Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13447
Peer-review started: May 27, 2015
First decision: August 26, 2015
Revised: September 12, 2015
Accepted: October 17, 2015
Article in press: October 20, 2015
Published online: December 28, 2015
Processing time: 212 Days and 12.9 Hours
AIM: To investigate the roles and interactions of mutT homolog (MTH)-1 and hypoxia-inducible factor (HIF)-1α in human colorectal cancer (CRC).
METHODS: The expression and distribution of HIF-1α and MTH-1 proteins were detected in human CRC tissues by immunohistochemistry and quantitative real-time polymerase chain reaction (qRT-PCR). SW480 and HT-29 cells were exposed to normoxia or hypoxia. Protein and mRNA levels of HIF-1α and MTH-1 were analyzed by western blotting and qRT-PCR, respectively. In order to determine the effect of HIF-1α on the expression of MTH-1 and the amount of 8-oxo-deoxyguanosine triphosphate (dGTP) in SW480 and HT-29 cells, HIF-1α was silenced with small interfering RNA (siRNA). Growth studies were conducted on cells with HIF-1α inhibition using a xenograft tumor model. Finally, MTH-1 protein was detected by western blotting in vivo.
RESULTS: High MTH-1 mRNA expression was detected in 64.2% of cases (54/84), and this was significantly correlated with tumor stage (P = 0.023) and size (P = 0.043). HIF-1α protein expression was correlated significantly with MTH-1 expression (R = 0.640; P < 0.01) in human CRC tissues. Hypoxic stress induced mRNA and protein expression of MTH-1 in SW480 and HT-29 cells. Inhibition of HIF-1α by siRNA decreased the expression of MTH-1 and led to the accumulation of 8-oxo-dGTP in SW480 and HT-29 cells. In the in vivo xenograft tumor model, expression of MTH-1 was decreased in the HIF-1α siRNA group, and the tumor volume was much smaller than that in the mock siRNA group.
CONCLUSION: MTH-1 expression in CRC cells was upregulated via HIF-1α in response to hypoxic stress, emphasizing the crucial role of HIF-1α-induced MTH-1 in tumor growth.
Core tip: Hypoxia is a common characteristic of solid tumors. However, the relationship between hypoxia-inducible factor (HIF)-1α and the human mutT homolog (MTH)-1 had not been clearly investigated. The present study revealed a new mechanism through which HIF-1α upregulates MTH-1 expression in colorectal cancer and provided evidence that hypoxia enhances the expression of MTH-1, likely by modulating HIF-1α protein level. These results emphasize the important role of HIF-1α-induced MTH-1 in tumor progression.
- Citation: Qiu Y, Zheng H, Sun LH, Peng K, Xiao WD, Yang H. Hypoxia-inducible factor-1 modulates upregulation of mutT homolog-1 in colorectal cancer. World J Gastroenterol 2015; 21(48): 13447-13456
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13447.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13447
Colorectal cancer (CRC) is one of the most lethal solid tumors of the gastrointestinal tract and is especially common in elderly populations. In 2008, there were an estimated 1.23 million new cases of CRC and 608700 deaths[1]. Early diagnosis results in a favorable prognosis, such that stage I and stage II disease have an 80%-90% 5-year survival. In contrast, 5-year survival is only 8.1% for stage IV[2]. The propensity for tumors to progress and metastasize reflects not only the oncogenic mutations in the cancer cells but also dynamic interactions between tumor cells and their local microenvironment[3]. Understanding these processes has advanced both our understanding of the underlying causes of CRC and our ability to innovate novel cancer treatment strategies.
Hypoxia is a common feature in most solid human tumors. It has been hypothesized that exposure to hypoxia is associated with oxidative stress and elevated levels of oxidatively damaged DNA [e.g., 8-oxo-deoxyguanosine triphosphate (dGTP)][4]. Accumulation of oxidized bases in either nuclear or mitochondrial DNA triggers various cellular dysfunctions, including mutagenesis and programmed cell death or senescence[5]. To offset oxidative damage to nucleic acids, tumor cells are equipped with antioxidant enzymes like human mutT homolog (MTH)-1. MTH-1 is able to eliminate oxidized deoxynucleotide triphosphates (dNTPs) before they are incorporated into DNA by hydrolyzing 8-oxo-dGTP and 2-hydroxy-dATP to the monophosphate[6]. Correlation between MTH-1 expression and clinical stage was previously discussed with regard to non-small cell lung carcinomas and breast tumors[7,8]. Furthermore, it has been suggested that MTH-1 represents a molecular marker of oxidative stress and can be used to explore the relationship between oxidative stress and genomic instability[9].
The search for factors affecting the progression and behavior of tumors has revealed the importance of the microenvironment surrounding the tumor cells. Hypoxia-inducible factor (HIF)-1 is a major transcriptional factor for tumor cells growing in a low-oxygen environment[10]. HIF-1 is a heterodimer composed of an inducible HIF-1α subunit and a HIF-1β subunit. HIF-1β is a nuclear protein that is constitutively expressed and functions independently of oxygen tension. HIF-1α, in contrast to HIF-1β, is a cytoplasmic protein responsive to oxygen levels. Therefore, all HIF-1 activity is determined by the intracellular level of HIF-1α[11]. Hypoxia induces HIF-1α, which binds to the hypoxia-response elements present in target genes, controlling glucose transport, angiogenesis, erythropoiesis, and intracellular homeostasis and potentially increasing the survival of tumor cells[12]. DNA microarray analysis suggests that more than 2% of all human genes in endothelial cells are regulated by HIF-1α[13]. It is well accepted that hypoxia in the depth of solid tumors and cell survival often coexist during tumor growth and that experimental hypoxia provokes base excision repair (BER) changes in CRC cells[14]. However, little is known about hypoxic status in vivo and its functional relationship with the expression of MTH-1 in CRCs. Therefore, we first examined the expression and localization of HIF-1α immunohistochemically in relation to MTH-1 in CRC. Based on the topological correlation between the two molecules, we hypothesized that MTH-1 expression may be upregulated by hypoxic conditions to facilitate colorectal tumor growth. In this case, regulation of HIF-1α-induced MTH-1 expression might represent a novel therapeutic target in CRC.
SW480 and HT-29 cells were maintained in Roswell Park Memorial Institute (RPMI)-1640 medium (HyClone; Thermo Fisher Scientific, Inc., Pittsburgh, PA, United States) supplemented with 10% fetal bovine serum (Gibco, Life Technologies, Carlsbad, CA, United States) and antibiotics (1% penicillin and 1% streptomycin) at 37 °C with 95% air and 5% CO2. To expose cells to a hypoxic environment, cells were placed in an airtight chamber with inflow and outflow valves infused with a gas mixture (1% O2, 5% CO2, and 94% N2).
Overall, 84 patients (58 males, 26 females) diagnosed with CRC at the Department of Pathology, Xinqiao Hospital, Third Military Medical University, China, were enrolled in the study. All specimens were resected surgically between 2012 and 2014, and the diagnoses were confirmed pathologically. No patient had received preoperative chemotherapy or radiotherapy. None of the patients had a known history of familial polyposis syndrome or hereditary nonpolyposis colorectal cancer syndrome. Tumor stage was defined according to the CRC staging standard by the International Union Against Cancer. All specimens were classified according to the differentiation degree: 15 cases were well differentiated, 39 were moderately well differentiated, and 30 were poorly differentiated. Each tissue was used with the approval of the Ethics Committee of the Xinqiao Hospital, Third Military Medical University, after obtaining written informed consent from the patients.
The tissues were fixed in 4% paraformaldehyde, cut into 4 μm sections, treated with 0.5% hydrogen peroxide in methanol, blocked for 45 min, and subsequently incubated with anti-hMTH-1 (1:250; Abcam, Cambridge, United Kingdom), anti-HIF-1α (1:350; Abcam), or purified rabbit immunoglobulin G (IgG) (10 mg/mL; negative control) overnight at 4 °C. Following incubation with biotinylated secondary goat anti-rabbit antibody (Zhongshan Golden Bridge, Beijing, China) and an avidin-biotin-peroxidase complex (Zhongshan Golden Bridge) for 45 min at 37 °C, respectively, slides were colored using diaminobenzidine, and nuclei were counterstained with Mayer’s modified hematoxylin and mounted with polyvinylpyrrolidone. The histological examination was performed under a light microscope (400 ×).
Human-specific HIF-1α small interfering RNA (siRNA) and a nontargeting control siRNA were synthesized and purified by Sangon Biotech (Shanghai, China). Target sequence for human HIF-1α siRNA was 5’-GGAAATGAGAGAAATGCTTAC-3’, and target sequence for nonsilencing siRNA (mock) was 5’-AATTCTCCGAACGTGTCACGT-3’. SW480 and HT-29 cells were plated at a concentration of 8 × 105 cells per well in six-well plates on the day before siRNA transfection. After 24 h, the cells were transfected with siRNA in Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) reagent. After incubation for 6 h, medium was replaced with fresh RPMI-1640. According to the indicated time, the cells were incubated for subsequent studies.
Samples of tumor and normal tissue were immediately immersed in RNA later solution (Qiagen, Hilden, Germany) and stored at -80 °C until processed. Total RNA from cells or tissues was extracted using the commercial RNeasy mini kit (Qiagen). Total RNA was transcribed with a PrimeScript RT reagent kit (Takara Bio, Shiga, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) assays were performed using the Thermal Cycler Dice Real Time System II (Takara Bio) and SYBR Premix Ex Taq II (Takara Bio). Primer sequences used in this study were designed as follows: HIF-1α (forward 5’-GCCGCTGGAGACACAATCATA-3’ and reverse 5’-GGTGAGGGGAGCATTACATCAT-3’), MTH-1 (forward 5’-TAGTCAGCTGTTAGACTCCCTGC-3’, reverse 5’-GTGGAAAGCACACCAACAGG-3’), β-actin (forward 5’-ATCATGTTTGAGACCTTCAA-3’, reverse 5’-CATCTCTTGCTCGAAGTCCA-3’). PCR denaturing was set at 94 °C for 5 min, and annealing/extending was set at 59 °C for 1 min, for a total of 40 cycles. The specificity of the amplified PCR products was assessed by a melting curve analysis. The expression of each gene was normalized to β-actin expression in the individual samples.
The cells were washed twice with phosphate-buffered saline (PBS) before lysis in cold RIPA buffer (PBS, 1% Nonidet P-40 (NP-40), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mg/mL (p-amidinophenyl) methanesulfonyl fluoride hydrochloride, 1.0 mmol/L sodium orthovandate, 1 ×mammalian protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, United States). The protein concentration was determined according to the Bradford method using bicinchoninic acid (BCA) assay reagent (Beyotime, Beijing, China). Samples (25 μg protein) were resolved on 8%-12% SDS-polyacrylamide gel electrophoresis (PAGE) and then electrophoretically transferred to a polyvinylidene fluoride membranes (Millipore, Bedford, MA, United States). Membranes were blocked in 5% bovine serum albumin for 1 h at room temperature and then incubated with antibodies overnight at 4 °C: anti-MTH-1 (1:500), anti-HIF-α (1:1000), and anti-β-actin (1:1000). The membranes were then washed three times in Tris-buffered saline with Tween (TBST) (50 mmol/L Tris-HCl pH 7.5, 140 mmol/L NaCl, 0.1% Tween) and incubated with secondary antibody at room temperature for 1 h. An enhanced chemiluminescence (ECL) reagent, ECL western blotting detection reagent (Amersham Life Sciences, Chalfont St. Giles, United Kingdom), was used to enable the labeled protein bands to be detected with Image Station 4000R (Kodak, New Haven, CT, United States).
The DNA hydrolysates were dissolved in high performance liquid chromatography (HPLC) grade water and filtered through a 0.2-μm syringe filter before applying the samples to a Waters ODS HPLC column (4.6 mm × 250 mm), 5-μm particle size (Milford, MA, United States). The running buffer for 8-oxo-dGTP from nuclear and mitochondrial DNA was 50 mmol/L potassium phosphate (pH 5.1) in 5% acetonitrile, and the retention time was 7.5 min. Detection of 8-oxo-dG required an electrochemical detector (Coulochem 5100H, Interscience, Breda, The Netherlands) with a 5021 conditioning cell and 5010 analytical cell. Standard samples of dGTP and 8-oxo-dGTP were analyzed to ensure their correct separation and to allow identification of those derived from cellular DNA. Three determinations were made on each hydrolyzed pooled sample, one of them including a spike of standard 8-oxo-dGTP, and the average of the two unspiked replicates was used in the calculation of mean values shown in the Tables.
Five-week-old female BALB/C nude mice were purchased from the Laboratory Animal Center of Third Military Medical University and were maintained in specific pathogen-free units under isothermal conditions. All animal use procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Third Military Medical University Animal Care Committee. SW480 and HT-29 cells (5 × 105) suspended in 0.1 mL serum-free medium were implanted subcutaneously into nude mice. After the tumor volume reached 50-70 mm3, which was calculated according to the formula V = 0.5 × L × S2 (L: long diameter; S: short diameter), the animals were divided into interference and mock groups. According to the method of Filleur et al[15], the mice were intraperitoneally injected with 3 μL siRNA or mock siRNA suspended in 50 μL saline three times per week. Tumors were monitored by measuring their volume with a caliper. After 48 d, animals were killed, and the tumor was dissected for further study.
All the data were repeated three times and analyzed by Graphpad Prism version 6.0 (La Jolla, CA, United States). Data of immunohistochemical staining were analyzed by using Fisher’s exact probability test. One-way analysis of variance (ANOVA) and the Mann-Whitney U test were used to analyze quantitative data, and P < 0.05 was considered to be statistically significant.
MTH-1 mRNA level was measured in 84 matched pairs of CRC/adjacent histologically normal mucosa tissue samples by qRT-PCR. The tumor/normal ratio of MTH-1 expression (T/N ratio) in each case was calculated. All of the normal colorectal mucosa expressed MTH-1 mRNA in low amounts. Relative overexpression of MTH-1 mRNA (T/N ratio ≥ 1.5) was observed in 54 of 84 (64.2%) CRCs (Table 1). The relationship between expression of the MTH-1 gene and clinicopathological features of the CRC was examined. Stage III/IV tumors exhibited significantly higher levels of MTH-1 mRNA relative to early-stage tumors. A significant difference was also observed for tumor size (P = 0.043, Table 1). No significant differences were observed in age, gender, and histological differentiation.
| Characteristics | n (%) | MTH-1 | |
| means ± SD | P value | ||
| Total | 84 (100) | ||
| Age (yr) | |||
| ≤ 60 | 39 (46.43) | 3.782 ± 4.017 | 0.3921 |
| > 60 | 45 (53.57) | 2.720 ± 1.993 | |
| Gender | |||
| Male | 58 (69.05) | 3.002 ± 2.609 | 0.2981 |
| Female | 26 (30.95) | 4.146 ± 3.449 | |
| Differentiation degree | |||
| Well and moderate | 52 (61.90) | 3.549 ± 2.971 | 0.2091 |
| Poor | 32 (39.10) | 2.666 ± 3.302 | |
| Tumor size (cm) | |||
| ≤ 3 | 32 (38.10) | 1.673 ± 1.342 | 0.0431 |
| > 3 | 52 (61.90) | 4.159 ± 3.593 | |
| Stage | |||
| I/II | 51 (60.71) | 2.098 ± 1.627 | 0.0232 |
| III/IV | 33 (29.29) | 4.935 ± 4.028 | |
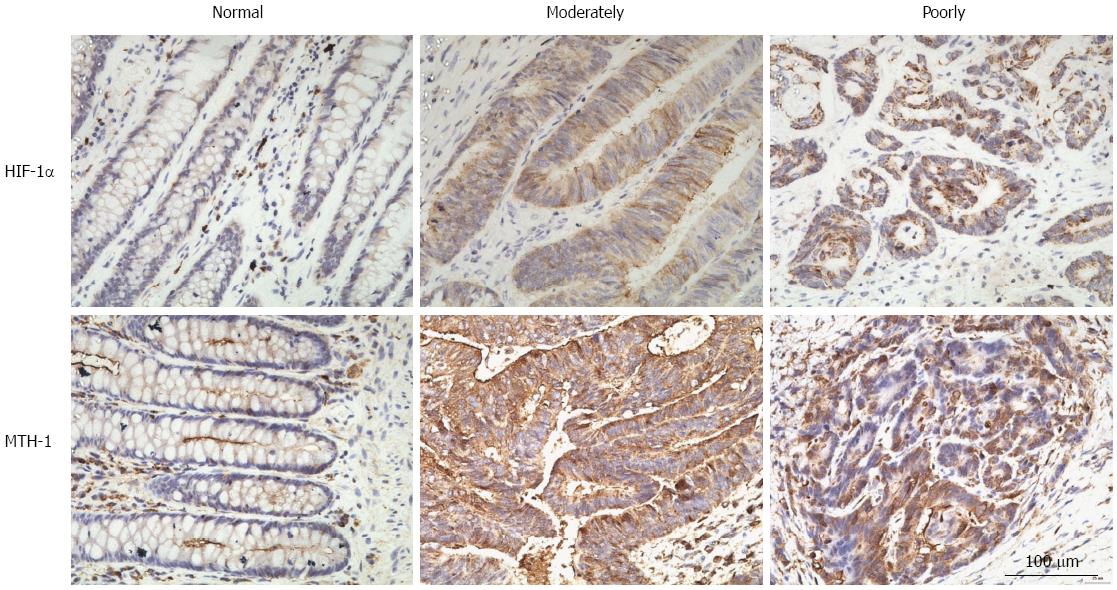
To investigate the association between HIF-1α and MTH-1 expression in CRC, we performed immunohistochemical staining for these proteins in tissue specimens from 84 CRC patients. Staining for HIF-1α and MTH-1 in representative clinical samples is shown in Figure 1. Among the 84 CRC specimens, 66.7% (56/84) and 60.0% (47/84) were positive for HIF-1α and MTH-1 expression, respectively. HIF-1α was predominantly expressed in the cytoplasm and nucleus of the tumor cells, while MTH-1 was largely expressed in the cytoplasm. However, there was no expression or only weak expression of both proteins in the corresponding normal tissue. The expression level of HIF-1α and MTH-1 was significantly higher in CRC tissues compared with corresponding normal tissues. Coexpression of HIF-1α and MTH-1 was detected in 43 (51.2%) patients. Spearman analysis showed that the expression level of HIF1-α was significantly associated with MTH-1 expression (R = 0.640, P < 0.01; Table 2).
| MTH-1 | HIF-1α(n) | Total | |
| Positive | Negative | ||
| Positive | 43 | 4 | 47 |
| Negative | 13 | 24 | 37 |
| Total | 56 | 28 | 84 |
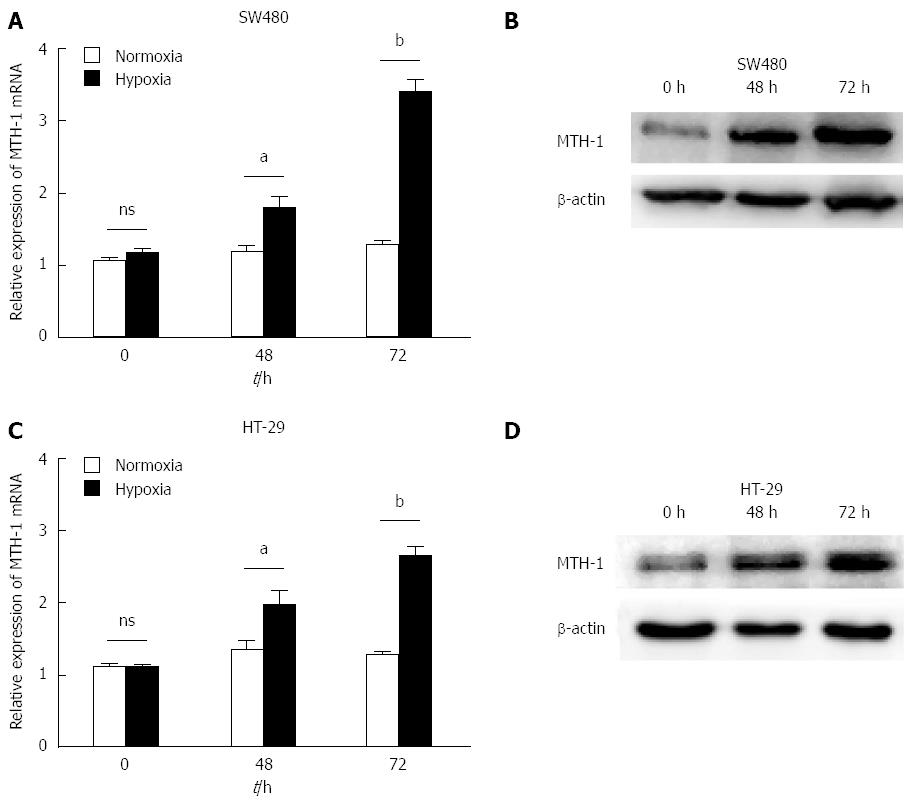
SW480 and HT-29 cells were incubated under normoxic and hypoxic conditions. mRNA levels of MTH-1 were determined by qRT-PCR from cells exposed to hypoxia for 0, 48, and 72 h. Hypoxia enhanced the levels of MTH-1 mRNA in a time-dependent manner (Figure 2A and C). Protein levels of MTH-1 were markedly increased after exposure to hypoxia for 48 h and 72 h (Figure 2B and D).
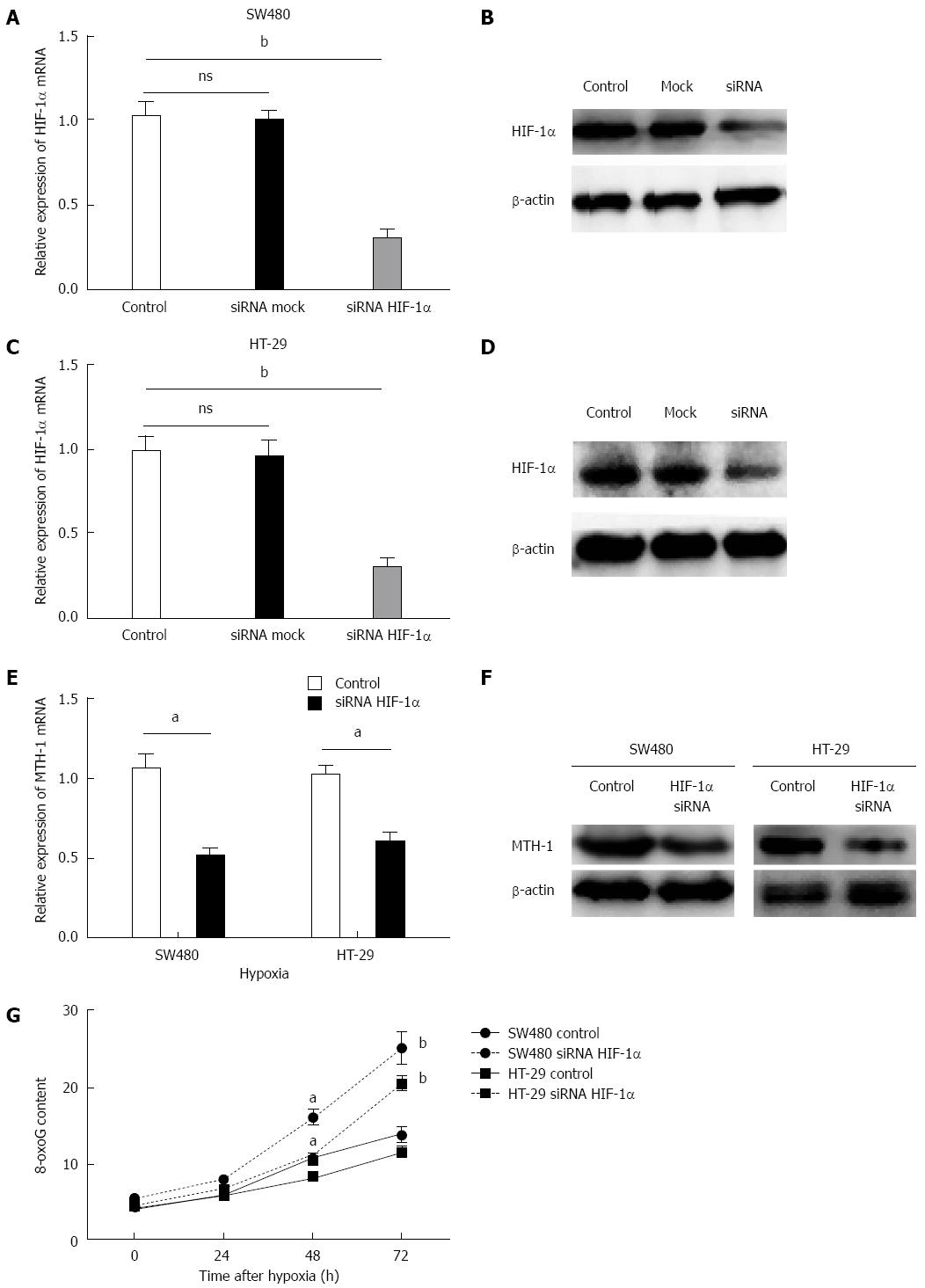
A major increase in MTH-1 was observed after 72 h of hypoxia, therefore, this time point was chosen for the following experiments. As shown in Figure 3A-D, transfection with HIF-1α siRNA markedly reduced the mRNA expression level of HIF-1α (P < 0.01) and expression of HIF-1α protein (P < 0.01). Upregulation of MTH-1 mRNA, induced by hypoxia, was significantly attenuated when HIF-1α expression was knocked down by siRNA (Figure 3E). Consistent with this observation, the HIF-1α siRNA also decreased MTH-1 protein expression in response to hypoxia (Figure 3F). To determine whether the downregulated expression of MTH-1 was involved in BER, levels of 8-oxo-dGTP were measured by HPLC/electrochemical detection in nucleoside mixtures prepared from DNA of two strains of CRC cells (SW480 and HT-29). These cells displayed elevated residual 8-oxo-dGTP base lesions at 48 and 72 h following hypoxia treatment. As shown in Figure 3G, transfection with HIF-1α siRNA markedly increased the level of 8-oxo-dGTP.
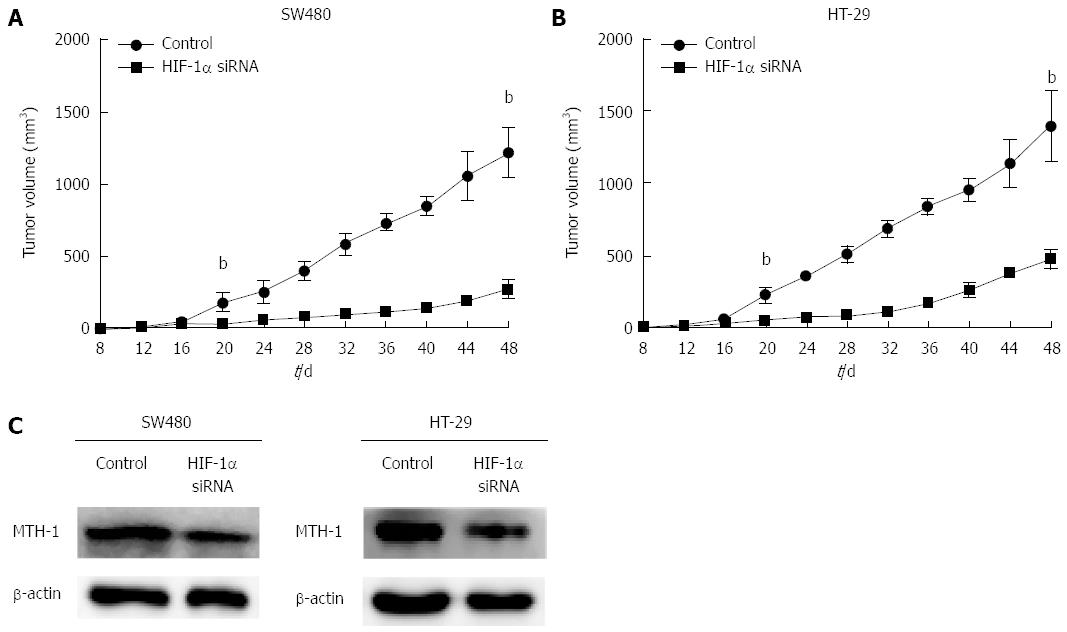
To determine the effect of HIF-1α on MTH-1 expression and CRC tumor growth in vivo, SW480 and HT-29 cells suspended in serum-free medium were subcutaneously implanted into nude mice, and siRNA was intraperitoneally injected. Growth of the xenograft tumor was studied by tumor growth curve. There was a significant difference in tumor growth between the HIF-1α interference group and the mock group after 20 d (Figure 4A and B). MTH-1 protein expression was analyzed in the xenograft tumor by western blotting. As shown in Figure 4C, MTH-1 proteins showed a marked reduction in the siHIF-1α group as compared with the mock group.
Regions of acute and chronic hypoxia exist within solid tumors and can lead to increased rates of mutagenesis and/or altered DNA damage and repair protein expression. The MTH-1 protein sanitizes oxidized dNTP pools to prevent incorporation of damaged bases during DNA replication[16]. Recent studies showed that MTH-1 protein may be a potent promoter of tumorigenic growth and that MTH-1 catalytic activity is markedly increased in many cancers[17,18], which is an example of non-oncogene addiction and probably a survival response to prevent cell death induced by oxidative DNA damage. In the present study, we analyzed the expression patterns of MTH-1 mRNA in CRC. We found a relative overexpression of MTH-1 mRNA (T/N ratio ≥ 1.5) in 64.2% of CRCs. In addition, we observed that advanced-stage tumors exhibited significantly higher levels of MTH-1 mRNA relative to early-stage tumors. Although the number of cases analyzed in this study was not sufficient to draw any definitive conclusion, these results suggest that the level of oxidative stress in CRC increases with the stage of the disease. Our data are consistent with previous reports in which expression of MTH-1 showed significant correlation with aggressive features of CRC, such as tumor size and advanced stage[6]. Thus, MTH-1 seems to be a highly valuable marker that is closely associated with survival and dissemination of CRC cells, conferring a survival advantage through the inhibition of oxidative-stress-induced DNA damage.
A recent study demonstrated that expression of BER proteins in CRC cells was adaptable and strongly dependent on the tumor microenvironment[14]. Thus, we focused on hypoxia, which has been documented in colorectal tissues, ranging from normal mucosa to benign adenoma and carcinoma[19,20]. In colonic adenocarcinoma, HIF-1α overexpression was associated with angiogenesis, invasion, and metastasis[21]. We found HIF-1α expression in 56 (66.7%) of 84 CRCs. Moreover, correlation analysis showed that expression of HIF-1α was positively correlated with that of MTH-1. In our study, expression of HIF-1α was mainly localized to the cytoplasm of tumor cells and was observed diffusely throughout the entire tumor tissue. MTH-1 immunoreactivity of normal colon mucosa was faint in the cytoplasm, and strong cytoplasmic immunostaining was observed in lymphoid cells. In contrast, MTH-1 immunoreactivity was markedly increased in the cytoplasm of tumor cells. Its immunoreactivity was stronger than that of normal mucosal epithelium. With respect to tumor biology, our results suggest that tumor cells that experience hypoxia may express MTH-1, leading to acquired genetic stability and contributing to tumor progression.
Based on the above findings, we hypothesized that hypoxia may directly or indirectly upregulate the expression of MTH-1, resulting in the tumor progression observed in CRC. In this study, we demonstrated that expression of MTH-1 was upregulated in SW480 and HT-29 cells that were exposed to hypoxic stress. Upregulation of MTH-1 mRNA and protein in SW480 and HT-29 cells was observed after exposure to 48 and 72 h of hypoxic stress. However, Chan et al[14] showed no significant changes in mRNA and protein levels of MTH-1 in RKO human CRC cell line after exposure to 72 h hypoxia of 0.2% O2. They also demonstrated that the expression of MTH-1 and other BER genes (MYH and OGG1) was independent of the p53 gene in the RKO cell line. Although difference in the types of cells may account for the different cellular responses in MTH-1 expression, these results suggest that expression of MTH-1 is dynamically regulated by hypoxic stress. To confirm that MTH-1 is induced by HIF-1α, siRNA was used to decrease the expression of HIF-1α. We found that hypoxia-induced MTH-1 expression was HIF-1α dependent, as demonstrated by the ability of HIF-1α siRNA to inhibit hypoxia-induced MTH-1 upregulation in SW480 and HT-29 cells. The effect of siHIF-1α on suppression of MTH-1 protein level was limited. This may have been due to the long half-life of the MTH-1 protein[22]. However, even a minor increase in BER protein levels may have important implications for carcinogenesis and tumor progression in the colon[23]. Notably, we found that the cells in the siHIF-1α groups displayed increased residual base damage at 72 h following hypoxia. This result suggests that hypoxic stress induces the corresponding defensive mechanisms, producing higher levels of defensive mechanisms against 8-oxo-dGTP accumulation in tumor cells, as previously suggested by Kennedy et al[24].
In the in vivo xenograft tumor model, siRNA was intraperitoneally injected. Twenty days later, the tumor volume of the siHIF-1α group was smaller than that in the mock group. We also found that the reduction in HIF-1α significantly reduced the expression of MTH-1 in CRC cells in vivo. These results support a role for HIF-1α in mediating MTH-1 expression in CRC and imply that MTH-1 and HIF-1α together play a role in the growth of CRC. Silencing of HIF-1α facilitated residual base damage and inhibited tumor growth in this study. However, whether or not HIF-1α is a good target for the development of cancer therapeutics requires further investigation. Since it has a large number of downstream genes, HIF-1α may play a complex role in cancer biology[25,26]. Results of a recent paper validated MTH-1 as an anti-cancer target in vivo and described certain small molecules as first-in-class nudix hydrolase family inhibitors that potently and selectively engage and inhibit the MTH-1 protein in tumor cells[27,28]. Together, these results exemplify the non-oncogene addiction concept for anti-cancer treatment and validate MTH-1 as a lethal cancer phenotype.
The present study revealed a new mechanism through which HIF-1α upregulates MTH-1 expression in CRC and provides evidence that hypoxia increases expression of MTH-1, likely by modulating HIF-1α protein level. These results emphasize the important role of HIF-1α-induced MTH-1 in tumor progression. The dissection of the multistep regulation of MTH-1 gene under hypoxic conditions is just beginning. Further studies are required to elucidate the mechanism by which HIF-1α drives MTH-1 upregulation.
We wish to thank Drs. Rao Jun and Wu Feng from Department of Pathology, Xi’nan Hospital for their valuable comments and advice regarding the pathological diagnosis.
Hypoxia-inducible factor (HIF)-1 is a major transcriptional factor for tumor cells. To offset oxidative damage to nucleic acids, tumor cells are equipped with mutT homolog (MTH)-1. Hypoxia in solid tumors and cell survival often coexist during tumor growth, and hypoxia provokes base excision repair changes in colorectal cancer (CRC) cells.
Recent studies have shown that MTH-1 protein may be a potent promoter of tumorigenic growth and that MTH-1 catalytic activity is markedly increased in many cancers. However, little is known about hypoxic status in vivo and its functional relationship with the expression of MTH-1 in CRC.
The present study revealed a new mechanism through which HIF-1α upregulates MTH-1 expression in CRC and provides evidence that hypoxia increases the expression of MTH-1, likely by modulating HIF-1α protein level. These results emphasize the important role of HIF-1α-induced MTH-1 in tumor progression.
Cancers have dysfunctional redox regulation resulting in reactive oxygen species production, damaging both DNA and free deoxynucleotide triphosphates (dNTPs). These results exemplify the non-oncogene addiction concept for anti-cancer treatment and validate MTH-1 as a lethal cancer phenotype.
HIF-1 is a major transcriptional factor for tumor cells growing in a low-oxygen environment. MTH-1 is able to eliminate oxidized dNTPs before they can be incorporated into DNA.
This interesting work demonstrates the upregulation of MTH-1 expression in colorectal cancer cells via HIF-1α in response to hypoxic stress. Under hypoxic conditions, the authors utilized small interfering RNA for HIF-1α to show the association between HIF-1α and MTH-1.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (11)] |
| 2. | O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1174] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 3. | Shehade H, Oldenhove G, Moser M. Hypoxia in the intestine or solid tumors: a beneficial or deleterious alarm signal? Eur J Immunol. 2014;44:2550-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 4. | Risom L, Lundby C, Thomsen JJ, Mikkelsen L, Loft S, Friis G, Møller P. Acute hypoxia and reoxygenation-induced DNA oxidation in human mononuclear blood cells. Mutat Res. 2007;625:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Nakabeppu Y, Oka S, Sheng Z, Tsuchimoto D, Sakumi K. Programmed cell death triggered by nucleotide pool damage and its prevention by MutT homolog-1 (MTH1) with oxidized purine nucleoside triphosphatase. Mutat Res. 2010;703:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Koketsu S, Watanabe T, Nagawa H. Expression of DNA repair protein: MYH, NTH1, and MTH1 in colorectal cancer. Hepatogastroenterology. 2004;51:638-642. [PubMed] |
| 7. | Kennedy CH, Pass HI, Mitchell JB. Expression of human MutT homologue (hMTH1) protein in primary non-small-cell lung carcinomas and histologically normal surrounding tissue. Free Radic Biol Med. 2003;34:1447-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Wani G, Milo GE, D’Ambrosio SM. Enhanced expression of the 8-oxo-7,8-dihydrodeoxyguanosine triphosphatase gene in human breast tumor cells. Cancer Lett. 1998;125:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Iida T, Furuta A, Kawashima M, Nishida J, Nakabeppu Y, Iwaki T. Accumulation of 8-oxo-2’-deoxyguanosine and increased expression of hMTH1 protein in brain tumors. Neuro Oncol. 2001;3:73-81. [PubMed] |
| 10. | Yoo YG, Christensen J, Gu J, Huang LE. HIF-1α mediates tumor hypoxia to confer a perpetual mesenchymal phenotype for malignant progression. Sci Signal. 2011;4:pt4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1381] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 12. | Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9 Suppl 5:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 604] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 13. | Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 880] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 14. | Chan N, Ali M, McCallum GP, Kumareswaran R, Koritzinsky M, Wouters BG, Wells PG, Gallinger S, Bristow RG. Hypoxia provokes base excision repair changes and a repair-deficient, mutator phenotype in colorectal cancer cells. Mol Cancer Res. 2014;12:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Filleur S, Courtin A, Ait-Si-Ali S, Guglielmi J, Merle C, Harel-Bellan A, Clézardin P, Cabon F. SiRNA-mediated inhibition of vascular endothelial growth factor severely limits tumor resistance to antiangiogenic thrombospondin-1 and slows tumor vascularization and growth. Cancer Res. 2003;63:3919-3922. [PubMed] |
| 16. | Yoshimura D, Sakumi K, Ohno M, Sakai Y, Furuichi M, Iwai S, Nakabeppu Y. An oxidized purine nucleoside triphosphatase, MTH1, suppresses cell death caused by oxidative stress. J Biol Chem. 2003;278:37965-37973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Speina E, Arczewska KD, Gackowski D, Zielińska M, Siomek A, Kowalewski J, Oliński R, Tudek B, Kuśmierek JT. Contribution of hMTH1 to the maintenance of 8-oxoguanine levels in lung DNA of non-small-cell lung cancer patients. J Natl Cancer Inst. 2005;97:384-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Gad H, Koolmeister T, Jemth AS, Eshtad S, Jacques SA, Ström CE, Svensson LM, Schultz N, Lundbäck T, Einarsdottir BO. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature. 2014;508:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 418] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 19. | Greijer AE, Delis-van Diemen PM, Fijneman RJ, Giles RH, Voest EE, van Hinsbergh VW, Meijer GA. Presence of HIF-1 and related genes in normal mucosa, adenomas and carcinomas of the colorectum. Virchows Arch. 2008;452:535-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Xue X, Taylor M, Anderson E, Hao C, Qu A, Greenson JK, Zimmermann EM, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 2012;72:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830-5835. [PubMed] |
| 22. | Obtulowicz T, Swoboda M, Speina E, Gackowski D, Rozalski R, Siomek A, Janik J, Janowska B, Ciesla JM, Jawien A. Oxidative stress and 8-oxoguanine repair are enhanced in colon adenoma and carcinoma patients. Mutagenesis. 2010;25:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Schafmayer C, Buch S, Egberts JH, Franke A, Brosch M, El Sharawy A, Conring M, Koschnick M, Schwiedernoch S, Katalinic A. Genetic investigation of DNA-repair pathway genes PMS2, MLH1, MSH2, MSH6, MUTYH, OGG1 and MTH1 in sporadic colon cancer. Int J Cancer. 2007;121:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kennedy CH, Cueto R, Belinsky SA, Lechner JF, Pryor WA. Overexpression of hMTH1 mRNA: a molecular marker of oxidative stress in lung cancer cells. FEBS Lett. 1998;429:17-20. [PubMed] |
| 25. | Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4764] [Cited by in RCA: 5118] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 26. | Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 1063] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 27. | Huber KV, Salah E, Radic B, Gridling M, Elkins JM, Stukalov A, Jemth AS, Göktürk C, Sanjiv K, Strömberg K. Stereospecific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. Nature. 2014;508:222-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 28. | Dominissini D, He C. Cancer: Damage prevention targeted. Nature. 2014;508:191-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hokama A, Kodaz H, Vlachostergios PJ S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH