Published online Dec 21, 2015. doi: 10.3748/wjg.v21.i47.13259
Peer-review started: August 1, 2015
First decision: August 26, 2015
Revised: August 31, 2015
Accepted: October 17, 2015
Article in press: October 20, 2015
Published online: December 21, 2015
Processing time: 137 Days and 2 Hours
AIM: To evaluate the epithelial-to-mesenchymal transition (EMT) of circulating tumor cells (CTCs) in gastric cancer patients.
METHODS: We detected tumor cells for expression of four epithelial (E+) transcripts (keratins 8, 18, and 19 and epithelial cell adhesion molecule) and two mesenchymal (M+) transcripts (Vimentin and Twist) by a quantifiable, dual-colorimetric RNA-in situ hybridization assay. Between July 2014 and October 2014, 44 patients with gastric cancer were recruited for CTC evaluation. Blood samples were obtained from selected patients during the treatment course [before surgery, after surgery and at the 6th cycle of XELOX based chemotherapy (about 6 mo postoperatively)].
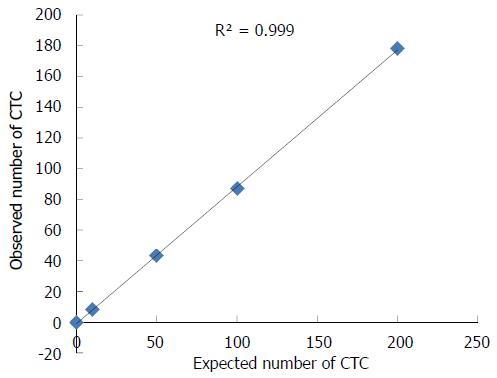
RESULTS: We found the EMT phenomenon in which there were a few biphenotypic E+/M+ cells in primary human gastric cancer specimens. Of the 44 patients, the presence of CTCs was reported in 35 (79.5%) patients at baseline. Five types of cells including from exclusively E+ CTCs to intermediate CTCs and exclusively M+ CTCs were identified (4 patients with M+ CTCs and 10 patients with M+ or M+ > E+ CTCs). Further, a chemotherapy patient having progressive disease showed a proportional increase of mesenchymal CTCs in the post-treatment blood specimens. We used NCI-N87 cells to analyze the linearity and sensitivity of CanPatrolTM system and the correlation coefficient (R2) was 0.999.
CONCLUSION: The findings suggest that the EMT phenomenon was both in a few cells of primary tumors and abundantly in CTCs from the blood of gastric cancer patients, which might be used to monitor therapy response.
Core tip: Epithelial-to-mesenchymal transition has been thought to play a critical role in tumor metastatic progression in preclinical models, however, characterizing the epithelial vs mesenchymal phenotypes of circulating tumor cells has been challenging. In this study, we aimed to evaluate epithelial-to-mesenchymal transition phenomenon in circulating gastric tumor cells by a combination of physical and biological methods. Our findings have provided evidence of the phenomenon both in rare cells within primary tumors and more abundantly in circulating tumor cells. Furthermore, we demonstrated that the evaluation of the mesenchymal circulating tumor cells in peripheral blood can be used to monitor therapy response in gastric cancer patients.
- Citation: Li TT, Liu H, Li FP, Hu YF, Mou TY, Lin T, Yu J, Zheng L, Li GX. Evaluation of epithelial-mesenchymal transitioned circulating tumor cells in patients with resectable gastric cancer: Relevance to therapy response. World J Gastroenterol 2015; 21(47): 13259-13267
- URL: https://www.wjgnet.com/1007-9327/full/v21/i47/13259.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i47.13259
Gastric cancer is a serious public health concern in East Asia, South America and Eastern Europe, accounting for more than 950000 new cases per year (China alone accounts for 42% of new cases worldwide), and it is the third cause of cancer death all around the world (GLOBOCAN 2012)[1]. Because mass screening is rarely practiced worldwide except Japan and Korea, gastric cancer is often diagnosed at an advanced stage. Like common cancers, most gastric cancer-related deaths result from metastasis[2], which is rarely predictable by standard imaging work-ups like positron emission tomography/computed tomography scans or tumor markers tests. Circulating tumor cells (CTCs) originating from solid tumors are related with the course of hematogenous metastatic spread to the distant sites[3], exemplifying the switch from localized to systemic disease. Therefore, evaluating CTCs has clinical relevance in the monitoring and the outcomes of metastatic tumors. The recent discoveries on CTCs demonstrate how these cells are related with hematogenous metastasis, with an opinion on the epithelial-mesenchymal transition (EMT) phenomenon[4]. The investigation by Yu et al[5] found dynamic changes in the number of epithelial and mesenchymal CTCs in breast cancer patients as well as the potential of monitoring therapy response.
It was thought that EMT phenomenon played a critical role in tumor metastatic progression in preclinical models[6,7], however, characterizing the epithelial vs mesenchymal phenotypes of CTCs has been challenging. Increasing evidence coming from clinical setting of CTCs supports the phenomenon of the EMT in human tumors. Accordingly, we are exploring the methods to identify the unique stem CTC subpopulation[7], and its significance is further emphasized by recent findings suggesting the occurrence of mesenchymal markers in tumor tissues as a poor prognostic factor in many cancers[5,8,9]. Furthermore, sequential analysis of CTCs, so called “liquid biopsy”, may provide clinical significance on the effectiveness and progression of systemic therapies and consequently would facilitate “tailor-made” therapeutic strategies[10,11].
To date, the CellSearch System is the only FDA-cleared CTC enumeration assay, which defines a CTC according to its size, positivity for epithelial cell adhesion molecule (EpCAM) and CK, and negativity of CD45 expression[12]. The current techniques besides the CellSearch System are able to isolate CTCs by epithelial markers, however, these epithelial markers based methods most likely overlook a subpopulation of CTCs undergoing EMT[13,14]. Thus, the new CTC capture systems should be essential to isolate the cell subpopulation with mesenchymal phenotype. To our knowledge, there have been few reports regarding the detecting methods and clinical significance of mesenchymal CTCs in cancer patients, specifically gastric cancer.
In the present study, we adopted two mesenchymal transcripts, Vimentin and Twist, to detect mesenchymal phenotypes of CTCs and tumor tissues in advanced gastric cancer, which have been reported as sensitive markers to detect them[12]. Accordingly, the EMT phenomenon of CTCs in advanced gastric cancer and its relationship with chemotherapy response were evaluated as well.
We used the NCI-N87 cell line for the analysis. NCI-N87 cells were cultured in DMEM medium supplemented with 10% fetal bovine serum, 100 U/mL of penicillin and 100 μg/mL of streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
Between July 2014 and October 2014, 44 patients with newly diagnosed gastric cancer in our institution, without receiving neoadjuvant chemo- or radiotherapy, were prospectively enrolled in the study. The blood samples from our selected patients were acquired during the treatment course [before surgery, after surgery and at the 6th cycle of XELOX based chemotherapy (about 6 mo postoperatively)[15]] and analyzed with CanPatrolTM System (Surexam Biotech, Guangzhou, China)[16]. The blood samples from ten healthy volunteers were used as controls.
For the gastric cancer patients, 5 mL peripheral blood samples were collected in EDTA tubes by venipuncture and filtered with a 8-μm diameter pores calibrated membrane (Millipore, Billerica, MA, United States)[16]. The required filtration system included a filtration tube containing the membrane (SurExam, Guangzhou, China), a manifold vacuum plate with valve settings (SurExam, Guangzhou, China), an E-Z 96 vacuum manifold (Omega, Norcross, GA, United States), and a vacuum pump (Auto Science, Tianjin, China). Before filtration, red blood cell lysis buffer was used to remove erythrocyte, then PBS with 4% formaldehyde (Sigma, St. Louis, MO, United States) was used to resuspend the remaining cells for 5 min. The pumping pressure is 0.08 MPa.
We established three groups of nucleic acid probes to identify and examine the expression levels of epithelial and mesenchymal genes in CTCs by a multiplex RNA-in situ hybridization (RNA-ISH) assay. Group 1 probes contained four pooled epithelial transcripts [keratins (KRT) 8, 18 and 19; EpCAM]. Group 2 probes had two mesenchymal transcripts (Vimentin and Twist). The last group only contained a CD45 transcript which was used to discriminate white blood cells and CTCs. The detail hybridization assay procedure followed the published literature[16]. Briefly, the cells retained on the filter were permeabilized and digested with protease. And then, the cells were subjected to a serial of hybridization reactions with a cocktail probe specific to the intended examined genes described above. Finally, we used 4’,6-diamidino-2-phenylindole (DAPI) to stain the cell nucleus. The samples were analyzed with a fluorescent microscope. The red and green dots of fluorescent signal observed in the cells represented the epithelial and mesenchymal gene expression, respectively. The purple fluorescent dots represented the CD45 gene expression (the markers of white blood cells). The assays were applied in both selected primary human gastric cancer specimens and all blood specimens.
Data are presented as median and range (or mean ± SD) for continuous variables or as frequencies and proportions for categorical variables. Mann-Whitney U test and the Kruskal-Wallis H test were used to analyze the relationship between CTC count at baseline and tumor stage. The relationship between CTC count and the molecular classification of primary tumor according to Her2 status was examined using Spearman’s rank correlation coefficient. All statistical calculations were performed with SPSS for Windows release 18.0. A P value < 0.05 was considered statistically significant.
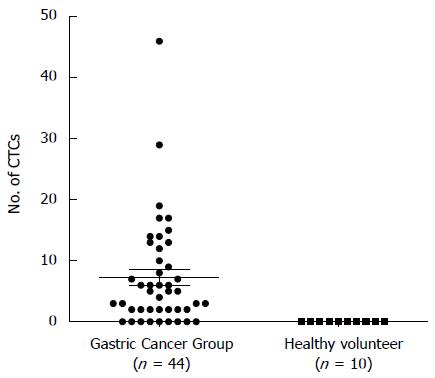
Totally 44 patients with gastric cancer (median age 56 years, range 25-87 years) were enrolled in this study. These patients consisted of 6 (13.6%) patients with gastric signet-ring cell carcinoma (SRCC) and 38 (86.4%) patients with gastric adenocarcinoma. Of these, 11 patients (7 men and 4 women) had early gastric cancer diagnosed pathologically as American Joint Committee on Cancer stage IA and IB. All 37 patients underwent laparoscopic gastrectomy with D2 lymphadenectomy; the remaining 7 patients with unresectable tumors were treated by laparoscopic exploration or palliative surgery. Thirty-six patients had no detectable overt metastasis (stage M0), and 5 patients presented with existing synchronous metastases in peritoneal carcinosis (Table 1). CTCs were detected in 35 (79.5%) patients from the gastric cancer group and the average count was 7.25 CTCs. No CTCs were detected in all of the samples from healthy volunteers (Figure 1). With regard to clinicopathological features, 11 (37.1%) patients had lymphatic emboli and elevated Her2 levels (+++ or ++) were detected in 9 (22%) gastric cancer patients.
| CanPatrol | |||
| All | Positive, n/total | P value | |
| Median age, yr (range) | 56 (25-87) | ||
| Gender | 0.780 | ||
| Male | 31 | 25/31 (80.6) | |
| Female | 13 | 10/13 (76.9) | |
| Post-TNM stage | |||
| Tumor | 0.799 | ||
| T1 | 11 | 9/11 (81.2) | |
| T2 | 4 | 3/4 (75) | |
| T3 | 5 | 4/5 (80) | |
| T4 | 21 | 19/21 (90.5) | |
| Node | 0.710 | ||
| N0 | 22 | 17/22 (77.3) | |
| N+ | 19 | 18/19 (94.7) | |
| Metastasis | 0.115 | ||
| M0 | 36 | 30/36 (83.3) | |
| M1 | 5 | 5/5 (100) | |
| Site of metastases | |||
| Peritoneal carcinosis | 5 | 5/5 (100) | |
| Histology | 0.400 | ||
| Adenocarcinoma | 38 | 31/38 (81.6) | |
| Signet-ring cell carcinoma | 6 | 4/6 (66.7) | |
| Lymphatic emboli | 0.461 | ||
| No | 21 | 17/21 (81) | |
| Yes | 11 | 10/11 (90.9) | |
| Her2 | 0.303 | ||
| +++ | 2 | 2/2 (100) | |
| ++ | 7 | 7/7 (100) | |
| + | 12 | 11/12 (91.7) | |
| 0 | 20 | 15/20 (75) | |
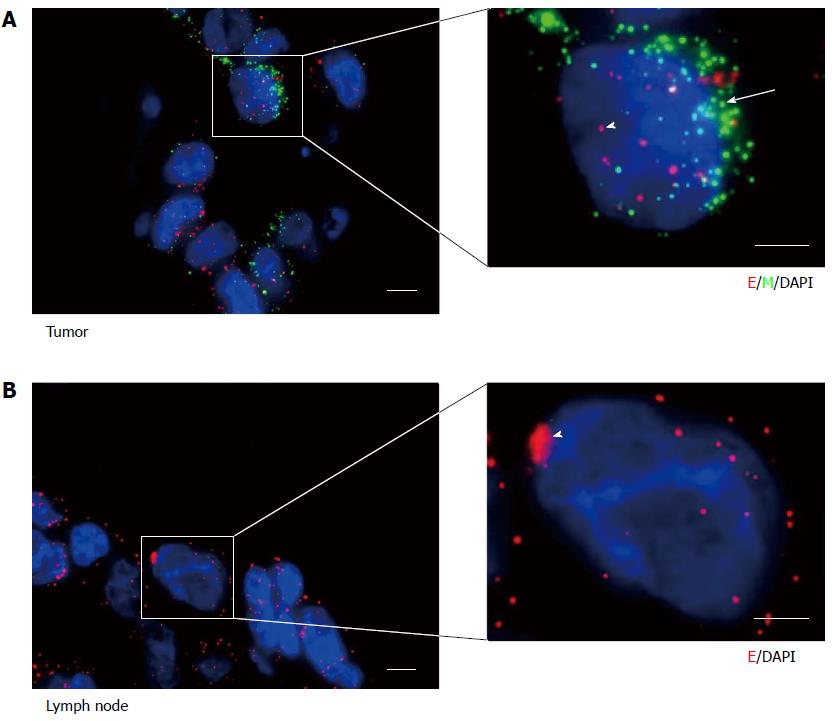
We first applied the RNA-ISH assays to primary human gastric cancer specimens. There were a small number of biphenotypic E+/M+ cells among the majority of E+ cancer cells with clear epithelial histology of primary tumors (Figure 2A). However, we only found E+ cancer cells in draining lymph nodes (Figure 2B).
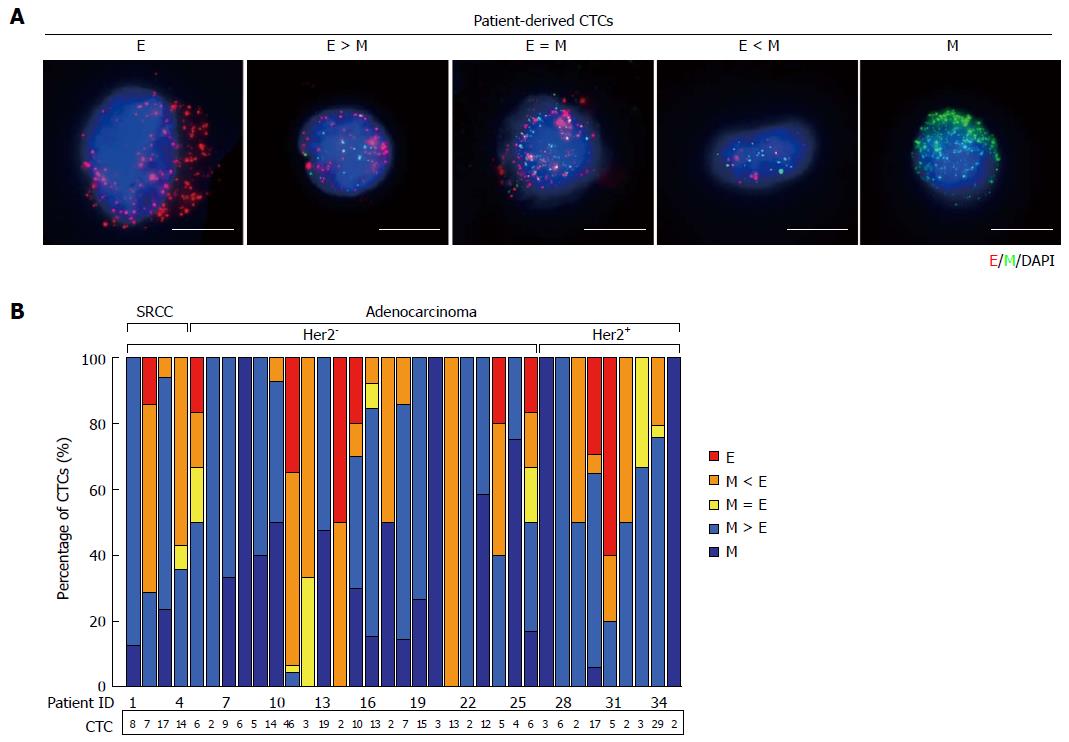
We also analyzed blood samples from 44 gastric cancer patients. Using the RNA-ISH assays, we defined five types of cells including from exclusively epithelial (E+) CTCs to intermediate (E+ > M+, E+ = M+, M+ > E+) CTCs and exclusively mesenchymal (M+) CTCs from blood samples (Figure 3A). Of 35 patients, four patients with M+ CTCs and ten patients with M+ or M > E CTCs were observed. CTCs had been captured in 35 (79.5%) patients, with EMT features varying according to histological subtype (SRCC and adenocarcinoma) and molecular classification (Her2- and Her2+) (Figure 3B). Among them, nine (25.7%) patients were detected with Her2+ gastric cancer, and 26 (74.3%) patients with Her2- gastric cancer. The CTCs from patients with both SRCC and adenocarcinoma were predominantly mesenchymal phenotypes. Furthermore, we compared the CTC counts from patients with Her2+vs Her2- in primary tumors but observed no statistically significant correlations with the number of E+/M+ cells.
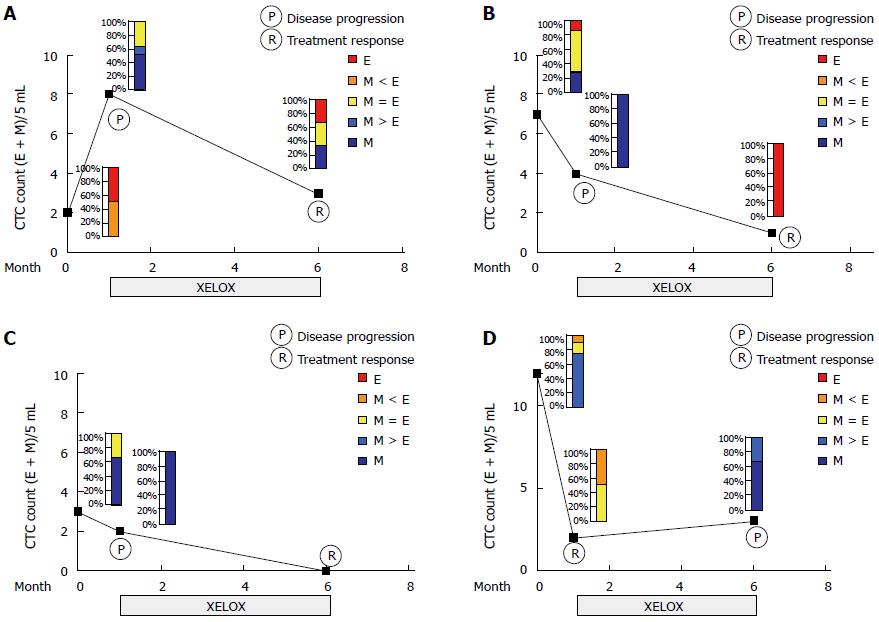
To test the possibility of the ratio of mesenchymal (M) to epithelial (E) content serving as an indicator of therapeutic response, we combined both the CTC counts and mesenchymal features of CTCs (Figure 4). Sequential blood samples were obtained from four patients undergoing adjuvant chemotherapy (XELOX regimen, capecitabine plus oxaliplatin). Three patients showed a decrease in CTC counts and/or a proportional decrease in mesenchymal feature in post-treatment samples at the 6th cycle adjuvant chemotherapy, compared with those in the post-surgery samples (Figure 4A-C). In contrast, one patient who had progressive disease evaluated by a CT scan showed an increased number or proportion of mesenchymal CTCs in the post-treatment specimen (Figure 4D).
We used NCI-N87 cells to analyze the sensitivity and linearity of the CanPatrolTM system. We spiked 10, 50, 100 and 200 NCI-N87 cells into 5 mL of blood to get the recovery of the cells (Figure 5). The correlation coefficient (R2) was 0.999.
Because of the minimally invasive feature of obtaining sequential blood specimens from cancer patients as well as potential clinical implications of the CTCs, seeking for methods to isolate and analyze CTCs for diagnosis, prognosis evaluation, and monitoring of cancer patients has been highlighted in recent years[17]. There are several underlying benefits for successful CTCs/circulating tumor DNA-based diagnostics, due to the ability to obtain sequential blood samples from cancer patients throughout the treatment course[18]. That is, characterizing CTCs may potentially provide clinicians with: (1) biomarkers predictive of therapy resistance[19,20]; (2) an independent biomarker of prognosis[3,21-23]; and (3) an indicator for early relapse[24], as well as materials for the evaluation of therapy resistance[17,25,26]. Currently, CTC enumerations have been widely admitted as an independent prognostic biomarker for a few tumors, however, their tentative roles as predictive biomarkers that may influence treatment decisions have been highly elusive and challenging to carry out, partially attributing to the extreme exiguity of CTCs in the peripheral circulation.
CellSearch System was used in the majority of published studies, which depends on epithelial tumor cell expression of KRT 8, 18, and 19 and EpCAM, presence of an intact nucleus (DAPI), and absence of the leukocyte marker CD45. However, because of the heterogeneity of CTCs, most current platforms overlooked mesenchymal-like CTCs, on which mesenchymal markers, such as Vimentin and N-cadherin, are upregulated[4]. Like a recent published report[5], a novel technology with a combination of epithelial and mesenchymal markers was used in our study, resulting in an increase of ten percent (4 patients with mesenchymal CTCs) in gastric CTC enumeration as well as identifying 10 more patients with intermediate phenotypes (biphenotypic E+/M+ cells). The approaches of CTC isolation or enrichment can be mainly categorized into two groups: physical methods and biological methods[14]. Perhaps using more than one technique, limited isolation may be improved by the inclusion of physical method-based isolation of CTCs, which may help to specifically target CTCs. Besides the biological method (immunological antibody-based capture of CTCs), we simultaneously used a filtration-based approach (a physical method, an 8 µm filtration tube in the present study)[16] for CTC isolation, further increasing sensitivity and specificity.
The vital technical challenge for CTC research comes from the rarity of tumor cells in blood. Most CTC technologies rely on the expression of epithelial markers (EpCAM-positive and keratins-positive cells) by tumor cells for their capture. But considerable disparity exists between the numbers of “epithelial” CTCs detected in different cancer types, perhaps because of a subpopulation of CTCs undergoing EMT, linked to their stemness[27]. Studies aiming to research the EMT phenomenon of CTCs have revealed high expression levels of mesenchymal markers such as AKT2, TWIST, PIK3α, N-cadherin and Vimentin[28]. Mesenchymal CTCs were correlated with cancer prognosis and therapy resistance in several cancer types[5,9,29]. While there exist few studies on the EMT phenomenon in CTCs specifically in gastric cancer, it is currently a distinct research focus. In the present study, we attempted at detecting and characterizing the EMT phenomenon in CTCs and gastric tumor tissues in clinical settings by using mesenchymal CTC markers (Vimentin and Twist). We found that human primary gastric tumor tissues contain scarce tumor cells that express epithelial and mesenchymal markers, but not in lymph node metastasis. In addition, the presence of CTCs bearing a mesenchymal phenotype has also been detected in the present study, which highlights the heterogeneity present in the circulating gastric tumor cells. Although there was an obvious increase in the number of mesenchymal CTCs in late-stage gastric cancer (data now shown), these data did not show a statistically significant difference in our analysis, which might be due to the relative small sample size. A large scale trial with higher statistic power is warranted.
CTCs may be the promise of serving as “liquid biopsies” for tumors with the potential for providing information predictive of response and chemotherapy resistance. Several reports have demonstrated the ratio of epithelial to mesenchymal markers on CTCs can be used to monitor the likeliness of therapy response. Yu and colleagues[5] found a subpopulation of CTCs with a mixed epithelial-mesenchymal phenotype at baseline and the mesenchymal phenotype was observed at stages of disease progression (suppressed at stages of treatment response), further implicating mesenchymal CTCs in the metastatic progression. Additionally, Satelli et al[9] suggested that CTC enumeration from a combination of EpCAM and Vimentin-based methods appeared to be a strong and reliable predictor for therapeutic outcomes in metastatic breast cancer with chemotherapy. Likewise, we compared CTC features in serial blood samples from four patients who underwent D2 gastrectomy (pre-operative, post-operative and post-adjuvant chemotherapy). One case who had progressive disease after 6 cycles of XELOX regimen showed the phenotypic changes in post-adjuvant chemotherapy specimen, compared with pre-treatment, showing an increased numbers of mesenchymal CTCs (Twist and Vimentin upregulated). The remaining three cases who responded to therapy showed a decrease in CTC counts and/or a proportional decrease in mesenchymal CTCs. Notably, one case who underwent curable resection surgery showed an increased number of mesenchymal CTCs in post-operative samples compared with pre-operative one, suggesting that surgical operation may play a critical role in the detachment of primary tumor cells to the peripheral circulation[30]. Therefore, adjuvant therapy should be highlighted to reduce the risk of hematogenous metastasis even after curable resection in selected patients.
A caveat has to be noted for the present study as well as all other studies which do not confirm the tumor cell identity by genomic markers. Because markers for mesenchymal-like CTCs are mostly not tumor-specific[31]. Furthermore, small sample size is another drawback when evaluating therapy response through obtaining sequential blood specimens.
In conclusion, our findings have provided evidence of the EMT phenomenon in human gastric cancer specimens, both in rare cells within primary tumors and more abundantly in CTCs by a combination of physical and biological methods. Furthermore, we demonstrated that the evaluation of the mesenchymal CTCs in peripheral blood can be used to monitor therapy response in gastric cancer patients. Clinical relevance of mesenchymal CTCs as a potential biomarker of therapeutic resistance and as a potential drug target in gastric cancer warrants further investigation.
The authors would like to thank Surexam Biotech, Guangzhou, China for the technical support.
Like common cancers, most gastric cancer-related deaths result from metastasis, which is rarely predictable by standard imaging work-ups like positron emission tomography/computed tomography scans or tumor marker tests. Circulating tumor cells (CTCs) originating from solid tumors are related with the course of hematogenous metastatic spread to the distant sites, exemplifying the switch from localized to systemic disease. Therefore, evaluating CTCs has clinical relevance in the monitoring and the outcomes of metastatic tumors.
The recent discoveries on CTCs demonstrate how these cells are involved in hematogenous metastasis, with a focus on the epithelial-mesenchymal transition (EMT). The investigation by Yu and colleagues found that dynamic changes in the number of epithelial and mesenchymal CTCs in breast cancer patients as well as the potential of monitoring therapy response.
So far, there have been few reports regarding the detecting methods and clinical significance of mesenchymal CTCs in cancer patients, specifically gastric cancer. This is the first study evaluating the mesenchymal CTCs in peripheral blood and therapy response in gastric cancer patients.
The EMT phenomenon in human gastric cancer specimens was found both in rare cells within primary tumors and more abundantly in CTCs by a combination of physical and biological methods. Furthermore, the evaluation of the mesenchymal CTCs in peripheral blood can be used to monitor therapy response in gastric cancer patients. Mesenchymal CTCs maybe is a potential biomarker of therapeutic resistance or a potential drug target in gastric cancer.
CTCs in peripheral circulation originating from solid tumors are involved in the process of hematogenous metastatic spreading to distant sites, exemplifying the switch from localized to systemic disease. Mesenchymal-to-epithelial transition (MET) is a crucial physiologic event that converts mesenchymal cells to epithelial cells. There is increasing evidence suggesting that MET maybe also regulate epithelial carcinogenesis.
The paper is a good contribution in investigating the role of mesenchymal-to-epithelial transition in circulating tumor cells of gastric cancer. The issue is not new but every new contribution confirming the feasibility and efficacy of a possible new marker is welcome.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20720] [Article Influence: 1883.6] [Reference Citation Analysis (23)] |
| 2. | Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1874] [Cited by in RCA: 2016] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 3. | Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3360] [Cited by in RCA: 3447] [Article Influence: 156.7] [Reference Citation Analysis (0)] |
| 4. | Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11:129-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 491] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 5. | Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1963] [Cited by in RCA: 1973] [Article Influence: 151.8] [Reference Citation Analysis (0)] |
| 6. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7903] [Article Influence: 464.9] [Reference Citation Analysis (1)] |
| 7. | Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 8. | Okabe H, Ishimoto T, Mima K, Nakagawa S, Hayashi H, Kuroki H, Imai K, Nitta H, Saito S, Hashimoto D. CD44s signals the acquisition of the mesenchymal phenotype required for anchorage-independent cell survival in hepatocellular carcinoma. Br J Cancer. 2014;110:958-966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Satelli A, Brownlee Z, Mitra A, Meng QH, Li S. Circulating tumor cell enumeration with a combination of epithelial cell adhesion molecule- and cell-surface vimentin-based methods for monitoring breast cancer therapeutic response. Clin Chem. 2015;61:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Tsujiura M, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Otsuji E. Liquid biopsy of gastric cancer patients: circulating tumor cells and cell-free nucleic acids. World J Gastroenterol. 2014;20:3265-3286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 821] [Article Influence: 63.2] [Reference Citation Analysis (3)] |
| 12. | Esmaeilsabzali H, Beischlag TV, Cox ME, Parameswaran AM, Park EJ. Detection and isolation of circulating tumor cells: principles and methods. Biotechnol Adv. 2013;31:1063-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Friedlander TW, Premasekharan G, Paris PL. Looking back, to the future of circulating tumor cells. Pharmacol Ther. 2014;142:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Harouaka R, Kang Z, Zheng SY, Cao L. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther. 2014;141:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 799] [Article Influence: 66.6] [Reference Citation Analysis (1)] |
| 16. | Wu S, Liu Z, Liu S, Lin L, Yang W, Xu J. Enrichment and enumeration of circulating tumor cells by efficient depletion of leukocyte fractions. Clin Chem Lab Med. 2014;52:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Pavese JM, Bergan RC. Circulating tumor cells exhibit a biologically aggressive cancer phenotype accompanied by selective resistance to chemotherapy. Cancer Lett. 2014;352:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 583] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 19. | Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1363] [Cited by in RCA: 1329] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 20. | Miyamoto DT, Lee RJ, Stott SL, Ting DT, Wittner BS, Ulman M, Smas ME, Lord JB, Brannigan BW, Trautwein J. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2012;2:995-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 21. | Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218-4224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 785] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 22. | de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302-6309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1786] [Cited by in RCA: 1723] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 23. | Muinelo-Romay L, Vieito M, Abalo A, Nocelo MA, Barón F, Anido U, Brozos E, Vázquez F, Aguín S, Abal M. Evaluation of Circulating Tumor Cells and Related Events as Prognostic Factors and Surrogate Biomarkers in Advanced NSCLC Patients Receiving First-Line Systemic Treatment. Cancers (Basel). 2014;6:153-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Pachmann K, Camara O, Kavallaris A, Krauspe S, Malarski N, Gajda M, Kroll T, Jörke C, Hammer U, Altendorf-Hofmann A. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol. 2008;26:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, Raghavan D, Heller G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 497] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 26. | Friedlander TW, Ngo VT, Dong H, Premasekharan G, Weinberg V, Doty S, Zhao Q, Gilbert EG, Ryan CJ, Chen WT. Detection and characterization of invasive circulating tumor cells derived from men with metastatic castration-resistant prostate cancer. Int J Cancer. 2014;134:2284-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6972] [Cited by in RCA: 6911] [Article Influence: 383.9] [Reference Citation Analysis (0)] |
| 28. | Satelli A, Mitra A, Brownlee Z, Xia X, Bellister S, Overman MJ, Kopetz S, Ellis LM, Meng QH, Li S. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin Cancer Res. 2015;21:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 29. | Mego M, Mani SA, Lee BN, Li C, Evans KW, Cohen EN, Gao H, Jackson SA, Giordano A, Hortobagyi GN. Expression of epithelial-mesenchymal transition-inducing transcription factors in primary breast cancer: The effect of neoadjuvant therapy. Int J Cancer. 2012;130:808-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Miyazono F, Natsugoe S, Takao S, Tokuda K, Kijima F, Aridome K, Hokita S, Baba M, Eizuru Y, Aikou T. Surgical maneuvers enhance molecular detection of circulating tumor cells during gastric cancer surgery. Ann Surg. 2001;233:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Bednarz-Knoll N, Alix-Panabières C, Pantel K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer Metastasis Rev. 2012;31:673-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Di Vita M, Furka A S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Zhang DN