Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1377
Peer-review started: November 4, 2014
First decision: November 14, 2014
Revised: November 24, 2014
Accepted: December 20, 2014
Article in press: December 22, 2014
Published online: February 7, 2015
Processing time: 97 Days and 6.9 Hours
Geographically the prevalence of duodenal ulceration is related to the staple foods in the diet in regions of developing countries where the diet is stable. It is higher in regions where the diet is based on milled rice, refined wheat or maize, yams, cassava, sweet potato, or green bananas, and is lower in regions where the staple diet is based on unrefined wheat or maize, soya, certain millets or certain pulses. Experiments on rat gastric and duodenal ulcer models showed that it was the lipid fraction in staple foods from low prevalence areas that was protective against both gastric and duodenal ulceration, including ulceration due to non-steroidal anti-inflammatory drugs (NSAIDs). It also promoted ulcer healing. The lipid from the pulse, Dolichos biflorus, horse gram which was highly protective was used to identify the fractions with protective activity in the lipid. The protective activity lay in the phospholipid, sterol and sterol ester fractions. In the phospholipid fraction phosphatidyl choline (lethicin) and phosphatidyl ethanolamine (cephalin) were predominant. In the sterol fraction the sub-fractions showing protective activity contained β-sitosterol, stigmasterol, and an unidentified isomer of β-sitosterol. The evidence from animal models shows that certain dietary phospholipids and phytosterols have a protective action against gastroduodenal ulceration, both singly and in combination. This supports the protective role of staple diets in areas of low duodenal ulcer prevalence and may prove to be of importance in the prevention and treatment of duodenal ulceration and management of recurrent ulcers. A combination of phospholipids and phytosterols could also play an important role in protection against ulceration due to NSAIDs.
Core tip: Geographically the prevalence of duodenal ulceration is low in regions where unrefined wheat or maize, certain millets and pulses and soya are staple foods. Experiments on rat gastric and duodenal ulcer models showed that the lipid fraction present in these staple foods was both protective against gastric and duodenal ulceration and also promoted ulcer healing. It was also protective against ulceration due to non-steroidal anti-inflammatory drugs. Further experiments showed that the protective activity lay in the phospholipid and sterol fractions present in the lipid fraction. These fractions were protective individually and in combination. The clinical application is discussed.
- Citation: Tovey FI. Role of dietary phospholipids and phytosterols in protection against peptic ulceration as shown by experiments on rats. World J Gastroenterol 2015; 21(5): 1377-1384
- URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1377.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1377
This research began in the 1950s when the author was working as a surgeon in South India. Duodenal ulceration especially in males was a major surgical problem, the majority of the cases coming from the area where rice was the staple crop and only a few from the area where the staple crops were a millet or pulse. This suggested that duodenal ulcer prevalence was related to the staple diet[1].
Information was then collected from all over India confirming a higher prevalence in areas where milled rice or cassava was the staple food and lower prevalence where the staple food was unrefined wheat, millet or a pulse. Later from 1970 to 1984, in conjunction with the Medical Research Council in the United Kingdom, more information was gathered from Sub-Saharan Africa, China, and other developing countries. A picture was built up by correspondence, by personal visits (two to China, three to Africa and two return visits to India), by assessing reports from many sources and publications, from the opinion of clinical workers as to whether duodenal ulcer was a major or minor problem in the area where they worked, from radiological and necropsy records, and from hospital admission figures.
The period 1951 to 1984 was an ideal time for collecting such information for two reasons. Firstly, the primary treatment for duodenal ulceration in developing countries was still surgical, and valuable information about local prevalence could be gathered from the number of surgical operations for chronic duodenal ulceration, or its complications, obtained from hospitals of similar size and with similar surgical facilities. Nowadays, with the widespread advent of medical treatment for duodenal ulceration, such information is no longer available. Secondly, in rural areas during this period staple diets were stable, whereas nowadays, with better communications, there is much more variety in the diets in many rural areas and any relationship to a staple diet is no longer clear. Information from urban areas was less valuable because of more social classes and a wide variation in diets.
A consistent pattern was established. The prevalence of duodenal ulcer was higher in areas where milled rice, wheat or maize, yams, sorghum, cassava (manioc), sweet potato and green bananas were the staple food. It was lower in regions where unrefined wheat or maize, soya and certain millets and pulses were the staple diet[2-8].
Pyloric Ligation: To determine the effect and nature of the protective factor(s) in the diet of low prevalence areas in India, experiments were carried out on rats with South Indian rice, or rice and cassava diet, with Punjabi unrefined wheat diet, and with millets or pulses from other areas of low duodenal ulcer prevalence, using the pyloric ligation model which produces ruminal ulceration. The rats were fed for two weeks with the food prior to the pyloric ligation and then sacrificed 6 h after ligation. These were compared with equal numbers of rats on a normal laboratory stock diet. The Punjabi diet gave marked protection whereas the South Indian rice diet showed a significant increase in ulceration and the rice plus cassava diet an even greater increase[9].
Rats fed on certain of the millets or pulses from low prevalence areas also showed significantly less ulceration than those fed on foodstuffs from high prevalence areas. Ethanol, ether and water extracts from these and unrefined wheat were then tested and the protective action was found to lie in the ether extracts showing it to be a lipid. Amongst these the pulse Dolichos biflorus (horse gram) and its lipid (HGL) gave the most protection[10].
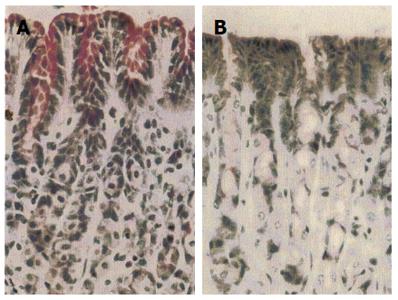
Histological studies of the gastric mucosa of rats fed on South Indian rice diet for 2 wk showed that a supplement of 4 mg HGL daily resulted in an increased content of mucus in the surface epithelial cells and a thicker surface mucous layer compared with those without the supplement (Figure 1).
In the pyloric ligation model the ulceration is due to a high concentration of acid. Further experiments were next conducted on models in which mucosal ulceration was produced by local irritants.
Rat ethanol model: This model was used to compare the protective activity of the lipids extracted from different protective staple foods, including wheat bran and fresh rice bran. Gastric ulceration was produced by the intragastric instillation of 1.0 mL of 100% (later 80%) ethanol into rats 1 h after they had been given a dose of 4.0 mg of the respective lipid. The rats were killed 15 min after the instillation of ethanol and the areas of mucosal ulceration were measured using a planimeter and compared with that of controls. All the lipid extracts gave marked protection and again this was most marked in the lipid obtained from horse gram. HGL also gave protection when given intramuscularly 24 h prior to the rats being given the ethanol. This lipid was selected for further experiments[10].
Aspirin model: To determine its activity against non-steroidal anti-inflammatory drugs (NSAIDs) induced ulceration experiments were done using acidified aspirin to produce gastric ulceration. Two milligram of HGL were instilled into the oesophagus 1 h prior to giving aspirin and resulted in a 90% reduction in the total length of ulceration[10].
Cystamine model: To determine the effect of HGL on duodenal ulceration a novel method of cysteamine induced duodenal ulceration was used. A slow release preparation of cysteamine given by injection to the rats 24 h beforehand produced uniform duodenal ulceration. HGL given either by intra-oesophageal installation or intramuscularly in doses ranging from 4 mg down to 50 μg gave complete protection[10].
HGL promoted the healing of ulcers in the ethanol model, the cysteamine model and in ulcers produced by topical application of acetic acid to the anterior surface of the rat’s stomach[10].
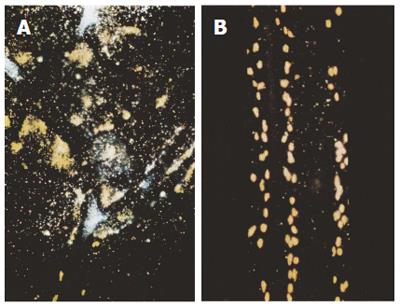
In the pyloric ligation experiments a diet of stored white rice was ulcerogenic when compared with controls on stock diet. Stored rice bran oil greatly increased the extent of the ulceration of rats on South Indian diet when 0.1 mL was instilled into the stomach at the time of pyloric ligation. In this model mucosal biopsies showed severe degranulation of mast cells, but this did not occur if 4 mg of HGL were instilled into the oesophagus prior to pyloric ligation[11-14] (Figure 2).
Fresh rice bran and rice bran oil however were protective in the pyloric ligation experiments. The protective action of fresh rice bran oil was also reported by Gimeno Forner et al[13] using several rat models, including ulceration due to indomethacin. The reason for this change on storage is that within a few days rice bran and rice bran oil become rancid. On milling the bran and germ of rice, unlike those of wheat, do not come separately the bran remaining attached to the germ. The bran is rich in enzymes, in particular lipases, and some oil, and the germ is rich in oil. The resultant bruising of milling causes the lipase in the bran to mix with the oil present in the germ. As a result the oil undergoes rapid lipolysis and peroxidation, with the production of ketoaldehydes which are ulcerogenic in animal models. These changes also occur on storage in the residual oil present in the grains of milled rice, which in India are said to make the rice more tasty. This may account for the high incidence of duodenal ulcer wherever refined or polished rice is the staple diet. This ulcerogenic activity can be reversed by α-tocopherol and by cysteine which inhibit the peroxidation. Also the ulcerogenic activity of rice bran oil disappeared when it was combined with whole wheat oil.
The oil from whole wheat was strongly protective in the pyloric ligation and alcohol models. As mentioned above the bran and germ of wheat come apart separately on milling. Unlike rice the germ is rich in lipase and not the bran. Wheat bran is fairly stable from lipolysis but lipolysis occurs in the germ if it is not treated. Wheat bran oil was strongly protective against ulceration in pyloric ligation and alcohol models but not wheat germ or its oil[11].
Horse gram lipid is rich in phospholipids and sterols. Thin layer chromatography of HGL showed the presence of free fatty acids and triglycerides, phospholipids, sterol esters and sterols. Different techniques were used to obtain fractions of HGL containing each of these components which in turn were tested on different animal models for protective activity[15].
Free fatty acids: Using the pyloric ligation model there was no significant difference between controls and rats on the rice diet plus 3 mg of the free fatty acid and triglyceride fraction, and in rats on a rice and cassava diet receiving 11 mg of the fraction daily.
Phospholipids: Different fractions obtained by different techniques gave protection in the pyloric ligation model in doses of 100 μg for 3 d prior to ligation, in the ethanol model in doses down to 1 μg, and in the cysteamine model in doses of 2 μg.
Sterols: Thin layer chromatography (TLC) of the sterol fraction from HGL showed three bands corresponding to different sterols. These bands were tested for protective activity. Of these the band corresponding to 60% pure β-sitosterol (Sigma) was highly protective in both the rat ethanol (P = 0.0019) and cysteamine models (P = 0.0357). It was effective in both models in very small doses (3.17 μg). It was not protective however in the pyloric ligation model (diet rice and cassava) given a daily dose for two weeks.
The difference may be explained by the fact that sterols when given directly into the stomach in the acute models probably act topically but only for a short time until gastric emptying when their activity ceases. They are poorly absorbed in the small intestine and hence would have no prolonged systemic action when given in daily doses.
Sterol esters: Two fractions shown on TLC were tested. One fraction, when tested using the long-term pyloric ligation model in rats fed on the ulcerogenic rice and cassava diet and given a daily doses 0.93 mg of the fraction for 14 d prior to the ligation, gave highly significant protection. However using the acute ethanol and cysteamine single dose models the fraction gave no protection suggesting that the activity was systemic and not topical.
TLC and high performance liquid chromatography (HPLC) analysis of the phospholipids in HGL showed the presence of phosphatidyl choline and ethanolamine, lyso-phosphatidyl choline and ethanolamine, phosphatidyl serine and inositol plus small amounts of other unidentified phospholipids[16].
Using the rat ethanol model 60% pure β-sitosterol derived from soya (Sigma) gave significant protection. On HPLC of the HGL the fraction corresponding to commercial 60% β-sitosterol showed high peaks corresponding to stigmasterol and β-sitosterol and smaller peaks corresponding to campesterol and ∆7-stigmasterol (Schottenol).
A preparation of 98% pure β-sitosterol was not protective. Likewise 90%-95% pure ∆7-stigmasterol (Schottenol) was not protective whereas 50% pure Schottenol gave highly significant protection. Stigmasterol (90%) gave insignificant protection (P = 0.15). This suggested that the protective activity may lie in a combination of sterols.
The HPLC fraction was now subjected to further analysis and 5 major sub-fractions were obtained and tested for protective activity using the ethanol model. Of these, two closely similar fractions were found showing highly significant activity (P = 0.0006). One of these was shown to contain β-sitosterol and stigmasterol and the other contained β-sitosterol and an unidentified isomer of β-sitosterol which might be У-sitosterol or δ7-stigmasterol (schottenol)[16,17].
The findings show that certain of the phospholipids and sterols/sterol esters present in the lipid of foods from low prevalence areas of duodenal ulcer are highly protective in both gastric and duodenal ulcer animal models, both when incorporated in the diet for 14 d in the long term pyloric ligation models and also acutely following a single dose in the ethanol, aspirin and cysteamine models. The effect therefore may be both systemic and topical.
Phospholipids have an important role in gastric and duodenal mucosa. They are integral components of the cell membranes and of the mucosal cells. They contribute to the viscosity of the surface layer of mucus[18] and prevent intracellular acidification. Unsaturated phospholipids supply unsaturated fatty acids for the synthesis of prostaglandins.
Lichtenberg and colleagues describe the surfactant action of Dipalmitoyl phosphatidyl choline (DPPC) and phosphatidyl choline, forming a hydrophobic barrier to the effect of gastric acid. The surfactant effect, particularly that of DPPC, is enhanced in the presence of neutral lipids allowing the formation of a microemulsion[19,20].
Romero and Lichtenberger[21] reported that the protective effect of unsaturated phospholipids was also enhanced by sterols (β-sitosterol and cholesterol), possibly due to enhancement of the packing of the unsaturated phospholipids in the cell membranes.
Lichtenberger et al[22], Anand et al[23] and Dunjic et al[24] have showed that in rats and humans DPPC and phosphatidyl choline are protective against the injurious effects of NSAIDs.
Phosphatidylcholine is present in plant sterols. DPPC is not found in plant material but is present in milk and animal fats.
Phytosterols regulate membrane fluidity and the activity of membrane bound enzymes[25]. They are active in reducing proton and sodium ion leaks from cell membranes[26] and are reported to be anti-inflammatory[27], anti-pyretic and to enhance immune response[28,29]. Phytosterols enhance the stability of phospholipid monolayers[30]). They are reported to reduce the water permeability of phosphatidyl-choline bilayers.
Experimentally, certain phytosterols present in sponges and coral, but derived from plankton, have been shown to inhibit histamine release from peritoneal mast cells in rats[31,32].
There are only a few reports of the ulceroprotective activity of phytosterols. The enhancement of the protective activity of phospholipids by sterols is mentioned above. Otherwise there are only two reports about the ulceroprotective activity of sitosterols alone in animal models comparing the protective activity of β-sitosterol with its glycoside. One[33] reports on the anti-gastroulcerative activity of β-sitosterol-glycoside and its aglycone in rats using acetic acid and cold stress models, the effect being greater with the aglycone. The second paper[34] reports gastroprotective activity of β-sitosterol and sitosterol-3-0-b-glucoside in rat ethanol, aspirin, histamine, pyloric ligation and 8 d glucose diet models, the sitosterol-glycoside in this report being more effective than sitosterol.
The sterols present in plant material always exist in combination with sterol esters and glycosides (sterolins).
Apart from the two papers quoted above three papers report on the protective activity of sterolins (sterol glycosides), two papers[35,36] report that unripe green banana (musa paradisiaca) in the Gangetic basin is protective against experimental ulceration in the rat aspirin model, between August and October when it contains a combination of four sitosterolins (sitoindoside I and II, sitosterol gentobioside and sitosterol myo-inosityl-b-D-glucoside). The other paper[37], using mice restraint models, reports the ulceroprotective activity of a mixture of steryl-b-D-glucosides.
As reported the free fatty acid fraction obtained from HGL when given as a 14 d supplement was not protective in the rat pyloric ligation model[15]. This may be because essential fatty acids of the ω6 series which are known to take a part in prostaglandin synthesis and have been shown to be protective are only found in certain seed oils. A low intake of linoleic acid has been associated with a high prevalence of duodenal ulceration[38-41].
There is irrefutable evidence that eradication of Helicobacter pylori (H. pylori) infection leads to healing of H. pylori positive duodenal ulcers, but this does not necessarily mean that H. pylori infection is the primary cause of the ulceration[42]. Infection with H. pylori is widespread yet only a minority of infected persons develop duodenal ulceration. There is evidence that H. pylori infection has been prevalent for several centuries yet duodenal ulceration only became common at the beginning of the twentieth century[42-47].
H. pylori infection could be a secondary and not a primary factor in duodenal ulceration. In support information gathered from India, Africa and China, countries with a high prevalence of H. pylori infection, showed no evidence of any correlation between the prevalence of duodenal ulceration and the prevalence of H. pylori infection or with virulence factors, the only correlation being that with the staple foods. In developed countries with a lower prevalence of H. pylori infection a number of duodenal ulcers not linked with NSAIDs, especially early ulcers, are H. pylori negative. Duodenal ulceration can also recur despite eradication of H. pylori infection. Duodenal ulcers heal with proton pump inhibitors without the eradication of H. pylori. As many as 50% of acute perforated duodenal ulcers have been reported as H. pylori negative[48-61].
This suggests that H. pylori may be a secondary factor in duodenal ulceration and the primary cause is high acid secretion linked with reduced mucosal protection. When ulceration occurs the presence of H. pylori could act like a secondary infection, interfering with the process of healing so that the ulcer becomes chronic. Eradication of the H. pylori infection then allows the ulcer to heal.
NSAIDs: NSAIDs are a common cause of peptic ulceration. The lipid fraction from horse gram (HGL) was highly protective against ulceration in the rat aspirin model[10]. Lichtenberger et al[22] and Anand et al[23] have shown that phosphatidyl choline and DPPC are effective against not only aspirin but also indomethacin, diclofenac and naproxen induced ulceration in rats and that the protective activity is enhanced by β-sitosterol[21]. In humans they showed that phosphatidylcholine reduces the toxicity of aspirin and ibuprofen[62]. In a recent clinical trial[63] they reported that a combination of phosphatidyl choline derived from soya lethicin with aspirin reduced the incidence of gastroduodenal ulcers from 17.6% to 5.1% (P = 00069)[63].
Conclusion with human applications: The lipid fractions present in the staple foods, unrefined wheat and maize, soya and certain millets and pulses from low prevalence areas of duodenal ulcer have been shown to be protective against gastric and duodenal ulceration in rat models, the lipid from the pulse horse gram being the most potent. The fraction from horse gram is protective not only against gastric ulceration in the pylorus ligated, ethanol and aspirin models but is also protective against duodenal ulceration induced by cysteamine. The lipid has also been shown to promote ulcer healing.
The experiments show that the protective activity of the lipid lies in its phospholipid and sterol fractions, which give protection both individually and in combination. The lipid was protective when given intramuscularly in both the pyloric ligation and cysteamine models[15] showing that it has a systemic as well as a topical effect. This systemic effect must be due just to the sterols and not the phospholipids present in the lipid, because phospholipids when give intramuscularly are absorbed locally and have no systemic action. This confirms the protective activity of sterols when acting individually. These dietary phospholipids and phytosterols may prove to be of great significance with regards to human duodenal ulceration, both in reducing the prevalence in areas where it is high and also in the management of cases of refractory duodenal ulceration, especially those which recur despite eradication of H. pylori.
The protective activity of the lipid may also be of clinical importance in giving protection against the ulcerogenic effect of NSAIDs. The clinical protection given by phospholipids alone against aspirin and ibuprofen as reported by Lichtenberger et al[22] may well be enhanced by the combination of phytosterols with phospholipid as present in the lipid[16]. A ready source of such a combination would be wheat bran or its oil.
I would like to acknowledge the enormous contribution to the earlier research made by the late Dr Paul Jayaraj MD, FRCPath, and more recently the contributions made by Dr Bruno Linclau, Reader at the School of Chemistry, University of Southampton in the preparation and analysis of the sterols, and Doga Capanoglu, Professors Omez Ozutemiz and Serhat Bor of the Ege University,Bornova , Turkey in conducting the animal bioassays.
| 1. | Tovey FI. Duodenal ulcer in Mysore. Characteristics and aetiological factors. Trop Geogr Med. 1972;24:107-117. [PubMed] |
| 2. | Tovey F. Peptic ulcer in India and Bangladesh. Gut. 1979;20:329-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Tovey FI, Tunstall M. Duodenal ulcer in black populations in Africa south of the Sahara. Gut. 1975;16:564-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Tovey FI, Hobsley M, Segal I, Jayaraj AP. Duodenal ulcer in South Africa: home-pounded versus milled maize. J Gastroenterol Hepatol. 2005;20:1008-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Tovey FI. Duodenal ulcer in China. J Gastroenterol Hepatol. 1992;7:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Wong BC, Ching CK, Lam SK, Li ZL, Chen BW, Li YN, Liu HJ, Liu JB, Wang BE, Yuan SZ. Differential north to south gastric cancer-duodenal ulcer gradient in China. China Ulcer Study Group. J Gastroenterol Hepatol. 1998;13:1050-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Tovey FI. Diet and duodenal ulcer. J Gastroenterol Hepatol. 1994;9:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Tovey FI. Staple diets and duodenal ulcer prevalence. Int Health. 2009;1:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Jayaraj AP, Tovey FI, Clark CG. Possible dietary protective factors in relation to the distribution of duodenal ulcer in India and Bangladesh. Gut. 1980;21:1068-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Jayaraj AP, Tovey FI, Lewin MR, Clark CG. Duodenal ulcer prevalence: experimental evidence for the possible role of dietary lipids. J Gastroenterol Hepatol. 2000;15:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Jayaraj AP, Tovey FI, Clark CG, Hobsley M. Dietary factors in relation to the distribution of duodenal ulcer in India as assessed by studies in rats. J Gastroenterol Hepatol. 2001;16:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Jayaraj AP, Tovey FI, Clark CG, Rees KR, White JS, Lewin MR. The ulcerogenic and protective action of rice and rice fractions in experimental peptic ulceration. Clin Sci (Lond). 1987;72:463-466. [PubMed] |
| 13. | Gimeno Forner L, Bolant Hernández B, Calvo Bermúdez MA, Ariño Matíez J, Palomar Pérez EF, Amorós Escolar JC, Lloris Carsí JM, Narbona B. [Antiulcerogenic properties of bran rice oil in rats]. Rev Esp Enferm Apar Dig. 1989;75:225-230. [PubMed] |
| 14. | Jayaraj AP, Rees KR, Tovey FI, White JS. A molecular basis of peptic ulceration due to diet. Br J Exp Pathol. 1986;67:149-155. [PubMed] |
| 15. | Paul Jayaraj A, Tovey FI, Hobsley M. Duodenal ulcer prevalence: research into the nature of possible protective dietary lipids. Phytother Res. 2003;17:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Tovey FI, Bardhan KD, Hobsley M. Dietary phosphilipids and sterols protective against peptic ulceration. Phytother Res. 2013;27:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Tovey FI, Capanoglu D. Langley JG, Herniman JM, Bor S, Ozutemiz O, Hobsley M, Bardhan KD, Linclau B. Dietary phytosterols protective against peptic ulceration. Gastroenterol Res. 2011;4:149-156. |
| 18. | Murty VL, Sarosiek J, Slomiany A, Slomiany BL. Effect of lipids and proteins on the viscosity of gastric mucus glycoprotein. Biochem Biophys Res Commun. 1984;121:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Lichtenberger LM, Graziani LA, Dial EJ, Butler BD, Hills BA. Role of surface-active phospholipids in gastric cytoprotection. Science. 1983;219:1327-1329. [PubMed] |
| 20. | Hills BA, Butler BD, Lichtenberger LM. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983;244:G561-G568. [PubMed] |
| 21. | Romero JJ, Lichtenberger LM. Sterol-dependence of gastric protective activity of unsaturated phospholipids. Dig Dis Sci. 1990;35:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Lichtenberger LM, Wang ZM, Romero JJ, Ulloa C, Perez JC, Giraud MN, Barreto JC. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med. 1995;1:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 214] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Anand BS, Romero JJ, Sanduja SK, Lichtenberger LM. Phospholipid association reduces the gastric mucosal toxicity of aspirin in human subjects. Am J Gastroenterol. 1999;94:1818-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Dunjic BS, Axelson J, Ar’Rajab A, Larsson K, Bengmark S. Gastroprotective capability of exogenous phosphatidylcholine in experimentally induced chronic gastric ulcers in rats. Scand J Gastroenterol. 1993;28:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Hennessey TM. Effects of membrane plant sterols on excitable cell functions. Comp Biochem Physiol C. 1992;101:1-8. [PubMed] |
| 26. | Haines TH. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog Lipid Res. 2001;40:299-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 256] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K, Bhargava KP. Anti-inflammatory and antipyretic activities of beta-sitosterol. Planta Med. 1980;39:157-163. [PubMed] |
| 28. | Bouic PJ, Lamprecht JH. Plant sterols and sterolins: a review of their immune-modulating properties. Altern Med Rev. 1999;4:170-177. [PubMed] |
| 29. | Navarro A, De las Heras B, Villar A. Anti-inflammatory and immunomodulating properties of a sterol fraction from Sideritis foetens Clem. Biol Pharm Bull. 2001;24:470-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Hac-Wydro K, Wydro P, Jagoda A, Kapusta J. The study on the interaction between phytosterols and phospholipids in model membranes. Chem Phys Lipids. 2007;150:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Shoji N, Umeyama A, Takei M, Arihara S. Potent inhibitors of histamine release: polyhydroxylated sterols from the Okinawan soft coral Sinularia abrupta. J Pharm Sci. 1994;83:761-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Takei M, Burgoyne DL, Andersen RJ. Effect of contignasterol on histamine release induced by anti-immunoglobulin E from rat peritoneal mast cells. J Pharm Sci. 1994;83:1234-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Xiao M, Yang Z, Jiu M, You J, Xiao R. [The antigastroulcerative activity of beta-sitosterol-beta-D-glucoside and its aglycone in rats]. Hua Xi Yi Ke Da Xue Xue Bao. 1992;23:98-101. [PubMed] |
| 34. | Navarrete A, Trejo-Miranda JL, Reyes-Trejo L. Principles of root bark of Hippocratea excelsa (Hippocrataceae) with gastroprotective activity. J Ethnopharmacol. 2002;79:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Ghosal S, Saini KS. Sitoindosides 1 and 11. Two new anti-ulcerogenic steryl acyl-glycosides from Musa Paradisiaca. J Chem Res. 1984;23:965-975. |
| 36. | Ghosal S. Steryl glycosides and acyl steryl glycosides from Musa paradisiaca. Phyochemistry. 1985;24:1807-1810. [DOI] [Full Text] |
| 37. | Okuyama E, Yamazaki M. [The principles of Tetragonia tetragonoides having an antiulcerogenic activity. I. Isolation and identification of sterylglucoside mixture (compound A)]. Yakugaku Zasshi. 1983;103:43-48. [PubMed] |
| 38. | Tarnawski A, Hollander D, Strachura J, Krause WJ, Zipoer RD, Gergely H. Cytoprotection of the gastric mucosa by essential fatty acids (EFA)- prostaglandin precursors. Dig Dis Sci. 1985;30:404. |
| 39. | Tarnawski A, Hollander D, Krause WJ. Is linoleic acid (dietary essential fatty acid) cytoprotective for the gastric mucosa? Gastroenterol. 1986;88:1610. |
| 40. | Tarnawski A, Hollander D, Gergely H. Protection of the gastric mucosa by linoleic acid--a nutrient essential fatty acid. Clin Invest Med. 1987;10:132-135. [PubMed] |
| 41. | Grant HW, Palmer KR, Riermesma RR, Oliver MF. Duodenal ulcer is associated with low dietary linoleic acid intake. Gut. 1990;31:997-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Weiner H, Shapiro AP. Is Helicobacter pylori really the cause of gastroduodenal disease? QJM. 1998;91:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Yamaoka Y, Orito E, Mizokami M, Gutierrez O, Saitou N, Kodama T, Osato MS, Kim JG, Ramirez FC, Mahachai V. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002;517:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 44. | Baron JH. Peptic ulcer. Mt Sinai J Med. 2000;67:58-62. [PubMed] |
| 45. | Baron JH, Sonnenberg A. Hospital admissions for peptic ulcer and indigestion in London and New York in the 19th and early 20th centuries. Gut. 2002;50:568-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Baron JH, Sonnenberg A. Alimentary diseases in the poor and middle class in London 1773-1815, and in New York poor 1797-1818. Aliment Pharmacol Ther. 2002;16:1709-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Kidd M, Modlin IM. A century of Helicobacter pylori: paradigms lost-paradigms regained. Digestion. 1998;59:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Tovey FI, Hobsley M. Is Helicobacter pylori the primary cause of duodenal ulceration? J Gastroenterol Hepatol. 1999;14:1053-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Hobsley M, Tovey FI. Helicobacter pylori: the primary cause of duodenal ulceration or a secondary infection? World J Gastroenterol. 2001;7:149-151. [PubMed] |
| 50. | Hobsley M, Tovey FI, Holton J. Precise role of H pylori in duodenal ulceration. World J Gastroenterol. 2006;12:6413-6419. [PubMed] |
| 51. | Hobsley M, Tovey FI, Holton J. Controversies in the Helicobacter pylori/duodenal ulcer story. Trans R Soc Trop Med Hyg. 2008;102:1171-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Segal I, Ally R, Mitchell H. Helicobacter pylori--an African perspective. QJM. 2001;94:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Ching CK, Lam SK. Helicobacter pylori epidemiology in relation to peptic ulcer and gastric cancer in south and north China. J Gastroenterol Hepatol. 1994;9 Suppl 1:S4-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Holcombe C, Omotara BA, Eldridge J, Jones DM. H. pylori, the most common bacterial infection in Africa: a random serological study. Am J Gastroenterol. 1992;87:28-30. [PubMed] |
| 55. | Bytzer P, Teglbjaerg PS. Helicobacter pylori-negative duodenal ulcers: prevalence, clinical characteristics, and prognosis--results from a randomized trial with 2-year follow-up. Am J Gastroenterol. 2001;96:1409-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Jyotheeswaran S, Shah AN, Jin HO, Potter GD, Ona FV, Chey WY. Prevalence of Helicobacter pylori in peptic ulcer patients in greater Rochester, NY: is empirical triple therapy justified? Am J Gastroenterol. 1998;93:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Borody TJ, George LL, Brandl S, Andrews P, Ostapowicz N, Hyland L, Devine M. Helicobacter pylori-negative duodenal ulcer. Am J Gastroenterol. 1991;86:1154-1157. [PubMed] |
| 59. | Ciociola AA, McSorley DJ, Turner K, Sykes D, Palmer JB. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol. 1999;94:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Pest P, Zárate J, Varsky C, Man F, Schraier M. Helicobacter pylori in recently-diagnosed versus chronic duodenal ulcer. Acta Gastroenterol Latinoam. 1996;26:273-276. [PubMed] |
| 61. | Boulos PB, Botha A, Hobsley M, Holton J, Oshowo AO, Tovey FI. Possible absence of Helicobacter pylori in the early stages of duodenal ulceration. QJM. 2002;95:749-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Lanza FL, Marathi UK, Anand BS, Lichtenberger LM. Clinical trial: comparison of ibuprofen-phosphatidylcholine and ibuprofen on the gastrointestinal safety and analgesic efficacy in osteoarthritic patients. Aliment Pharmacol Ther. 2008;28:431-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Cryer B, Bhatt DL, Lanza FL, Dong JF, Lichtenberger LM, Marathi UK. Low-dose aspirin-induced ulceration is attenuated by aspirin-phosphatidylcholine: a randomized clinical trial. Am J Gastroenterol. 2011;106:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Rodrigo L S- Editor: Qi Y L- Editor: A E- Editor: Wang CH