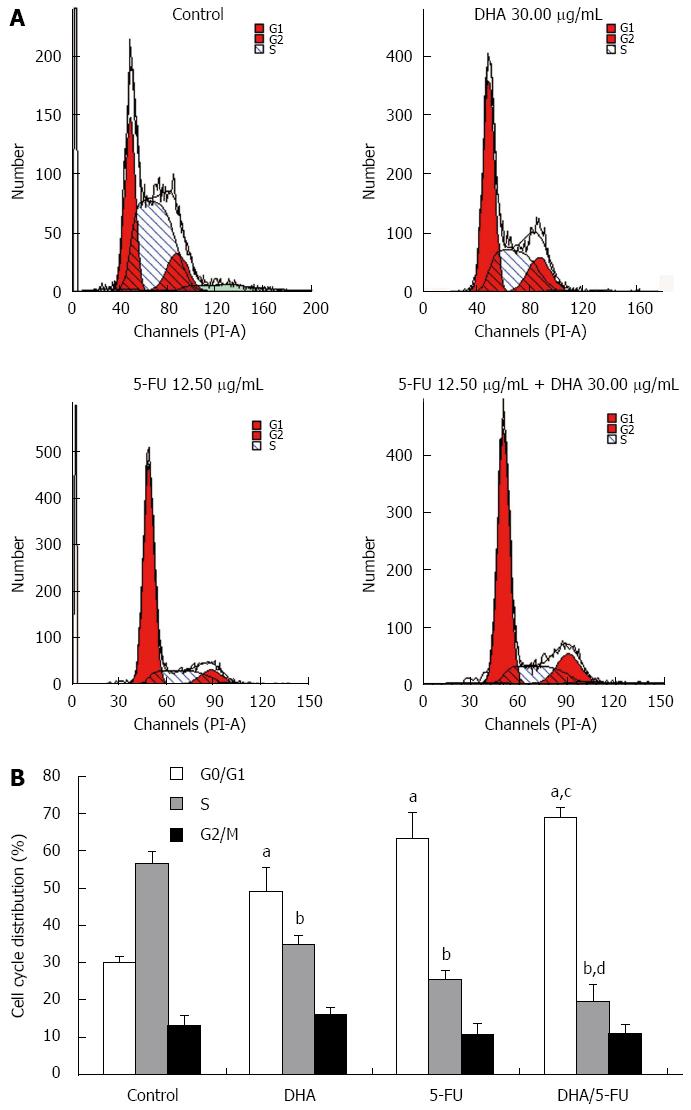
The effect of DHA and 5-FU on the cell cycle distribution was evaluated by flow cytometry. The cell cycle distribution of AGS cells in the control group showed a percentage of cells of 30.04% ± 1.54% in the G0/G1 phase, and 56.76% ± 3.14% in the S phase. However, exposure of the cells to DHA or 5-FU brought about a significant increase in the percentage of cells in the G0/G1 phase (49.05% ± 6.41% and 63.39% ± 6.83%) while the proportion of cells in the S phase sharply decreased (34.75% ± 2.35% and 25.63% ± 2.21%) as compared to the control cells. On the other hand, the combination treatment led to a further accumulation of cells in G0/G1 phase (69.06% ± 2.63%) and reduction in S phase (19.80% ± 4.30%) compared to DHA and 5-FU alone (Figure 3).
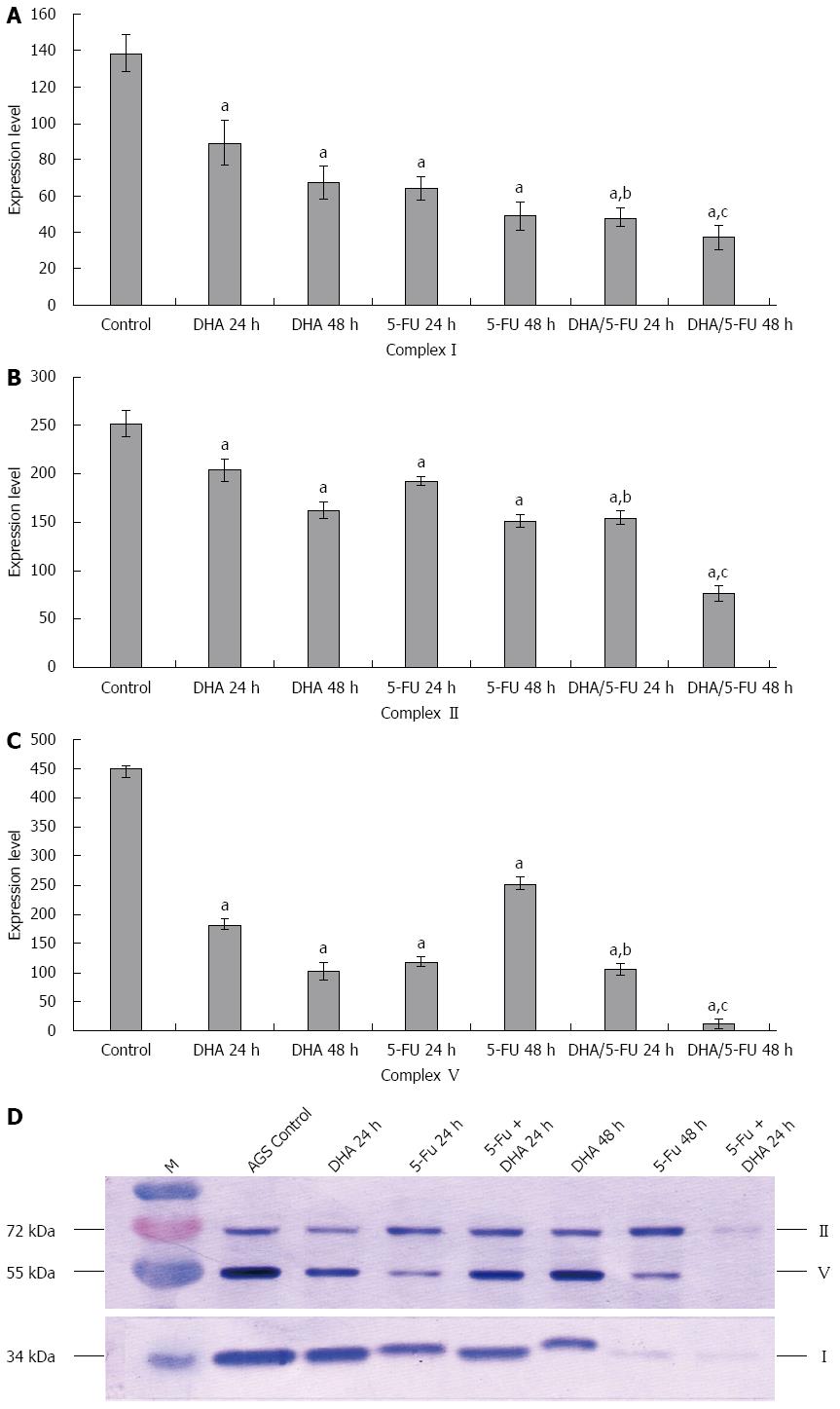
The effect of DHA and 5-FU on the expression of METCs I, II and V in AGS cells was investigated by Western blot analysis. As shown in Figure 4, incubation of the cells with DHA for 48 h resulted in a significant decrease in the expression of METCs I, II and V by 2.0-fold, 1.55-fold and 4.41-fold, respectively, as compared with control cells. Treatment with 5-FU for 48 h also led to a remarkable reduction in the expression of METCs I, II and V by 2.81-fold, 1.67-fold and 4.94-fold, respectively, as compared with control cells. The combination treatment of DHA with 5-FU further exhibited a statistically significant decrease in the expression of METCs compared to the individual treatments.
Improving the efficacy of chemotherapy in gastric cancer is a necessity for increasing the overall survival rate and quality of life for patients[3]. In spite of progress in traditional cancer chemotherapy, the prognosis for patients with advanced gastric cancer is still very poor. The antineoplastic agent 5-FU is the first-line chemotherapy which has a broad activity in gastrointestinal cancers; however, as with most cancer drugs, the major disadvantage of 5-FU is its high toxicity and low therapeutic response[26]. Therefore, the development of new treatment modalities for gastric cancer is urgently needed. The present study investigated the therapeutic potential of DHA as an adjuvant for 5-FU in gastric cancer. The present results demonstrated that DHA worked synergistically with 5-FU to inhibit the growth of gastric carcinoma cells. As a result, the addition of DHA decreased the effective concentration of 5-FU which could translate to a decreased in vivo dose, thus mitigating the inherent toxicity concerns.
Various studies have reported that DHA itself exhibits therapeutic properties across a broad spectrum of cancer cell lines. However, the effects of DHA on gastric cancer are notably limited. Initial research suggests that DHA has the potential to suppress cancer cell proliferation and induce apoptosis in cultured human gastric cancer cells[17,27,28], but only at markedly high concentrations of 100-150 μmol/L (32.85-49.28 μg/mL) or 180-200 μmol/L (59.13-65.70 μg/mL). These concentrations were considerably higher than those used in the present study. For typical in vitro drug combination studies, it is necessary to approximate the IC50 values for each compound to estimate a working dose range[29]. Therefore, in the current study a series of concentrations of 5-FU and DHA were first tested to determine their individual IC50 values against AGS cells. The results showed that the IC50 values for 5-FU and DHA administered in combination for 24 h dropped remarkably to 12.90 μg/mL and 34.17 μg/mL, respectively. As a result, the optimal treatment concentrations for DHA and 5-FU were determined to be 30.00 μg/mL and 12.50 μg/mL, respectively, in different groups. Meanwhile, the results also demonstrated that DHA decreased the IC50 value for 5-FU by 3.56-2.15-fold. The findings indicate DHA and 5-FU alone or in combination markedly suppress the proliferation of AGS in both a time and concentration-dependent manner. These results are consistent with previous reports[18,19]. The combination of two compounds was also found to be synergistic in a statistically significant manner.
Cancer cells are often characterized by cell cycle abnormalities which lead to the unregulated proliferation. Various experimental studies have reported that DHA itself could arrest melanoma cells[30] and colon cancer cell line Caco-2[31] in the S phase, while FM3A mammary cancer cells[32] were arrested in the G0/G1 phase. In the present study, cell cycle analysis showed that treatment of the AGS cells with DHA brought about an increase of the percentage of cells in the G0/G1 phase with a concurrent decrease of cells in the S phase. These findings accounted, at least in part, for the negative effect DHA has on the growth of gastric cancer cells. These observations are consistent with findings from the human colon adenocarcinoma cell line HT-29[33] and human hepatocellular carcinoma cell lines[34]. Our results from the flow cytometric analysis of cells exposed to 5-FU showed a marked reduction in the cells in S phase. After the combined treatment of 5-FU with DHA the specific G0/G1 phase accumulation was markedly increased which strongly supports the ability of DHA to enhance the effects of 5-FU. As a result, the cell cycle arrest in G0/G1 phase was probably one of underlying mechanisms of synergistic interactions between DHA and 5-FU.
Mitochondria are the important site for cellular energy metabolism. The mitochondrial inner membrane contains the enzyme complexes of the electron transport chain. These enzymes play a crucial role in energy metabolism. The mitochondrial electron transfer chain consists of five multiprotein complex, NADH-ubiquinone oxidoreductase (complex I), succinate-ubiquinone oxidoreductase (complex II), ubiquinone-cytochrome C oxidoreductase (complex III), cytochrome C oxidase (complex IV) and ATP synthase (complex V). Among them, complexes I and II are the major entry points for electron transfer, and complex V is the exit. Normal cells depend principally on mitochondrial oxidative phosphorylation to produce ATP for their metabolic activities. It has been hold that the anaerobic glycolysis in cancer cell is due to a lasting damage of mitochondrial oxidative phosphorylation as suggested by Otto Warburg. However, at present this opinion is challenged by recent studies which have demonstrated that mitochondria are indeed functional in most cancer cells and still chiefly contribute to the ATP supply of cancer cells[22-25]. Research by Colquhoun et al[35] had indicated that eicosapentaenoic acid, one member of polyunsaturated fatty acid family, could cause a significant decrease in the activity of mitochondrial respiratory chain complexes I, III and IV in the Walker 256 rat carcinosarcoma in vitro. This would have been the major cause of the decrease in mitochondrial energy. The results of the present study suggest the expression of complexes I, II and V was markedly reduced in AGS cells following treatment with DHA and 5-FU alone or in combination. In other words, the two test compounds could inhibit both the major entry and exit points for electron transfer in the mitochondrial electron transfer chain, which interfered with mitochondrial oxidative phosphorylation and cellular energy metabolism.
To sum up, the combination of 5-FU with DHA showed synergistically enhanced therapeutic anticancer properties through cell cycle arrest in G0/G1 phase and interference with the energy metabolism of AGS cells. The therapeutic combination of these two compounds is a promising candidate for further in vivo studies as they offer greater potential outcomes for progression free survival with an improved toxicity profile.
Postoperative chemotherapy is an essential part of multi-disciplinary therapeutic strategies for gastric cancer. 5-fluorouracil (5-FU) is the first-line chemotherapeutic agent recommended for this malignancy. However, its considerable adverse effects often limit the dosage to a sub-effective range. Therefore, it is imperative to find a better solution to improve the efficacy of 5-FU.
Current studies have demonstrated that docosahexaenoic acid (DHA) has the potential to augment the efficacy of chemotherapeutics and DHA supplementation can act as a new solution to chemosensitize cancer cells. The associated mechanisms involve enhancing the induction of apoptosis of cancer cells.
Co-administration of DHA with 5-FU synergistically enhanced anticancer properties against AGS human gastric cancer cell lines. The underlying mechanism may involve interference with energy production of AGS cells through downregulation of mitochondrial electron transfer chain complexes, which is firstly demonstrated, and cell cycle arrest in G0/G1 phase.
This study presents new therapeutic clues to improve the efficacy of chemotherapy in gastric cancer. The therapeutic combination of these two compounds is a promising candidate for further in vivo studies as they offer greater potential outcomes for progression free survival with an improved toxicity profile.
DHA, one of the most important members of the omega-3 polyunsaturated fatty acids, has 22 carbon atoms and 6 double bounds. Evidence has shown that DHA can act as an adjuvant to improve the efficacy of anticancer treatment.
The present study is an informative work. Data show that synergistic anticancer properties of DHA and 5-FU may involve interference with energy production of AGS cells through downregulation of mitochondrial electron transfer chain complexes and cell cycle arrest. Further studies in vivo about the therapeutic combination of these two compounds may be worth pursuing.
| 1. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2655] [Cited by in RCA: 2655] [Article Influence: 132.8] [Reference Citation Analysis (1)] |
| 2. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10466] [Article Influence: 654.1] [Reference Citation Analysis (2)] |
| 3. | Jiang Y, Ajani JA. Multidisciplinary management of gastric cancer. Curr Opin Gastroenterol. 2010;26:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 4. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 614] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 5. | Sastre J, Garcia-Saenz JA, Diaz-Rubio E. Chemotherapy for gastric cancer. World J Gastroenterol. 2006;12:204-213. [PubMed] |
| 6. | Granci V, Cai F, Lecumberri E, Clerc A, Dupertuis YM, Pichard C. Colon cancer cell chemosensitisation by fish oil emulsion involves apoptotic mitochondria pathway. Br J Nutr. 2013;109:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Rani I, Vaiphei K, Agnihotri N. Supplementation of fish oil augments efficacy and attenuates toxicity of 5-fluorouracil in 1,2-dimethylhydrazine dihydrochloride/dextran sulfate sodium-induced colon carcinogenesis. Cancer Chemother Pharmacol. 2014;74:309-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Siddiqui RA, Harvey KA, Xu Z, Bammerlin EM, Walker C, Altenburg JD. Docosahexaenoic acid: a natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors. 2011;37:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Simopoulos AP. Omega-6/omega-3 essential fatty acids: biological effects. World Rev Nutr Diet. 2009;99:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Sun SN, Jia WD, Chen H, Ma JL, Ge YS, Yu JH, Li JS. Docosahexaenoic acid (DHA) induces apoptosis in human hepatocellular carcinoma cells. Int J Clin Exp Pathol. 2013;6:281-289. [PubMed] |
| 11. | Yang T, Fang S, Zhang HX, Xu LX, Zhang ZQ, Yuan KT, Xue CL, Yu HL, Zhang S, Li YF. N-3 PUFAs have antiproliferative and apoptotic effects on human colorectal cancer stem-like cells in vitro. J Nutr Biochem. 2013;24:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Parada B, Reis F, Cerejo R, Garrido P, Sereno J, Xavier-Cunha M, Neto P, Mota A, Figueiredo A, Teixeira F. Omega-3 fatty acids inhibit tumor growth in a rat model of bladder cancer. Biomed Res Int. 2013;2013:368178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Xue M, Wang Q, Zhao J, Dong L, Ge Y, Hou L, Liu Y, Zheng Z. Docosahexaenoic acid inhibited the Wnt/β-catenin pathway and suppressed breast cancer cells in vitro and in vivo. J Nutr Biochem. 2014;25:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Yao QH, Zhang XC, Fu T, Gu JZ, Wang L, Wang Y, Lai YB, Wang YQ, Guo Y. ω-3 polyunsaturated fatty acids inhibit the proliferation of the lung adenocarcinoma cell line A549 in vitro. Mol Med Rep. 2014;9:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Wu S, Liang J, Zhang L, Zhu X, Liu X, Miao D. Fish consumption and the risk of gastric cancer: systematic review and meta-analysis. BMC Cancer. 2011;11:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, Lim YW, Traina SB, Hilton L, Garland R. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006;295:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 339] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Lee SE, Lim JW, Kim H. Activator protein-1 mediates docosahexaenoic acid-induced apoptosis of human gastric cancer cells. Ann N Y Acad Sci. 2009;1171:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Zhuo Z, Zhang L, Mu Q, Lou Y, Gong Z, Shi Y, Ouyang G, Zhang Y. The effect of combination treatment with docosahexaenoic acid and 5-fluorouracil on the mRNA expression of apoptosis-related genes, including the novel gene BCL2L12, in gastric cancer cells. In Vitro Cell Dev Biol Anim. 2009;45:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Wu Q, Yu JC, Liu YQ, Kang WM, Guo WD. [Effect of combination of docosahexaenoic acid and fluorouracil on human gastric carcinoma cell strain MGC803]. Zhongguo Yixue Kexueyuan Xuebao. 2010;32:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Frezza C, Gottlieb E. Mitochondria in cancer: not just innocent bystanders. Semin Cancer Biol. 2009;19:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788-8793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1413] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 23. | Moreno-Sánchez R, Rodríguez-Enríquez S, Saavedra E, Marín-Hernández A, Gallardo-Pérez JC. The bioenergetics of cancer: is glycolysis the main ATP supplier in all tumor cells? Biofactors. 2009;35:209-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Upadhyay M, Samal J, Kandpal M, Singh OV, Vivekanandan P. The Warburg effect: insights from the past decade. Pharmacol Ther. 2013;137:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Calviello G, Di Nicuolo F, Serini S, Piccioni E, Boninsegna A, Maggiano N, Ranelletti FO, Palozza P. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother Pharmacol. 2005;55:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res. 2001;7:2168-2181. [PubMed] |
| 27. | Sheng H, Li P, Chen X, Liu B, Zhu Z, Cao W. Omega-3 PUFAs induce apoptosis of gastric cancer cells via ADORA1. Front Biosci (Landmark Ed). 2014;19:854-861. [PubMed] |
| 28. | Dai J, Shen J, Pan W, Shen S, Das UN. Effects of polyunsaturated fatty acids on the growth of gastric cancer cells in vitro. Lipids Health Dis. 2013;12:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Chou TC. Preclinical versus clinical drug combination studies. Leuk Lymphoma. 2008;49:2059-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 30. | Albino AP, Juan G, Traganos F, Reinhart L, Connolly J, Rose DP, Darzynkiewicz Z. Cell cycle arrest and apoptosis of melanoma cells by docosahexaenoic acid: association with decreased pRb phosphorylation. Cancer Res. 2000;60:4139-4145. [PubMed] |
| 31. | Jordan A, Stein J. Effect of an omega-3 fatty acid containing lipid emulsion alone and in combination with 5-fluorouracil (5-FU) on growth of the colon cancer cell line Caco-2. Eur J Nutr. 2003;42:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Khan NA, Nishimura K, Aires V, Yamashita T, Oaxaca-Castillo D, Kashiwagi K, Igarashi K. Docosahexaenoic acid inhibits cancer cell growth via p27Kip1, CDK2, ERK1/ERK2, and retinoblastoma phosphorylation. J Lipid Res. 2006;47:2306-2313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Chen ZY, Istfan NW. Docosahexaenoic acid, a major constituent of fish oil diets, prevents activation of cyclin-dependent kinases and S-phase entry by serum stimulation in HT-29 cells. Prostaglandins Leukot Essent Fatty Acids. 2001;64:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Lee CY, Sit WH, Fan ST, Man K, Jor IW, Wong LL, Wan ML, Tan-Un KC, Wan JM. The cell cycle effects of docosahexaenoic acid on human metastatic hepatocellular carcinoma proliferation. Int J Oncol. 2010;36:991-998. [PubMed] |
| 35. | Colquhoun A, Schumacher RI. gamma-Linolenic acid and eicosapentaenoic acid induce modifications in mitochondrial metabolism, reactive oxygen species generation, lipid peroxidation and apoptosis in Walker 256 rat carcinosarcoma cells. Biochim Biophys Acta. 2001;1533:207-219. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Djuric Z S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S