Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.89
Peer-review started: June 3, 2015
First decision: July 14, 2015
Revised: August 8, 2015
Accepted: October 13, 2015
Article in press: October 13, 2015
Published online: January 7, 2016
Processing time: 216 Days and 8.8 Hours
Since its introduction in the 1970’s, magnetic resonance imaging (MRI) has become a standard imaging modality. With its broad and standardized application, it is firmly established in the clinical routine and an essential element in cardiovascular and abdominal imaging. In addition to sonography and computer tomography, MRI is a valuable tool for diagnosing cardiovascular and abdominal diseases, for determining disease severity, and for assessing therapeutic success. MRI techniques have improved over the last few decades, revealing not just morphologic information, but functional information about perfusion, diffusion and hemodynamics as well. Four-dimensional (4D) flow MRI, a time-resolved phase contrast-MRI with three-dimensional (3D) anatomic coverage and velocity encoding along all three flow directions has been used to comprehensively assess complex cardiovascular hemodynamics in multiple regions of the body. The technique enables visualization of 3D blood flow patterns and retrospective quantification of blood flow parameters in a region of interest. Over the last few years, 4D flow MRI has been increasingly performed in the abdominal region. By applying different acceleration techniques, taking 4D flow MRI measurements has dropped to a reasonable scanning time of 8 to 12 min. These new developments have encouraged a growing number of patient studies in the literature validating the technique’s potential for enhanced evaluation of blood flow parameters within the liver’s complex vascular system. The purpose of this review article is to broaden our understanding of 4D flow MRI for the assessment of liver hemodynamics by providing insights into acquisition, data analysis, visualization and quantification. Furthermore, in this article we highlight its development, focussing on the clinical application of the technique.
Core tip: Liver cirrhosis is one of the leading cause of morbidity and mortality in the United States, Europe and Asia. Advanced stages of liver cirrhosis are accompanied by hemodynamic changes of the hepatic vascular system. Four-dimensional (4D) flow magnetic resonance imaging (MRI) has been validated for the clinical assessment of the liver blood flow in patients with advanced liver cirrhosis. It represents a method that supplements Doppler ultrasound and provides important additional information on the vessel system in difficult patients. The purpose of this review is to provide insights into 4D flow MRI for blood flow visualization and quantification in patients with advanced liver cirrhosis.
- Citation: Stankovic Z. Four-dimensional flow magnetic resonance imaging in cirrhosis. World J Gastroenterol 2016; 22(1): 89-102
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/89.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.89
Liver cirrhosis is a leading cause of serious morbidity and the 9th most often cause of death in the United States and Europe with a mortality of more than 35000 deaths per year[1,2]. The most common causes of liver cirrhosis in developed countries are alcoholic liver disease and hepatitis C, while in most parts of the sub-African continent and in Asia, hepatitis B dominates as the predominant cause[3-5]. Liver cirrhosis reflects the histological development of regenerative nodules and fibrotic bands as a response to chronic liver injury. In the cascade of liver damage, it appears at advanced stages of liver fibrosis and is accompanied by distortion of the hepatic vascular system. The hemodynamic changes in the liver result in the shunting of the portal venous and arterial blood supply directly into the central hepatic veins, bypassing the exchange between hepatic sinusoids and the adjacent hepatocytes[6,7]. The major clinical effects of the liver damage and liver cirrhosis are impaired liver function, portal hypertension with increased intrahepatic resistance, and potential development of malignant hepatocellular carcinoma. The hemodynamic changes in liver cirrhosis are closely associated with portal hypertension and vascular alterations in the liver parenchyma[8,9].
Over the last 3 decades, phase contrast (PC) MRI has become established in the clinical routine for diagnosing hemodynamic alterations in the heart, aorta and large thoracic and abdominal vessel systems[10-12]. Further improvements in PC-MRI led to time-resolved (CINE) 3-dimensional (3D) PC-MRI technique with the feasibility of three-directional velocity encoding [four-dimensional (4D) flow MRI]. 4D flow MRI enables 3D volumetric coverage and the means of assessing hemodynamic changes in a specific region over time[13-17].
The purpose of this review article is to provide insights into the 4D flow MRI imaging technique for blood flow visualization and flow quantification in patients with advanced liver cirrhosis and in healthy volunteers. I also aim to describe the clinical advantages and disadvantages of the method, illustrating recent developments of the technique in the liver’s vascular system as well as presenting several very recent clinical applications of the method for evaluating hemodynamic anomalies.
A perfect non-invasive test for patients with liver cirrhosis would be simple to perform, safe, inexpensive, accurate, reproducible, yield numeric results in real time, reflect therapy-induced changes, enable prognostic stratification, and predict potential long-term outcomes related to liver cirrhosis[18-20].
Liver biopsy is acknowledged as the gold standard when diagnosing liver cirrhosis and assessing the stage and grade of chronic hepatitis[21-24]. The histological sub-classification and quantitative evaluation of hepatic fibrosis reveals a correlation with the disease’s clinical stage and prognosis, and is valuable in validating other non-invasive markers[25,26]. The repeatability of liver biopsy and its clinical acceptance by the patients is limited by its potential for complications, sampling errors, inter- and intra-observer variability, and invasiveness[27,28]. In patients with liver cirrhosis, measurements of the hepatic venous pressure gradient (HVPG) provide an accurate estimate of portal pressure and offer a solid clinical and prognostic marker for chronic liver disease[29-32]. It enables us to assess the development of clinical complications, e.g. esophageal varices and bleeding and the risk of decompensation, and provides independent prognostic information on patients’ survival and future mortality risk[33-37]. Similar to liver biopsy, HVPG’s main limitation is its invasiveness and limited availability[32]. Much effort has been made to identify a non-invasive alternative offering comparable reliability to the invasive interventions described above.
An important clinical role is played by laboratory tests such as biochemical and hematological serum markers with the benefits of simple, non-invasive and repetitive testing and results reflecting the entire liver. Serum markers are “indirect” and “direct”: the “indirect markers” reveal the degree and stage of fibrosis, while “direct markers” indicate the enzymes playing a key role in matrix regulation or the hepatic matrix components and their deposition or removal[38,39]. Studies evaluating serum markers have demonstrated their predictive efficiency for fibrosis and cirrhosis[40,41] although a meta-analysis describes their accuracy as limited in assessing the fibrosis stage[42]. To optimize reliability, a combination of serum markers is used instead of a single marker[43]. Nevertheless, non-invasive assessment of the dynamic changes in portal hypertension, liver fibrosis and cirrhosis by laboratory serum markers remains inadequate[42,43].
Diagnostic imaging modalities have a fundamental function in managing patients with chronic liver disease and in diagnosing the malignant transformation to hepatocellular carcinoma. The clinical gold standard and most comprehensive available and evaluated imaging test is ultrasound (US)[44-48] and accompanied techniques including transient elastography (TE)[49,50], acoustic radiation force impulse (ARFI)[51] and supersonic shear-wave elastography (SSWE)[52] imaging. Based on its broad availability, non-invasive character and easy applicability, Doppler US is well established in the clinical routine for the follow-up and assessment of patients with chronic liver disease and liver cirrhosis[44-48]. In advanced stages of liver fibrosis, morphological alterations are accompanied by changes in the liver’s blood flow[53]. Assessment by US include liver and spleen size measurements, liver parenchyma evaluation, but also measurements of portal vein (PV) velocities, PV blood flow, liver perfusion, and resistance indices in splanchnic arteries[53-57]. Ultrasound is reported to demonstrate very low sensitivity and specificity for liver fibrosis, and no correlation was detected between US results and liver biopsies when staging liver fibrosis[58,59]. Better assessment of hemodynamic changes in the liver has been made possible by contrast-enhanced ultrasound (CEUS)[60,61]. There is evidence that regional hepatic perfusion parameters correlate with the severity of liver failure and are increased in patients with liver cirrhosis[61]. The usual US limitations are involved in this technique, as the involvement of different contrast agents with different kinetics and drug design, varying operator skills and inconsistent availability of the method. Other limitations are the patient-related and operator-dependent conditions. US is influenced by the stage of NPO (nothing per mouth), patient respiration, ascites, bowel gas, differences in equipment and inter-observer variability in the measurements[62-66].
Other ultrasound-based techniques focus on the mechanical properties of liver tissue and measure differences in viscoelasticity in patients with liver fibrosis[51]. The modalities in evaluating liver stiffness in hepatic fibrosis are useful to reduce invasive pressure measurements, predict lethal complications or improve patient’s prognoses and risk stratification. The two most frequent modalities are either real-time based elastography or shear-wave elastography. TE is the most widely applied and tested modality, followed by ARFI[51] and SSWE[52]. Numerous studies have shown that TE results correlate significantly with the histological stage of liver fibrosis and are very diagnostically accurate[67-69]. Nevertheless, TE has a high measurement failure rate of up to 20% due to limitations like severe obesity, ascites and subcutaneous fat[49,60,70]. Further restrictions for its broad clinical use are its wide range of cut-off values and results variability across different studies[71-74].
Conventional CT and MRI are well-suited for evaluating morphological anomalies in patients with chronic liver disease, as they show the degree of liver injury from cirrhosis and accompanying complications. Alterations result from portal hypertension, hepatic insufficiency and portosystemic shunting, which result in ascites, gastrointestinal bleeding, coagulopathy, encephalopathy, and the formation of collateral vessels and portosystemic shunts[75-77]. Predisposing locations of the collateral vessels are distal esophageal, the gastric fundus, paraumbilical, splenorenal, retroperitoneal, abdominal wall and hemorrhoidal[78,79]. These morphologic changes in liver hemodynamics are usually visible in advanced stages of liver cirrhosis. As a result, CT and MRI are unsuitable for diagnosis of the early stages of liver cirrhosis, including functional evaluation of the liver blood flow. An MRI-based assessment of liver stiffness by MR elastography (MRE) offers better contrast between different body tissues than Ultrasound[80-82]. Further advantages of the technique is its potential to assess the whole liver, observer-independence, and no influence by the body habitus[83,84]. MRE has become standard for assessing liver fibrosis as it offers generally high sensitivity and specificity for different histological gradings[81-86]. Factors associated with this technique that limit its use in the daily clinical routine are its expense and restricted availability[83,84].
Much experience has been gained over the last two decades in using MRI to hemodynamically assess the liver blood flow[87-90]. Most published studies relied on 2D PC-MRI measurements, a robust and reliable technique for hemodynamic assessment of the liver. It has revealed low intra- and inter-observer variability and high reproducibility compared to Doppler US[87-90]. 2D PC-MRI is, however, limited by the application and positioning of 2D planes. Flow measurements and flow quantification results obtained via 2D PC-MRI can be unreliable because of difficulties in precise orthogonal positioning of the measurement plane within the complex liver vascular system[91]. Another large group of studies assessing liver hemodynamics relied on contrast-enhanced MRI. Since nephrogenic systemic fibrosis (NSF) has been observed with its potential connection to the injection of gadolinium-based contrast medium, contrast medium is being more carefully applied[92-96].
Until now, no non-invasive diagnostic modality has been able to determine the changes in and exact stage of chronic liver disease and portal hypertension. Nevertheless, new modalities reveal promising results in hemodynamic assessments of the liver. Further clinical trials examining different disease stages are needed to validate the reproducibility and long-term prognostic values of these non-invasive diagnostic modalities. These tests have the potential to lead to unknown and new paradigms in the specific management of patients with chronic liver disease.
During data acquisition with 4D flow MRI, velocity is encoded along all three spatial directions throughout the entire heart cycle. This results in a time-resolved volumetric velocity field from the scanned body region, e.g. heart, aorta, lung, intracranial vessels or liver[10,97,98]. To take quantitative velocity measurements in one spatial direction, two data acquisitions and the following subtraction are needed for velocity encoding. Seeking velocity encoding in all three spatial directions, one needs a reference image and three velocity-encoded images acquired along the three orthogonal directions X, Y and Z[99-101]. The data is acquired over several cardiac cycles, while data acquisition at the same time is synchronized with the heart cycle using the k-space segmentation technique. The 4D flow MRI data is thus measured only partially during one heart cycle; the entire data is acquired ongoing over several heart cycles. After completing 4D flow MRI data acquisition, 4 time-resolved 3D flow datasets are generated: one dataset with the magnitude information containing anatomic information and three flow datasets giving the velocities in each spatial direction X, Y and Z. The extended amount of data encoding for three spatial dimensions, three velocity directions, and the time information over the cardiac cycle is reflected in the scan time. Several recent examinations in 4D flow MRI sequencing addressed this limitation and tried to find a potential solution to shorten the scan time. While the manufacturers continuously improve the hardware aspect of the scanners, others are working on software improvements.
Fast imaging techniques like radial imaging 3D PC-VIPR (vastly undersampled isotropic projection reconstruction)[102,103], spiral techniques without acceleration[104], compressed sensing[59,105] or parallel imaging enable 4D flow MRI scans within 8 to 20 min[15,106]. Conventional acceleration techniques like GRAPPA (generalized auto-calibrating partially parallel acquisitions)[57] or SENSE (sensitivity encoding)[55] can usually achieve a scan time reduction by factor 2 or 3. Applying higher values could negatively influence the quantification of velocities with a reduced signal-to-noise ratio[107]. Further advancements in spatial-temporal parallel imaging techniques such as k-t PCA (k-t principal component analysis)[108], k-t GRAPPA[109-111], k-t SENSE[112,113] or k-t BLAST[113,114] facilitate higher acceleration factors and represent promising imaging techniques for scan time reduction using 4D flow MRI. Several studies have already used a radial data acquisition technique combined with under sampling of the data (PC-VIPR) for assessing the arterial and portal venous system within the liver and splanchnic vessels[115-117]. This imaging technique offered shorter scan times together with broad volumetric coverage and enhanced spatial resolution, fewer motion artifacts and self-gating. A recent study evaluated scan time savings using a k-t GRAPPA accelerated Cartesian 4D flow MRI to visualize and quantify liver hemodynamics[118]. Three different acceleration factors were used, with additional focus on temporal resolution. All k-t GRAPPA acceleration factors displayed significant scan time savings, R = 3 and R = 5 over 40% to almost 8 min and R = 8 over 70% with a 4D flow MRI scan time of almost 4 min. While acceleration factors R = 3 and R = 5 showed quantitative blood flow and velocity results comparable to standard GRAPPA R = 2, acceleration factor R = 8 revealed increased noise and artifacts with significantly lower measurement results in the arterial system according to the calculated blood flow parameters[118]. Another current study is evaluating a combined-spiral-sampling and dynamic-compressed-sensing approach for faster acquisition of 4D flow MRI[119]. In a study cohort with 10 subjects, investigators have demonstrated the feasibility of applying 4D flow MRI within an average scan time of 24 s (18-25 s range), comparable to 2D PC-MRI measurements. Moderate to substantial agreement was observed in the delineation of arterial and venous vessel borders between the spiral 4D flow MRI and the Cartesian 4D flow MRI approach. Quantitative results revealed good agreement and a 95% confidence interval between 60% and 77% for the flow parameters acquired[119]. These recent studies addressing the acceleration of 4D flow MRI for abdominal imaging show the potential for this technique to be accelerated to last a few seconds while enabling comprehensive evaluation of liver hemodynamics. For thoracic or abdominal applications of the 4D flow MRI technique, breathing control and ECG-gating is needed to reduce consequent artifacts. In addition to breathing bellows or navigator gating, self-gating methods have been reported[120-123]. Upcoming studies will continue to validate the scan-time savings results using various acceleration techniques and offering broad clinical application for patients with advanced liver cirrhosis as well as better understanding of complex blood flow changes in the liver.
Many technical aspects must be considered when performing 4D flow MRI data acquisition and validating the data acquired. A detailed description and discussion of these technical aspects is beyond the scope of this clinically-focused review article, and I offer only an overview of the most important facts. For further detailed information, please consult comprehensive review articles for 4D flow MRI, e.g.[16,17].
Velocity encoding sensitivity (Venc) represents the peak flow velocity that can be acquired. If the peak velocity of the blood flow in the vessels exceeds the preset setting for the Venc, the accuracy of flow visualization and quantification of the liver hemodynamics may be compromised and anti-aliasing corrections are necessary[124]. To obtain optimal image quality, the Venc should represent the hemodynamic conditions in the hepatic area and be as high as necessary to avoid anti-aliasing, but as low as possible to reduce velocity noise. A typical Venc setting for the portal venous system is 50 cm/s, for the arteries 100 cm/s, and values even above 150 cm/s within a TIPS stent[16,17]. An interesting approach for the liver with different flow velocities is a dual-venc, already discussed in other regions of the body[125].
Many errors in 4D flow MRI can impair image quality and trigger inaccuracies in quantitative flow measurements. The most common errors are phase offset errors based on gradient field nonlinearity[106], eddy currents[126] and Maxwell terms[127]. Before further processing the 4D flow MRI data, it is important to take such errors into account and consider appropriate correction strategies.
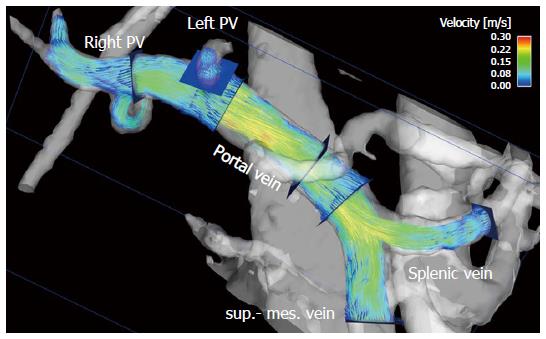
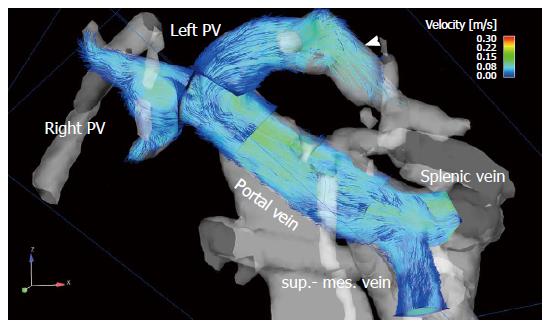
To visualize the 4D flow MRI data acquired, many options are available on the market, usually using 2D analysis planes which must be positioned in the vessel of interest[11,128-133] (Figures 1 and 2). Time-resolved particle traces and 3D streamlines are emitted from these analysis planes. Time-resolved particle traces display the temporal evolution of blood flow over one or more cardiac cycles[132]. Velocity changes can be visualized or the flow pattern traced to its origin by color-coding the particle-traces. Streamlines represent 3D traces that track the spatial distribution of 3D velocities within an individual cardia time frame. Color-coding the streamlines enables visualization of the spatial distribution and orientation, especially of peak blood flow velocities.
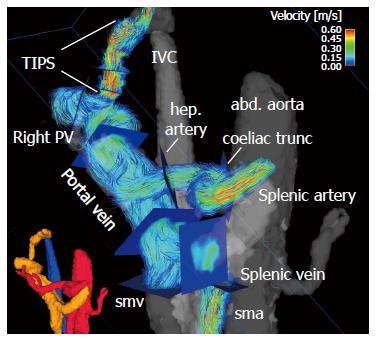
4D flow MRI has the potential to retrospectively quantify hemodynamic parameters of the liver at any location within the 3D data volume following data acquisition[134,135]. 2D analysis planes can be freely positioned in the interesting arterial or portal venous vessel to quantify standard flow parameters like peak and mean velocities, flow volume over the cardiac cycle, vessel area, shunt fraction or flow reversal (Figures 2 and 3). Several studies report excellent agreement between 2D PC-MRI and 4D flow MRI in flow quantifications[136,137]. Good scan-rescan reproducibility and low inter- and intra-observer variability have been shown in conjunction with 4D flow MRI flow quantification in the intracranial, cervical, thoracic and abdominal vessel systems[136,138,139]. New strategies are also discussed in the literature for evaluating more advanced hemodynamic parameters, e.g. wall shear stress, pressure difference, turbulent kinetic energy and pulse wave velocity[135,140-143].
4D flow MRI represents a non-invasive and observer-independent technique for comprehensive 3D volumetric evaluation of the liver and splanchnic region, as well as the means of assessing the liver’s arterial and portal venous system, collateral vessels, and potential shunts. In addition to initial studies evaluating flow in the portal vein[89,144-146] the focus of the first feasibility studies using 4D flow MRI in the liver was on the entire portal venous system[147,148]. These studies addressed the inflow from the splenic and superior mesenteric vein, the splenic-mesenteric confluence, the intrahepatic portal vein and right and left intrahepatic branches[147,148]. Initial studies comparing healthy volunteers and patients with liver cirrhosis applied a velocity encoding of 50 cm/s and attained complete visualization of the extrahepatic vessels in over 94% of their subjects (Figure 2). The small intrahepatic branches presented a limitation, however, as complete visualization was only possible in about 80% of the subjects[147,148]. Another group applied radial 4D flow MRI acquisition[102,103] and demonstrated good to excellent visualization in patients with liver cirrhosis, identifying all arterial vessels and 86% of the portal venous circulation[115,116]. Subsequent studies using Cartesian 4D flow MRI and a velocity encoding of 100 cm/s were also able to evaluate the liver’s arterial and portal venous system[118,149]. Complete visualization of the arterial system was accomplished in liver cirrhosis patients and volunteers in almost 100% of the cases. The limiting factor in the volunteer studies was the left intrahepatic branches (complete visualization in 50% to 60% of the cases)[118,149]. In the patient cohort studies the limitations in the portal venous system were more obvious as complete visualization of the extrahepatic vessels was only possible in in 60% to 90% of the cases, while the small intrahepatic branches of the portal vein were completely visible in only 20% to 60% of the cases[150,151]. A possible reason for these limitations might be in the spatial resolution of the 4D flow MRI sequence and the quite long scan time under free breathing during acquisition, resulting in partial volume effects and signal blurring due to organ motion and reduced effective spatial resolution in the Z-direction. In addition to visualization of the hepatic hemodynamic system, a major advantage of the 4D flow MRI sequence is its ability to evaluate the blood flow direction. Qualitative results in a feasibility study using radial 4D flow MRI depicted reverse hepatofugal flow in 5% of the portal venous vessels[115]. Using Cartesian 4D flow MRI, that study group showed a reopened umbilical vein in 6 out of 20 liver cirrhosis patients with portosystemic shunts[148]. In another patient-cohort study addressing more advanced stages of liver cirrhosis portosystemic collateral vessel systems were visualized prior to TIPS revision in 8 out of 11 cases[151]. The shunt was not visible in the follow-up 4D flow MRI examination after insertion of a TIPS stent graft[151,152]. The successful occlusion of the shunt was proven during the intervention. The results of these studies also highlight one of the limitations of the 4D flow MRI sequence. Due to the 100 cm/s velocity encoding favoring high blood flow velocities and the applied spatial resolution, it can be difficult to distinguish the collateral vessels having a small diameter and low blood flow velocities in advanced stages of the liver disease. Precise differentiation of the regressive development of collateral vessels might be difficult when referring to 4D flow MRI using only the actual sequence parameter. Better spatial resolution would improve visualization of the portal venous vessel system and the collateral vessels with low blood flow velocities and small vessel size in advanced stages of liver disease. A further potential limiting factor is the signal-to-noise ratio. The above-mentioned studies involving radial acquisition applied contrast medium, while the Cartesian-based studies did not. The influence of the contrast medium, field strength and better respiratory triggering on the 4D flow MRI for the liver hemodynamics should be examined in future studies with larger patient cohorts.
As well as visualizing 3D blood flow characteristics and clinically illustrating complex alterations in patients with advanced liver disease, 4D flow MRI enables the comprehensive quantification of blood flow parameters from the same dataset. Several working groups have conducted a quantitative evaluation of flow parameters in the portal venous system using Doppler US or MRI, while most of the MRI results are based on 2D PC-MRI measurements[144,145,153-156]. Initial studies using 4D flow MRI within the portal venous system displayed moderate, but significant correlations among 4D flow MRI, 2D PC-MRI and Doppler US values[147,148]. The peak-velocity results were between 23-27 cm/s, slightly lower than in other studies reporting 28 cm/s using 2D PC-MRI and Doppler US[89]. Mean velocity values between 10-12 cm/s resemble those in other studies based on 2D PC-MRI (11-14 cm/s)[144,145,154]. However, published values from studies applying Doppler US reveal higher mean velocities between 15-17 cm/s[156,157]. The peak and mean velocities of blood flow in the liver tended to be underestimated in conjunction with 4D flow MRI (between 35% and 38%)[147,148]. Another 4D flow MRI study evaluating blood flow in the carotid bifurcation offered a similar underestimation of flow velocities (between 31% and 39%) via 4D flow MRI compared to Doppler US[158]. An explanation for MRI’s tendency to underestimate velocities is related to the data acquisition method. The velocity data is acquired over several cardiac cycles, resulting in an average velocity progression. Velocity changes within the different heart cycles and short time fluctuations are not displayed. In comparison: Doppler ultrasound measurements represent real-time velocity data[144,156]. MRI’s underestimation of velocities therefore has to do with velocity averaging. Another reason might be partial volume effects due to lower spatial resolution in MRI than in Doppler US.
A 4D flow MRI study detected lower flow-volume values after measuring mean velocities and vessel area (mean 0.7 ± 0.4 L/min)[148] compared to 2D PC-MRI studies (between 1.0 and 1.3 L/min)[89,145,155]. Doppler US studies, however, yielded a wide range of flow volumes (between 0.3 and 1.3 L/min)[159,160]. One reason for these different flow volumes could be hemodynamic changes after ingestion. A Doppler US-based study evaluating postprandial hyperemia in patients with liver cirrhosis revealed an average increase in portal venous flow velocities of 29% and a 38% increase in the portal venous blood flow[161]. Most recent studies evaluating reproducibility and the postprandial effect via 4D flow MRI report good to excellent short-term reproducibility in the fasting state[162,163]. Portal venous flow parameters were significantly higher in the postprandial state, confirming the large impact of caloric intake on portal venous flow[162]. Portal venous flow regulation might also be impaired after a meal challenge in patients with liver cirrhosis[163].
Recent 4D flow MRI studies measuring the vessel area report higher values compared to Doppler US in correlation to earlier studies with 2D PC-MRI and Doppler US[144,145,147,148,164]. One reason for the differences between these two modalities might be the location and angle of the ultrasound transducer during vessel diameter assessment, which would compromise reliable measurements not just in small vessels. The differences are inter-observer and intra-observer variability[157].
A recent reproducibility study examined the reliability of 4D flow MRI data acquired from the thoracic aorta with high flow velocities[138] showing good scan-rescan reliability and low inter- and intra-observer variability in the acquired and clinically-relevant blood-flow parameters and in wall shear stress (WSS)[138]. Via radial acquisition, another study group confirmed the results from quantifying hepatic and splanchnic hemodynamics using 4D flow MRI[116]. Investigating patients with portal hypertension, that study revealed good internal data consistency and low inter- and intra-observer variability in 4D flow MRI data from the liver’s vascular system[116]. They validated their results indirectly by being internally consistent at three different locations within the vascular system. Taking measurements at three different locations in the portal vein revealed an average absolute error of 4.2% ± 3.9%. Comparison of flow into the portal confluence coming from the splenic and superior mesenteric veins with the flow in the portal vein yielded an error of 5.9% ± 2.5%. Assessment of flow in the portal bifurcation and the right and left intrahepatic portal vein branches showed an error of 5.8% ± 3.1%[116]. Another study applying Cartesian 4D flow MRI revealed similarly good results with small errors in the internal consistency validation of the flow parameters[150]. Those authors performed additionally a real scan-rescan validation of 4D flow MRI with a rescan of all volunteers at least 5 mo later on the same scanner. They reported good reproducibility of 4D flow MRI quantification in the portal venous system with low blood flow velocities offering a mean average difference of 2% between the two scans for peak velocities and 5% for the mean velocities[150]. Quantitative evaluation of arterial blood flow velocities using 4D flow MRI yielded robust results with mean average differences of 3% for peak velocities and 7% for mean velocities[150]. Reproducibility of flow volume assessments showed low error for mean average differences of 6% in the portal vein, while the arterial flow volume evaluation was limited by an error of 14%. Inter-observer variability of between 16% and 26% is described in the literature in association with the evaluation of portal venous blood flow velocities using the clinical gold standard, Doppler US[165]. 4D flow MRI has yielded similar results: a scan-rescan-variability between 25% and 26% in the assessment of flow velocities[150]. A possible reason for these variations is the manual segmentation of vessel borders. A semi- or full-automatic segmentation method could improve the reproducibility of such calculated parameters and those derived from the vessel area[166].
Doppler US-based studies describe significantly low flow velocities and flow volumes in patients with advanced liver cirrhosis compared to healthy volunteers[159,167]. In a study taking the radial 4D flow MRI approach, the MELD score was calculated to estimate disease severity and correlated with image quality[115], yielding no correlation between image quality and the MELD score. Another study analyzed their patient cohort applying the Child-Pugh score to assess the degree of liver failure compared to the quantitative flow parameters derived from 4D flow MRI data and Doppler US measurements[148]. They detected no relevant correlation between disease severity and changes in liver hemodynamics; the expected changes were only visible in few hepatic vessels. Those 4D flow MRI findings might be associated with the study patients they recruited. In both 4D flow MRI studies, most of the cohorts’ liver cirrhosis patients presented an early stage of the disease and a low MELD score or Child-Pugh stage A with subsequently few anomalies in liver hemodynamics[115,148]. The Doppler US studies reveal upon closer inspection to have included patients with mainly advanced liver cirrhosis (Child-Pugh stage C) and more advanced impairments in the hepatic vessel system[159,167]. To further validate 4D flow MRI data, additional patient studies are needed with larger patient cohorts and involving different stages of disease severity.
The most interesting novelty represented by 4D flow MRI is that it allows us to reliably assess both the portal venous system in patients with altered liver hemodynamics, as well as small arteries in the liver and splanchnic system[115,116,118,149,151] (Figure 3). It is a technique that offers quantitative equilibrium in the patient’s blood flow between the arterial inflow to the liver and splanchnic system and the portal venous outflow to the liver parenchyma. We can thus calculate the shunt fraction in patients with advanced liver cirrhosis and a portosystemic shunt[151,152]. An increase in liver perfusion of 424 mL/min can be verified after TIPS intervention by assessing the different flow rates in the hepatic arteries, portal vein and TIPS stent-graft[151]. Doppler US studies reveal an increase in flow velocities after a TIPS intervention by a factor of 2 to 4[168,169]. 4D flow MRI data has demonstrated results similar to Doppler US’s in peak velocities in the portal vein before TIPS insertion (4D flow MRI: 19 ± 5 cm/s vs Doppler US 10-20 cm/s), but revealed lower values in the portal vein during TIPS follow-up (4D flow MRI: 28 ± 7 cm/s vs Doppler US 40-60 cm/s)[151,169]. Normally-functioning stent-shunts yielded in-stent values via 4D flow MRI comparable to those in Doppler US with peak velocities measuring between 50 cm/s and 200 cm/s (range 58-194 cm/s)[151,169]. Stenoses within the stents were reliably depicted and confirmed by invasive catheter pressure gradient measurements during stent shunt revision[151]. These recent studies evaluating 4D flow MRI for abdominal imaging after TIPS placement show the potential for this technique to be an additional tool for interventional radiologists while enabling pre-procedure mapping and planning of the optimal stent graft configurations[151,152]. As a result, ideal outcome after TIPS placement can be obtained including pressure gradient reduction and long-term stent graft patency.
There is ample evidence that 4D flow MRI has potential to image the hemodynamics in patients with advanced liver cirrhosis and to measure altered blood flow parameters.
One of the main limitations of 4D flow MRI investigations addressing the liver and visceral blood flow is the small size of the patient and control cohorts in the clinical studies. This has a lot to do with the advanced clinical stages of patients suffering from worsening liver cirrhosis. It is difficult to obtain accurate data in patients with severe ascites, moreover, the compliance of patients with advanced stages of the disease is often poor. The imaging potential and therapeutic tools are sometime only palliative when treating patients with advanced liver cirrhosis, and we often have access to too few patients (e.g. Child-Pugh stage C). Their high mortality rate also makes a longitudinal study design with longer follow-up controls more difficult than, for example, examining patients with heart diseases. Nevertheless, our aim should be to carry out large multicenter cohort studies reflecting different manifestations of liver cirrhosis in order to further validate the 4D flow MRI technique in a clinical setting.
Validation of the 4D flow MRI method is another limitation: studies have already compared 4D flow MRI to Doppler US, the clinical gold standard, in many body regions[158,170] including hepatic and visceral blood flow[116,149]. 4D flow MRI provides good scan-rescan variability and low inter- intra-observer variability[116,149,158,170], although most of those studies’ subjects were healthy volunteers, and few patients with liver cirrhosis were monitored during follow-up. We will need further clinical cohort studies to assess the accuracy of the quantitative 4D flow MRI results in comparison with invasive measurements of hemodynamic parameters.
A further limiting factor of the 4D flow MRI sequence is still the spatial and temporal resolution. The Cartesian 4D flow MRI approach has particular limitations in capturing the small intrahepatic vessels and hepatic arteries. With its superior resolution, the radial 4D flow MRI sequence visualizes the vessel system impressively. 4D flow MRI methods require further improvements to enhance the accuracy of quantitative results in the small vessels. Another limitation of 4D flow MRI compared to Doppler US is the acquisition of data over several cardiac cycles averaging over time. This issue is a concern in the arteries especially, less so in the portal venous system, whose peak velocities and flow volume values are slightly lower and do not reveal brief time variations in blood flow. The 4D flow MRI sequence is still being researched. The shortage of freely-available software packages, the time it takes to perform the pre- and post-processing, and the lack of a standardized approach for data evaluation are additional factors that limit the broader clinical application of this method. A collective endeavor is needed from clinicians, researchers and manufacturers to pave the way for greater availability of the 4D flow MRI sequence and consequent increased clinical applications. Further developments should focus on refining the clinical workflow, on presenting the acquired data to clinical colleagues, and on improving the accessibility of results from within existing patient archives.
4D flow MRI has been validated for the clinical assessment of the liver blood flow in patients with advanced liver cirrhosis. It is an MRI technique that can examine the patient from a functional perspective as a part of “one-stop-shopping”. More importantly, 4D flow MRI is a method that supplements Doppler US. It provides important additional information on the vessel system in difficult patients. The potential of 4D flow MRI is growing; the more advanced it becomes, the better we will understand the pathophysiology of liver cirrhosis and the dynamic alterations it causes. That, in turn, will ensure better patient management and more accurate risk stratification.
P- Reviewer: Thimmappa ND S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Groszmann RJ. Hyperdynamic circulation of liver disease 40 years later: pathophysiology and clinical consequences. Hepatology. 1994;20:1359-1363. [PubMed] |
| 2. | Pagliaro L, D’Amico G, Luca A, Pasta L, Politi F, Aragona E, Malizia G. Portal hypertension: diagnosis and treatment. J Hepatol. 1995;23 Suppl 1:36-44. [PubMed] |
| 3. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [PubMed] |
| 4. | Bellentani S, Pozzato G, Saccoccio G, Crovatto M, Crocè LS, Mazzoran L, Masutti F, Cristianini G, Tiribelli C. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut. 1999;44:874-880. [PubMed] |
| 5. | Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5-10. [PubMed] |
| 6. | Schaffner F, Poper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239-242. [PubMed] |
| 7. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1605] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 8. | Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol. 2004;40:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Pelc NJ, Herfkens RJ, Shimakawa A, Enzmann DR. Phase contrast cine magnetic resonance imaging. Magn Reson Q. 1991;7:229-254. [PubMed] |
| 11. | Kilner PJ, Yang GZ, Mohiaddin RH, Firmin DN, Longmore DB. Helical and retrograde secondary flow patterns in the aortic arch studied by three-directional magnetic resonance velocity mapping. Circulation. 1993;88:2235-2247. [PubMed] |
| 12. | Kvitting JP, Ebbers T, Wigström L, Engvall J, Olin CL, Bolger AF. Flow patterns in the aortic root and the aorta studied with time-resolved, 3-dimensional, phase-contrast magnetic resonance imaging: implications for aortic valve-sparing surgery. J Thorac Cardiovasc Surg. 2004;127:1602-1607. [PubMed] |
| 13. | Bogren HG, Mohiaddin RH, Yang GZ, Kilner PJ, Firmin DN. Magnetic resonance velocity vector mapping of blood flow in thoracic aortic aneurysms and grafts. J Thorac Cardiovasc Surg. 1995;110:704-714. [PubMed] |
| 14. | Wigström L, Sjöqvist L, Wranne B. Temporally resolved 3D phase-contrast imaging. Magn Reson Med. 1996;36:800-803. [PubMed] |
| 15. | Markl M, Chan FP, Alley MT, Wedding KL, Draney MT, Elkins CJ, Parker DW, Wicker R, Taylor CA, Herfkens RJ. Time-resolved three-dimensional phase-contrast MRI. J Magn Reson Imaging. 2003;17:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging. 2012;36:1015-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 534] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 17. | Stankovic Z, Allen BD, Garcia J, Jarvis KB, Markl M. 4D flow imaging with MRI. Cardiovasc Diagn Ther. 2014;4:173-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 120] [Reference Citation Analysis (0)] |
| 18. | Schalm SW. The diagnosis of cirrhosis: clinical relevance and methodology. J Hepatol. 1997;27:1118-1119. [PubMed] |
| 19. | Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol. 2009;50:17-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (3)] |
| 20. | Kim MY, Jeong WK, Baik SK. Invasive and non-invasive diagnosis of cirrhosis and portal hypertension. World J Gastroenterol. 2014;20:4300-4315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Ludwig J. The nomenclature of chronic active hepatitis: an obituary. Gastroenterology. 1993;105:274-278. [PubMed] |
| 22. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3123] [Article Influence: 104.1] [Reference Citation Analysis (1)] |
| 23. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] |
| 24. | Hytiroglou P, Thung SN, Gerber MA. Histological classification and quantitation of the severity of chronic hepatitis: keep it simple! Semin Liver Dis. 1995;15:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Kim MY, Cho MY, Baik SK, Park HJ, Jeon HK, Im CK, Won CS, Kim JW, Kim HS, Kwon SO. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol. 2011;55:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 26. | Kim SU, Oh HJ, Wanless IR, Lee S, Han KH, Park YN. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol. 2012;57:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1760] [Article Influence: 70.4] [Reference Citation Analysis (1)] |
| 28. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 422] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Perelló A, Escorsell A, Bru C, Gilabert R, Moitinho E, García-Pagán JC, Bosch J. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology. 1999;30:1393-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111-117. [PubMed] |
| 31. | Kumar M, Sakhuja P, Kumar A, Manglik N, Choudhury A, Hissar S, Rastogi A, Sarin SK. Histological subclassification of cirrhosis based on histological-haemodynamic correlation. Aliment Pharmacol Ther. 2008;27:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 542] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 33. | Lebrec D, De Fleury P, Rueff B, Nahum H, Benhamou JP. Portal hypertension, size of esophageal varices, and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology. 1980;79:1139-1144. [PubMed] |
| 34. | Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419-424. [PubMed] |
| 35. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 661] [Article Influence: 31.5] [Reference Citation Analysis (1)] |
| 36. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2209] [Article Influence: 110.5] [Reference Citation Analysis (3)] |
| 37. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 844] [Article Influence: 44.4] [Reference Citation Analysis (1)] |
| 38. | Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006;12:3682-3694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 190] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 39. | Berzigotti A, Ashkenazi E, Reverter E, Abraldes JG, Bosch J. Non-invasive diagnostic and prognostic evaluation of liver cirrhosis and portal hypertension. Dis Markers. 2011;31:129-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (1)] |
| 40. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1037] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 41. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3335] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 42. | Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol. 2006;44:462-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 43. | Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, El-Kamary SS, Sulkowski M, Bass EB. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36:S161-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aubé C, Gallois Y, Rifflet H, Maïga MY, Penneau-Fontbonne D. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609-1616. [PubMed] |
| 45. | Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D’Errico A, Zironi G, Grigioni W, Bolondi L. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27:979-985. [PubMed] |
| 46. | Nicolau C, Bianchi L, Vilana R. Gray-scale ultrasound in hepatic cirrhosis and chronic hepatitis: diagnosis, screening, and intervention. Semin Ultrasound CT MR. 2002;23:3-18. [PubMed] |
| 47. | Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 48. | Shen L, Li JQ, Zeng MD, Lu LG, Fan ST, Bao H. Correlation between ultrasonographic and pathologic diagnosis of liver fibrosis due to chronic virus hepatitis. World J Gastroenterol. 2006;12:1292-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111-134. [PubMed] |
| 50. | Talwalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology. 2008;135:299-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 51. | Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 52. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 53. | Baik SK. Haemodynamic evaluation by Doppler ultrasonography in patients with portal hypertension: a review. Liver Int. 2010;30:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Iwao T, Toyonaga A, Oho K, Tayama C, Masumoto H, Sakai T, Sato M, Tanikawa K. Value of Doppler ultrasound parameters of portal vein and hepatic artery in the diagnosis of cirrhosis and portal hypertension. Am J Gastroenterol. 1997;92:1012-1017. [PubMed] |
| 55. | Aubé C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, Valsesia E, Pilette C, Rousselet MC, Bedossa P. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol. 1999;30:472-478. [PubMed] |
| 56. | Haktanir A, Cihan BS, Celenk C, Cihan S. Value of Doppler sonography in assessing the progression of chronic viral hepatitis and in the diagnosis and grading of cirrhosis. J Ultrasound Med. 2005;24:311-321. [PubMed] |
| 57. | Iliopoulos P, Vlychou M, Margaritis V, Tsamis I, Tepetes K, Petsas T, Karatza C. Gray and color Doppler ultrasonography in differentiation between chronic viral hepatitis and compensated early stage cirrhosis. J Gastrointestin Liver Dis. 2007;16:279-286. [PubMed] |
| 58. | Kutcher R, Smith GS, Sen F, Gelman SF, Mitsudo S, Thung SN, Reinus JF. Comparison of sonograms and liver histologic findings in patients with chronic hepatitis C virus infection. J Ultrasound Med. 1998;17:321-325. [PubMed] |
| 59. | Chen CH, Lin ST, Yang CC, Yeh YH, Kuo CL, Nien CK. The accuracy of sonography in predicting steatosis and fibrosis in chronic hepatitis C. Dig Dis Sci. 2008;53:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Klibanov AL. Ultrasound molecular imaging with targeted microbubble contrast agents. J Nucl Cardiol. 2007;14:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Berzigotti A, Nicolau C, Bellot P, Abraldes JG, Gilabert R, García-Pagan JC, Bosch J. Evaluation of regional hepatic perfusion (RHP) by contrast-enhanced ultrasound in patients with cirrhosis. J Hepatol. 2011;55:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 62. | Burns PN, Jaffe CC. Quantitative flow measurements with Doppler ultrasound: techniques, accuracy, and limitations. Radiol Clin North Am. 1985;23:641-657. [PubMed] |
| 63. | de Vries PJ, van Hattum J, Hoekstra JB, de Hooge P. Duplex Doppler measurements of portal venous flow in normal subjects. Inter- and intra-observer variability. J Hepatol. 1991;13:358-363. [PubMed] |
| 64. | Paulson EK, Kliewer MA, Frederick MG, Keogan MT, DeLong DM, Nelson RC. Doppler US measurement of portal venous flow: variability in healthy fasting volunteers. Radiology. 1997;202:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Bernatik T, Strobel D, Hahn EG, Becker D. Doppler measurements: a surrogate marker of liver fibrosis? Eur J Gastroenterol Hepatol. 2002;14:383-387. [PubMed] |
| 66. | Lim AK, Patel N, Eckersley RJ, Kuo YT, Goldin RD, Thomas HC, Cosgrove DO, Taylor-Robinson SD, Blomley MJ. Can Doppler sonography grade the severity of hepatitis C-related liver disease? AJR Am J Roentgenol. 2005;184:1848-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 397] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 68. | Kim do Y, Kim SU, Ahn SH, Park JY, Lee JM, Park YN, Yoon KT, Paik YH, Lee KS, Chon CY. Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig Dis Sci. 2009;54:1758-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (2)] |
| 69. | Kim SU, Kim do Y, Park JY, Lee JH, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Choi EH. How can we enhance the performance of liver stiffness measurement using FibroScan in diagnosing liver cirrhosis in patients with chronic hepatitis B? J Clin Gastroenterol. 2010;44:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 70. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 934] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 71. | Kazemi F, Kettaneh A, N’kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 72. | Lemoine M, Katsahian S, Ziol M, Nahon P, Ganne-Carrie N, Kazemi F, Grando-Lemaire V, Trinchet JC, Beaugrand M. Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment Pharmacol Ther. 2008;28:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 74. | Reiberger T, Ferlitsch A, Payer BA, Pinter M, Homoncik M, Peck-Radosavljevic M. Non-selective β-blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J Gastroenterol. 2012;47:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 75. | Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, Matsumoto T. Cirrhosis: modified caudate-right lobe ratio. Radiology. 2002;224:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Aguirre DA, Behling CA, Alpert E, Hassanein TI, Sirlin CB. Liver fibrosis: noninvasive diagnosis with double contrast material-enhanced MR imaging. Radiology. 2006;239:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 77. | Yu JS, Shim JH, Chung JJ, Kim JH, Kim KW. Double contrast-enhanced MRI of viral hepatitis-induced cirrhosis: correlation of gross morphological signs with hepatic fibrosis. Br J Radiol. 2010;83:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Brancatelli G, Federle MP, Ambrosini R, Lagalla R, Carriero A, Midiri M, Vilgrain V. Cirrhosis: CT and MR imaging evaluation. Eur J Radiol. 2007;61:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Vilgrain V. Ultrasound of diffuse liver disease and portal hypertension. Eur Radiol. 2001;11:1563-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Bolognesi M, Sacerdoti D, Mescoli C, Bombonato G, Cillo U, Merenda R, Giacomelli L, Merkel C, Rugge M, Gatta A. Different hemodynamic patterns of alcoholic and viral endstage cirrhosis: analysis of explanted liver weight, degree of fibrosis and splanchnic Doppler parameters. Scand J Gastroenterol. 2007;42:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Oliphant TE, Manduca A, Ehman RL, Greenleaf JF. Complex-valued stiffness reconstruction for magnetic resonance elastography by algebraic inversion of the differential equation. Magn Reson Med. 2001;45:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207-1213.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 720] [Article Influence: 37.9] [Reference Citation Analysis (1)] |
| 83. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 543] [Article Influence: 30.2] [Reference Citation Analysis (2)] |
| 84. | Hong WK, Kim MY, Baik SK, Shin SY, Kim JM, Kang YS, Lim YL, Kim YJ, Cho YZ, Hwang HW. The usefulness of non-invasive liver stiffness measurements in predicting clinically significant portal hypertension in cirrhotic patients: Korean data. Clin Mol Hepatol. 2013;19:370-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Asbach P, Klatt D, Schlosser B, Biermer M, Muche M, Rieger A, Loddenkemper C, Somasundaram R, Berg T, Hamm B. Viscoelasticity-based staging of hepatic fibrosis with multifrequency MR elastography. Radiology. 2010;257:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 86. | Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: A meta-analysis. Hepatology. 2012;56:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 87. | Burkart DJ, Johnson CD, Morton MJ, Wolf RL, Ehman RL. Volumetric flow rates in the portal venous system: measurement with cine phase-contrast MR imaging. AJR Am J Roentgenol. 1993;160:1113-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Hara AK, Burkart DJ, Johnson CD, Felmlee JP, Ehman RL, Ilstrup DM, Harmsen WS. Variability of consecutive in vivo MR flow measurements in the main portal vein. AJR Am J Roentgenol. 1996;166:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 89. | Yzet T, Bouzerar R, Allart JD, Demuynck F, Legallais C, Robert B, Deramond H, Meyer ME, Balédent O. Hepatic vascular flow measurements by phase contrast MRI and doppler echography: a comparative and reproducibility study. J Magn Reson Imaging. 2010;31:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Gouya H, Vignaux O, Sogni P, Mallet V, Oudjit A, Pol S, Legmann P. Chronic liver disease: systemic and splanchnic venous flow mapping with optimized cine phase-contrast MR imaging validated in a phantom model and prospectively evaluated in patients. Radiology. 2011;261:144-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Buonocore MH, Bogren H. Factors influencing the accuracy and precision of velocity-encoded phase imaging. Magn Reson Med. 1992;26:141-154. [PubMed] |
| 92. | Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 619] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 93. | Thomsen HS, Morcos SK, Dawson P. Is there a causal relation between the administration of gadolinium based contrast media and the development of nephrogenic systemic fibrosis (NSF)? Clin Radiol. 2006;61:905-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 94. | Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1350] [Cited by in RCA: 1331] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 95. | Swaminathan S, Horn TD, Pellowski D, Abul-Ezz S, Bornhorst JA, Viswamitra S, Shah SV. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. N Engl J Med. 2007;357:720-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 96. | Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 647] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 97. | Atkinson DJ, Edelman RR. Cineangiography of the heart in a single breath hold with a segmented turboFLASH sequence. Radiology. 1991;178:357-360. [PubMed] |
| 98. | Thomsen C, Cortsen M, Söndergaard L, Henriksen O, Ståhlberg F. A segmented K-space velocity mapping protocol for quantification of renal artery blood flow during breath-holding. J Magn Reson Imaging. 1995;5:393-401. [PubMed] |
| 99. | Bernstein MA, Shimakawa A, Pelc NJ. Minimizing TE in moment-nulled or flow-encoded two- and three-dimensional gradient-echo imaging. J Magn Reson Imaging. 1992;2:583-588. [PubMed] |
| 100. | Pelc NJ, Bernstein MA, Shimakawa A, Glover GH. Encoding strategies for three-direction phase-contrast MR imaging of flow. J Magn Reson Imaging. 1991;1:405-413. [PubMed] |
| 101. | Johnson KM, Markl M. Improved SNR in phase contrast velocimetry with five-point balanced flow encoding. Magn Reson Med. 2010;63:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 102. | Gu T, Korosec FR, Block WF, Fain SB, Turk Q, Lum D, Zhou Y, Grist TM, Haughton V, Mistretta CA. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR Am J Neuroradiol. 2005;26:743-749. [PubMed] |
| 103. | Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med. 2008;60:1329-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 104. | Dyvorne HA, Jajamovich GH, Besa C, Cooper N, Taouli B. Simultaneous measurement of hepatic and splenic stiffness using MR elastography: preliminary experience. Abdom Imaging. 2015;40:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 105. | Tariq U, Hsiao A, Alley M, Zhang T, Lustig M, Vasanawala SS. Venous and arterial flow quantification are equally accurate and precise with parallel imaging compressed sensing 4D phase contrast MRI. J Magn Reson Imaging. 2013;37:1419-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 106. | Markl M, Bammer R, Alley MT, Elkins CJ, Draney MT, Barnett A, Moseley ME, Glover GH, Pelc NJ. Generalized reconstruction of phase contrast MRI: analysis and correction of the effect of gradient field distortions. Magn Reson Med. 2003;50:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Thunberg P, Karlsson M, Wigström L. Accuracy and reproducibility in phase contrast imaging using SENSE. Magn Reson Med. 2003;50:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 108. | Pedersen H, Kozerke S, Ringgaard S, Nehrke K, Kim WY. k-t PCA: temporally constrained k-t BLAST reconstruction using principal component analysis. Magn Reson Med. 2009;62:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 109. | Huang F, Akao J, Vijayakumar S, Duensing GR, Limkeman M. k-t GRAPPA: a k-space implementation for dynamic MRI with high reduction factor. Magn Reson Med. 2005;54:1172-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 110. | Jung B, Honal M, Ullmann P, Hennig J, Markl M. Highly k-t-space-accelerated phase-contrast MRI. Magn Reson Med. 2008;60:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 111. | Jung B, Stalder AF, Bauer S, Markl M. On the undersampling strategies to accelerate time-resolved 3D imaging using k-t-GRAPPA. Magn Reson Med. 2011;66:966-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 112. | Tsao J, Boesiger P, Pruessmann KP. k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med. 2003;50:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 559] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 113. | Baltes C, Kozerke S, Hansen MS, Pruessmann KP, Tsao J, Boesiger P. Accelerating cine phase-contrast flow measurements using k-t BLAST and k-t SENSE. Magn Reson Med. 2005;54:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 114. | Stadlbauer A, van der Riet W, Crelier G, Salomonowitz E. Accelerated time-resolved three-dimensional MR velocity mapping of blood flow patterns in the aorta using SENSE and k-t BLAST. Eur J Radiol. 2010;75:e15-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 115. | Frydrychowicz A, Landgraf BR, Niespodzany E, Verma RW, Roldán-Alzate A, Johnson KM, Wieben O, Reeder SB. Four-dimensional velocity mapping of the hepatic and splanchnic vasculature with radial sampling at 3 tesla: a feasibility study in portal hypertension. J Magn Reson Imaging. 2011;34:577-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 116. | Roldán-Alzate A, Frydrychowicz A, Niespodzany E, Landgraf BR, Johnson KM, Wieben O, Reeder SB. In vivo validation of 4D flow MRI for assessing the hemodynamics of portal hypertension. J Magn Reson Imaging. 2013;37:1100-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 117. | Landgraf BR, Johnson KM, Roldán-Alzate A, Francois CJ, Wieben O, Reeder SB. Effect of temporal resolution on 4D flow MRI in the portal circulation. J Magn Reson Imaging. 2014;39:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 118. | Stankovic Z, Fink J, Collins JD, Semaan E, Russe MF, Carr JC, Markl M, Langer M, Jung B. K-t GRAPPA-accelerated 4D flow MRI of liver hemodynamics: influence of different acceleration factors on qualitative and quantitative assessment of blood flow. MAGMA. 2015;28:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Dyvorne H, Knight-Greenfield A, Jajamovich G, Besa C, Cui Y, Stalder A, Markl M, Taouli B. Abdominal 4D flow MR imaging in a breath hold: combination of spiral sampling and dynamic compressed sensing for highly accelerated acquisition. Radiology. 2015;275:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 120. | Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology. 1989;173:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 562] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 121. | Markl M, Harloff A, Bley TA, Zaitsev M, Jung B, Weigang E, Langer M, Hennig J, Frydrychowicz A. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging. 2007;25:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 122. | Uribe S, Beerbaum P, Sørensen TS, Rasmusson A, Razavi R, Schaeffter T. Four-dimensional (4D) flow of the whole heart and great vessels using real-time respiratory self-gating. Magn Reson Med. 2009;62:984-992. [PubMed] |
| 123. | van Ooij P, Semaan E, Schnell S, Giri S, Stankovic Z, Carr J, Barker AJ, Markl M. Improved respiratory navigator gating for thoracic 4D flow MRI. Magn Reson Imaging. 2015;33:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 124. | Bock J, Kreher BW, Hennig J, Markl M. Optimized pre-processing of time-resolved 2D and 3D Phase Contrast MRI data. Berlin, Germany: Proceedings of the 15th Annual Meeting of ISMRM 2007; Abstract 3138. |
| 125. | Nilsson A, Bloch KM, Carlsson M, Heiberg E, Ståhlberg F. Variable velocity encoding in a three-dimensional, three-directional phase contrast sequence: Evaluation in phantom and volunteers. J Magn Reson Imaging. 2012;36:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 126. | Walker PG, Cranney GB, Scheidegger MB, Waseleski G, Pohost GM, Yoganathan AP. Semiautomated method for noise reduction and background phase error correction in MR phase velocity data. J Magn Reson Imaging. 1993;3:521-530. [PubMed] |
| 127. | Bernstein MA, Zhou XJ, Polzin JA, King KF, Ganin A, Pelc NJ, Glover GH. Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med. 1998;39:300-308. [PubMed] |
| 128. | Wigström L, Ebbers T, Fyrenius A, Karlsson M, Engvall J, Wranne B, Bolger AF. Particle trace visualization of intracardiac flow using time-resolved 3D phase contrast MRI. Magn Reson Med. 1999;41:793-799. [PubMed] |
| 129. | Kozerke S, Hasenkam JM, Pedersen EM, Boesiger P. Visualization of flow patterns distal to aortic valve prostheses in humans using a fast approach for cine 3D velocity mapping. J Magn Reson Imaging. 2001;13:690-698. [PubMed] |
| 130. | Napel S, Lee DH, Frayne R, Rutt BK. Visualizing three-dimensional flow with simulated streamlines and three-dimensional phase-contrast MR imaging. J Magn Reson Imaging. 1992;2:143-153. [PubMed] |
| 131. | Bogren HG, Mohiaddin RH, Kilner PJ, Jimenez-Borreguero LJ, Yang GZ, Firmin DN. Blood flow patterns in the thoracic aorta studied with three-directional MR velocity mapping: the effects of age and coronary artery disease. J Magn Reson Imaging. 1997;7:784-793. [PubMed] |
| 132. | Buonocore MH. Visualizing blood flow patterns using streamlines, arrows, and particle paths. Magn Reson Med. 1998;40:210-226. [PubMed] |
| 133. | Markl M, Draney MT, Hope MD, Levin JM, Chan FP, Alley MT, Pelc NJ, Herfkens RJ. Time-resolved 3-dimensional velocity mapping in the thoracic aorta: visualization of 3-directional blood flow patterns in healthy volunteers and patients. J Comput Assist Tomogr. 2004;28:459-468. [PubMed] |
| 134. | Valverde I, Simpson J, Schaeffter T, Beerbaum P. 4D phase-contrast flow cardiovascular magnetic resonance: comprehensive quantification and visualization of flow dynamics in atrial septal defect and partial anomalous pulmonary venous return. Pediatr Cardiol. 2010;31:1244-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 135. | Stalder AF, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M. Quantitative 2D and 3D phase contrast MRI: optimized analysis of blood flow and vessel wall parameters. Magn Reson Med. 2008;60:1218-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 136. | Wentland AL, Grist TM, Wieben O. Repeatability and internal consistency of abdominal 2D and 4D phase contrast MR flow measurements. Acad Radiol. 2013;20:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 137. | Meckel S, Leitner L, Bonati LH, Santini F, Schubert T, Stalder AF, Lyrer P, Markl M, Wetzel SG. Intracranial artery velocity measurement using 4D PC MRI at 3 T: comparison with transcranial ultrasound techniques and 2D PC MRI. Neuroradiology. 2013;55:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 138. | Markl M, Wallis W, Harloff A. Reproducibility of flow and wall shear stress analysis using flow-sensitive four-dimensional MRI. J Magn Reson Imaging. 2011;33:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 139. | Nordmeyer S, Riesenkampff E, Crelier G, Khasheei A, Schnackenburg B, Berger F, Kuehne T. Flow-sensitive four-dimensional cine magnetic resonance imaging for offline blood flow quantification in multiple vessels: a validation study. J Magn Reson Imaging. 2010;32:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 140. | Lum DP, Johnson KM, Paul RK, Turk AS, Consigny DW, Grinde JR, Mistretta CA, Grist TM. Transstenotic pressure gradients: measurement in swine--retrospectively ECG-gated 3D phase-contrast MR angiography versus endovascular pressure-sensing guidewires. Radiology. 2007;245:751-760. [PubMed] |
| 141. | Bock J, Frydrychowicz A, Lorenz R, Hirtler D, Barker AJ, Johnson KM, Arnold R, Burkhardt H, Hennig J, Markl M. In vivo noninvasive 4D pressure difference mapping in the human aorta: phantom comparison and application in healthy volunteers and patients. Magn Reson Med. 2011;66:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 142. | Dyverfeldt P, Gårdhagen R, Sigfridsson A, Karlsson M, Ebbers T. On MRI turbulence quantification. Magn Reson Imaging. 2009;27:913-922. [PubMed] |
| 143. | Bolster BD, Atalar E, Hardy CJ, McVeigh ER. Accuracy of arterial pulse-wave velocity measurement using MR. J Magn Reson Imaging. 1998;8:878-888. [PubMed] |
| 144. | Nanashima A, Shibasaki S, Sakamoto I, Sueyoshi E, Sumida Y, Abo T, Nagasaki T, Sawai T, Yasutake T, Nagayasu T. Clinical evaluation of magnetic resonance imaging flowmetry of portal and hepatic veins in patients following hepatectomy. Liver Int. 2006;26:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 145. | Lycklama à Nijeholt GJ, Burggraaf K, Wasser MN, Schultze Kool LJ, Schoemaker RC, Cohen AF, de Roos A. Variability of splanchnic blood flow measurements using MR velocity mapping under fasting and post-prandial conditions--comparison with echo-Doppler. J Hepatol. 1997;26:298-304. [PubMed] |
| 146. | Zekanovic D, Ljubicic N, Boban M, Nikolic M, Delic-Brkljacic D, Gacina P, Klarin I, Turcinov J. Doppler ultrasound of hepatic and system hemodynamics in patients with alcoholic liver cirrhosis. Dig Dis Sci. 2010;55:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 147. | Stankovic Z, Frydrychowicz A, Csatari Z, Panther E, Deibert P, Euringer W, Kreisel W, Russe M, Bauer S, Langer M. MR-based visualization and quantification of three-dimensional flow characteristics in the portal venous system. J Magn Reson Imaging. 2010;32:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Stankovic Z, Csatari Z, Deibert P, Euringer W, Blanke P, Kreisel W, Abdullah Zadeh Z, Kallfass F, Langer M, Markl M. Normal and altered three-dimensional portal venous hemodynamics in patients with liver cirrhosis. Radiology. 2012;262:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 149. | Stankovic Z, Jung B, Collins J, Russe MF, Carr J, Euringer W, Stehlin L, Csatari Z, Strohm PC, Langer M. Reproducibility study of four-dimensional flow MRI of arterial and portal venous liver hemodynamics: influence of spatio-temporal resolution. Magn Reson Med. 2014;72:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 150. | Stankovic Z, Csatari Z, Deibert P, Euringer W, Jung B, Kreisel W, Geiger J, Russe MF, Langer M, Markl M. A feasibility study to evaluate splanchnic arterial and venous hemodynamics by flow-sensitive 4D MRI compared with Doppler ultrasound in patients with cirrhosis and controls. Eur J Gastroenterol Hepatol. 2013;25:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 151. | Stankovic Z, Rössle M, Euringer W, Schultheiss M, Salem R, Barker A, Carr J, Langer M, Markl M, Collins JD. Effect of TIPS placement on portal and splanchnic arterial blood flow in 4-dimensional flow MRI. Eur Radiol. 2015;25:2634-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 152. | Stankovic Z, Blanke P, Markl M. Usefulness of 4D MRI flow imaging to control TIPS function. Am J Gastroenterol. 2012;107:327-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Edelman RR, Zhao B, Liu C, Wentz KU, Mattle HP, Finn JP, McArdle C. MR angiography and dynamic flow evaluation of the portal venous system. AJR Am J Roentgenol. 1989;153:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 154. | Tamada T, Moriyasu F, Ono S, Shimizu K, Kajimura K, Soh Y, Kawasaki T, Kimura T, Yamashita Y, Someda H. Portal blood flow: measurement with MR imaging. Radiology. 1989;173:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 155. | Sugano S, Yamamoto K, Sasao K, Watanabe M. Portal venous blood flow while breath-holding after inspiration or expiration and during normal respiration in controls and cirrhotics. J Gastroenterol. 1999;34:613-618. [PubMed] |
| 156. | Zoli M, Marchesini G, Cordiani MR, Pisi P, Brunori A, Trono A, Pisi E. Echo-Doppler measurement of splanchnic blood flow in control and cirrhotic subjects. J Clin Ultrasound. 1986;14:429-435. [PubMed] |
| 157. | O’Donohue J, Ng C, Catnach S, Farrant P, Williams R. Diagnostic value of Doppler assessment of the hepatic and portal vessels and ultrasound of the spleen in liver disease. Eur J Gastroenterol Hepatol. 2004;16:147-155. [PubMed] |
| 158. | Harloff A, Albrecht F, Spreer J, Stalder AF, Bock J, Frydrychowicz A, Schöllhorn J, Hetzel A, Schumacher M, Hennig J. 3D blood flow characteristics in the carotid artery bifurcation assessed by flow-sensitive 4D MRI at 3T. Magn Reson Med. 2009;61:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 159. | Tziafalia C, Vlychou M, Tepetes K, Kelekis N, Fezoulidis IV. Echo-Doppler measurements of portal vein and hepatic artery in asymptomatic patients with hepatitis B virus and healthy adults. J Gastrointestin Liver Dis. 2006;15:343-346. [PubMed] |
| 160. | Shi BM, Wang XY, Mu QL, Wu TH, Xu J. Value of portal hemodynamics and hypersplenism in cirrhosis staging. World J Gastroenterol. 2005;11:708-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 161. | Ozdogan O, Atalay H, Cimsit C, Tahan V, Tokay S, Giral A, Imeryuz N, Baltacioglu F, Tuney D, Erzen C. Role of echo Doppler ultrasonography in the evaluation of postprandial hyperemia in cirrhotic patients. World J Gastroenterol. 2008;14:260-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 162. | Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One. 2014;9:e97355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 163. | Roldán-Alzate A, Frydrychowicz A, Said A, Johnson KM, Francois CJ, Wieben O, Reeder SB. Impaired regulation of portal venous flow in response to a meal challenge as quantified by 4D flow MRI. J Magn Reson Imaging. 2015;42:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 164. | Ohnishi K, Saito M, Nakayama T, Iida S, Nomura F, Koen H, Okuda K. Portal venous hemodynamics in chronic liver disease: effects of posture change and exercise. Radiology. 1985;155:757-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 81] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 165. | Sabbà C, Merkel C, Zoli M, Ferraioli G, Gaiani S, Sacerdoti D, Bolondi L. Interobserver and interequipment variability of echo-Doppler examination of the portal vein: effect of a cooperative training program. Hepatology. 1995;21:428-433. [PubMed] |
| 166. | van der Geest RJ, Niezen RA, van der Wall EE, de Roos A, Reiber JH. Automated measurement of volume flow in the ascending aorta using MR velocity maps: evaluation of inter- and intraobserver variability in healthy volunteers. J Comput Assist Tomogr. 1998;22:904-911. [PubMed] |
| 167. | Taourel P, Blanc P, Dauzat M, Chabre M, Pradel J, Gallix B, Larrey D, Bruel JM. Doppler study of mesenteric, hepatic, and portal circulation in alcoholic cirrhosis: relationship between quantitative Doppler measurements and the severity of portal hypertension and hepatic failure. Hepatology. 1998;28:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 168. | Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 169. | Foshager MC, Ferral H, Nazarian GK, Castañeda-Zúñiga WR, Letourneau JG. Duplex sonography after transjugular intrahepatic portosystemic shunts (TIPS): normal hemodynamic findings and efficacy in predicting shunt patency and stenosis. AJR Am J Roentgenol. 1995;165:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 170. | Meckel S, Stalder AF, Santini F, Radü EW, Rüfenacht DA, Markl M, Wetzel SG. In vivo visualization and analysis of 3-D hemodynamics in cerebral aneurysms with flow-sensitized 4-D MR imaging at 3 T. Neuroradiology. 2008;50:473-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |