Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2358
Peer-review started: August 23, 2014
First decision: September 15, 2014
Revised: October 7, 2014
Accepted: November 11, 2014
Article in press: November 11, 2014
Published online: February 28, 2015
Processing time: 191 Days and 0.4 Hours
AIM: To investigate whether the use of synchronous hepatectomy and splenectomy (HS) is more effective than hepatectomy alone (HA) for patients with hepatocellular carcinoma (HCC) and hypersplenism.
METHODS: From January 2007 to March 2013, 84 consecutive patients with HCC and hypersplenism who underwent synchronous hepatectomy and splenectomy in our center were compared with 84 well-matched patients from a pool of 268 patients who underwent hepatectomy alone. The short-term and long-term outcomes of the two groups were analyzed and compared.
RESULTS: The mean time to recurrence was 21.11 ± 12.04 mo in the HS group and 11.23 ± 8.73 mo in the HA group, and these values were significantly different (P = 0.001). The 1-, 3-, 5-, and 7-year disease-free survival rates for the patients in the HS group and the HA group were 86.7%, 70.9%, 52.7%, and 45.9% and 88.1%, 59.4%, 43.3%, and 39.5%, respectively (P = 0.008). Platelet and white blood cell counts in the HS group were significantly increased compared with the HA group one day, one week, one month and one year postoperatively (P < 0.001). Splenectomy and micro-vascular invasion were significant independent prognostic factors for disease-free survival. Gender, tumor number, and recurrence were independent prognostic factors for overall survival.
CONCLUSION: Synchronous hepatectomy and hepatectomy potentially improves disease-free survival rates and alleviates hypersplenism without increasing the surgical risks for patients with HCC and hypersplenism.
Core tip: The optimal approach for treating patients suffering from hepatocellular carcinoma (HCC) and hypersplenism is not well established. In the present study, synchronous hepatectomy and hepatectomy improved disease-free survival rates and alleviated hypersplenism without increasing the surgical risk for patients with HCC and hypersplenism.
- Citation: Zhang XY, Li C, Wen TF, Yan LN, Li B, Yang JY, Wang WT, Jiang L. Synchronous splenectomy and hepatectomy for patients with hepatocellular carcinoma and hypersplenism: A case-control study. World J Gastroenterol 2015; 21(8): 2358-2366
- URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2358.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2358
Hypersplenism, secondary to portal hypertension, is commonly associated with hepatocellular carcinoma (HCC) in cirrhotic patients, resulting in anemia, leucopenia, and thrombocytopenia[1-3]. Given the poor preoperative situation, increased surgical risks and poor long-term survival, hypersplenism is considered a contraindication for HCC patients undergoing liver resection[4-6]. The guidelines of the American Association for the Study of Liver Disease recommended that splenectomy be performed in cirrhotic patients with preserved liver function[7]. However, splenic immune function decreases with the development of HCC[8]. Nomura et al[9] reported that splenectomy improved liver fibrosis and led to beneficial immunological changes in cirrhotic patients with hepatitis, and splenectomy was also suggested to improve antitumor responses. In addition, splenectomy is advocated as an effective treatment for hypersplenism and portal hypertension, as this procedure improves low white blood cell (WBC) and platelet counts and reduce portal vein pressure[1,10,11]. The selection criteria and surgical techniques have been refined, and the outcome of patients undergoing liver resection has improved[12-14], with 5-year survival rates after resection reported to exceed 50%[7,15-18]. However, the best method for treating patients suffering from HCC and hypersplenism is currently not well established.
We carefully performed synchronous hepatectomy and splenectomy (HS) in patients with HCC and hypersplenism with preserved liver function. The aim of this case-control study was to investigate whether the use of synchronous hepatectomy and splenectomy was better than hepatectomy alone (HA) for patients with HCC and hypersplenism.
From January 2007 to March 2013, 84 consecutive patients with HCC and hypersplenism who underwent hepatectomy and splenectomy (the HS group) in the Department of Liver Surgery and Liver Transplantation Center of the West China Hospital of Sichuan University were retrospectively enrolled.
The HA group consisted of 84 patients selected from a pool of 268 patients who underwent HA for HCC and hypersplenism during the same period and met the selection criteria for hepatectomy and splenectomy. The cases and controls were well matched at a 1:1 ratio between the HS group and the HA group for the following variables: age, gender, hepatitis B surface antigen (HBsAg), alpha-fetoprotein (AFP), WBC and platelet (PLT) counts, splenomegaly, number of tumors, tumor size and micro-vascular invasion (MVI). To reduce bias, contemporary case controls were selected in a consecutive manner, and patients who received splenectomy after primary hepatectomy were excluded from the case-control study. The mean duration of follow-up was 35.87 ± 16.16 mo in the HS group and 33.45 ± 17.94 mo in the HA group. All of the enrolled patients were categorized as having Child-Pugh A liver function. The demographic data of the two patient groups are presented in Table 1.
| Variable | HS group | HA group | P value |
| Number of patients (n) | 84 | 84 | |
| Age (yr) | 49.32 ± 10.485 | 51.01 ± 10.830 | 0.306 |
| Gender (M/F) | 69:15 | 69:15 | 1.000 |
| HBsAg (positive/negative) | 71:13 | 72:12 | 0.828 |
| AFP (ng/mL) | 0.633 | ||
| ≤ 400 | 54 | 51 | |
| > 400 | 30 | 33 | |
| White blood cell count (109/L) | 3.96 ± 4.11 | 4.60 ± 4.41 | 0.332 |
| Platelet count (109/L) | 61.43 ± 42.113 | 68.93 ± 17.677 | 0.134 |
| Splenomegaly(mild/moderate/severe) | 12:46:36 | 14:53:27 | 0.380 |
| Chlid-Pugh class (A) | 84 | 84 | |
| Number of tumors | |||
| Single | 11 | 11 | 1.000 |
| Multiple | 73 | 73 | |
| Diameter of tumor (cm) | |||
| Mean | 4.18 ± 2.134 | 4.26 ± 1.996 | 0.809 |
| ≤ 5 | 13 | 18 | 0.320 |
| > 5 | 71 | 66 | |
| Micro-vascular invasion | |||
| Yes | 20 | 22 | 0.722 |
| No | 64 | 62 |
The following data were collected for all cases and controls: length of hospital stay, follow-up time, minor and major complications (Dindo et al[19] classification of surgical complications), type of liver resection (major vs minor), intraoperative bleeding, number of transfusions and time to recurrence. Data regarding total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and WBC and PLT counts were collected one day, one week, one month, and one year after the operation. The short- and long-term outcomes of the two groups were analyzed and compared.
All study subjects were informed of the benefits and risks of surgery in detail. Written informed consent for patient information to be stored in the hospital database and used for research was obtained from all patients.
Hypersplenism was defined as: (1) the portal vein greater than or equal to 1.0 cm in diameter; (2) the presence of hepatic cirrhosis and splenic thickness greater than or equal to 4.0 cm, as measured by radiographic examination; and (3) PLT and WBC counts less than 80 × 109/L and 3.0 × 109/L, respectively, obtained the week prior to surgery. Patients who suffered from splenomegaly with hypersplenism and/or splenomegaly that was classified as greater than class I (spleen enlarged beyond left subcostal margin and palpable) were treated by splenectomy.
All of the patients received follow-up monitoring 1 mo after the operation, every 3 mo thereafter during the first 3 years and then every 6 mo in subsequent years. All enrolled patients received regular follow-up monitoring until death or termination of the study. Disease status was assessed according to serum liver biochemistries, AFP levels, hepatitis B virus (HBV)-DNA levels and radiological examination. One or two types of radiological examination, such as ultrasound, contrast-enhanced ultrasound, contrast-enhanced computed tomography (CT), and magnetic resonance imaging, were chosen based on the specific situation. Tumor recurrence was diagnosed based on the identification of a new lesion in at least two radiological examinations and increased AFP levels. The final follow-up visit occurred at the end of July 2014, unless the patient had died prior to that time. The overall median follow-up time was 33 mo (5-86 mo).
Continuous variables are expressed as mean ± standard deviation and were compared between groups using the t-test or Mann-Whitney U test for variables with an abnormal distribution. Categorical data were compared using the χ2 test or Fisher’s exact test. The overall survival rates were analyzed using the Kaplan-Meier method, and the differences were analyzed using the log-rank test. The Cox proportional hazard model was used for univariate and multivariate analyses of prognostic factors after surgery. Two-tailed P values ≤ 0.05 were considered statistically significant. Calculations were performed using the SPSS package (SPSS, Inc. 1989-1995, Chicago, IL).
According to the case-match design, age, gender, HBsAg, AFP, WBC and PLT counts, splenomegaly, tumor number, tumor size and MVI were similar between the two groups of patients (Table 1). A total of 33 patients died in the HA group, whereas 26 patients died in the HS group. The length of hospital stay, follow-up time, type of liver resection (major vs minor), intraoperative bleeding, and number of transfusions during the perioperative period did not significantly differ between the two groups (Table 2). The mean time to recurrence was 21.11 ± 12.04 mo in the HS group and 11.23 ± 8.73 mo in the HA group, and these values were significantly different (P = 0.001). Subgroup analyses indicated that 8 of 35 patients suffered early recurrence (time to recurrence less than 12 mo) in the HA group, whereas 31 of 48 patients in the HA group experienced early recurrence (P < 0.001).
| Variable | HS group (n = 84) | HA group (n = 84) | P value |
| Hospital stays (d) | 9.71 ± 3.514 | 9.57 ± 5.267 | 0.899 |
| Type of resection1 (major/minor) | 20:64 | 32:52 | 0.066 |
| Intraoperative bleeding (mL) | 553.57 ± 281.316 | 498.21 ± 220.094 | 0.157 |
| Transfusion (Y/N) | 11/73 | 8/76 | 0.627 |
| Recurrence | 35 | 48 | 0.045 |
| Time to recurrence (mo) | |||
| Mean time | 21.11 ± 12.04 | 11.23 ± 8.73 | 0.001 |
| ≤ 12 mo | 8 | 31 | < 0.001 |
| > 12 mo | 27 | 17 | |
| Follow-up time (mo) | 35.87 ± 16.16 | 33.45 ± 17.94 | 0.360 |
| Upper gastrointestinal hemorrhage after operation | 4 | 7 | 0.349 |
No treatment-related mortality was noted in either group (Table 3). Complications above grade II were defined as severe complications and were analyzed. In the HS group, two patients developed pleural effusion and received pleurocentesis. In addition, two patients were diagnosed with intra-abdominal bleeding and underwent re-laparotomy. As a result of hypotension and shock, two patients were resuscitated and required ICU management. In the HA group, one patient developed pleural effusion and received pleurocentesis. Two patients suffered type I respiratory failure, and one patient suffered hypotension and shock; all of these conditions required ICU management. However, no significant difference in the incidence rate or classification of complications was noted between the two groups (P = 0.532).
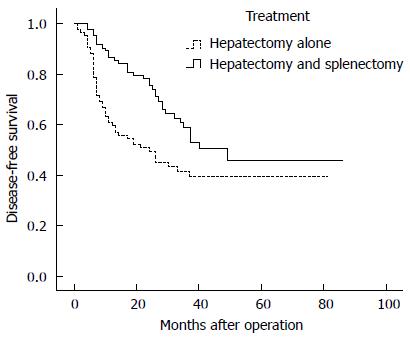
Disease-free survival rate: The 1-, 3-, 5-, and 7-year disease-free survival rates for patients in the HS group and HA group were 86.7%, 70.9%, 52.7%, and 45.9% and 88.1%, 59.4%, 43.3%, and 39.5%, respectively. Disease-free survival was enhanced in the HS group compared with the HA group (P = 0.008; Figure 1).
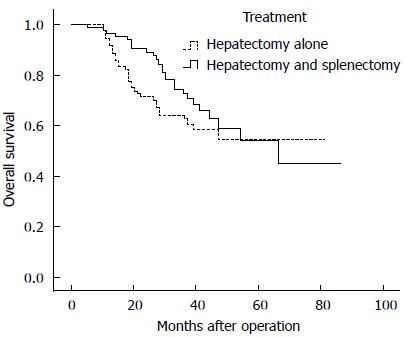
Overall survival rate: The 1-, 3-, 5-, and 7-year overall survival rates for patients in the HS group and HA group were 90.4%, 77.9%, 65.9%, and 45.2% and 85.7%, 70.0%, 58.3%, and 54.7%, respectively. No significant difference in overall survival was noted between the groups (P = 0.187) (Figure 2).
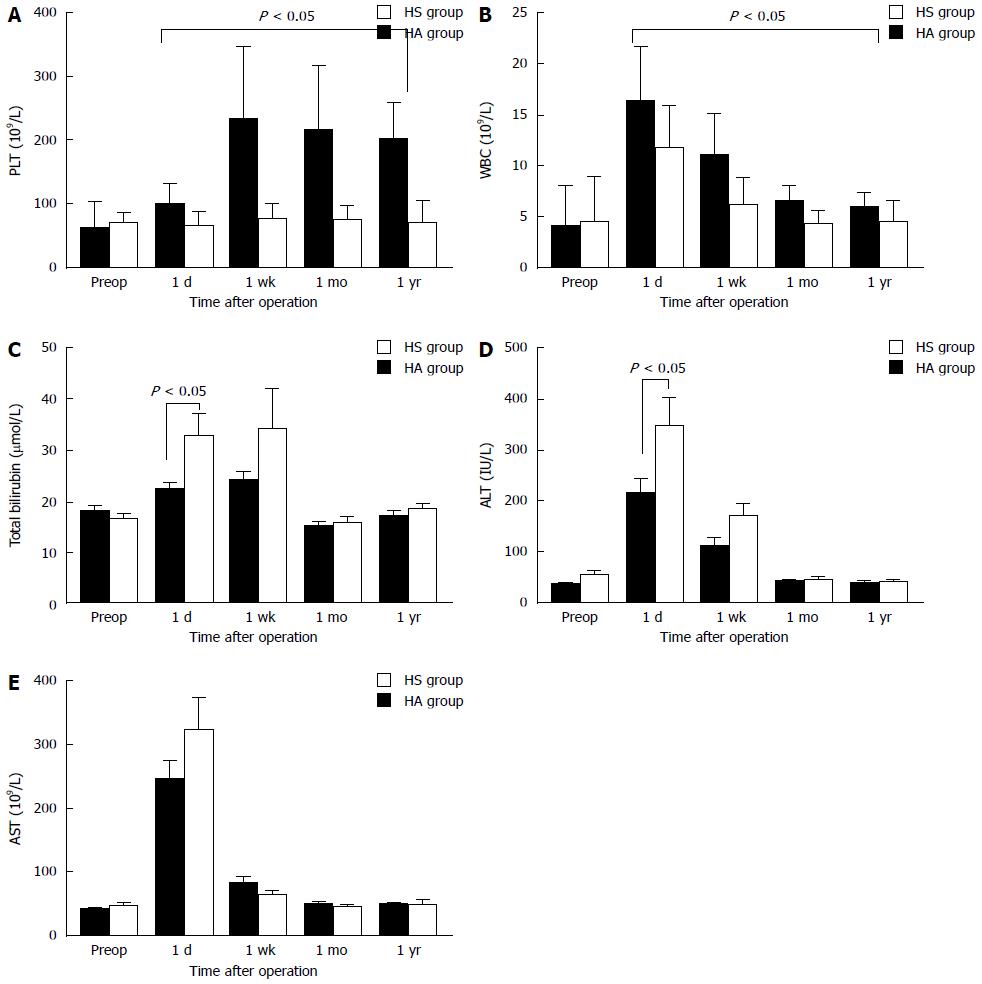
Total bilirubin, ALT, AST, and PLT and WBC counts were similar in both groups prior to surgery (Table 4). After surgery, the PLT and WBC counts in the HS group were significantly increased compared with the HA group (P < 0.001) (Figure 3A and B). The levels of TBIL and ALT were significantly increased in the HA group compared with the HS group on postoperative day one (P < 0.05) but then decreased to levels similar to those observed in the HS group at one week, one month and one year after the operation (Figure 3C and D). In addition, the serum AST levels did not differ between the two groups in the short-term or long-term period after surgery (P > 0.05).
| Variable | Pre-operation | 1 d | 1 wk | 1 mo | 1 yr | ||||||||||
| HS | HA | P value | HS | HA | P value | HS | HA | P value | HS | HA | P value | HS | HA | P value | |
| TBIL (μmol/L) | 17.94 ± 7.34 | 16.62 ± 6.63 | 0.415 | 22.34 ± 8.52 | 32.72 ± 27.14 | 0.026 | 24.04 ± 10.90 | 34.06 ± 48.89 | 0.216 | 15.12 ± 5.28 | 15.87 ± 6.70 | 0.858 | 17.20 ± 6.21 | 18.61 ± 5.82 | 0.314 |
| ALT (IU/L) | 35.90 ± 16.89 | 39.24 ± 14,54 | 0.519 | 213.85 ± 187.27 | 348.00 ± 342.49 | 0.036 | 112.00 ± 101.69 | 169.57 ± 154.49 | 0.058 | 43.49 ± 23.78 | 49.41 ± 21.62 | 0.860 | 39.82 ± 17.85 | 41.11 ± 34.53 | 0.838 |
| AST (IU/L) | 39.92 ± 15.69 | 47.16 ± 27.63 | 0.162 | 244.49 ± 186.49 | 321.81 ± 314.90 | 0.194 | 80.64 ± 77.53 | 64.19 ± 37.23 | 0.246 | 49.41 ± 21.62 | 44.22 ± 22.31 | 0.306 | 47.69 ± 27.73 | 48.00 ± 48.75 | 0.973 |
| PLT count (109/L) | 61.43 ± 42.11 | 68.93 ± 17.68 | 0.134 | 98.54 ± 33.27 | 67.11 ± 21.67 | < 0.001 | 230.95 ± 114.95 | 74.65 ± 26.05 | < 0.001 | 214.18 ± 102.03 | 73.24 ± 24.12 | < 0.001 | 199.97 ± 58.19 | 68.08 ± 36.52 | < 0.001 |
| WBC count (109/L) | 3.96 ± 4.11 | 4.60 ± 4.41 | 0.332 | 16.30 ± 5.38 | 11.79 ± 4.07 | < 0.001 | 10.93 ± 4.27 | 6.14 ± 2.74 | < 0.001 | 6.48 ± 1.71 | 4.25 ± 1.41 | < 0.001 | 5.96 ± 1.46 | 4.48 ± 2.02 | < 0.001 |
Disease-free survival: Splenectomy (HR = 0.531; 95%CI: 0.341-0.828; P = 0.005) and MVI (HR = 2.642, 95%CI: 1.685-4.143; P < 0.001) were identified as significant independent prognostic factors for disease-free survival using univariate and multivariate analyses (Table 5).
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Splenectomy | 0.498 (0.314-0.792) | 0.003 | 0.531 (0.341-0.828) | 0.005 |
| Gender, F | 0.873 (0.476-1.601) | 0.661 | ||
| Age, > 60 yr | 0.836 (0.395-1.769) | 0.639 | ||
| HBsAg, positive | 1.634 (0.716-3.727) | 0.243 | ||
| AFP, > 400 ng/mL | 1.386 (0.881-2.180) | 0.158 | ||
| Platelet count | 0.994 (0.985-1.003) | 0.221 | ||
| White blood cell count | 1.037 (0.993-1.083) | 0.1 | ||
| Tumor number, multiple | 1.668 (0.862-3.226) | 0.129 | ||
| Tumor size, > 5 cm | 0.785 (0.446-1.381) | 0.401 | ||
| Micro-vascular invasion | 2.766 (1.700-4.498) | <0.001 | 2.642 (1.685-4.143) | < 0.001 |
Overall survival: Univariate analysis indicated that the following four variables were statistically significant prognostic factors associated with overall survival in patients with HCC and hypersplenism: gender (female, P = 0.020), tumor number (multiple, P = 0.041), tumor size (> 5 cm, P = 0.027) and recurrence (P < 0.001). Furthermore, based on the multivariate analysis, gender (HR = 0.774; 95%CI: 1.164-4.041; P = 0.015), tumor number (HR = 0.801; 95%CI: 1.071-4.633; P = 0.032), and recurrence (HR = 2.344; 95%CI: 4.961-21.882; P < 0.001) were independent prognostic factors for long-term survival in patients with HCC and hypersplenism (Table 6).
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Splenectomy | 0.862 (0.497-1.492) | 0.595 | ||
| Gender, F | 0.466 (1.125-4.083) | 0.020 | 0.774 (1.164-4.041) | 0.015 |
| Age, > 60 yr | 0.876 (0.384-1.998) | 0.752 | ||
| HBsAg, positive | 0.552 (0.259-1.176) | 0.124 | ||
| AFP, > 400 ng/mL | 0.986 (0.563-1.728) | 0.962 | ||
| Platelet count | 1.004 (0.995-1.013) | 0.397 | ||
| White blood cell count | 0.975 (0.901-1.055) | 0.533 | ||
| Tumor number, multiple | 2.216 (1.033-4.752) | 0.041 | 0.801 (1.071-4.633) | 0.032 |
| Tumor size, > 5 cm | 2.067 (1.087-3.930) | 0.027 | 0.572 (0.991-3.169) | 0.054 |
| Micro-vascular invasion | 0.624 (0.329-1.182) | 0.148 | ||
| Recurrence | 14.376 (6.233-33.160) | < 0.001 | 2.344 (4.961-21.882) | < 0.001 |
Perioperative bleeding control and postoperative liver failure are the major concerns for cirrhotic patients with HCC and hypersplenism[4,5,7]. However, splenectomy has been suggested as a method to overcome these problems, as this procedure can reduce serum bilirubin levels and improve liver function[1,11]. In addition, PLT counts increase immediately after splenectomy, thereby potentially reducing intraoperative bleeding and surgical risk[20,21]. In the present case-control study, TBIL and ALT levels were reduced at one day and one week postoperatively in patients undergoing synchronous hepatectomy and splenectomy compared to those treated by hepatectomy alone. In addition, intraoperative bleeding and severe surgical complications were similar between the two groups. Furthermore, the disease-free survival was significantly increased in the HS group compared with the HA group, suggesting that synchronous hepatectomy and splenectomy are safe and beneficial for patients with HCC and hypersplenism.
The immunophysiology of the spleen in cirrhotic patients and the long-term outcomes of such patients after splenectomy are not fully understood. Aoe et al[22] reported that a large number of activated macrophages accumulated in the spleens of tumor-bearing hosts, which led to an abnormal T cell receptor-CD3 complex and suppressed the immune function of T cells. In addition, Ugel et al[23] recently reported that the spleen was fundamentally important for tumor-induced tolerance. Splenic CD11b+Gr-1intLy6Chi cells, which are mostly composed of proliferating CCR2+ inflammatory monocytes with myeloid progenitor features, expand in the marginal zone of the spleen, where these cells alter the normal tissue cytoarchitecture and cross-present tumor antigens to memory CD8+ T cells, resulting in tolerization[23]. Accordingly, splenectomy was shown to restore lymphocyte function and induce tumor regression when coupled with immunotherapy. Shimada et al[11] and Karakantza et al[24] reported that splenectomy increased the number of natural killer (NK) cells. In addition, the immune response against cancer is altered after splenectomy due to the modulation of CD4+ and CD8+ T cells[9,25,26]. Splenectomy results in a reduction of transforming growth factor (TGF)-β1, which is produced and secreted by the spleen, thereby significantly improving liver regeneration and ameliorating liver cirrhosis[27-29]. Thus, we hypothesized that splenectomy may play a prophylactic role against HCC recurrence after liver resection, and our results showed that disease-free survival and the time interval before recurrence were enhanced in the HS group compared with the HA group, which is consistent with a previous study[26].
Interestingly, synchronous hepatectomy and splenectomy decreased tumor recurrence and prolonged the interval to recurrence; however, this technique was not beneficial to the overall survival of patients with HCC and hypersplenism. The reduction of TGF-β levels after splenectomy was shown to lead to decreased recruitment of T regulatory (Treg) cells via the TGF-β-miRNA-34a-CCL22 pathway[30,31], which may reduce the ability of HCC cells to evade immune defenses and inhibit tumor metastasis. Intricate immunosuppressive mechanisms, such as abnormal T cell receptor-CD3 complex[22], tumor-induced tolerance[23], suppression of NK cells[11,24], and impaired T cell function, may also be altered by splenectomy, thereby temporarily inducing tumor regression. However, sustained hepatitis viral infection results in inflammation and inflammatory microenvironments that promote fibrosis, cirrhosis and even permanent oncogenesis. As demonstrated in the present study, most patients were characterized as hepatitis b antigen-positive; thus, the outcomes of the case-control groups were not significantly different (P = 0.187).
In addition, numerous reports suggest that patients can significantly benefit from splenectomy. Hepatic resection increases portal hypertension, which is potentially associated with upper gastrointestinal hemorrhage[32]. Splenectomy reduces 20%-30% of the portal vein inflow, thereby greatly decreasing portal hypertension[33,34]. A total of four patients in the HS group experienced an episode of upper gastrointestinal hemorrhage after liver resection, whereas seven patients in the HA group experienced hemorrhage. In addition, WBC and PLT counts were significantly elevated after splenectomy. Thus, it was possible to administer adjuvant chemotherapy, which may prevent HCC recurrence[26,35,36].
This study had several limitations. First, given that it was not a randomized study, selection bias may be inherent to the study. To reduce this bias, we selected contemporary case controls in a consecutive manner and excluded patients who received splenectomy after primary hepatectomy. Second, the number of subjects was relatively small. A large randomized control study is thus needed to confirm the role of HS in improving disease-free survival for patients with HCC and hypersplenism.
In conclusion, our results suggest that synchronous hepatectomy and hepatectomy may improve disease-free survival rates and alleviate hypersplenism without an increased surgical risk for patients with HCC and hypersplenism.
Given its poor preoperative characteristics, increased surgical risks and poor long-term survival, hypersplenism is considered a contraindication for hepatocellular carcinoma (HCC) patients undergoing liver resection. Moreover, the optimal method to treat patients suffering from HCC and hypersplenism is not well established. Therefore, the authors conducted this case-control study to investigate whether the use of synchronous hepatectomy and splenectomy was better than hepatectomy alone for patients with HCC and hypersplenism.
The immunophysiology of the spleen in cirrhotic patients has not been fully characterized. Splenectomy potentially restores lymphocyte function and induces tumor regression when coupled with immunotherapy.
Synchronous splenectomy may play a prophylactic role against hepatocellular carcinoma recurrence after liver resection.
The use of synchronous hepatectomy and splenectomy may improve disease-free survival and alleviate hypersplenism without increasing the surgical risk for patients with HCC and hypersplenism.
The immunosuppressive mechanisms of HCC, including defective antigen presentation, tumor-induced tolerance, impaired CD4+ T cell function, suppression of natural killer cells, recruitment of immunosuppressive myeloid and lymphoid cell populations and the up-regulation of immune checkpoint pathways, are intricate and further interfere with the development of a meaningful anti-tumor response.
This is an interesting study that aims to determine the role of splenectomy and hepatectomy for patients with hepatocellular carcinoma and hypersplenism.
| 1. | Sugawara Y, Yamamoto J, Shimada K, Yamasaki S, Kosuge T, Takayama T, Makuuchi M. Splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg. 2000;190:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Lau WY. Management of hepatocellular carcinoma. J R Coll Surg Edinb. 2002;47:389-399. [PubMed] |
| 3. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 802] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 4. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 471] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 5. | Dimick JB, Cowan JA, Knol JA, Upchurch GR. Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg. 2003;138:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 197] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Looke DF, Runnegar NJ. Splenectomy and sepsis. Med J Aust. 2012;196:587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6631] [Article Influence: 442.1] [Reference Citation Analysis (1)] |
| 8. | Jasnis MA, Elján AM, Oisgold-Dagá S. Regulation of tumor growth by soluble spleen factors: effect of tumor resection. J Surg Oncol. 1987;35:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Nomura Y, Kage M, Ogata T, Kondou R, Kinoshita H, Ohshima K, Yano H. Influence of splenectomy in patients with liver cirrhosis and hypersplenism. Hepatol Res. 2014;44:E100-E109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Amin MA, el-Gendy MM, Dawoud IE, Shoma A, Negm AM, Amer TA. Partial splenic embolization versus splenectomy for the management of hypersplenism in cirrhotic patients. World J Surg. 2009;33:1702-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Shimada M, Hashizume M, Shirabe K, Takenaka K, Sugimachi K. A new surgical strategy for cirrhotic patients with hepatocellular carcinoma and hypersplenism. Performing a hepatectomy after a laparoscopic splenectomy. Surg Endosc. 2000;14:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, Kubota K, Zucchi A. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 353] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526-1529. [PubMed] |
| 14. | Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, Mazziotti A. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 276] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (5)] |
| 16. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 591] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 17. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1277] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 18. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 539] [Article Influence: 20.7] [Reference Citation Analysis (10)] |
| 19. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26150] [Article Influence: 1188.6] [Reference Citation Analysis (2)] |
| 20. | Wang Q, Sun K, Li XH, Peng BG, Liang LJ. Surgical treatment for hepatocellular carcinoma and secondary hypersplenism. Hepatobiliary Pancreat Dis Int. 2006;5:396-400. [PubMed] |
| 21. | Paquet KJ. Surgery for cirrhotic patients with hepatocellular carcinoma and hypersplenism. Surg Endosc. 2001;15:104-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Aoe T, Okamoto Y, Saito T. Activated macrophages induce structural abnormalities of the T cell receptor-CD3 complex. J Exp Med. 1995;181:1881-1886. [PubMed] |
| 23. | Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2:628-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Karakantza M, Mouzaki A, Theodoropoulou M, Bussel JB, Maniatis A. Th1 and Th2 cytokines in a patient with Evans’ syndrome and profound lymphopenia. Br J Haematol. 2000;110:968-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Hashimoto N, Shimoda S, Kawanaka H, Tsuneyama K, Uehara H, Akahoshi T, Kinjo N, Taketomi A, Shirabe K, Akashi K. Modulation of CD4+ T cell responses following splenectomy in hepatitis C virus-related liver cirrhosis. Clin Exp Immunol. 2011;165:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Chen XP, Wu ZD, Huang ZY, Qiu FZ. Use of hepatectomy and splenectomy to treat hepatocellular carcinoma with cirrhotic hypersplenism. Br J Surg. 2005;92:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Morinaga A, Ogata T, Kage M, Kinoshita H, Aoyagi S. Comparison of liver regeneration after a splenectomy and splenic artery ligation in a dimethylnitrosamine-induced cirrhotic rat model. HPB (Oxford). 2010;12:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Akahoshi T, Hashizume M, Tanoue K, Shimabukuro R, Gotoh N, Tomikawa M, Sugimachi K. Role of the spleen in liver fibrosis in rats may be mediated by transforming growth factor beta-1. J Gastroenterol Hepatol. 2002;17:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Ueda S, Yamanoi A, Hishikawa Y, Dhar DK, Tachibana M, Nagasue N. Transforming growth factor-beta1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Lab Invest. 2003;83:1595-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577-594.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 652] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 31. | Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 461] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 32. | Akimaru K, Onda M, Tajiri T, Yoshida H, Yokomuro S, Mamada Y, Taniai N. Hypersplenism induced by hepatectomy. Hepatogastroenterology. 2001;48:1170-1175. [PubMed] |
| 33. | Ferraz AA, Bacelar TS, Silveira MJ, Coelho AR, Câmara Neto RD, de Araújo Júnior JG, Ferraz EM. Surgical treatment of schistosomal portal hypertension. Int Surg. 2001;86:1-8. [PubMed] |
| 34. | Lacerda CM, Freire W, Vieira de Melo PS, Lacerda HR, Carvalho G. Splenectomy and ligation of the left gastric vein in schistosomiasis mansoni: the effect on esophageal variceal pressure measured by a non-invasive technique. Keio J Med. 2002;51:89-92. [PubMed] |
| 35. | Huang YH, Wu JC, Lui WY, Chau GY, Tsay SH, Chiang JH, King KL, Huo TI, Chang FY, Lee SD. Prospective case-controlled trial of adjuvant chemotherapy after resection of hepatocellular carcinoma. World J Surg. 2000;24:551-555. [PubMed] |
| 36. | Ishikawa T, Higuchi K, Kubota T, Seki K, Honma T, Yoshida T, Kamimura T. Prevention of intrahepatic distant recurrence by transcatheter arterial infusion chemotherapy with platinum agents for stage I/II hepatocellular carcinoma. Cancer. 2011;117:4018-4025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Pan MX, Tsoulfas G S- Editor: Yu J L- Editor: Wang TQ E- Editor: Wang CH