Published online Feb 21, 2015. doi: 10.3748/wjg.v21.i7.2254
Peer-review started: August 4, 2014
First decision: August 27, 2014
Revised: September 22, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: February 21, 2015
Processing time: 192 Days and 20.3 Hours
Mixed adenoneuroendocrine carcinoma (MANEC) is a malignant tumor with adenocarcinoma and neuroendocrine components, with ≥ 30% of each component required. MANEC of the ampulla is rare. To the best of our knowledge, only 15 cases of MANEC of the ampulla have been reported in the English-language literature. Here, we report two cases of MANEC of the ampulla in two women aged 43 and 60 years, which was confirmed by histology after pancreaticoduodenectomy. These tumors contained neuroendocrine and adenocarcinoma components. The neuroendocrine components were positive for chromogranin A (CgA), synaptophysin (Syn) and CD56 by immunostaining. The adenocarcinoma components were negative for CgA, Syn and CD56. Both cases were T3N0M0 (Stage IIIA). They survived for 15 and 20 mo after surgery, respectively. A brief discussion about the histopathological features, clinical behavior and treatment of MANEC of ampulla, and review of the relevant literature are presented.
Core tip: The authors report two cases of mixed adenoneuroendocrine carcinoma (MANEC) of the ampulla, which is an extremely rare disease. Both patients received pancreaticoduodenectomy and chemotherapy after surgery. The cases provide a brief discussion about the histopathological features, clinical behavior and treatment of MANEC of the ampulla based on previously published studies.
- Citation: Huang Z, Xiao WD, Li Y, Huang S, Cai J, Ao J. Mixed adenoneuroendocrine carcinoma of the ampulla: Two case reports. World J Gastroenterol 2015; 21(7): 2254-2259
- URL: https://www.wjgnet.com/1007-9327/full/v21/i7/2254.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i7.2254
According to the 2010 edition of the World Health Organization (WHO) classification of tumors of the digestive system, tumors with two malignant components: neuroendocrine and gland-forming epithelial cells, and each component exceeding 30%, are classified as mixed adenoneuroendocrine carcinoma (MANEC)[1]. These tumors have non-specific symptoms and imaging characteristics, and are mainly diagnosed histopathologically and immunohistochemically after surgery. MANEC can occur in various organs like the gallbladder[2], bile duct[3], stomach[4], colon[5] and cecum[6], and location in the ampullary region is rare. To the best of our knowledge, only 15 cases of MANEC of the ampulla have been reported in the English-language literature[7]. Here, we present two cases of MANEC of the ampulla and review the literature.
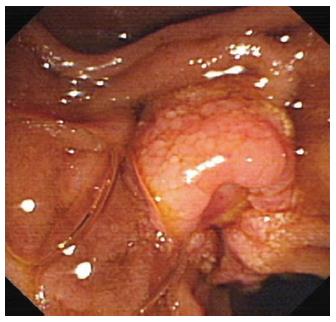
A 43-year-old woman presented with upper abdominal pain in November 2011. Physical examination revealed mild yellow sclera and right upper abdominal tenderness. Laboratory test results included total bilirubin 2.2 mg/dL [normal range (NR): 0.2-1.1 mg/dL], conjugated bilirubin 0.87 mg/dL (NR: 0-0.3 mg/dL), unconjugated bilirubin 1.33 mg/dL (NR: 0.1-1 mg/dL), aspartate aminotransferase 118 U/L (NR: < 35 U/L), alanine aminotransferase 285 U/L (NR: < 35 U/L), alkaline phosphatase 515 U/L (NR: 35-105 U/L). Serum levels of tumor marker carcinoembryonic antigen (CEA), carbohydrate antigen (CA)19-9 and CA12-5 were normal. Magnetic resonance cholangiopancreatography found a soft tissue signal shadow located in the ampulla region, associated with dilation of the common bile duct. Endoscopic retrograde cholangiopancreatography showed a cauliflower-like mass at the duodenal papilla (Figure 1), and the biopsy showed poorly differentiated adenocarcinoma.
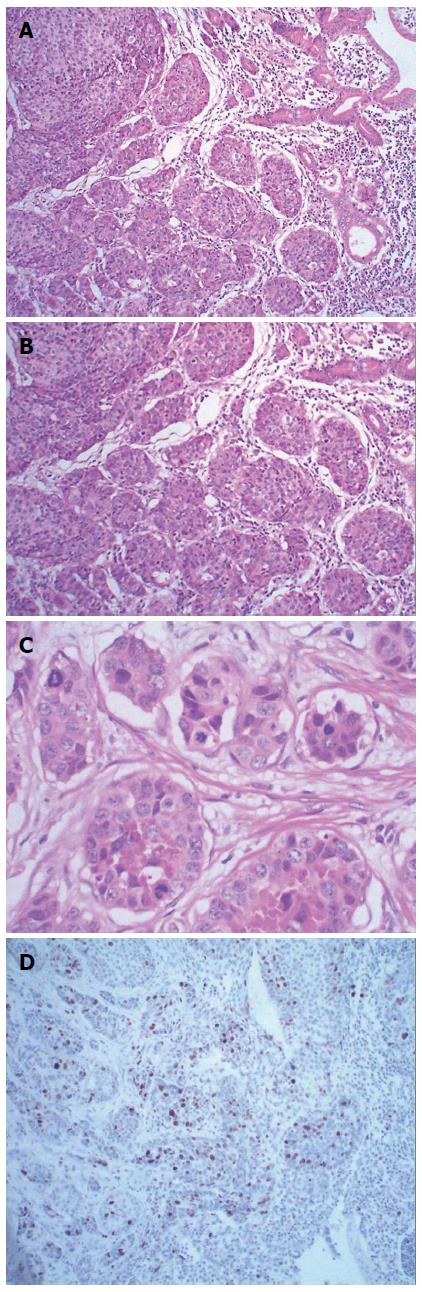
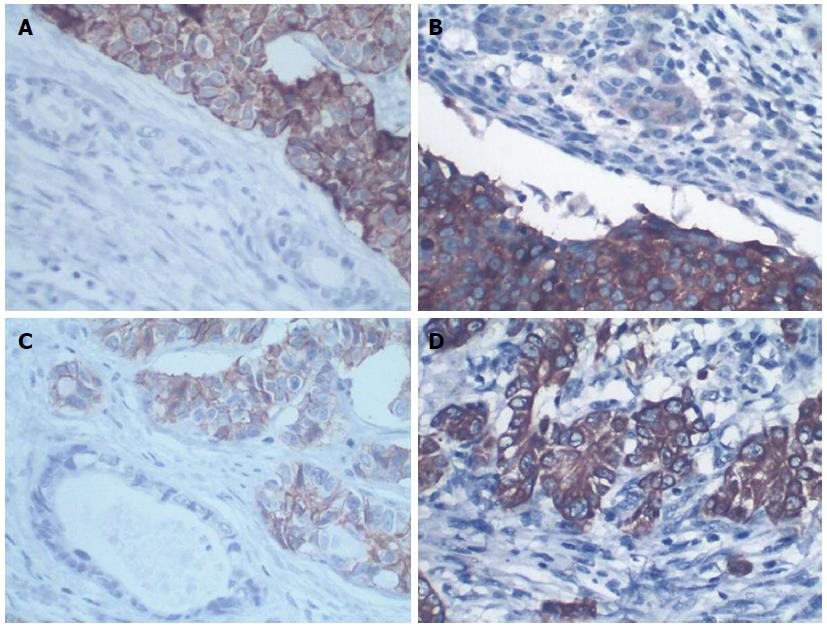
The patient underwent pancreaticoduodenectomy. A cauliflower-like mass at the duodenal papilla measuring 1.5 cm × 2.0 cm in diameter was presented as the surgical specimen. Histological examination showed that the tumor comprised two cell populations (Figure 2A). One was poorly differentiated tubular adenocarcinoma. The adenocarcinoma cells were arranged into an irregular pattern and accounted for 40% of the whole tumor. The second component was arranged in a nest and the tumor cells were consistent in size, with round nuclei, abundant cytoplasm and coarse chromatin (Figure 2B). The mitotic rate was 30/10 high-powered fields (HPF) (Figure 2C) and the Ki67 labeling index was 25% (Figure 2D). Immunohistochemically, the neuroendocrine components were positive for expression of chromogranin A (CgA), synaptophysin (Syn), and CD56 (Figure 3A-C), but negative for insulin, glucagon, gastrin and somatostatin. The adenocarcinoma cells were immunoactive for cytokeratin (CK)8 (Figure 3D) and CK18, but negative for CgA, Syn and CD56. Altogether, the cytomorphology and immunophenotype were consistent with the diagnosis of MANEC of the ampulla. The serosa and pancreas were infiltrated by the tumor, and the peripheral lymph nodes were free from tumor involvement and without distant metastasis. According to the 7th edition of the TNM system, the patient was T3N0M0 (Stage IIIA). The patient underwent adjuvant chemotherapy after surgery, consisting of a combination of oxaliplatin, calcium folinate and tegafur, and the patient died of tumor recurrence after 20 mo.
A 60-year-old woman was admitted to our department in September 2012 with symptoms of abdominal pain, nausea and vomiting for 4 mo, with a background of gastrorrhagia. The findings of physical examination were unremarkable. The laboratory tests showed a hemoglobin level of 6.5 g/dL and hematocrit of 22.5%. The liver function tests and tumor marker (CEA, CA19-9 and CA12-5) levels were normal. Computed tomography (CT) of the abdomen revealed a mass located in the lower common bile duct, with dilation of the intrahepatic and common bile ducts. Gastroduodenoscopy revealed a mass at the duodenal papilla, and biopsy showed poorly differentiated adenocarcinoma.
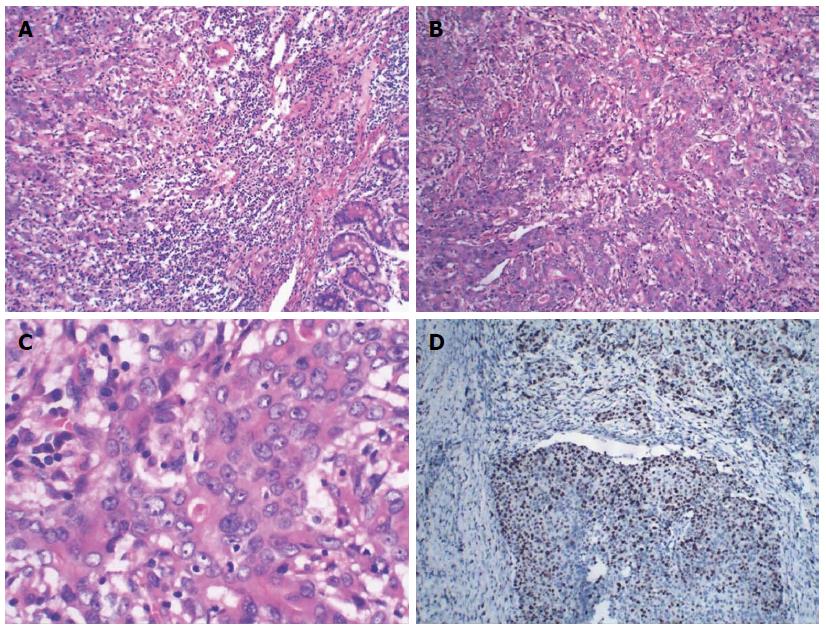
Pancreaticoduodenectomy was performed. An ulcerating mass at the ampulla of Vater measuring 1.5 cm × 1.7 cm in diameter was presented as the surgical specimen. Histological examination showed the tumor comprised two elements: poorly differentiated glandular adenocarcinoma and non-adenocarcinoma (Figure 4A). Approximately 55% of the tumor was composed of non-adenocarcinoma components. The adenocarcinoma cells were arranged into an irregular pattern. The non-adenocarcinoma components were arranged in a nest and the cells had abundant cytoplasm and nuclear hyperchromatism (Figure 4B). The mitotic rate was 25/10 HPF (Figure 4C) and the Ki67 index was 40% (Figure 4D). Immunohistochemical staining showed that the adenocarcinoma components were positive for CK8 and CK18, but negative for CgA, Syn and CD56. The non-adenocarcinoma cells were positive for CgA, Syn and CD56, but negative for CD34 and D2-40. Taking the morphological and immunohistochemical features into account, the diagnosis of MANEC of the ampulla was confirmed. The tumor penetrated the serosa and pancreas, but none of the regional lymph nodes showed metastasis and no distant metastasis was detected (T3N0M0, Stage IIIA). The patient underwent adjuvant chemotherapy after surgery, consisting of a combination of oxaliplatin and gemcitabine, and the patient died of tumor recurrence after 15 mo.
According to the WHO classification (2010), neuroendocrine neoplasms in the digestive system were categorized into NET G1 (carcinoid, mitotic count of < 2 per 10 HPF and/or ≤ 2% Ki67 index); NET G2 (mitotic count 2-20 per 10 HPF and/or 3%-20% Ki67 index); NET G3 (neuroendocrine carcinoma, mitotic count of > 20 per 10 HPF and/or > 20% Ki67 index); and MANEC[1]. MANEC can be divided into three subtypes: composite carcinoma, collision tumor and amphicrine tumor[8]. According to this subtype scheme, the two cases we presented here were both considered to be a composite carcinoma.
Patients with MANEC of the ampulla often complain of nonspecific symptoms such as abdominal pain, nausea, vomiting, epigastric discomfort, and jaundice. Most of the patients have no carcinoid syndrome. Examination such as endoscopy, ultrasound, CT and magnetic resonance imaging may provide useful information, but the confirmed diagnosis mainly depends on histopathological and immunohistochemical analysis after surgery. MANEC usually comprises two elements: a variable grade of differentiated adenocarcinoma and a neuroendocrine component. Each component should occupy ≥ 30% of the tumor. The adenocarcinoma components usually include intestinal type, goblet carcinoid, signet ring carcinoma, and acinar cell carcinoma. The neuroendocrine components contain small, intermediate and large neuroendocrine cells. The neuroendocrine cells usually show multifocal necrosis and high proliferation. Immunohistochemically, the neuroendocrine components are usually positive for CgA, Syn and CD56, and are also immunoreactive for neuron-specific enolase (NSE). To confirm the neuroendocrine nature of the tumor, it is recommended to rely on convincing immunoreactivity for at least two markers[9]. Adenocarcinoma components are usually negative for CgA, Syn, NSE and CD56. Moreover, the endothelial markers such as CD34 and D2-40 are used to confirm whether there is vascular invasion involved in the neuroendocrine tumors. As in our cases, the neuroendocrine components were positive for CgA, Syn and CD56. The Ki67 index was 25% and 40% respectively. The mitotic rates both exceeded 20/10 HPF, which is in accordance with the classification standard of neuroendocrine carcinoma. The adenocarcinoma components were positive for CK8 and CK18, but negative for CgA, Syn and CD56.
The histological origin of MANEC is unclear. The ultrastructural features of both neuroendocrine and glandular differentiation were at the single level, suggesting that the two components of MANEC arise from multipotential stem cells[2]. Moreover, Kim et al[10] have proposed that the common genetics of the glandular and neuroendocrine components of mixed tumor differentiation concurrently arise from a single precursor, by analyzing the genome-wide loss of heterozygosity. So, we suggest that the two components of MANEC arise from multipotential stem cells and show bi-phenotypic differentiation after carcinogenesis is initiated. The clinical behavior of this tumor is also still unclear due to the rarity of these tumors. Harada et al[11] have suggested that the neuroendocrine components more easily infiltrate the stromal, vascular and lymph node than the adenocarcinoma components. Some authors have also proposed that the neuroendocrine components show more aggressive features and influence the prognosis more easily than the adenocarcinoma components[12,13]. However, Volante et al[14] have reported that the clinical behavior of those tumors depends on the adenocarcinoma component if the associated endocrine component is well differentiated, and upon the neuroendocrine component if it is poorly differentiated. However, to date, there are no systematic data to illustrate why the percentage of each component of the MAENC is 30%, or whether this value represents the real threshold that somehow influences prognosis[1].
Pancreaticoduodenectomy is the optimal treatment for carcinoma of the ampulla. However, for patients who are unable to tolerate radical excision, local excision may be a better choice. After radical resection, multimodal treatment with adjuvant radiotherapy and/or chemotherapy should be adopted for patients with carcinoma of the ampulla. MANEC of the ampulla is a rare subtype of ampullary tumor with aggressive behavior and poor prognosis. Lee et al[4] have proposed that treatment should focus on the more aggressive cells of the tumor since the clinical outcome of this mixed tumor follows that of a more aggressive cell type. Wu et al[15] have proposed that the postoperative survival rate of neuroendocrine neoplasm significantly affected by tumor diameter, histological grade, lymph node metastasis, distant metastasis, radical surgery and muscular layer invasion. The present two patients underwent pancreaticoduodenctomy with no surgical complications and received adjuvant chemotherapy after surgery, but both died of tumor recurrence within 2 years. MANEC of the ampulla is considered as a highly malignant tumor, and the standardization of its treatment including surgery, chemotherapy and radiotherapy, biological targeted therapy requires further investigations.
The two female patients presented with non-specific symptoms; one with abdominal pain, and the other with abdominal pain, nausea and vomiting.
The physical signs of the two cases were also dissimilar; upon physical examination, one case revealed mild yellow sclera and right upper abdominal tenderness, and the other had no remarkable findings upon physical examination.
Duodenal adenocarcinoma, ampulla carcinoma, and pancreatic cancer. Pathological examination and immunohistochemical staining for chromogranin A (CgA), synaptophysin (Syn) and CD56 are the major methods for differential diagnosis.
The first patient showed total bilirubin 2.2 mg/dL, conjugated bilirubin 0.87 mg/dL, unconjugated bilirubin 1.33 mg/dL, aspartate aminotransferase 118 U/L, alanine aminotransferase 285 U/L and alkaline phosphatase 515 U/L. The second patient showed hemoglobin 6.5 g/dL and hematocrit 22.5%, and serum levels of tumor marker carcinoembryonic antigen, carbohydrate antigen (CA)19-9 and CA12-5 were both within NR: limits.
Imaging of the two patients revealed a space-occupying lesion located in the ampulla area with the bile duct dilating.
The tumors included neuroendocrine and adenocarcinoma components and each component exceeded 30%; the neuroendocrine components were positive for CgA, Syn and CD56, and the adenocarcinoma components were negative for CgA, Syn and CD56.
Both cases underwent pancreaticoduodenectomy and adjuvant chemotherapy after surgery.
Mixed adenoneuroendocrine carcinoma of the ampulla, which is a malignant tumor, is rare. The histological and clinical behavior is still not well understood.
Imaging and biopsy may provide some useful diagnostic information, but the diagnoses were all based on histology and immunostaining after surgery.
The presented cases are very rare. The article provides insights into the histopathology of this tumor and introduced the treatment, and can add more data in the treatment of this disease.
| 1. | Rindi G, Petrone G, Inzani F. The 2010 WHO classification of digestive neuroendocrine neoplasms: a critical appraisal four years after its introduction. Endocr Pathol. 2014;25:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 2. | Paniz Mondolfi AE, Slova D, Fan W, Attiyeh FF, Afthinos J, Reidy J, Pang Y, Theise ND. Mixed adenoneuroendocrine carcinoma (MANEC) of the gallbladder: a possible stem cell tumor? Pathol Int. 2011;61:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Onishi I, Kitagawa H, Harada K, Maruzen S, Sakai S, Makino I, Hayashi H, Nakagawara H, Tajima H, Takamura H. Intraductal papillary neoplasm of the bile duct accompanying biliary mixed adenoneuroendocrine carcinoma. World J Gastroenterol. 2013;19:3161-3164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Lee HH, Jung CK, Jung ES, Song KY, Jeon HM, Park CH. Mixed exocrine and endocrine carcinoma in the stomach: a case report. J Gastric Cancer. 2011;11:122-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Ito H, Kudo A, Matsumura S, Ban D, Irie T, Ochiai T, Nakamura N, Tanaka S, Tanabe M. Mixed adenoneuroendocrine carcinoma of the colon progressed rapidly after hepatic rupture: report of a case. Int Surg. 2014;99:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Jain A, Singla S, Jagdeesh KS, Vishnumurthy HY. Mixed adenoneuroendocrine carcinoma of cecum: a rare entity. J Clin Imaging Sci. 2013;3:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Zhang L, DeMay RM. Cytological features of mixed adenoneuroendocrine carcinoma of the ampulla: two case reports with review of literature. Diagn Cytopathol. 2014;42:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel). 2012;4:11-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Rindi G, Bordi C, La Rosa S, Solcia E, Delle Fave G. Gastroenteropancreatic (neuro)endocrine neoplasms: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S356-S360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Kim KM, Kim MJ, Cho BK, Choi SW, Rhyu MG. Genetic evidence for the multi-step progression of mixed glandular-neuroendocrine gastric carcinomas. Virchows Arch. 2002;440:85-93. [PubMed] |
| 11. | Harada K, Sato Y, Ikeda H, Maylee H, Igarashi S, Okamura A, Masuda S, Nakanuma Y. Clinicopathologic study of mixed adenoneuroendocrine carcinomas of hepatobiliary organs. Virchows Arch. 2012;460:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Makino A, Serra S, Chetty R. Composite adenocarcinoma and large cell neuroendocrine carcinoma of the rectum. Virchows Arch. 2006;448:644-647. [PubMed] |
| 13. | Kim TY, Chae HD. Composite neuroendocrine carcinoma with adenocarcinoma of the stomach misdiagnosed as a giant submucosal tumor. J Gastric Cancer. 2011;11:126-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449:499-506. [PubMed] |
| 15. | Wu F, Li P, Zhao H, Liu S, Guo C, Wang Y, Zhao D. [Prognostic factor analysis of surgical treatment in patients with rectal neuroendocrine neoplasms]. Zhonghua Yixue Zazhi. 2014;94:1237-1240. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Behzatoglu K, Gurzu S, Le Bian AZ S- Editor: Gou SX L- Editor: A E- Editor: Liu XM