Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1851
Peer-review started: August 5, 2014
First decision: September 15, 2014
Revised: September 29, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: February 14, 2015
Processing time: 190 Days and 8.1 Hours
AIM: To analyze tumor regression grade (TRG) for prognosis of locally advanced rectal adenocarcinoma (LARA) treated with preoperative radiotherapy.
METHODS: One hundred and ninety patients with clinical stage II/III LARA were studied. All patients underwent radical surgery (between 2004 and 2010) after 30-Gy/10-fraction preoperative radiotherapy (pre-RT). All 190 patients received a short course of pre-RT and were reassessed for disease recurrence and survival; the slides of surgical specimens were reviewed and classified according to Mandard TRG. We compared patients with good response (Mandard TRG1 or TRG2) vs patients with bad/poor response (Mandard TRG3-5). Outcomes evaluated were 5-year overall survival (OS), 5-year disease-free survival (DFS), and local, distant and mixed recurrence. Fisher’s exact test or χ2 test, log-rank test and proportional hazards regression analysis were used to calculate the probability that Mandard TRG was associated with patient outcomes.
RESULTS: One hundred and sixty-six of 190 patients (87.4%) were identified as Mandard bad responders (TRG3-5). High Mandard grade was correlated with tumor height (41.7% < 6 cm vs 58.3% ≥ 6 cm, P = 0.050), ypT stage (75% ypT0-2 vs 25% ypT3-4, P = 0.000), and ypN stage (75% ypN0 vs 25% ypN1, P = 0.031). In univariate survival analysis, Mandard grade bad responders had significantly worse OS and DFS than good responders (TRG1/2) (OS, 83.1% vs 96.4%, P = 0.000; DFS, 72.3% vs 92.0%, P = 0.002). In multivariate survival analysis, Mandard bad responders had significantly worse DFS than Mandard good responders (DFS 3.8 years (95%CI: 1.2-12.2 years, P = 0.026).
CONCLUSION: Mandard grade good responders had a favorable prognosis. TRG may be a potential predictor for DFS in LARA after pre-RT.
Core tip: We report that Mandard tumor regression grade (TRG) predicted the outcome of locally advanced rectal adenocarcinoma after preoperative radiotherapy. We examined the correlation of TRG in the prognosis of rectal adenocarcinoma. We found that high Mandard grade was correlated with tumor height, ypT stage, and ypN stage in Mandard poor responders. In univariate survival analysis, Mandard bad responders had significantly worse overall survival and disease-free survival (DFS) compared with Mandard good responders. In multivariate survival analysis, Mandard bad responders had significantly worse DFS than Mandard good responders. Mandard good responders had a favorable prognosis.
- Citation: Peng YF, Yu WD, Pan HD, Wang L, Li M, Yao YF, Zhao J, Gu J. Tumor regression grades: Potential outcome predictor of locally advanced rectal adenocarcinoma after preoperative radiotherapy. World J Gastroenterol 2015; 21(6): 1851-1856
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1851.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1851
Rectal cancer is a worldwide health concern[1]. In China, the incidence of rectal cancer is increasing at a rate of 4.2% annually, and is the fifth leading cause of cancer mortality[2]. Surgery remains the primary therapeutic tool for rectal cancer, and locoregional recurrence has been reduced by total mesorectal excision (TME) of cancers of the middle and lower rectum[3]. Preoperative radiotherapy (pre-RT), including short- or long-term courses, followed by TME can induce tumor regression and facilitate subsequent resection, resulting in improved local control and survival[4].
Although short- and long-term pre-RT regimens are considered standard for rectal cancer in western countries, they are not widely recommended in Asian countries such as Japan and China, where surgeons are more likely to perform extended operations to minimize local recurrence[5]. In China, a modified 30-Gy protocol for pre-RT was recommended by the Chinese Anti-Cancer Association in 2001 to minimize side effects and to increase flexibility without compromising therapeutic efficacy[6]. Our previous study[6] showed that, compared with surgery alone, the modified 30-Gy protocol was associated with significantly reduced local recurrence and complication rates. Patients had improved survival and downstaging, and clinical outcome was equivalent to standard pre-RT regimens. However, pre-RT (including the modified 30-Gy protocol) does not achieve benefit in all patients[4]. To quantify the response to pre-RT, different systems can be used that are particularly important in situations where the pathological response is not complete. Most of them have a 5-grade system, allowing the creation of groups according to the response[7,8]. In the present study, we aimed to assess the prognostic value of Mandard tumor regression grade (TRG)[9] in patients with locally advanced rectal adenocarcinoma (LARA) treated with 30-Gy/10-fraction pre-RT.
This study was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved ethically by Peking University Cancer Hospital Institutional Review Board. All patients provided written informed consent.
Data from 190 patients with resectable rectal adenocarcinoma treated in our hospital from June 2004 to August 2010 were collected. Eligible patients were selected according to the following criteria[10,11]: (1) resectable rectal cancer ≤ 10 cm from the anal verge; (2) evaluated by endorectal ultrasound (ERUS) or magnetic resonance imaging (MRI) before treatment; (3) primary carcinoma of the rectum identified histologically; (4) no clinical evidence of distant metastases; (5) having undergone transabdominal radical resection based on the principle of TME; and (6) having undergone R0 resection. Exclusion criteria were as follows: (1) patients who underwent concurrent neoadjuvant radiochemotherapy; (2) synchronous tumors or history of other malignant tumors within 5 years; (3) familial adenomatous polyposis and/or hereditary non-polyposis colorectal carcinoma; and (4) patients who died of complications or other non-cancer-related reasons.
All included patients underwent ERUS or MRI to evaluate tumor size, invasion depth and extent (T stage). All patients were identified as having involvement of the pararectal lymph nodes and were diagnosed as clinical stage II/III rectal cancer. Serum carcinoembryonic antigen was measured, and abdominal computed tomography (CT) and chest radiography were also routinely performed before treatment. Pre-RT with a total dose of 30 Gy/10 fractions was adopted, as recommended by the Chinese Anti-Cancer Association, based on high-level clinical evidence[12-14]. Surgical resection was performed 2-4 wk after full-dose RT.
Standard pathological tumor staging of the resected specimen was performed in accordance with the guidelines of the American Joint Committee on Cancer. Evidence of pathological complete response (ypCR) was defined as absence of viable adenocarcinoma in the surgical specimen or the presence of lakes of mucus without tumor cells. The histology of all surgical specimens was reviewed and confirmed independently and was classified based on the Mandard TRG system[9]. Grade 1: complete regression (fibrosis without detectable tumor tissue); Grade 2: fibrosis with scattered tumor cells; Grade 3: fibrosis and tumor cells with preponderance of fibrosis; Grade 4: fibrosis and tumor cells with preponderance of tumor cells; and Grade 5: tumor tissue without changes of regression.
All patients in the pre-RT group were given adjuvant chemotherapy for six to eight cycles, using the standard regimens based on 5-fluorouracil or capecitabine, such as FOLFOX, CapeOX, or capecitabine alone. Patients were followed at 3-mo intervals for the first 2 years and then at 6-mo intervals for the next 3 years. Evaluations consisted of physical examination, serum carcinoembryonic antigen, complete blood count, and blood chemical analysis. Proctoscopy, abdominal ultrasonography, CT of the abdomen and pelvis, and chest radiography were also routinely performed every 6-12 mo. Endpoints of this study were 5-year overall survival (OS) and 5-year disease-free survival (DFS).
Statistical analyses were performed using SPSS version 16.0 software. The categorical variables were analyzed with Pearson χ2 or Fisher’s exact test. Survival curves were plotted using the Kaplan-Meier method, and log-rank tests were performed to evaluate prognostic differences between groups. The Cox proportional hazards model was used for multivariate analysis. For all analyses, two-sided tests of significance were used, and P < 0.05 was considered significant.
We studied 190 patients (118 male, 62 female) with mid-low rectal adenocarcinoma treated with pre-RT. The median patient age was 58 years (range: 28-85 years). The median distance of the tumor from the anal verge was 5 cm (range: 1-10 cm). One hundred and twenty-nine patients had sphincter preservation, while the other 58 received abdominoperineal resection, and three underwent the Hartmann procedure. The morbidity of the series was 22.6% (Table 1).
| Variables | n (%) |
| Sex | |
| Male | 118 (62.1) |
| Female | 72 (37.9) |
| Age (yr) | |
| < 65 | 129 (67.9) |
| ≥ 65 | 61 (32.1) |
| Tumor height (cm) | |
| < 6 | 113 (59.5) |
| ≥ 6 | 77 (40.5) |
| Pre-TNM | |
| II | 30 (15.8) |
| III | 160 (84.2) |
| Pre-RT | 190 |
| Surgical procedure | |
| LAR | 127 (66.8) |
| APR | 58 (30.6) |
| Other | 5 (2.6) |
| Perioperative complications | |
| Morbidity | 43 (22.6) |
| Abdominal or pelvic abscess | 8 |
| Anastomosis leak | 11 |
| Reoperation | 11 |
The distribution of the proportions of ypTNM stages (Table 2) were as follows: complete response (no microscopic residual tumor cell), 2.6% (n = 5); Stage I, 25.8% (n = 49); Stage II, 27.4% (n = 52); and Stage III, 44.2% (n = 84). The median follow-up duration was 56 mo (range 3-125 mo), and the follow-up rate was 100%. Response to neoadjuvant therapy is outlined in Table 2. Classification of TRG according to the Mandard system allowed us to define two groups as previously described[3]: TRG1/2 and TRG3-5. We verified a good response to 30 Gy/10 fractions pre-RT in 24 patients (ypCR in 5%-12.6%) and a bad response in 166 patients (87.4%). The two groups of patients (good vs bad Mandard response) were comparable with respect to age (P = 0.284), sex (P = 0.379), clinical stage (P = 0.547), and surgical procedures performed (P = 0.173), with the exception of tumor height (P = 0.050), ypN-stage (ypN0/ypN+) (P = 0.031), and ypT-stage (ypT0-2/ypT3-4) (P < 0.001) (Table 3).
| Variables | |
| Postoperative stage | |
| 0 | 5 (2.6) |
| I | 49 (25.8) |
| II | 52 (27.4) |
| III | 84 (44.2) |
| Mandard TRG | |
| Good response (1 or 2) | 24 (12.6) |
| Bad response (3-5) | 166 (87.4) |
| Overall recurrence of disease | |
| Local | 17 (8.9) |
| Distant | 66 (39.8) |
| Local and distant | 7 (3.7) |
| 5-yr OS | 65.3% ± 2.2% |
| 5-yr DFS | 61.4% ± 4.4% |
| Parameter | TRG1 + 2 | TRG3 + 4 + 5 | P value |
| Sex | |||
| Male | 17 (70.8) | 101 (60.8) | 0.379 |
| Female | 7 (29.2) | 65 (39.2) | Fisher's Test |
| Age (yr) | |||
| < 65 | 15 (62.5) | 114 (68.7) | 0.284 |
| ≥ 65 | 9 (37.5) | 52 (32.3) | Fisher's Test |
| Tumor height (cm) | |||
| < 6 | 10 (41.7) | 104 (62.7) | 0.0501 |
| ≥ 6 | 14 (58.3) | 62 (27.3) | χ2 =3.847 |
| Clinical stage | |||
| II | 5 (20.8) | 25 (15.1) | 0.547 |
| III | 19 (79.2) | 141 (84.9) | Fisher's Test |
| Surgical procedure | |||
| LAR | 20 (83.3) | 107 (64.5) | 0.173 |
| APR + other | 4 (16.7) | 59 (35.5) | Fisher's Test |
| Pathological N-stage | |||
| ypN0 | 18 (75.0) | 85 (51.2) | 0.0311 |
| ypN1 | 6 (25.0) | 81 (48.8) | Fisher's Test |
| Pathological T-stage | |||
| ypT0-2 | 18 (75.0) | 47 (28.3) | 0.0001 |
| ypT3-4 | 6 (25.0) | 119 (71.7) | Fisher's Test |
Seventeen patients (8.9%) had local recurrence, 66 (34.7%) had distant recurrence, and seven (3.7%) had mixed recurrence.
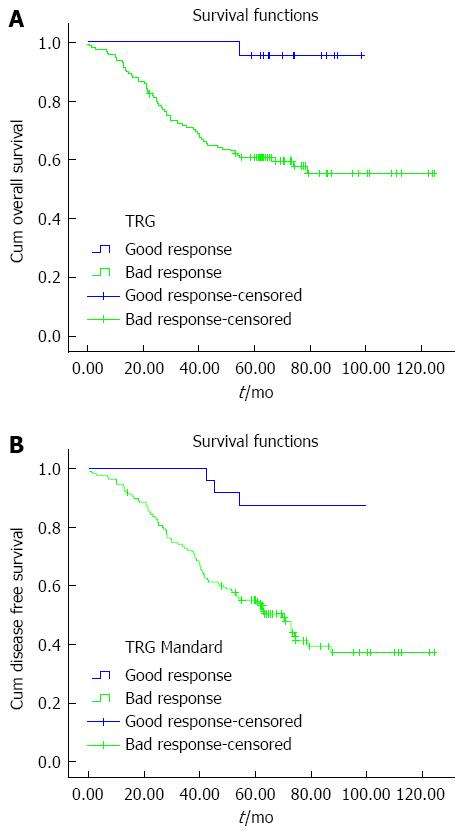
In univariate analysis, the mean follow-up was 56 mo (range: 3-125 mo). The 5-year OS and DFS was 65.3% and 61.4%, respectively (Table 2). In the different subsets, survival at 5 years was matched (Table 4). The 5-year OS and DFS in the patients who showed a bad and good response on Mandard TGR were 96.4% vs 83.1% (P = 0.002) and 92.0% vs 72.3% (P < 0.000), respectively (Table 4 and Figure 1). In multivariate Cox regression, DFS (3.8 years, 95%CI: 1.2-12.2 years, P = 0.026) in patients with bad Mandard response was significantly worse than in those with a good response after the following variables were entered: ypN stage (ypN0/ypN+), ypT stage, and tumor height. There was no significant survival difference in OS (6.5 years, 95%CI: 0.9-48.2 years, P = 0.066) between patients with bad and good Mandard response when comparing patients with complete (ypCR or Mandard TRG1) and partial (Mandard TRG2) pathological response (OS, P = 0.691; DFS, P = 0.502) (Table 4).
| Variables | P value | |
| 5-yr OS | ||
| Mandard good response (TRG1/2) | 96.4% ± 2.0% | 0.0021 |
| Mandard good response (TRG3-5) | 83.1% ± 4.2% | |
| ypCR (Mandard TRG1) | 100.00%2 | 0.6911 |
| Mandard partial response (TRG2) | 86.68%2 | |
| 5-yr DFS | ||
| Mandard good response (TRG1/2) | 92.0% ± 3.5% | 0.0001 |
| Mandard good response (TRG3-5) | 72.3% ± 3.8% | |
| ypCR (Mandard TRG1) | 100.00%2 | 0.5021 |
| Mandard partial response (TRG2) | 86.68%2 |
Neoadjuvant therapy, whether chemo-RT or RT alone, shows a significant improvement in local control and sphincter preservation[11,14-16]. Histological changes after pre-RT for rectal carcinoma vary considerably, with some entities showing complete absence of tumor cells, whereas others exhibit a mass of tumor cells with little or no regressive changes[17,18]. Compared with tumor downstaging or volume shrinkage, TRG can more accurately reflect tumor response at a cellular level[8]. Unlike the United States, in China, modified pre-RT (30-Gy/10 fractions) is widely adopted[19], thus our study addressed the clinical value of TRG following this particular RT regimen.
Our data confirmed that histological regression is closely coordinated with pathological T and N stage. As stated above, a significant proportion of cases with poor histological response contributed to the nodal-positive group, which indicated that TRG is an effective supplement to the TNM classification. A majority of studies support the view that patients with complete or partial response to preoperative treatment had better DFS than those with poor response[17,20,21]. Our data further demonstrated this conclusion by dividing TRG into poor response and TRG into good response in the Mandard system. This could guide the clinical decision making for postoperative adjuvant chemotherapy. For example, among patients with stage II rectal cancer who had no response, intensive chemotherapy might be considered in the adjuvant setting. In contrast, patients with a partial, but not complete response might not need further adjuvant chemotherapy and may benefit from fewer adverse drug effects.
Although the prognostic value of TRG has been well demonstrated, the clinical application of TRG still faces many problems. First, there are several TRG systems besides Mandard, and each has its own characteristics and indications[22,23]; however, which TRG system is more suitable for China is still in question. Our study suggests that the Mandard system is a potential candidate for tumor regression evaluation. Second, some studies have reported that the prognostic significance of TRG is not as crucial as ypTNM stage[24]. Thus, the association and role of TRG and TNM classification in prognostic evaluation needs to be addressed further.
Our study had some limitations. First, this study was retrospective, thus it might have had selection bias. Second, we did not compare the efficiency of the Mandard and other TRG systems in predicting tumor progression, thus, we could not conclude whether other TRG systems were better than Mandard. Third, the case number in some TRG subgroups was small, which may have influenced the reliability of the statistics.
In summary, our study demonstrates that TRG is a significant prognostic system for tumor progression and survival. It is a promising criterion for clinical decision making in adjuvant therapy.
This study showed that Mandard tumor regression grade (TRG) predicted the outcome of locally advanced rectal adenocarcinoma (LARA) after 30-Gy/10-fraction preoperative radiotherapy (pre-RT).
Neoadjuvant therapy, whether chemo-RT or RT alone, results in a significant improvement in local control and sphincter preservation. Histological changes after pre-RT for rectal carcinoma vary considerably, with some entities showing complete absence of tumor cells, whereas others exhibit a mass of tumor cells with little or no regressive changes. Compared with tumor downstaging or volume shrinkage, TRG could more accurately reflect tumor response at a cellular level.
The authors examined the correlation of TRG in the prognosis of rectal adenocarcinoma. They found that high Mandard grade was correlated with tumor height, ypT stage, and ypN stage in Mandard poor responders. In univariate survival analysis, Mandard bad responders had significantly worse overall survival and disease-free survival (DFS) than Mandard good responders (TRG1/2). In multivariate survival analysis, Mandard bad responders had significantly worse DFS than Mandard good responders. Mandard good responders had a favorable prognosis. Together, these studies suggest that TRG may be used as a potential predictor for DFS in Stage II/III LARA after short-course RT.
Mandard good responders had a favorable prognosis. TRG may be used as a potential predictor for DFS in Stage II/III LARA after 30-Gy/10-fraction pre-RT.
The TRG was first introduced by Mandard et al following the management of a patient with esophageal cancer. The TRG can categorize the cancer cell ratio from tissue with fibrosis or inflammation in numerous stages throughout chemoradiotherapy. TRG was quantitated in five grades: TRG 1 (complete regression) showed absence of residual cancer and fibrosis extending through the different layers of the esophageal wall; TRG 2 was characterized by the presence of rare residual cancer cells scattered through the fibrosis; TRG 3 was characterized by an increase in the number of residual cancer cells, but fibrosis still predominated; TRG 4 showed residual cancer outgrowing fibrosis; and TRG 5 was characterized by the absence of regressive changes.
This is a very interesting study about Mandard TRG in patients with stage II-III LARA. In this manuscript, the authors analyzed the prognostic value of Mandard TRG in patients with stage II-III locally advanced rectal adenocarcinoma treated with 30-Gy/10-fraction preoperative radiotherapy followed by radical surgery.
| 1. | Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3032] [Cited by in RCA: 3131] [Article Influence: 208.7] [Reference Citation Analysis (0)] |
| 2. | Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;11:4685-4688. [PubMed] |
| 3. | Santos MD, Silva C, Rocha A, Matos E, Nogueira C, Lopes C. Tumor regression grades: can they influence rectal cancer therapy decision tree? Int J Surg Oncol. 2013;2013:572149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1512] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 5. | Terashima M, Hoshino Y, Gotoh M. [Comparisons of standard treatments for colorectal cancer between Japan and Western Countries]. Gan To Kagaku Ryoho. 2007;34:694-699. [PubMed] |
| 6. | Zhan T, Gu J, Li M, Du C. Intermediate-fraction neoadjuvant radiotherapy for rectal cancer. Dis Colon Rectum. 2013;56:422-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Rule W, Meyer J. Current status of radiation therapy for the management of rectal cancer. Crit Rev Oncog. 2012;17:331-343. [PubMed] |
| 8. | Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol. 2013;3:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [PubMed] |
| 10. | Peng Y, Wang L, Du C, Gu J. Expression of vascular endothelial growth factor can predict distant metastasis and disease-free survival for clinical stage III rectal cancer following 30-Gy/10-f preoperative radiotherapy. Int J Colorectal Dis. 2012;27:1555-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Wang L, Zhong XG, Peng YF, Li ZW, Gu J. Prognostic value of pretreatment level of carcinoembryonic antigen on tumour downstaging and early occurring metastasis in locally advanced rectal cancer following neoadjuvant radiotherapy (30 Gy in 10 fractions). Colorectal Dis. 2014;16:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Campa D, Vodicka P, Pardini B, Naccarati A, Carrai M, Vodickova L, Novotny J, Hemminki K, Försti A, Barale R. A gene-wide investigation on polymorphisms in the taste receptor 2R14 (TAS2R14) and susceptibility to colorectal cancer. BMC Med Genet. 2010;11:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1131] [Article Influence: 66.5] [Reference Citation Analysis (6)] |
| 14. | Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008-1015. [PubMed] |
| 15. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [PubMed] |
| 16. | Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701. [PubMed] |
| 17. | Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, Ghadimi M, Mrak K, Merkel S. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 18. | Benzoni E, Intersimone D, Terrosu G, Bresadola V, Cojutti A, Cerato F, Avellini C. Prognostic value of tumour regression grading and depth of neoplastic infiltration within the perirectal fat after combined neoadjuvant chemo-radiotherapy and surgery for rectal cancer. J Clin Pathol. 2006;59:505-512. [PubMed] |
| 19. | Du CZ, Li J, Cai Y, Sun YS, Xue WC, Gu J. Effect of multidisciplinary team treatment on outcomes of patients with gastrointestinal malignancy. World J Gastroenterol. 2011;17:2013-2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Lindebjerg J, Spindler KL, Ploen J, Jakobsen A. The prognostic value of lymph node metastases and tumour regression grade in rectal cancer patients treated with long-course preoperative chemoradiotherapy. Colorectal Dis. 2009;11:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Losi L, Luppi G, Gavioli M, Iachetta F, Bertolini F, D’Amico R, Jovic G, Bertoni F, Falchi AM, Conte PF. Prognostic value of Dworak grade of regression (GR) in patients with rectal carcinoma treated with preoperative radiochemotherapy. Int J Colorectal Dis. 2006;21:645-651. [PubMed] |
| 22. | Santos MD, Silva C, Rocha A, Matos E, Nogueira C, Lopes C. Prognostic value of mandard and dworak tumor regression grading in rectal cancer: study of a single tertiary center. ISRN Surg. 2014;2014:310542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688-8696. [PubMed] |
| 24. | Kim TH, Chang HJ, Kim DY, Jung KH, Hong YS, Kim SY, Park JW, Oh JH, Lim SB, Choi HS. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys. 2010;77:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Rozzini R S- Editor: Yu J L- Editor: Webster JR E- Editor: Ma S