Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13542
Peer-review started: August 1, 2015
First decision: August 26, 2015
Revised: September 9, 2015
Accepted: October 17, 2015
Article in press: October 20, 2015
Published online: December 28, 2015
Processing time: 145 Days and 9 Hours
AIM: To determine the feasibility and effectiveness of endoscopic resection for the treatment of colorectal granular cell tumors (GCTs).
METHODS: This was a retrospective study performed at a single institution. From January 2008 to April 2015, we examined a total of 11 lesions in 11 patients who were treated by an endoscopic procedure for colorectal GCTs in the Endoscopy Center, Zhongshan Hospital of Fudan University, Shanghai, China. Either endoscopic mucosal resection or endoscopic submucosal dissection (ESD) was performed by three surgeons with expertise in endoscopic treatment. The pre- and post-operative condition and follow-up of these patients were evaluated by colonoscopy and endoscopic ultrasonography (EUS).
RESULTS: Of these 11 lesions, 2 were located in the cecum, 3 were in the ileocecal junction, 5 were in the ascending colon, and 1 was in the rectum. The median maximum diameter of the tumors was 0.81 cm (range 0.4-1.2 cm). The en bloc rate was 100%, and the complete resection rate was 90.9% (10/11). Post-operative pathology in one patient showed a tumor at the cauterization margin. However, during ESD, this lesion was removed en bloc, and no tumor tissue was seen in the wound. No perforations or delayed perforations were observed and emergency surgery was not required for complications. All patients were followed up to May 2015, and none had recurrence, metastasis, or complaints of discomfort.
CONCLUSION: Endoscopic treatment performed by endoscopists with sufficient experience appears to be feasible and effective for colorectal GCTs.
Core tip: Granular cell tumors (GCTs) are asymptomatic and are potentially malignant, which can pose a significant diagnostic and therapeutic challenge for endoscopists. The development of endoscopic techniques has had a marked influence on the diagnosis and treatment of colorectal submucosal tumors. We determined the feasibility and effectiveness of endoscopic resection for the treatment of colorectal GCTs. We conclude that endoscopic resection is safe and effective for treating colorectal GCTs, which allows en bloc resection in one visit, with a clear histological diagnosis, provides patients with a greater degree of comfort and leads to a better compliance.
- Citation: Take I, Shi Q, Qi ZP, Cai SL, Yao LQ, Zhou PH, Zhong YS. Endoscopic resection of colorectal granular cell tumors. World J Gastroenterol 2015; 21(48): 13542-13547
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13542.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13542
Granular cell tumor (GCT) is an uncommon, potentially malignant, asymptomatic mesenchymal tumor arising from Schwann cells[1,2]. In the gastrointestinal tract, GCTs often appear as a round, yellowish submucosal nodule covered by normal mucosa during endoscopy[3-5]. The development of endoscopic techniques and immunohistochemical analysis has had a marked influence on the diagnosis and treatment of gastrointestinal tract submucosal tumors (SMT)[6-9]. Recently, there have been many reports on upper gastrointestinal GCTs[3,9-11]; however, there are few studies on colorectal GCTs. The present study was conducted to evaluate the feasibility and effectiveness of endoscopic resection for the treatment of colorectal GCTs.
During a 7-year period between January 1, 2008 and April 30, 2015, a total of 531 colorectal SMTs were treated at the Endoscopy Center of Zhongshan Hospital of Fudan University, Shanghai, China. A computer search of the SMT pathology files was carried out to determine GCTs arising in the colon, and 11 cases were identified. Patient demographics, pathological data and surgery reports were obtained for all cases (Table 1). All the GCTs were discovered incidentally during endoscopy performed as a screening examination or for unrelated indications. All patients were informed about the option of endoscopic surgery and provided written informed consent.
| Patient number | Gender | Age (yr) | Location | Maximum tumor size (cm) | Treatment methods | Length of stay (d) | En bloc resection | Margin | S-100 |
| 1 | F | 27 | Cecum | 1.0 | EMR | 0 | Yes | Negative | + |
| 2 | F | 33 | Ascending colon | 0.4 | EMR | 1 | Yes | Negative | ++ |
| 3 | M | 42 | Ileocecal junction | 0.6 | EMR | 0 | Yes | Negative | + |
| 4 | M | 47 | Cecum | 0.6 | ESD | 1 | Yes | Negative | ++ |
| 5 | F | 51 | Ascending colon | 0.6 | EMR | 0 | Yes | Negative | + |
| 6 | M | 49 | Rectum | 0.8 | ESD | 1 | Yes | Negative | + |
| 7 | M | 48 | Ascending colon | 0.7 | ESD | 2 | Yes | Positive | + |
| 8 | F | 37 | Ascending colon | 0.8 | ESD | 1 | Yes | Negative | ++ |
| 9 | M | 49 | Ileocecal junction | 1.0 | ESD | 0 | Yes | Negative | ++ |
| 10 | F | 45 | Ileocecal junction | 1.2 | ESD | 1 | Yes | Negative | ++ |
| 11 | M | 60 | Ascending colon | 1.2 | ESD | 1 | Yes | Negative | +++ |
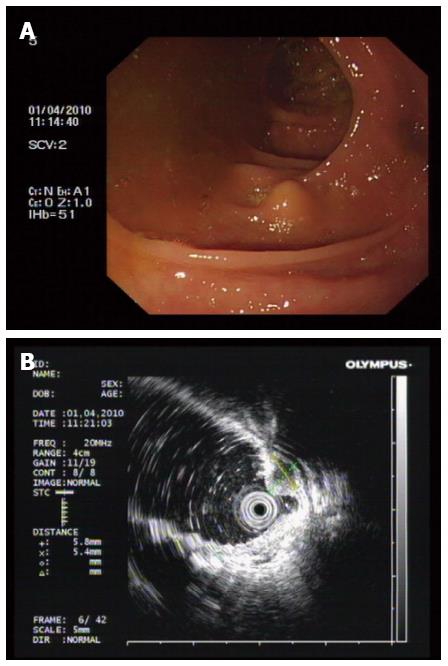
Lesion size, location, color, and surface conditions, with or without ulceration were recorded. Endoscopic ultrasonography (EUS) (Figure 1) was performed to evaluate the depth and echo of the lesion. All patients were asked to finish bowel preparation according to established principles for colorectal surgery.
Standard single-accessory-channel endoscopy (GIT-H260; Olympus, Tokyo, Japan), and AQ100 (Aohua, Shanghai, China) were used during the procedures. A short, transparent cap (ND-201–11802; Olympus) was attached to the front of the endoscope to provide a constant endoscopic view and to apply tension to the connective tissues during dissection. An IT-knife and/or a hook-knife (KD-611 and KD-620LR; Olympus) was used to dissect the submucosal layer and to peel the tumor. The high-frequency generator used was the HybridKnife system (ERBE, Tuebingen, Germany). Other equipment included injection needles (NM-4L-1), snares (SD-230U-20), hot biopsy forceps (FD-410LR), and clips (HX-610–135) (all from Olympus).
All procedures were performed under general anesthesia by three surgeons with expertise in endoscopic treatment (Zhou PH, Yao LQ and Zhong YS).
Endoscopic mucosal resection (EMR) procedures were performed in a standardized manner as follows: (1) Marking; marking dots were made approximately 5-10 mm from the lesion by argon plasma coagulation (APC); (2) Submucosal injection; several milliliters of solution (100 mL saline, 5 mL 0.8% indigo carmine, and 1 mL of epinephrine) were injected around the lesion using a 23-gauge disposable needle; (3) Snare resection; an endoloop was used to snare and ligate the lesion with the aid of suction, and then the lesion was completely resected; and (4) Closure; exposed vessels on the artificial ulcer were coagulated with APC or hot biopsy forceps to prevent delayed bleeding, and metallic clips were always used to close the deeply dissected areas.
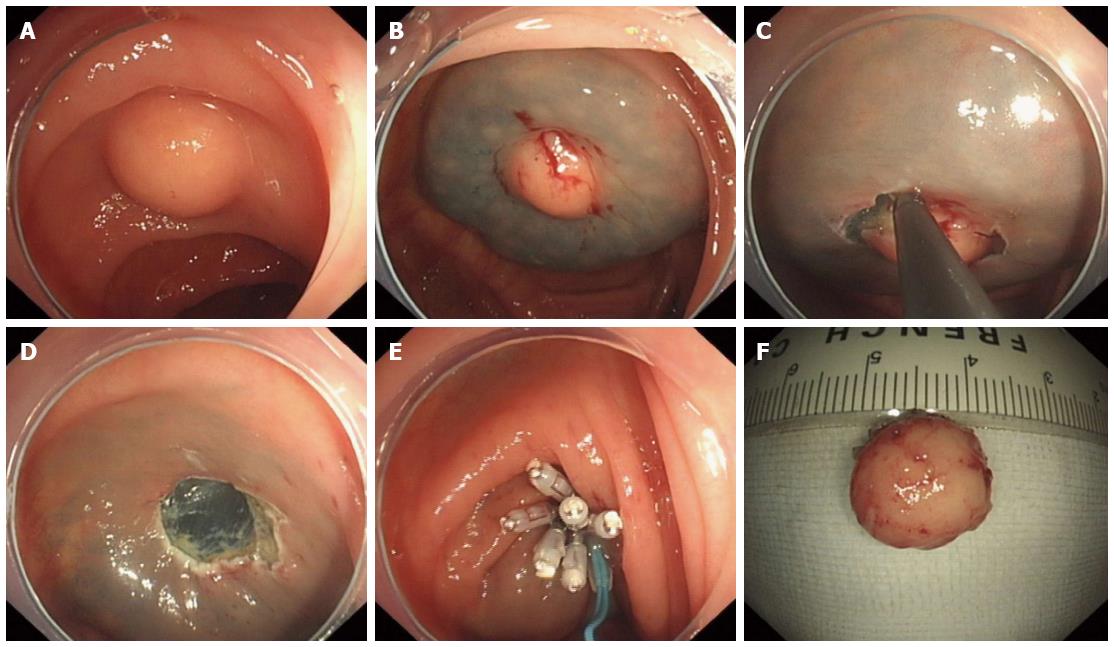
The following steps were used in this procedure (Figure 2): (1) Marking; (2) Submucosal injections were the same as for EMR; (3) Circumferential incision; we used an IT knife or a hook knife to cut the mucosa initially along the marked points; (4) Strip lesions; the submucosal connective tissue beneath the lesion was gradually dissected with the aid of the transparent cap; the above-mentioned solution was injected repeatedly during the dissection when necessary; direct dissection of the submucosal layer was carried out until complete removal was achieved; and (5) Closure was the same as for EMR.
Patients were allowed oral intake from the second day unless serious complications occurred. Antibiotics (second-generation cephalosporins, such as celaclor or cefuroxime) and hemocoagulase injections (ethamsylate or P-aminomethybenzoic acid) were routinely administered after the procedure.
Tissue specimens were fixed to a plastic foam plate using thin needles along their edges and were then fixed in formalin solution. Processing of the resected specimens and histopathological evaluations were performed after endoscopic resection by highly experienced pathologists. A resection with a tumor-free margin in which both the lateral and basal margins were free of tumor cells was considered a complete resection. A resection in which the tumor extended into the lateral or basal margin or in which the margins were indeterminate due to artificial burn effects was considered an incomplete resection and recommended for surgical intervention.
Of the 11 colorectal GCTs in 11 patients, 2 (18.2%) were located in the cecum, 3 (27.3%) were in the ileocecal junction, 5 (45.4%) were in the ascending colon, and 1 (9.1%) was in the rectum. The median maximum diameter of the tumors was 0.81 cm (range 0.4-1.2 cm).
All patients underwent endoscopic resection, including 4 cases of EMR and 7 cases of endoscopic submucosal dissection (ESD). Each lesion was removed by en bloc resection, which was defined as no tumor identified at the resection site by endoscopy. All lesions were submucosal without muscularis layer invasion. There were 10 (90.9%) complete resections, which were defined as no granular cell tissue seen microscopically at the resection margin. Post-operative pathology of one lesion showed that the tumor was seen at the cauterization margin, indicating incomplete resection. However, during ESD, this lesion was removed en bloc, and no tumor tissue was seen in the wound. The patient refused surgery or any other additional treatment after a full discussion with his physician. There was no recurrence in this patient during close follow-up.
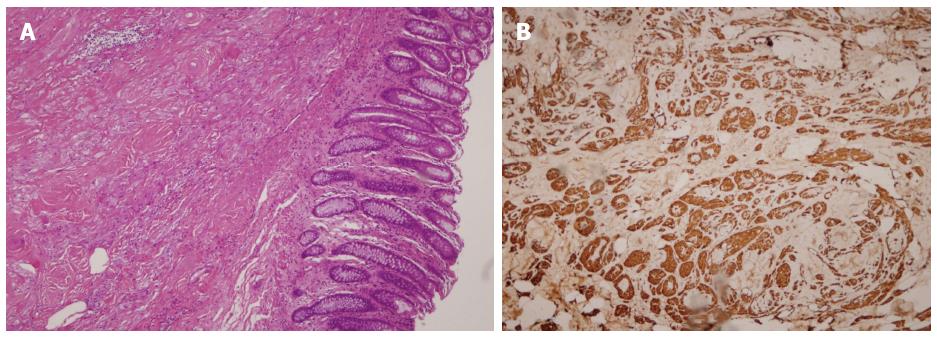
The procedures were successful, with no perforations, endoscopically uncontrolled bleeding, or any other severe adverse events requiring emergency surgery. The patients did not experience abdominal pain, abdominal distension, or any sign of peritonitis after endoscopic surgery. All samples were diagnosed as GCTs by hematoxylin and eosin (HE) staining and immunohistochemical (IHC) analysis (Figure 3).
One rectal GCT patient received a follow-up endoscopy one week after ESD. In the examination up to the rectum, wound healing was satisfactory, dry, and no bleeding was seen. In May 2015, all patients were followed up by telephone, and none complained of discomfort, including hematochezia or changed bowel habits. All 11 patients underwent complete colonoscopy up to the ileocecal junction annually after endoscopic treatment and no abnormalities were observed.
GCTs often occur in the tongue and the skin, and approximately 1%-8% of GCTs occur in the gastrointestinal tract[2,12], one-third of which occur in the esophagus[3,9,11]. Colorectal GCTs are even rarer. With improvements in endoscopy and IHC, there is the potential for an increase in the identification of GCTs in the gastrointestinal tract. Based on our experience, colorectal GCTs are found more frequently in the ileocecal junction and ascending colon. Of the 11 colorectal GCTs in the present study, 2 (18.2%) were located in the cecum, 3 (27.3%) were in the ileocecal junction, 5 (45.4%) were in the ascending colon, and 1 (9.1%) was in the rectum.
Most GCTs are asymptomatic and are clinically insignificant. However, 1%-3% of all GCTs have malignant potential[13,14], thus it is essential to distinguish them from other benign or malignant lesions. Malignant GCTs are often larger than 5 cm, have an unclear margin with the surrounding tissue, invade fat and/or muscle tissue, and are strongly positive for S-100 and neuron-specific enolase on IHC. Neither biopsies nor EUS can reliably distinguish benign GCTs from malignant GCTs; therefore, GCTs can pose a significant diagnostic and therapeutic challenge for endoscopists.
For asymptomatic and relatively small gastrointestinal SMTs, clinical follow-up is standard care. This usually requires patients to undergo multiple endoscopies, which often leads to noncompliance. Furthermore, histopathological diagnoses of SMTs are unobtainable unless the lesion is removed. Endoscopic resection has the advantage of being a minimally invasive technique, which typically results in a shorter operation time, minor post-operative pain, shorter hospital stay, and lower cost than traditional surgery[15,16]. EMR and ESD are both safe and effective for treating superficial gastrointestinal SMTs[17-19].
All our 11 colorectal GCTs evaluated were submucosal without muscularis layer involvement. In view of our results[17,18,20] and those in other studies[21,22], ESD and EMR are both suitable for GCTs less than 2 cm, as their complication rates and long-term outcomes are equivalent. In the present study, the lesions were approximately 1 cm; and the median maximum diameter of the tumors was 0.81 cm (range 0.4-1.2 cm). Eleven lesions were removed en bloc by both ESD and EMR, and the en bloc resection rate was 100%. Post-operative pathology showed a complete resection rate of 100% (4/4) for EMR, and 85.7% (6/7) for ESD. None of the patients in the two groups experienced bleeding, perforation, or disease progression. For lesions larger than 1 cm and/or closely related with the muscularis propria, we suggest performing ESD. During excavation, using the knife to dissect the submucosal connective tissue, the complete tumor capsule must be preserved and perforation avoided. Post-operative pathology showed a positive margin in only one patient; however, ESD achieved en bloc resection, and no tumor tissue was seen in the wound. The patient refused surgery after a full discussion with his physician. No recurrence was observed in this patient during close follow-up.
The main complication of endoscopic treatment for colorectal SMTs is perforation; this is also the bottleneck which limits endoscopic treatment of colorectal SMTs. With the widespread use of endoscopic full-thickness resection for gastric SMTs and improvements in suturing techniques[7,8,17], colorectal defect repair skills have also significantly improved. Common endoscopic suture methods suitable for colorectal defect repair include: (1) clipping; (2) clipping then strengthening with the endoloop; (3) purse-string suture with metallic clips and endoloop; and (4) interrupted suture with endoloop and metallic clips[23]. Furthermore, we have reported the submucosal tunneling endoscopic resection for the treatment of rectal SMTs[7], which solves the problem of difficulties in complete closure using the endoscopic suture technique. By mastering such techniques, breaking through the bottleneck can lead to expanded indications for endoscopic treatment, and the successful treatment of colorectal SMTs using minimally invasive endoscopic techniques.
Endoscopic resection is a safe, feasible and effective treatment for colorectal GCTs, allows en bloc resection in one visit, and a clear histological diagnosis.
In conclusion, endoscopic treatment performed by endoscopists with sufficient experience appears to be feasible and effective for colorectal GCTs.
Colorectal granular cell tumors (GCTs) are often asymptomatic and potentially malignant. As the tumor grows, patients present with different symptoms depending on the tumor location and size. Diagnosis and management of colorectal GCTs remain challenging.
Due to the rarity of these tumors, few studies have focused on colorectal GCTs. Therefore, this study described the authors’ experience in the diagnosis and surgical treatment of patients with colorectal GCTs.
Based on this study, the use of endoscopy, endoscopic ultrasonography and immunohistochemistry are effective means of diagnosing colorectal GCTs. The authors focused on the treatment of colorectal GCTs by endoscopic resection, which proved to be a feasible and effective treatment.
Endoscopic resection is a feasible and effective treatment for colorectal GCTs. Future randomized controlled trials comparing endoscopic resection and surgery are needed to address quality of life issues.
Endoscopic submucosal dissection and endoscopic mucosal resection are two treatment modalities used for gastrointestinal submucosal tumors.
Early detection and simultaneous removal of colorectal granular cell tumors with endoscopy are safe and effective which provide patients with a greater degree of comfort and lead to a better compliance.
| 1. | Stefansson K, Wollmann RL. S-100 protein in granular cell tumors (granular cell myoblastomas). Cancer. 1982;49:1834-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, Chun B. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 366] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Lu W, Xu MD, Zhou PH, Zhang YQ, Chen WF, Zhong YS, Yao LQ. Endoscopic submucosal dissection of esophageal granular cell tumor. World J Surg Oncol. 2014;12:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Irisawa A, Hernandez LV, Bhutani MS. Endosonographic features of a granular cell tumor of the colon. J Ultrasound Med. 2001;20:1241-1243. [PubMed] |
| 5. | An S, Jang J, Min K, Kim MS, Park H, Park YS, Kim J, Lee JH, Song HJ, Kim KJ. Granular cell tumor of the gastrointestinal tract: histologic and immunohistochemical analysis of 98 cases. Hum Pathol. 2015;46:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Shi Q, Xu MD, Chen T, Zhong YS, Zhou PH, Wu HF, Yao LQ. Endoscopic diagnosis and treatment of calcifying fibrous tumors. Turk J Gastroenterol. 2014;25 Suppl 1:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Hu JW, Zhang C, Chen T, Zhou PH, Zhong YS, Zhang YQ, Chen WF, Li QL, Yao LQ, Xu MD. Submucosal tunneling endoscopic resection for the treatment of rectal submucosal tumors originating from the muscular propria layer. J Cancer Res Ther. 2014;10 Suppl:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc. 2012;75:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Komori K, Akahoshi K, Tanaka Y, Motomura Y, Kubokawa M, Itaba S, Hisano T, Osoegawa T, Nakama N, Iwao R. Endoscopic submucosal dissection for esophageal granular cell tumor using the clutch cutter. World J Gastrointest Endosc. 2012;4:17-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Chen WS, Zheng XL, Jin L, Pan XJ, Ye MF. Novel diagnosis and treatment of esophageal granular cell tumor: report of 14 cases and review of the literature. Ann Thorac Surg. 2014;97:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Nakajima M, Kato H, Muroi H, Sugawara A, Tsumuraya M, Otsuka K, Domeki Y, Onodera S, Sasaki K, Tsubaki M. Esophageal granular cell tumor successfully resected by endoscopic submucosal dissection. Esophageal granular cell tumor successfully resected by endoscopic submucosal dissection. Esophagus. 2011;8:203-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Kahng DH, Kim GH, Park do Y, Jeon MS, Yi JW, Choi YY, Song GA. Endoscopic resection of granular cell tumors in the gastrointestinal tract: a single center experience. Surg Endosc. 2013;27:3228-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Saleh H, El-Fakharany M, Frankle M. Multiple synchronous granular cell tumors involving the colon, appendix and mesentery: a case report and review of the literature. J Gastrointestin Liver Dis. 2009;18:475-478. [PubMed] |
| 14. | Thacker MM, Humble SD, Mounasamy V, Temple HT, Scully SP. Case report. Granular cell tumors of extremities: comparison of benign and malignant variants. Clin Orthop Relat Res. 2007;455:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Zhong YS, Shi Q, Wu HF, Yao LQ, Zhou PH, Xu MD, Chen SY. Endoscopic resection for the treatment of duodenal Brunner’s adenomas. J Laparoendosc Adv Surg Tech A. 2012;22:904-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Zhou PH, Shi Q, Zhong YS, Yao LQ. New progress in endoscopic treatment of esophageal diseases. World J Gastroenterol. 2013;19:6962-6968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Shi Q, Zhong YS, Yao LQ, Zhou PH, Xu MD, Wang P. Endoscopic submucosal dissection for treatment of esophageal submucosal tumors originating from the muscularis propria layer. Gastrointest Endosc. 2011;74:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Zhou PH, Yao LQ, Xu MD, Zhong YS, Zhang YQ, Chen WF. Endoscopic ultrasonography and submucosal resection in the diagnosis and treatment of rectal carcinoid tumors. Chin Med J (Engl). 2007;120:1938-1939. [PubMed] |
| 20. | Zhou PH, Yao LQ, Qin XY, Xu MD, Zhong YS, Chen WF, Ma LL, Zhang YQ, Qin WZ, Cai MY. Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors: a retrospective study. Surg Endosc. 2010;24:2607-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Hoteya S, Yahagi N, Iizuka T, Kikuchi D, Kawano K, Noguchi T, Mizuno H, Hashimoto M. [Endoscopic resection for early gastric cancers by EMR/ESD]. Gan To Kagaku Ryoho. 2007;34:16-20. [PubMed] |
| 22. | Goto O, Uraoka T, Horii J, Yahagi N. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014;24:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z, Xu MD, Yao LQ. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy. 2013;45:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Arolfo S, Reshetnyak VI S- Editor: Gong ZM L- Editor: Webster JR E- Editor: Wang CH