Published online Dec 21, 2015. doi: 10.3748/wjg.v21.i47.13294
Peer-review started: June 3, 2015
First decision: July 19, 2015
Revised: August 17, 2015
Accepted: October 23, 2015
Article in press: October 26, 2015
Published online: December 21, 2015
Processing time: 200 Days and 9.8 Hours
AIM: To examine the quality of surgical care and long-term oncologic outcome after D2 gastrectomy for gastric cancer.
METHODS: From 1999 to 2008, a total of 109 consecutive patients underwent D2 gastrectomy without routine pancreaticosplenectomy in a multimodal setting at our institution. Oncologic outcomes together with clinical and histopathologic data were analyzed in relation to the type of surgery performed. Staging was carried out according to the Union for International Cancer Control criteria of 2002. Patients were followed-up for five years at the outpatient clinic. The primary measure of outcome was long-term survival with the quality of surgery as a secondary outcome measure. Clinical data were retrospectively collected from the patient records, and causes of death were obtained from national registries.
RESULTS: A total of 109 patients (58 men) with a mean age of 67.4 ± 11.2 years underwent total gastrectomy or gastric resection with D2 lymph node dissection. The tumor stage distribution was as follows: stage I, (27/109) 24.8%; stage II, (31/109) 28.4%; stage III, (41/109) 37.6%; and stage IV, (10/109) 9.2%. Forty patients (36.7%) received chemotherapy or chemoradiotherapy. The five-year overall survival rate for all 109 patients was 45.0%, and was 47.1% for the 104 patients treated with curative R0 resection. The five-year disease-specific survival rates were 53.0% and 55.8%, respectively. In a multivariate analysis, body mass index and tumor stage were independent prognostic factors for overall survival (both P < 0.01), whereas body mass index, tumor stage, tumor site, Lauren classification, and lymph node invasion were prognostic factors for cancer-specific survival (all P < 0.05). Postoperative 30-d mortality was 1.8% and 30-d, surgical (including three anastomotic leaks, two of which were treated conservatively), and general morbidities were 26.6%, 12.8%, and 14.7%, respectively.
CONCLUSION: D2 dissection is a safe surgical option for gastric cancer, providing quality surgical care and long-term oncologic outcomes that are in line with current Western standards.
Core tip: Gastric cancer remains one of the most lethal malignancies worldwide. Although radical surgery with adequate lymphadenectomy is the cornerstone of curative treatment, whether D2 lymphadenectomy is applicable in Western hospitals is not clear, despite the low reported morbidity and mortality rates and survival benefit. This single-center study of 109 patients demonstrates that D2 lymphadenectomy can be performed with relatively low mortality (1.8%) and morbidity (26.6%). Five-year survival rates were 45.0% (overall) and 53.0% (disease-specific). Therefore, D2 gastrectomy can be considered as a safe surgical option for gastric cancer.
- Citation: Mrena J, Mattila A, Böhm J, Jantunen I, Kellokumpu I. Surgical care quality and oncologic outcome after D2 gastrectomy for gastric cancer. World J Gastroenterol 2015; 21(47): 13294-13301
- URL: https://www.wjgnet.com/1007-9327/full/v21/i47/13294.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i47.13294
Gastric cancer is the sixth most common cancer and the third leading cause of cancer-related death worldwide[1,2]. The cornerstone of treatment involves adequate surgery, though it is not curative, as most patients present with advanced disease. As nodal involvement is indicative of a poor prognosis, more aggressive surgical approaches with lymph node removal have gained popularity[3]. D2 lymph node dissection is currently the gold standard in gastric cancer surgery in Asia with regard to long-term survival and local recurrence[4]. In the Western world, however, the role of D2 lymphadenectomy for the treatment of gastric cancer is controversial[5,6].
Large European randomized studies report higher mortality and morbidity with no survival benefit from D2 dissection with splenic and/or pancreatic resection compared to D1 dissection[7,8]. However, an Italian study found similar rates of postoperative complications (18% vs 12%) and mortality (2% vs 3%) when comparing D2 (with preservation of the spleen and pancreas) and D1 dissections[9]. Another small single-center randomized trial comparing D1 and D3 (current D2) dissections showed a better overall survival after D2/3 dissection, but no significant difference in disease-specific survival[10]. Finally, a Japanese randomized trial showed that extended D2 dissection with removal of para-aortic lymph nodes did not improve survival compared with standard D2 dissection[11].
D2 lymphadenectomy with preservation of the spleen and pancreas is now regarded as the standard surgical technique for locally advanced gastric cancer. Patient outcome has been further improved by the centralization of procedures to experienced high-volume units and the use multimodal treatments[12], such as chemotherapy and/or radiotherapy. An American study using postoperative chemoradiation[13] and European studies using pre- and postoperative chemotherapy[14,15] have shown improved survival with adjuvant therapies. Furthermore, a recent meta-analysis showed that adjuvant chemotherapy based on 5-fluorouracil (FU) regimens was associated with improved survival compared to surgery alone[16]. However, whether to use pre- and postoperative chemotherapy, postoperative chemoradiotherapy, or adjuvant chemotherapy remains unclear.
Due to the availability of multiple individually tailored treatment options, it is important to monitor the quality of care, which also affects outcomes. In Finland, for example, there is no national quality audit for gastric cancer. Therefore, the aim of this study was to assess the long-term oncologic outcome and quality of surgical care for patients who underwent D2 gastrectomy at our institution between 1998 and 2008.
This study examined 109 consecutive patients with histologically proven gastric adenocarcinoma that underwent D2 gastrectomy with curative intent at the Central Hospital of Central Finland in Jyväskylä between January 1998 and December 2008. Preoperative diagnosis and staging was performed via endoscopy and thoracoabdominal CT; laparoscopic staging was not routinely conducted during the study period. Clinical and follow-up patient data were collected retrospectively. Patients who underwent other surgical procedures (D1 dissection or palliative resections) were excluded. The study was approved by the ethics committee of the Central Hospital of Central Finland.
D2 lymph node dissections were conducted by senior upper gastrointestinal surgeons who also performed hepatobiliary and pancreatic surgeries. Resection of the pancreas was performed only when invasion by the gastric cancer was suspected. Indications for splenectomy were gastric carcinomas of the greater curvature in the upper and middle part of the stomach. Cholecystectomy was performed routinely, and additional organ resections were performed when deemed appropriate. The nodal dissection of the removed surgical specimen was performed by surgeons on the bench in the operating room in a standardized fashion following the JRSGC classification system[17]. R0 gastric resection was defined by the following parameters: negative resection margins, en bloc resection of adherent organs, and en bloc resection of the greater and lesser omentum.
Perioperative care during the study period included the assessment and optimization of medical risk factors, thromboprophylaxis with low-molecular-weight heparin and elastic stockings, prophylactic antibiotics, standard anesthesia with epidural analgesia, avoidance of hypothermia, and increased oxygen concentrations. Nasogastric tubes were removed in the operation theatre.
Tumors were staged by pathologists according to the 2002 Union for International Cancer Control/Tumor-node-metastasis categories[18]. Lymph node ratios were defined as the proportion of metastatic lymph nodes from the total number studied, and classified as follows: ratio, 0%, 1%-9%, 10%-25%, and > 25%[19]. The histopathologic tumor type was evaluated according to Lauren classification[20].
A selective approach for neoadjuvant and adjuvant oncologic treatments was used. Adjuvant postoperative chemotherapy consisted of FU-based regimens combined with epirubicin and cisplatin or oxaliplatin. Chemoradiotherapy (40-45 Gy and FU or capecitabine) was generally used in the postoperative setting, but two patients received preoperative chemoradiation (Table 1). None of the patients had perioperative chemotherapy[14,15].
| Characteristic | n (%) |
| Male gender | 51 (46.8) |
| Body mass index (kg/m2), mean (SD) | 25.6 (4.9) |
| ASA score III-IV | 67 (61.5) |
| Tumor site | |
| Upper | 21 (19.3) |
| Middle | 43 (39.4) |
| Lower | 38 (34.9) |
| All levels | 7 (6.4) |
| UICC tumor stage | |
| I | 27 (24.8) |
| II | 31 (28.4) |
| III | 41 (37.6) |
| IV | 10 (9.2) |
| Tumor type | |
| Intestinal | 50 (45.9) |
| Diffuse | 56 (51.4) |
| Mixed | 3 (2.8) |
| Lymph node invasion | |
| No | 45 (41.2) |
| Yes | 64 (58.7) |
| Type of surgery | |
| Total gastrectomy | 103 (94.5) |
| Subtotal gastric resection | 6 (5.5) |
| D2-lymph node dissection | 109 (100) |
| Splenectomy | 48 (44.0) |
| Cholecystectomy | 19 (17.4) |
| Additional organ resection due to cancer invasion | 10 (9.2) |
| Radicality | |
| R0 | 104 (95.4) |
| Neoadjuvant/adjuvant treatment | |
| No | 69 (63.3) |
| Neoadjuvant chemoradiotherapy | 2 (1.8) |
| Adjuvant chemotherapy | 26 (23.9) |
| Adjuvant chemoradiotherapy | 12 (11.0) |
Patients were followed at the surgical outpatient clinic every six months for two years, and then once a year for up to five years from the operation. Tumor recurrence was defined as a recurrent tumor in the tumor bed or distant organs and diagnosed by CT, magnetic resonance imaging, or endoscopy. Histopathologic confirmation was not mandatory. The end of follow-up was June 30, 2014. The causes of death were obtained from the National Cause of Death Registry.
Statistical analyses were conducted using SPSS (version 15.0 for Windows; SPPS Inc., Chicago, IL, United States) and STATA (version 11; StatCorp LP, College Station, TX, United States) software. Results are given as mean ± SD or median (interquartile range). Pearson’s χ2 or Fisher’s exact tests were used to compare frequencies, and Student’s t and Mann-Whitney U tests were used for continuous variables. The Kaplan-Meier method was used to calculate the survival, and the differences between groups were compared with the log-rank test. Survival times were calculated from the date of surgery until the time of death or the end of follow-up. Factors affecting survival were analyzed with univariate and multivariate Cox proportional hazards regression models; only variables with P < 0.20 were entered in the multivariate analysis. All statistical tests were two-sided, with a P < 0.05 considered as statistically significant.
Baseline patient and tumor characteristics are shown in Table 1. A total of 109 patients with a mean age of 67.4 ± 11.2 years (median, 69 years; range: 59-77 years) underwent total gastrectomy or subtotal gastric resection with D2 lymph node dissection; 5/109 (4.6%) patients had residual microscopic disease upon histopathologic examination. Ten patients received multivisceral resections for locally invasive or metastatic disease, including resection of the colon (n = 2), pancreas (n = 1), liver (n = 1), esophagus (n = 2), kidney (n = 2), and ovaries (n = 2).
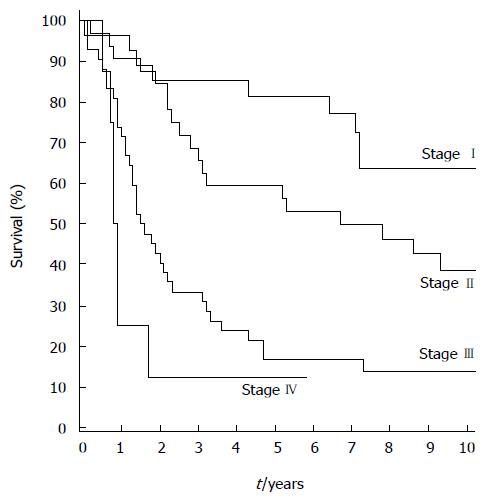
The overall median follow-up time was 3.3 (1.5-5.1) years, and 9.1 (7.9-12.3) years for the 37 surviving patients. No patients were lost to follow-up. The five- and 10-year overall survival rates were 45.0% [95%CI: 35.5%-54.0%] and 32.9% (95%CI: 23.9%-42.2%), respectively. The five- and 10-year disease-specific survival rates were 53.0% (95%CI: 42.7%-62.3%) and 47.8% (95%CI: 37.4%-57.5%). For the 104 patients with R0 resection, the five- and 10-year overall survival rates were 47.1% (95%CI: 37.3%-56.3%) and 34.5% (95%CI: 25.1%-44.0%) and disease-specific survival rates were 55.8% (95%CI: 45.2%-65.2%) and 50.3% (95%CI: 39.5%-60.2t), respectively. For tumor stages I, II, and III, the five-year overall survival rates were 81.5% (95%CI: 61.1%-91.8%), 61.3% (95%CI: 40.5%-74.0%), and 17.1% (95%CI: 7.3%-29.3%) (Figure 1), and disease-specific survival rates were 95.8% (95%CI: 73.9%-99.4%), 63.8% (95%CI: 44.2%-78.1%), and 22.6% (95%CI: 10.4%-37.7%), respectively.
Univariate and multivariate analyses were performed to identify prognostic factors of overall and cancer-specific survival. The results of the univariate analysis are shown in Table 2. In the multivariate analysis, body mass index (BMI) and tumor stage were the independent prognostic factors of overall survival (both P < 0.01), and BMI, tumor stage, tumor site, lymph node invasion, and Lauren’s classification (intestinal vs diffuse) were independent prognostic factors of cancer-specific survival (all P < 0.05). Of note, significantly more patients with BMI < 25 had metastatic lymph nodes than those with BMI > 25 (67.9% vs 49.1%, P < 0.05).
| Variables | OS | DSS | ||||
| Events/patients | HR (95%CI) | P value | Events/patients | HR (95%CI) | P value | |
| Age (yr) | 0.151 | 0.721 | ||||
| < 60 | 16/29 | 1 | 13/29 | 1 | ||
| 60-75 | 31/47 | 1.33 (0.73-2.44) | 23/47 | 1.22 (0.62-2.42) | ||
| > 75 | 25/33 | 1.59 (0.85-2.97) | 15/33 | 1.23 (0.54-2.79) | ||
| Sex | 0.83 | 0.67 | ||||
| Male | 34/51 | 1 | 26/51 | 1 | ||
| Female | 38/58 | 1.05 (0.66-1.67) | 25/58 | 0.89 (0.51-1.54) | ||
| BMI (kg/m2) | 0.02 | 0.01 | ||||
| < 25 | 43/56 | 1 | 33/56 | 1 | ||
| > 25 | 29/53 | 0.56 (0.35-0.90) | 18/53 | 0.47 (0.27-0.84) | ||
| ASA | 0.61 | 0.43 | ||||
| I-II | 25/42 | 1 | 22/42 | 1 | ||
| III-IV | 47/67 | 1.13 (0.70-1.85) | 29/67 | 0.80 (0.46-1.39) | ||
| Operation type | 0.05 | 0.041 | ||||
| D2 | 27/51 | 1 | 20/51 | 1 | ||
| D2 + splenectomy | 38/48 | 1.59 (0.97-2.61) | 24/48 | 1.44 (0.81-2.56) | ||
| D2 + adjacent organ resection | 7/10 | 1.80 (0.78-4.16) | 7/10 | 3.67 (1.25-10.80) | ||
| Radicality | 0.02 | < 0.01 | ||||
| R0 | 67/104 | 1 | 46/104 | 1 | ||
| R1 | 5/5 | 3.18 (1.24-8.14) | 5/5 | 4.43 (1.69-11.59) | ||
| Neoadjuvant/adjuvant therapy | 0.021 | < 0.011 | ||||
| No | 41/69 | 1 | 20/69 | 1 | ||
| Neoadjuvant | 1/2 | 0.75 (0.10-5.48) | 1/2 | 1.53 (0.21-11.40) | ||
| Adjuvant | 30/38 | 1.82 (1.13-2.93) | 30/38 | 3.55 (2.01-6.28) | ||
| Tumor site | 0.071 | < 0.011 | ||||
| Upper third | 14/21 | 1 | 8/21 | 1 | ||
| Middle third | 26/43 | 0.91 (0.48-1.75) | 15/43 | 0.95 (0.40-2.25) | ||
| Lower third | 25/38 | 1.04 (0.54-2.01) | 21/38 | 1.54 (0.68-3.48) | ||
| All levels | 7/7 | 2.87 (1.14-7.21) | 7/7 | 4.74 (1.70-13.23) | ||
| Tumor stage | < 0.011 | 0.031 | ||||
| I | 13/38 | 1 | 4/38 | 1 | ||
| II | 19/27 | 2.98 (1.47-6.05) | 12/27 | 5.96 (1.92-18.50) | ||
| III | 31/34 | 5.99 (3.09-11.62) | 26/34 | 15.34 (5.30-44.38) | ||
| IV | 9/10 | 10.15 (4.18-24.66) | 9/10 | 30.67 (9.17-102.65) | ||
| Lauren classification | 0.66 | 0.03 | ||||
| Intestinal | 34/50 | 1 | 18/50 | 1 | ||
| Diffuse2 | 38/59 | 1.11 (0.66-1.77) | 33/59 | 1.87 (1.05-3.33) | ||
| Lymph node invasion | < 0.01 | |||||
| No | 20/45 | 1 | 9/45 | 1 | ||
| Yes | 52/64 | 2.90 (1.72-4.89) | 42/64 | 4.95 (2.40-10.23) | ||
| Lymph node ratio (%) | < 0.011 | < 0.01 | ||||
| 0 | 20/45 | 1 | 9/45 | 1 | ||
| 1-9 | 9/15 | 1.88 (0.85-4.14) | 7/15 | 3.03 (1.12-8.16) | ||
| 10-25 | 16/20 | 2.69 (1.37-5.28) | 12/20 | 4.11 (1.70-9.95) | ||
| > 25 | 27/29 | 3.74 (2.09-6.70) | 23/29 | 7.00 (3.22-15.20) | ||
The parameters reflecting the quality of care are shown in Table 3. Overall 30-d mortality was 1.8%, including one death from cerebral stroke and one from severe pneumonia. The overall complication rate was 26.6% (29/109) with 12.8% surgical and 14.7% general morbidity rates. The 30-d reoperation rate was 3.7% due to postoperative hemorrhage (n = 1), pleural empyema (n = 1), esophagojejunal anastomotic leak in a cirrhotic patient (n = 1), and colon obstruction (n = 1). The median postoperative hospital stay was 10 (8-12) d. Seven patients had a late reoperation because of bowel obstruction caused by locoregional recurrence or intra-abdominal carcinosis.
| Parameter | Value |
| Duration of surgery (min) | 230 (200-255) |
| Operative blood loss (mL) | 500 (300-900) |
| Hospital stay (d) | 10 (8-12) |
| Number of lymph nodes studied | 19 (11-25) |
| 30-d mortality | 2 (1.8) |
| 30-d morbidity1 | 29 (26.6) |
| General morbidity | |
| Cardiac | 1 (0.9) |
| Pleural effusion | 8 (7.3) |
| Pneumonia | 3 (1.8) |
| Pleural empyema | 1 (0.9) |
| Urinary tract infection | 2 (1.8) |
| Thromboembolism | 1 (0.9) |
| Cerebral infarction | 1 (0.9) |
| Surgical morbidity | |
| Bleeding | 1 (0.9) |
| Anastomotic leak2 | 3 (2.8) |
| Abdominal abscess | 7 (6.4) |
| Wound infection | 1 (0.9) |
| Common bile duct injury | 1 (0.9) |
| Bowel obstruction | 1 (0.9) |
| Dindo-Clavien classification[32], severe morbidity | 9 (8.3) |
| Grade III | 3 (2.8) |
| Grade IV | 3 (2.8) |
| Grade V | 3 (2.8) |
The treatment of gastric cancer is complex and has evolved from surgical management to a multidisciplinary model[12]. Consequently, overall five-year survival rates across all tumor stages range from 33%-47% as reported in European randomized trials[7,8]. In the present cohort of patients undergoing D2 gastrectomy for gastric cancer without routine pancreaticosplenectomy and multimodal treatment, the five-year overall and cancer-specific survival rates were 45% and 53%, and slightly higher, at 47% and 56%, respectively, for those having R0 resection. Asian studies have reported higher survival rates ranging from 59% up to 70% depending on the extent of lymphadenectomy, with minor survival benefit gained by D3 or D4 lymphadenectomy compared with less radical dissection[10,11,21].
Variability in survival rates among studies may, in part, depend upon tumor stage distribution, use of neoadjuvant and adjuvant treatments, and differences in the quality of the surgical care. The reported five-year survival rates for curative surgical resection ranges from 60%-90% for patients with stage I, 30%-50% for patients with stage II disease, and 10%-25% for patients with stage III disease[22], which is in line with what was observed in the present study. The results indicate that tumor stage and a BMI < 25 are predictive of overall survival, with additional factors of tumor site, Lauren classification, and lymph node invasion predictive of cancer-free survival. Although the lymph node ratio is a prognostic tool that can be used to stratify patients with gastric cancer undergoing limited lymph node dissection and to reduce stage migration[19], this was only a significant prognostic factor for survival in the univariate, but not multivariate, analysis in the present study.
Data from Asian studies show that D2 dissection provides better survival rates compared with D1 dissection[10,11,21], and that a more extended lymphadenectomy does not improve survival. However, European randomized studies failed to demonstrate any survival benefit from D2 over D1 dissection. Five-year survival rates were 35% (D1) and 33% (D2) in the MRC trial[7], and 45% and 47%, respectively, in the Dutch trial[8]. This, in combination with high morbidity and mortality rates, has led to a reserved attitude against D2 surgery in Western centers. However, results from a 15-year follow-up of the Dutch trial showed a significant survival benefit for D2 without pancreaticosplenectomy compared to D1 (35% vs 22%)[8]. Moreover, a recent meta-analysis including eight randomized controlled trials with 2044 patients confirmed that there is no overall survival benefit for D2 lymphadenectomy, but found a benefit among patients who had resection without a splenectomy and/or pancreatectomy[6]. Importantly, some previous randomized trials have been scrutinized based on the inclusion of a large number of operating surgeons and heterogeneous surgical techniques. Thus, universal standardization and surgical quality control of D2 dissection is needed to improve evaluation of the oncologic outcome[23,24]. Currently, radical D2 gastrectomy is indicated for resectable stage IB-III disease, provided that patients are medically fit and the procedure is carried out in specialized, high-volume centers with appropriate surgical expertise and postoperative care[12].
Despite radical surgery, a substantial proportion of patients relapse, which indicates that there is a need for additional therapeutic modalities, such as radiation and chemotherapy, though more evidence is needed with regard to the (neo)adjuvant setting[25]. A randomized phase III study performed in the United States (Intergroup 0116) demonstrated a survival benefit with postoperative chemoradiotherapy compared with surgery alone based primarily on patients undergoing D1 gastrectomy[26]. The European UK MRC MAGIC trial demonstrated a survival benefit when patients with resectable stage II and III gastric cancers undergoing either D1 or D2 surgery were treated with three cycles of preoperative chemotherapy (epirubicin, cisplatin, and FU) followed by an additional three cycles of postoperative chemotherapy compared with surgery alone[15]. As a result, perioperative chemotherapy has been widely adopted as the standard of care throughout the United Kingdom and Europe. However, additional real-life data is needed, as a recent Norwegian study showed that perioperative chemotherapy was completed in less than half of the patients in line with the MAGIC trial, and the tumor response did not afford any long-term survival benefit[27]. Nevertheless, a recent meta-analysis containing data from 17 randomized trials confirmed an overall survival benefit for FU-based chemotherapy for gastric cancer compared with surgery alone (55.3% vs 49.6%, HR = 0.82, 95%CI: 0.76-0.90)[28]. Particularly, data from the Japanese ACTS-GC[29] and the Korean CLASSIC[30] trials showed that adjuvant chemotherapy improves overall survival after D2 gastrectomy. Recent European guidelines recommend either chemoradiotherapy or chemotherapy delivered in the adjuvant setting for patients with ≥ stage IB gastric cancer who undergo surgery without preoperative chemotherapy[12]. Although neoadjuvant and adjuvant treatments were used in our cohort of patients, the protocol was not uniform during the study period due to the lack of an international consensus regarding various treatment strategies.
Additional parameters can be used to assess the quality of surgical care, including postoperative mortality, reoperation rate, and 30-d readmission rate. The mortality rate within 30 d after a surgical procedure for gastric cancer has been reduced substantially over the last decades. Indeed, the postoperative mortality rate in this patient series (1.8%) is within the reported range of 0%-2%[9-11], and is similar to earlier European multicenter randomized trials[7,8]. Furthermore, the 30-d morbidity (26.6%) is in line with other reports: 20.9%-28.1% in the JCOG trial[11] and 17.9% by Degiuli et al[9]. Early postoperative complications typically include bleeding, ileus, cholecystitis, pancreatitis, pulmonary infections, thromboembolism, and anastomotic leakage, which is the most significant complication after total gastrectomy. In our report, the rate of symptomatic leakage was 2.8%, within the commonly reported incidence range of 1.9%-4.5%. The reoperation rate (3.7%) also compares well with the figures reported from other centers[10,11]. Asian studies show that, even in high-volume centers, there is a certain amount of morbidity associated with major gastric surgery. Overall, the current international standards of care were well met in this series of patients treated with D2 gastrectomy and combined modality therapy for gastric cancer.
This single-center audit should be interpreted with some caution. The number of patients is relatively small due to a declining incidence of gastric cancer in Western Europe. In addition, some changes in the management were made during the study period, including the addition of multidisciplinary team meetings and some modifications in chemotherapy regimens. However, the mortality, reoperation, and local recurrence rates are low. Currently, endoscopic ultrasound can be used to determine the proximal and distal extent of the tumor and provide further assessment of the T and N stages. Moreover, laparoscopy with or without peritoneal washings for malignant cells is now recommended to exclude occult metastatic disease in all stage IB-III stomach cancers considered potentially resectable[31].
In conclusion, the present audit indicates that D2 lymphadenectomy with pancreatic preservation and selective splenectomy is a safe surgical strategy for patients with locally advanced gastric cancer. The oncologic outcome is largely determined by the stage of the disease at presentation, which can be improved through the use of neoadjuvant and adjuvant chemotherapy regimens.
Morbidity and mortality rates after D2 gastrectomy are relatively high in European randomized studies, and evidence of a long-term survival benefit has been controversial. Nevertheless, D2 lymphadenectomy has been adopted for treatment protocols for gastric cancer, despite the lack of quality audits in some countries.
Asian studies show that D2-3 lymphadenectomy improves survival and can be performed with acceptable complication rates. The present study evaluates the long-term oncologic outcomes of 109 consecutive D2 gastrectomy patients with gastric adenocarcinoma at the authors’ institution. Clinicopathologic factors affecting survival were assessed, and the quality of surgery was a secondary outcome measure.
The overall five-year and disease-specific survivals for the 109 patients in this cohort were 45.0% and 53.0%, respectively, which are in line with European studies. The mortality associated with D2 lymphadenectomy without routine pancreatectomy and/or splenectomy was only 1.8%, and morbidity was 26.6%. Low body mass index, advanced stage, tumor site, Lauren classification, and lymph node invasion were independent prognostic factors for poor survival.
Large randomized studies are essential for defining clinical guidelines. However, it is important to report how these guidelines are implemented in clinical practice. In this series, the Western standards for long-term oncologic outcome and quality of surgical care were well met.
The authors examined long-term oncologic outcome and quality of surgical care in gastric cancer patients treated with D2 lymphadenectomy without routine pancreaticosplenectomy. Clinicopathologic factors affecting survival were assessed. Their results show that in this consecutive series, survival and morbidity rates were comparable with European studies. Mortality was low. To this end, D2 gastrectomy is a safe surgical option for gastric cancer, and can be well implemented in clinical practice.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25605] [Article Influence: 1707.0] [Reference Citation Analysis (11)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20722] [Article Influence: 1883.8] [Reference Citation Analysis (23)] |
| 3. | Sasako M. Principles of surgical treatment for curable gastric cancer. J Clin Oncol. 2003;21:274s-275s. [PubMed] |
| 4. | Shimada Y. JGCA (The Japan Gastric Cancer Association). Gastric cancer treatment guidelines. Jpn J Clin Oncol. 2004;34:58. [PubMed] |
| 5. | Verlato G, Giacopuzzi S, Bencivenga M, Morgagni P, De Manzoni G. Problems faced by evidence-based medicine in evaluating lymphadenectomy for gastric cancer. World J Gastroenterol. 2014;20:12883-12891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Jiang L, Yang KH, Chen Y, Guan QL, Zhao P, Tian JH, Wang Q. Systematic review and meta-analysis of the effectiveness and safety of extended lymphadenectomy in patients with resectable gastric cancer. Br J Surg. 2014;101:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. [PubMed] |
| 8. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1338] [Article Influence: 83.6] [Reference Citation Analysis (1)] |
| 9. | Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [PubMed] |
| 11. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [PubMed] |
| 12. | Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [PubMed] |
| 14. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1551] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 15. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [PubMed] |
| 16. | Ge L, Wang HJ, Yin D, Lei C, Zhu JF, Cai XH, Zhang GQ. Effectiveness of 5-flurouracil-based neoadjuvant chemotherapy in locally-advanced gastric/gastroesophageal cancer: a meta-analysis. World J Gastroenterol. 2012;18:7384-7393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Sayegh ME, Sano T, Dexter S, Katai H, Fukagawa T, Sasako M. TNM and Japanese staging systems for gastric cancer: how do they coexist? Gastric Cancer. 2004;7:140-148. [PubMed] |
| 18. | Sobin LH, Wittekind C. TNM classification of malignant tumours. 6th edition. New York: Wiley-Liss 2002; . |
| 19. | Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 318] [Article Influence: 16.7] [Reference Citation Analysis (2)] |
| 20. | Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 21. | Yonemura Y, Wu CC, Fukushima N, Honda I, Bandou E, Kawamura T, Kamata T, Kim BS, Matsuki N, Sawa T. Randomized clinical trial of D2 and extended paraaortic lymphadenectomy in patients with gastric cancer. Int J Clin Oncol. 2008;13:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Seevaratnam R, Bocicariu A, Cardoso R, Mahar A, Kiss A, Helyer L, Law C, Coburn N. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer. 2012;15 Suppl 1:S60-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 23. | Dikken JL, van Sandick JW, Allum WH, Johansson J, Jensen LS, Putter H, Coupland VH, Wouters MW, Lemmens VE, van de Velde CJ. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg. 2013;100:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Kim HI, Hur H, Kim YN, Lee HJ, Kim MC, Han SU, Hyung WJ. Standardization of D2 lymphadenectomy and surgical quality control (KLASS-02-QC): a prospective, observational, multicenter study [NCT01283893]. BMC Cancer. 2014;14:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Dikken JL, van Sandick JW, Maurits Swellengrebel HA, Lind PA, Putter H, Jansen EP, Boot H, van Grieken NC, van de Velde CJ, Verheij M. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer. 2011;11:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 636] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 27. | Bringeland EA, Wasmuth HH, Fougner R, Mjønes P, Grønbech JE. Impact of perioperative chemotherapy on oncological outcomes after gastric cancer surgery. Br J Surg. 2014;101:1712-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 614] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 29. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1119] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 30. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1333] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 31. | Yoon H, Lee DH. New approaches to gastric cancer staging: beyond endoscopic ultrasound, computed tomography and positron emission tomography. World J Gastroenterol. 2014;20:13783-13790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Clavien PA, Strasberg SM. Severity grading of surgical complications. Ann Surg. 2009;250:197-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Abdel-Wahab M S- Editor: Yu J L- Editor: A E- Editor: Liu XM