Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2433
Peer-review started: August 20, 2014
First decision: September 27, 2014
Revised: November 6, 2014
Accepted: December 22, 2014
Article in press: December 22, 2014
Published online: February 28, 2015
Processing time: 193 Days and 5.4 Hours
AIM: To investigate whether MYC and BCL-2 coexpression has prognostic significance in primary gastrointestinal diffuse large B-cell lymphoma (PGI-DLBCL) patients, and explore its associations with patients’ clinical parameters.
METHODS: Fresh and paraffin-embedded tumor tissue samples from 60 PGI-DLBCL patients who had undergone surgery at the Tianjin Medical University Cancer Institute and Hospital from January 2005 to May 2010 were obtained, and 30 lymphoid tissue samples from reactive lymph nodes of age- and sex-matched patients represented control samples. Staging and diagnostic procedures were conducted according to the Lugano staging system. All patients had been treated with three therapeutic modalities: surgery, chemotherapy, or radiotherapy. Expression of MYC and BCL-2 were detected at both protein and mRNA levels by immunohistochemistry and real-time RT-PCR.
RESULTS: Positive expression levels of MYC and BCL-2 proteins were detected in 35% and 45% of patients, respectively. MYC+/BCL-2+ protein was present in 30% of patients. MYC and BCL-2 protein levels were correlated with high MYC and BCL-2 mRNA expression, respectively (both P < 0.05). We found that advanced-stage disease (at IIE-IV) was associated with MYC and BCL-2 coexpression levels (P < 0.05). In addition, MYC+/BCL-2+ patients had more difficulty in achieving complete remission than others (P < 0.05). Presence of MYC protein expression only affected overall survival and progression-free survival (PFS) when BCL-2 protein was coexpressed. The adverse prognostic impact of MYC+/BCL-2+ protein on PFS remained significant (P < 0.05) even after adjusting for age, Lugano stage, international prognostic index, and BCL-2 protein expression in a multivariable model.
CONCLUSION: MYC+/BCL-2+ patients have worse chemotherapy response and poorer prognosis than patients who only express one of the two proteins, suggesting that assessment of MYC and BCL-2 expression by immunohistochemistry has clinical significance in predicting clinical outcomes of PGI-DLBCL patients.
Core tip: We investigated MYC and BCL-2 coexpression in primary gastrointestinal diffuse large B-cell lymphoma (PGI-DLBCL) and explored its associations with patients’ clinical parameters. In contrast to previously published results about MYC/BCL-2 coexpression in DLBCL, this study focused on PGI-DLBCL. Although PGI-DLBCL is rare, we had a large collection of 60 PGI-DLBCL cases to test the protein and mRNA levels of MYC and BCL-2. We found that MYC+/BCL-2+ patients have worse chemotherapy response and poorer prognosis than patients who only express one of the two proteins, suggesting that assessment of MYC and BCL-2 expression has clinical significance in predicting clinical outcomes of PGI-DLBCL patients.
-
Citation: Xia B, Zhang L, Guo SQ, Li XW, Qu FL, Zhao HF, Zhang LY, Sun BC, You J, Zhang YZ. Coexpression of
MYC andBCL-2 predicts prognosis in primary gastrointestinal diffuse large B-cell lymphoma. World J Gastroenterol 2015; 21(8): 2433-2442 - URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2433
Primary gastrointestinal (PGI) lymphoma is the most common type of extranodal lymphoma, comprising about 30%-40% of all extranodal lymphomas, but only accounts for 1%-8% of all gastrointestinal (GI) malignancies[1]. The most frequent pathological type is mucosa-associated lymphoid tissue lymphoma and diffuse large B-cell lymphoma (DLBCL)[2]. The most common primary site is the stomach (60%-70%), followed by the small bowel (20%-30%), colorectum (5%-10%), and esophagus (< 1%)[2,3]. In China, DLBCL is the most common type of lymphoma as well as a highly heterogeneous disease, comprising approximately 30%-40% of adult non-Hodgkin lymphoma patients. PGI-DLBCL is a relatively rare disease, comprising only 1%-4% of those with gastrointestinal malignancies. Given the location of the GI tract and GI lymphoma association with infections such as H. pylori infection, celiac disease, inflammatory bowel disease and autoimmune diseases, we consider PGI-DLBCL as a distinct disease since their evaluation, diagnosis, management and prognosis are different from DLBCL of lymph node origin. Thus, our study here is focused on primary gastrointestinal DLBCL. MYC, an oncogenic transcription factor, has been recognized as one of the most frequently affected genes in human malignancies, with about 70% of all human malignancies showing overexpression of MYC[4]. MYC is also a critical player during lymphoma development, and its potential role in oncogenesis of hematopoietic cells was first demonstrated during the mid-1980s. Burkitt lymphoma is the first known example of MYC-induced lymphomagenesis[4]. In the past decade, our understanding of MYC-induced lymphomagenesis has been greatly improved.
BCL-2, an anti-apoptotic gene, has been implicated in conferring chemotherapy resistance in non-Hodgkin’s lymphoma and has been extensively studied as a prognostic biomarker in DLBCL[5]. It was found to have an adverse effect on the prognosis of a subgroup of patients with germinal center B-cell-like (GCB) DLBCL who had been treated with CHOP-like therapy and rituximab[6].
MYC translocations, with or without BCL-2 translocations, have been associated with inferior prognosis in DLBCL. We, in this study, focus on examining MYC and BCL2 protein expression by using recently developed and commercially available monoclonal antibodies. The MYC antibody targets the N-terminus of the MYC protein and has been shown to predict MYC rearrangements and has been validated for use in FFPE tissues. We believe that molecular confirmation of the immunohistochemistry for MYC and BCL-2 is more practical, relevant and closely representative. This is based on the concept that, in addition to translocations, MYC can also be deregulated by amplifications, mutations, or by microRNA-dependent mechanisms. Although MYC translocations can be detected by karyotype and fluorescence in situ hybridization (FISH), FISH fails to detect MYC deregulation caused by mechanisms other than translocation. This suggests that mechanisms other than gene rearrangements are responsible for elevated protein expression in a considerable proportion of DLBCL cases.
DLBCL patients with both MYC and BCL-2 protein coexpression have shown inferior overall survival (OS) and progression-free survival (PFS)[7]. This concurrent expression of MYC and BCL-2 proteins in patients with PGI-DLBCL has thus far not been clearly understood. In the present study, we used real-time quantitative PCR to measure expression levels of MYC and BCL-2 mRNA and used our results to investigate whether the coexpression of MYC and BCL-2 proteins has prognostic significance in patients with PGI-DLBCL. Furthermore, we explored associations among these coexpression levels and patients’ clinical parameters.
We obtained fresh and paraffin-embedded tumor tissue samples from 60 PGI-DLBCL patients who had undergone surgery at the Tianjin Medical University Cancer Institute and Hospital from January 2005 to May 2010. In addition, our study included 30 lymphoid tissue samples from reactive lymph nodes of age- and sex-matched patients, which represented control samples. This study was approved by the hospital-based ethics committee. The diagnoses of all patients were reevaluated by at least two experienced hematopathologists. Patients provided informed consent to allow complete clinical information and follow-up data to be obtained.
All patients were staged according to the Lugano staging system[8], which is modified from the Ann Arbor criteria for primary gastrointestinal non-Hodgkin’s lymphoma. The staging system included the patients’ history, physical examination, chest X-ray, gastrointestinal endoscopy, abdominal ultrasound, bone marrow aspiration, biopsy, and computed tomography (CT) scans of the neck, chest, abdomen, and pelvis. Routine laboratory tests included the measurement of hemoglobin and serum lactate dehydrogenase (LDH) levels. Low hemoglobin was defined as < 120 g/L in men and < 110 g/L in women. High LDH was defined as > 245 U/L.
All patients had been treated with three therapeutic modalities: surgery, chemotherapy, or radiotherapy. All patients had been suspected as having other gastrointestinal malignancies but were diagnosed with PGI-DLBCL by postoperative pathology. None of the patients had preoperative chemotherapy, radiotherapy, or other treatment history, and all patients received at least four cycles of a standard-dose R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)-like regimen. The status of chemotherapy response was evaluated by Cheson’s standard criteria.
The fresh tissue specimens had been immediately frozen in liquid nitrogen until use. Approximately 25 mg of fresh tissue was used for RNA extraction, pulverized under liquid nitrogen with a pestle and mortar. Total RNA was isolated from tissue samples using RNeasy (Qiagen, Valencia, CA) following the manufacturer’s instructions (Qiagen), and the concentration was determined by 260 nm/280 nm absorbance using a Nanodrop® ND-1000 spectrophotometer (Thermo Scientific). The reverse transcription reaction for the mRNA of MYC and BCL-2 was done using Takara Kit (TaKaRa Bio Inc.) according to the manufacturer’s protocol. cDNA were stored at -20°C until use (Table 1).
| Genes | Forward sequence (5’→3’) | Reverse sequence (5’→3’) |
| MYC | CCTCCACTCGGAAGGACTATC | TGTTCGCCTCTTGACATTCTC |
| BCL-2 | GTGGATGACTGAGTACCTGAACC | AGACAGCCAGGAGAAATCAAAC |
| β-actin | CCTGGCACCCAGCACAAT | GGGCCGGACTCGTCATAC |
Amplification for the cDNA of MYC and BCL-2 was performed in a total volume of 20 μL containing 10 μL of kit-supplied QuantiTect™ SYBR® Green RT-PCR Master mix (Applied-Biosystems), 0.4 μL of each primer (Table 1), 2 μL of cDNA, and 7.2 μL ddH2O. The PCR cycling parameters were set as follows: 95 °C for 30 s followed by 40 cycles of PCR reaction at 95 °C for 5 s and 60 °C for 34 s. The reactions were performed in the Bio-Rad CM9600 real-time PCR detection system (Bio-Rad, Hercules, CA). β-Actin was chosen as an internal standard. All PCR reactions were repeated 3 times. The relative amount of the mRNA was calculated by subtracting the average Ct value of the internal standard from the average Ct value of the target mRNA, yielding a ΔCt value. The ΔΔCt value was then calculated by subtracting the average ΔCt value of the controls from the respective ΔCt values of each patient sample. Relative expression levels were expressed as 2-ΔΔCt.
All tissue biopsies were fixed in 10% buffered formalin, embedded in paraffin, and cut into 4-μm sections. After deparaffinization, heat-induced antigen retrieval techniques were used. Endogenous peroxidase activity was then blocked with 0.5% H2O2. After being washed in phosphate-buffered saline, the sections were stained for the following antibodies: MYC (ab51154, Abcam), BCL-2 (ZM0010, ZSGB-BIO), CD10 (ZM0283, ZSGB-BIO), BCL-6 (ZM-0011,ZSGB-BIO), and MUM-1 (ZA-0583, ZSGB-BIO), CD5 (ZM0283, ZSGB-BIO), CD20 (ZM0039,ZSGB-BIO). The reaction was carried out at 4 °C overnight. After the sections were washed in phosphate-buffered saline again, the secondary antibodies (PV6000, ZSGB-BIO) were dropped. The cell nucleus was restained with hematoxylin after diaminobenzidine showed color, the results were judged as positive if 30% or more were stained.
SPSS 17.0 was used for statistical analysis. The results of MYC and BCL-2 mRNA expression are presented as mean ± SD. The relative expression levels between patients with PGI-DLBCL and healthy controls were analyzed by the nonparametric Mann-Whitney U-test. Fisher’s exact test and χ2 test were used to determine differences in proportions regarding different clinical characteristics of the same groups. Unpaired t-test was used to find statistical significances between the PGI-DLBCL group and the normal control group. Univariate analysis of survival was done with the Kaplan-Meier method, which was carried out on PFS times and OS times. PFS was measured from the time of diagnosis to the date of treatment failure, clinical relapse, metastasis, disease progression, or death due to any cause. OS was calculated from the date of diagnosis until date of last follow-up or death. The significant factors (P < 0.05) of univariate analysis were included into the Cox regression model for multivariate analysis, in order to decide which factor could be the independent prognostic factor for survival. P < 0.05 was judged as statistically significant.
Patients had a median age of 57 years (range: 23-79 years), with similar distribution of both sexes (32 males and 28 females; ratio = 1.1). Using the Lugano staging system, 36 patients (60%) presented with stage I-II2 and 24 patients (40%) presented with stage IIE-IV (advanced stage). Patients were divided into subgroups according to clinical parameters, such as age, primary site, LDH level, Lugano staging system, international prognostic index (IPI) score, anemia, and DLBCL pathological type. Table 2 shows the clinical characteristics of the 60 patients with PGI-DLBCL.
| Clinical characteristics | n | DP | Non-DP | χ2 | P |
| Patients | 60 | 18 | 42 | ||
| Age (yr) | 2.534 | 0.111 | |||
| ≤ 60 | 34 | 13 | 21 | ||
| > 60 | 26 | 5 | 21 | ||
| Gender | 0.051 | 0.821 | |||
| Male | 32 | 10 | 22 | ||
| Female | 28 | 8 | 20 | ||
| Primary site | 0.013 | 0.908 | |||
| Stomach | 36 | 11 | 25 | ||
| Intestinal | 24 | 7 | 17 | ||
| Lugano staging system | 22.781 | < 0.0001 | |||
| I-II2 | 36 | 2 | 34 | ||
| IIE-IV | 24 | 16 | 8 | ||
| LDH | 0.207 | 0.649 | |||
| Normal | 34 | 11 | 23 | ||
| Elevated | 26 | 7 | 19 | ||
| B symptoms | 0.277 | 0.599 | |||
| Positive | 7 | 1 | 6 | ||
| Negative | 53 | 17 | 36 | ||
| IPI | 2.286 | 0.131 | |||
| 0-2 | 50 | 13 | 37 | ||
| 3-5 | 10 | 5 | 5 | ||
| Pathological type | 2.188 | 0.139 | |||
| Non-GCB | 48 | 17 | 31 | ||
| GCB | 12 | 1 | 11 | ||
| CD10 status | 0.013 | 0.908 | |||
| Positive | 36 | 117 | 25 | ||
| Negative/NA | 24 | 17 | |||
| CD5 status | 2.265 | 0.127 | |||
| Positive | 49 | 13 | 36 | ||
| Negative/NA | 11 | 5 | 6 | ||
| CD20 status | 0.207 | 0.649 | |||
| Positive | 34 | 11 | 23 | ||
| Negative/NA | 26 | 7 | 19 | ||
| Anemia | 0.003 | 0.955 | |||
| Present | 33 | 10 | 23 | ||
| Absent | 27 | 8 | 19 | ||
| Treatment | 2.017 | 0.156 | |||
| ST + CT | 51 | 13 | 38 | ||
| ST + CT + RT | 9 | 5 | 4 | ||
| Therapeutic evaluation | |||||
| CR | 48 | 9 | 39 | 11.910 | 0.001 |
| PR/SD/PD | 12 | 9 | 3 | ||
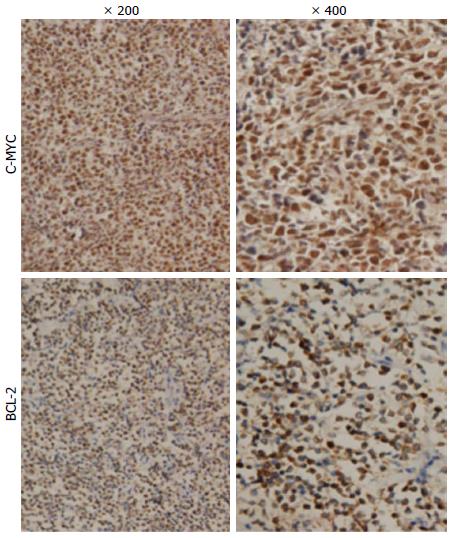
In our patient group, 21 (35%) samples were positive for MYC and 27 (45%) samples were positive for BCL-2 (Figure 1). Concurrent expression of MYC and BCL-2 proteins was present in 18 (30%) patients. When we grouped patients according to the clinical characteristics, concurrent expression of MYC and BCL-2 proteins was significantly closely related to Lugano staging system, with a higher proportion of patients with stage IIE-IV showing both MYC and BCL-2 expression (P < 0.05; Table 2). No significant differences were observed in other clinical characteristic subgroups. Distribution by gender, age, origin, LDH, B symptoms, IPI, and anemia was similar among the MYC and BCL-2 protein coexpression group, as well as for the other expression groups (P > 0.05; Table 2). When we grouped patients into germinal center B-cell-like (GCB) DLBCL and non-GCB DLBCL groups based on the immunophenotypic profile, we found that the proportion of GCB and non-GCB did not differ significantly (P > 0.05).
All patients had been treated with surgery and chemotherapy with or without radiotherapy. After the initial chemotherapy, 48 (80%) patients achieved complete remission (CR), with 12 (20%) patients not achieving CR or partial remission (PR), stable disease (SD), and progressive disease (PD). The distribution of patients treated with or without radiotherapy was similar in the MYC and BCL-2 protein coexpression group and in all other groups (P > 0.05, Table 2). In addition, we found that MYC and BCL-2 coexpression was associated with inferior chemotherapy response in patients with PGI-DLBCL. Patients who had coexpression of MYC and BCL-2 were less likely to achieve CR than other patient groups, with the difference being statistically significant (P < 0.05; Table 2).
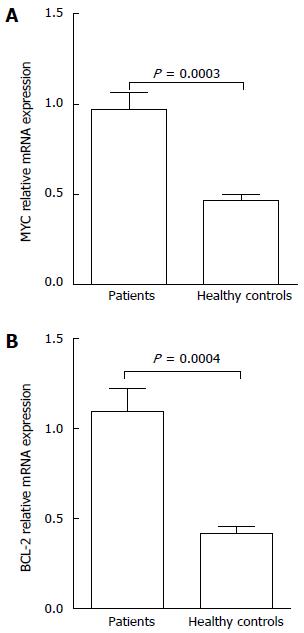
The results of real-time PCR in patient groups showed that the expression level of MYC mRNA was 0.97 ± 0.72 and BCL-2 mRNA was 1.10 ± 0.98, with both significantly higher than those shown in normal controls (P < 0.05) (Figure 2). We also observed a significant correlation between BCL-2 mRNA and BCL-2 protein expression in PGI-DLBCL as a group (Spearman correlation of 0.64, P < 0.05; Table 3). MYC protein was also correlated with presence of high MYC mRNA (Spearman correlation of 0.43, P < 0.05; Table 4).
| BCL-2 | BCL-2 mRNA | r | P | |
| High | Low | |||
| Positive | 18 | 9 | 0.640 | < 0.0001 |
| Negative | 2 | 31 | ||
| MYC | MYC mRNA | r | P | |
| High | Low | |||
| Positive | 17 | 4 | 0.430 | 0.0001 |
| Negative | 14 | 25 | ||
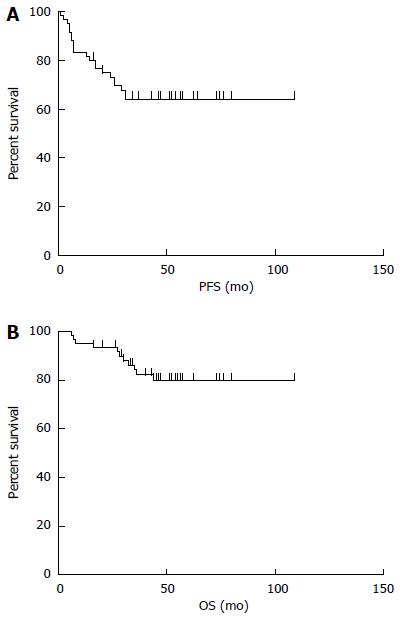
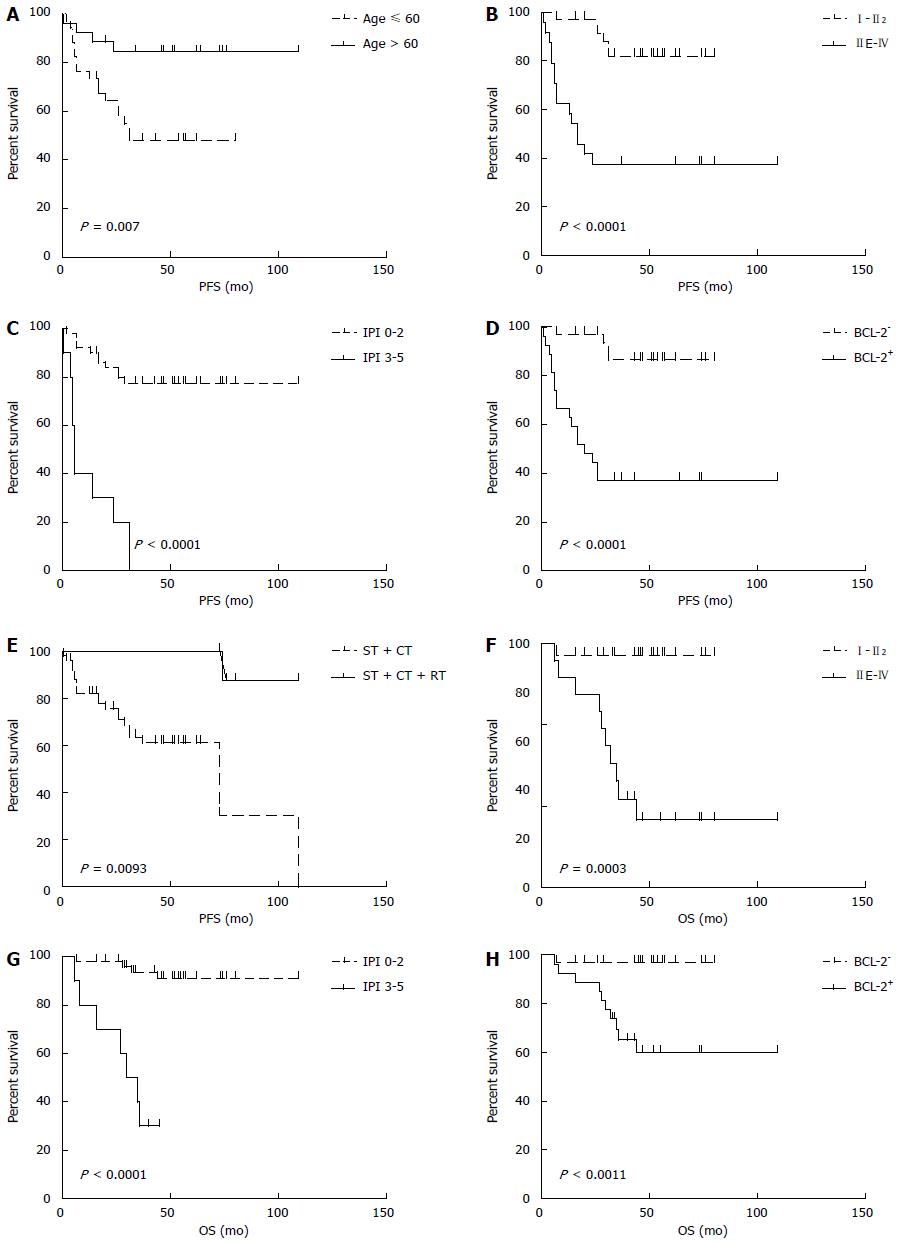
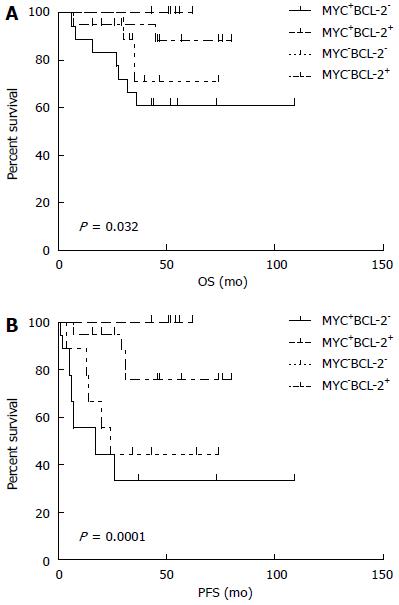
The median follow-up time was 48 mo (range: 6-109 mo). The median PFS was 44.5 mo (95%CI: 63.4-86.8, range: 1-109 mo), and the median OS was 49 mo (95%CI: 83.4-101.2, range: 6-109 mo). The 5-year PFS and OS estimates for all patients were 65% and 82%, respectively (Figure 3). In univariate analysis, advanced-stage disease (IIE-IV), IPI (3-5), BCL-2 protein expression, and coexpression of MYC and BCL-2 proteins were all associated with inferior OS, whereas age (> 60 years), advanced-stage disease (IIE-IV), IPI (3-5), BCL-2 protein expression, and coexpression of MYC and BCL-2 proteins adversely affected PFS. However, radiotherapy could improve PFS significantly (Figure 4, Table 5). Gender, primary site, LDH, B symptoms, pathological type, anemia, and MYC protein expression did not significantly affect prognosis (P > 0.05; Table 5). Presence of MYC protein expression only affected OS and PFS when BCL-2 protein was coexpressed; the negative prognostic impact of MYC and BCL-2 was amplified when both variables were present (Figure 5). Prognosis for coexpression of MYC and BCL-2 proteins was worse than for the MYC+/BCL-2- group and MYC-/BCL-2- group. In addition, in the Cox multivariant model, the adverse prognostic impact of concurrent expression of MYC/BCL-2 protein and IPI on PFS existed even after adjusting for age, Lugano stage, IPI, and BCL-2 protein expression, whereas IPI was the only impact factor for OS (Table 6).
| Prognostic factors | n | OS | PFS | ||
| χ2 | P | χ2 | P | ||
| Age (yr) | 0.376 | 0.540 | 7.377 | 0.007 | |
| ≤ 60 | 34 | ||||
| > 60 | 26 | ||||
| Gender | 0.059 | 0.808 | 0.189 | 0.664 | |
| Male | 32 | ||||
| Female | 28 | ||||
| Primary site | 0.132 | 0.717 | 1.211 | 0.271 | |
| Stomach | 36 | ||||
| Intestinal | 24 | ||||
| Lugano staging system | 13.355 | < 0.0001 | 17.581 | < 0.0001 | |
| I-II2 | 36 | ||||
| IIE-IV | 24 | ||||
| LDH | 0.029 | 0.864 | 0.118 | 0.731 | |
| Normal | 34 | ||||
| Elevated | 26 | ||||
| B symptoms | 1.667 | 0.197 | 0.787 | 0.375 | |
| Positive | 7 | ||||
| Negative | 53 | ||||
| IPI | 27.098 | < 0.0001 | 39.737 | < 0.0001 | |
| 0-2 | 50 | ||||
| 3-5 | 10 | ||||
| Pathological type | 0.033 | 0.856 | 0.020 | 0.888 | |
| Non-GCB | 48 | ||||
| GCB | 12 | ||||
| Anemia | 0.526 | 0.468 | 0.101 | 0.751 | |
| Present | 33 | ||||
| Absent | 27 | ||||
| Treatment | 0.249 | 0.618 | 6.758 | 0.0093 | |
| ST + CT | 51 | ||||
| ST + CT + RT | 9 | ||||
| MYC protein expression | 0.347 | 0.556 | 0.078 | 0.780 | |
| MYC+ | 21 | ||||
| MYC- | 39 | ||||
| BCL-2 protein expression | 10.701 | 0.001 | 19.463 | < 0.0001 | |
| BCL-2+ | 27 | ||||
| BCL-2- | 33 | ||||
| MYC/BCL-2 coexpression | 10.956 | 0.001 | 15.198 | < 0.0001 | |
| MYC+/BCL-2+ | 18 | ||||
| All others | 42 | ||||
| Prognostic factors | OS | PFS | ||||
| HR | 95%CI | P | HR | 95%CI | P | |
| Age | 1.570 | 0.384-6.430 | 0.530 | 0.403 | 0.132-1.224 | 0.109 |
| Lugano staging system | 0.253 | 0.009-6.990 | 0.417 | 1.091 | 0.243-4.893 | 0.910 |
| IPI | 13.246 | 2.929-59.903 | 0.001 | 15.302 | 4.119-56.845 | < 0.0001 |
| Treatment | 1.374 | 0.247-7.625 | 0.717 | 1.542 | 0.522-4.555 | 0.434 |
| BCL-2 protein expression | 1.056 | 0.029-37.972 | 0.976 | 1.773 | 0.154-20.434 | 0.646 |
| MYC/BCL-2 coexpression | 4.435 | 0.728-26.994 | 0.106 | 11.371 | 1.264-102.295 | 0.030 |
PGI-DLBCL is an aggressive lymphoma that may arise de novo or transform from other lymphoma, mostly transforming from MALT lymphoma. It most commonly occurs in men with a median age of 50-60 years old[9]. The International Prognostic Index (IPI), which incorporates 5 clinical parameters (age 60 years or older, disease stage III/IV, high LDH level, 2 or more extranodal sites of disease, and an Eastern Cooperative Oncology Group performance status of 2 or more), is recognized as the gold standard for predicting prognosis in patients with DLBCL. However, the IPI evaluation system only integrates some clinical characteristics, and the molecular biology of cancer is not included. Research on gene expression profiling has shown the presence of DLBCL subtypes associated with different cells of origin and clinical outcomes[10,11]. This implies that potential prognostic biomarkers and different pathogenetic pathways may exist among the subtypes.
The proto-oncogene MYC is a member of common abnormalities in human malignancies and is a critical player in the development of lymphomas[12-15]. BCL-2 is an important anti-apoptotic gene that inhibits apoptosis by encoding a mitochondrial protein. In addition, in vitro studies suggest that BCL-2 has a role in drug resistance[16,17]. MYC aberrations in DLBCL are usually concurrent with other genetic lesions, such as rearrangements of BCL-2 and/or BCL-6. Patients with these tumors, named as double-hit or triple-hit lymphomas, have an extremely poor prognosis, generally with survival of only 6 mo[18-22]. Moreover, many recent studies have suggested that the coexpression of MYC and BCL-2 proteins contributes to the inferior survival of DLBCL patients[7,23,24]. DLBCL patients with concurrent expression of MYC and BCL-2 proteins have been reported to have a poor prognosis, no matter whether the MYC or BCL-2 gene is rearranged or not[7,25,26].
Johnson et al[7] reported a study using c-Myc antibody in combination with an antibody for BCL-2 in a training cohort of 167 and a validation set of another 140 patients with DLBCL. They found MYC translocations, high MYC mRNA, and MYC protein expression in 11%, 11%, and 33% of the samples, respectively. MYC protein expression was associated with an inferior progression-free and overall survival only when BCL-2 protein was coexpressed. MYC/BCL-2 protein coexpression was observed in 21% of the DLBCL cases, and the negative impact on prognosis remained significant after adjusting for the presence of high-risk features in a multivariable model that included elevated IPI score. Here, we observed the rate of concurrent expression (MYC+/BCL-2+) is higher in PGI-DLBCL than in all of DLBCL (30% vs 21%), suggesting that concurrent expression of MYC and BCL-2 is more frequently in PGI-DLBCL. Moreover, our study showed that MYC+/BCL-2+ patients had more difficulty in achieving complete remission than others (P < 0.05). Presence of MYC protein expression only affected OS and PFS when BCL-2 protein was coexpressed. The adverse prognostic impact of MYC+/BCL-2+ protein on PFS remained significant (P < 0.05) even after adjusting for age, Lugano stage, IPI, and BCL-2 protein expression in a multivariable model. MYC+/BCL-2+ patients have worse chemotherapy response and poorer prognosis than patients who only express one of the two proteins, suggesting that assessment of MYC and BCL-2 expression by immunohistochemistry has clinical significance in predicting prognosis clinical outcomes of PGI-DLBCL patients. Therefore, we speculated that MYC and BCL-2 expression, particularly MYC-/BCL-2 protein coexpression, may play a role in the aggressive pathogenesis of PGI-DLBCL.
Hu et al[24] reported that MYC/BCL-2 protein coexpression in DLBCL associated with poor prognosis occurred more frequently in the ABC subgroup. They further confirmed that, after excluding patients with MYC/BCL-2 coexpression, the clinical outcome of patients with ABC-DLBCL was similar to that of patients with GCB subgroup. Their results suggested MYC/BCL-2 coexpression appears to account for the inferior prognosis of patients with ABC-DLBCL, and MYC/BCL-2 coexpression may be a better predictor of prognosis than the cell-of-origin classification.
In summary, our results suggested that PGI-DLBCL with concurrent expression of MYC and BCL-2 proteins characterizes a subset of PGI-DLBCL patients with poor prognosis. Previous reports have suggested that chemotherapy regimens that include BCL-2-targeted drugs, such as BH3 mimetics, have shown efficacy in murine MYC+/BCL-2+ lymphomas[27-29]. BCL-2-targeted therapy may represent a promising new apoptosis-modulating strategy for MYC+/BCL-2+ patients with PGI-DLBCL.
We thank Hong Zheng, Hai-Xing Li, Ke-Xin Chen, Wen-Feng Cao, Qiong-Li Zhai for their excellent assistance.
Primary gastrointestinal (PGI) lymphoma is the most common type of extranodal lymphoma, comprising about 30%-40% of all extranodal lymphomas. The most frequent pathological type is mucosa-associated lymphoid tissue lymphoma and diffuse large B-cell lymphoma (DLBCL). In China, DLBCL is the most common type of lymphoma as well as a highly heterogeneous disease, comprising approximately 30%-40% of adult non-Hodgkin lymphoma patients. PGI-DLBCL is a relatively rare disease, comprising only 1%-4% of those with gastrointestinal (GI) malignancies. We consider PGI-DLBCL as a distinct disease, since their evaluation, diagnosis, management and prognosis are different from DLBCL of lymph node origin. Thus, this study here is focused on PGI-DLBCL. MYC, an oncogenic transcription factor, has been recognized as one of the most frequently affected genes in human malignancies, with about 70% of all human malignancies showing overexpression of MYC. BCL-2, an anti-apoptotic gene, has been implicated in conferring chemotherapy resistance in non-Hodgkin’s lymphoma and has been extensively studied as a prognostic biomarker in DLBCL. DLBCL patients with both MYC and BCL-2 protein coexpression have shown inferior overall survival (OS) and progression-free survival (PFS). This concurrent expression of MYC and BCL-2 proteins in patients with PGI-DLBCL has thus far not been clearly understood.
Lymphomas with recurrent chromosomal breakpoints activating multiple oncogenes, including MYC and BCL-2 are often referred to as “Dual Hit” or “Double Hit” lymphomas (DHL). DHL make up an important part of this novel WHO category and represent heterogeneous cases of aggressive B-cell lymphoma. PGI-DLBCL is a relatively rare disease. Herein, the authors focus on PGI-DLBCL, and observed that the rate of concurrent expression (MYC+/BCL-2+) is higher in PGI-DLBCL than all of DLBCL (30% vs 21%), suggesting that concurrent expression of MYC and BCL-2 is more frequently in PGI-DLBCL.
Johnson and colleagues reported a study that used c-Myc antibody in combination with an antibody for BCL2 in a training cohort of 167 and a validation set of another 140 patients with DLBCL. They found MYC translocations, high MYC mRNA, and MYC protein expression in 11%, 11%, and 33% of the samples, respectively. MYC protein expression was associated with an inferior progression-free and overall survival only when BCL-2 protein was coexpressed. However, most of the DLBCL cases in this study are nodal DLBCL. PGI-DLBCL in this series are relatively small and the incidence and significance of MYC or/BCL-2 expression was not mentioned in this study and has not been reported in the literature. Given the specialized location of the GI tract and GI lymphoma associations with infections such as H. pylori infection, celiac disease, inflammatory bowel disease and autoimmune diseases, authors have considered PGI-DLBCL as a distinct entity, since their evaluation, diagnosis, management and prognosis are different from DLBCL of lymph node origin (nodal). Thus, this study is performed to focus on PGI-DLBCL, and it was observed that the rate of concurrent expression (MYC+/BCL-2+) was higher in PGI-DLBCL than in all of DLBCL (30% vs 21%).
MYC/BCL-2 double-hit lymphoma is a highly aggressive lymphoma with generally poor response to first line and salvage treatment, with a median OS of 0.2-1.5 years. These results suggest that PGI-DLBCL with concurrent expression of MYC and BCL-2 proteins characterizes a subset of PGI-DLBCL patients with poor prognosis. Previous reports have suggested that chemotherapy regimens that include BCL-2-targeted drugs, such as BH3 mimetics, have shown efficacy in murine MYC+/BCL-2+ lymphomas. BCL-2-targeted therapy may represent a promising new apoptosis-modulating strategy for MYC+/BCL-2+ patients with PGI-DLBCL.
MYC/BCL-2 double-hit lymphoma: Lymphomas with recurrent chromosomal breakpoints activating multiple oncogenes, including MYC and BCL-2 are often referred to as “Dual Hit” or “Double Hit” lymphomas (DHL). PGI-DLBCL: Primary gastrointestinal lymphoma is the most common type of extranodal lymphoma, comprising about 30%-40% of all extranodal lymphomas, but only accounts for 1%-8% of all gastrointestinal malignancies. The most frequent pathological type is mucosa-associated lymphoid tissue lymphoma and diffuse large B-cell lymphoma.
The paper demonstrated the significance of MYC and BCL-2 co-expression on the prognosis of PGI-DLBCL. Multivariate analysis revealed that MYC and BCL-2 double positive phenotype was the independent factor for poorer response to chemotherapy and poorer prognosis. The presentation of the data was clear and experiments were performed carefully. The precise identification of MYC/BCL-2 expression in PGI-DLBCL is quite important for clinical settings.
| 1. | Koch P, del Valle F, Berdel WE, Willich NA, Reers B, Hiddemann W, Grothaus-Pinke B, Reinartz G, Brockmann J, Temmesfeld A. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001;19:3861-3873. [PubMed] |
| 2. | Nakamura S, Matsumoto T. Gastrointestinal lymphoma: recent advances in diagnosis and treatment. Digestion. 2013;87:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer. 2003;97:2462-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Klapproth K, Wirth T. Advances in the understanding of MYC-induced lymphomagenesis. Br J Haematol. 2010;149:484-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Iqbal J, Neppalli VT, Wright G, Dave BJ, Horsman DE, Rosenwald A, Lynch J, Hans CP, Weisenburger DD, Greiner TC. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Iqbal J, Meyer PN, Smith LM, Johnson NA, Vose JM, Greiner TC, Connors JM, Staudt LM, Rimsza L, Jaffe E. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res. 2011;17:7785-7795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, Scott DW, Tan KL, Steidl C, Sehn LH. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452-3459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 766] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 8. | Rohatiner A, d’Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397-400. [PubMed] |
| 9. | Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3:209-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7015] [Cited by in RCA: 6396] [Article Influence: 246.0] [Reference Citation Analysis (10)] |
| 11. | Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2802] [Cited by in RCA: 2719] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 12. | van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 752] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 13. | Hoffman B, Liebermann DA. Apoptotic signaling by c-MYC. Oncogene. 2008;27:6462-6472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 14. | Kerosuo L, Piltti K, Fox H, Angers-Loustau A, Häyry V, Eilers M, Sariola H, Wartiovaara K. Myc increases self-renewal in neural progenitor cells through Miz-1. J Cell Sci. 2008;121:3941-3950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1064] [Cited by in RCA: 1052] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 16. | Simonian PL, Grillot DA, Nuñez G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood. 1997;90:1208-1216. [PubMed] |
| 17. | Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151-157. [PubMed] |
| 18. | Johnson NA, Savage KJ, Ludkovski O, Ben-Neriah S, Woods R, Steidl C, Dyer MJ, Siebert R, Kuruvilla J, Klasa R. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273-2279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 470] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 19. | Le Gouill S, Talmant P, Touzeau C, Moreau A, Garand R, Juge-Morineau N, Gaillard F, Gastinne T, Milpied N, Moreau P. The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14; 18) and 8q24/c-MYC rearrangement. Haematologica. 2007;92:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Kanungo A, Medeiros LJ, Abruzzo LV, Lin P. Lymphoid neoplasms associated with concurrent t(14; 18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol. 2006;19:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Niitsu N, Okamoto M, Miura I, Hirano M. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14; 18) and 8q24/c-MYC translocations. Leukemia. 2009;23:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 22. | Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102:83-87. [PubMed] |
| 23. | Horn H, Ziepert M, Becher C, Barth TF, Bernd HW, Feller AC, Klapper W, Hummel M, Stein H, Hansmann ML. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 414] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 24. | Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, Liu WM, Visco C, Li Y, Miranda RN. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021-431; quiz 4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 545] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 25. | Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, Nielsen O, Gadeberg OV, Mourits-Andersen T, Frederiksen M. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460-3467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 532] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 26. | Schmitt CA, Lowe SW. Bcl-2 mediates chemoresistance in matched pairs of primary E(mu)-myc lymphomas in vivo. Blood Cells Mol Dis. 2001;27:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2612] [Cited by in RCA: 2756] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 28. | Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421-3428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1594] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 29. | Mason KD, Vandenberg CJ, Scott CL, Wei AH, Cory S, Huang DC, Roberts AW. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad Sci USA. 2008;105:17961-17966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kitagawa M S- Editor: Yu J L- Editor: Logan S E- Editor: Zhang DN