Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12457
Peer-review started: June 19, 2015
First decision: July 19, 2015
Revised: August 7, 2015
Accepted: September 13, 2015
Article in press: September 14, 2015
Published online: November 21, 2015
Processing time: 154 Days and 5.2 Hours
AIM: To evaluate effects of dietary supplementation of sulforaphane (SF)-rich broccoli sprout (BS) extract on hepatic abnormalities in Japanese male participants.
METHODS: In a randomized, placebo-controlled, double blind trial, male participants with fatty liver received either BS capsules containing glucoraphanin [GR; a precursor of SF (n = 24)] or placebo (n = 28) for 2 mo. Liver function markers, serum levels of aspartate and alanine aminotransferases (AST and ALT, respectively) and γ-glutamyl transpeptidase (γ-GTP) and an oxidative stress marker, urinary levels of 8-hydroxydeoxyguanosine (8-OHdG), were measured and compared in participants before and after the trial period. In an animal model, chronic liver failure was induced in Sprague-Dawley rats by successive intraperitoneal injection with N-nitrosodimethylamine (NDMA) for 4 wk. Concomitantly, rats received AIN-76 diets supplemented with or without BS extract. Thereafter, rats were sacrificed, and their sera and livers were collected to measure serum liver function markers and hepatic levels of thiobarbituric acid reactive substances (TBARS) levels and hepatic glutathione S-transferase (GST) activity, a prototypical phase 2 antioxidant enzyme.
RESULTS: Dietary supplementation with BS extract containing SF precursor GR for 2 mo significantly decreased serum levels of liver function markers, ALT [median (interquartile range), before: 54.0 (34.5-79.0) vs after supplementation: 48.5 (33.3-65.3) IU/L, P < 0.05] and γ-GTP [before: 51.5 (40.8-91.3) vs after: 50.0 (37.8-85.3) IU/L, P < 0.05], as well as the alkali phosphatase activity. Placebo showed no significant effects on the markers. The urinary level of 8-OHdG, an established oxidative stress marker, was significantly reduced in participants who had received BS capsules but not the placebo [before: 6.66 (5.51-9.03) vs after: 5.49 (4.89-6.66) ng/mg-creatinine, P < 0.05]. The reduction of urinary 8-OHdG was significantly correlated with decreased levels of both ALT and γ-GTP [∆8-OHdG and ∆ALT: Spearman r (r) 0.514 and P = 0.012, ∆8-OHdG and ∆γ-GTP: r = 0.496 and P = 0.016]. Intake of BS extract prevented NDMA-induced chronic liver failure in rats, which was attributable to the suppression of the increase in TBARS through induction of hepatic phase 2 antioxidant enzymes including hepatic GST (86.6 ± 95.2 vs 107.8 ± 7.7 IU/g, P < 0.01).
CONCLUSION: Dietary supplementation with BS extract containing the SF precursor GR is likely to be highly effective in improving liver function through reduction of oxidative stress.
Core tip: A randomized, placebo-controlled, double blind trial was conducted to assess the efficacy of dietary supplementation with broccoli sprout extract containing glucoraphanin (GR), a sulforaphane (SF) precursor, on hepatic abnormalities in Japanese men without changing their lifestyle or habits. Supplementation for 2 mo significantly decreased serum levels of liver function markers such as alanine aminotransferase and γ-glutamyl transpeptidase. The effect was associated with a reduction of urinary 8-hydroxydeoxyguanosine, an oxidative stress marker. Dietary supplementation with SF precursor GR is effective in improving liver function, and represents a potent method for maintaining good liver condition.
- Citation: Kikuchi M, Ushida Y, Shiozawa H, Umeda R, Tsuruya K, Aoki Y, Suganuma H, Nishizaki Y. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J Gastroenterol 2015; 21(43): 12457-12467
- URL: https://www.wjgnet.com/1007-9327/full/v21/i43/12457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i43.12457
The prevalence of viral hepatitis has rapidly decreased alongside the notable improvements in the development therapeutic agents against viral hepatitis over the past 25 years. Meanwhile, the incidence of lifestyle-related “obesity and hepatic abnormalities” has increased and thus, has focused the attention of many researchers and clinicians involved in the care and management of liver diseases[1]. Non-alcoholic fatty liver disease (NAFLD) is a lifestyle-related liver disease that occurs in patients without or with almost no history of alcohol intake and has a high risk of accompanying necrosis/inflammation and fibrosis of the liver. This so-called non-alcoholic steatohepatitis (NASH) is one of the leading causes of cirrhosis and liver cancer[2]. In 2010, the Japanese Society of Hepatology published “Guidelines for the management of NASH/NAFLD 2010”, but there are only a few established consensuses for the treatment of NASH and NAFLD. A diet and exercise therapy is preferentially chosen for the purpose of weight loss; however, it is difficult to encourage patients to change their lifestyles and habits. Prolonged medical treatment has some risk of adverse effects. Alcoholic liver disease (ALD), which comprises a spectrum of diseases including alcoholic fatty liver, hepatitis, cirrhosis, and hepatocellular carcinoma, remains a major health problem worldwide[3]. Stopping alcohol consumption is the most effective strategy for the improvement of ALD, but it is not always easy to preach temperance to patients who thrive on alcohol consumption. We therefore have been keen on developing dietary methods for improving liver health and function of patients without adverse effects and drastic modification of lifestyle patterns including excessive diet restriction and ergotherapy.
Sulforaphane (SF), identified in broccoli[4], is one of the most fascinating phytochemicals in the world because it protects aerobic cells against carcinogens and toxic DNA-damaging electrophiles and oxidants by induction of a network of phase 2 detoxification and antioxidant enzymes, and by suppressing inflammatory responses[5,6]. Major cytoprotective effects of SF are mediated by the transcriptional upregulation of the Kelch-like ECH-associated protein 1 (Keap1)-NF-E2-related factor 2 (Nrf2) pathway and other anti-inflammatory mechanisms including inhibition of the NF-κB pathway[7,8]. Nrf2 has recently been suggested to play a critical role in protecting liver health not only from hepatotoxic chemicals but also from lifestyle-related factors such as high-energy food consumption[9-11]. Based on these mechanisms, in animal experimental models, dietary SF has been demonstrated to protect against a wide variety of liver diseases caused by hepatotoxic chemicals[12-15], alcohol[16], and high energy diets[17,18]. To our knowledge, however, the hepatoprotective effects of SF in human subjects have never been reported so far.
Several clinical trials[19-21] have been conducted with the focus on prevention or therapeutic effects of SF against various types of cancer, because SF was originally expected to be used as a cancer chemopreventive agent. In previous trials, doses of glucoraphanin (GR), a glucosinolate (GSL) precursor of SF, were set at more than 400 μmol/d, which appears to be much higher compared to the estimated daily intake of GSLs (less than 100 μmol/d) according to previous surveys[22,23]. From the aspect of safety based on eating experience, a lower dose is suitable for dietary supplementation with GR.
We herein describe the effects of dietary supplementation with a lower dose of SF precursor GR (approximately 69 μmol/d) on hepatic abnormalities in Japanese men who did not undergo any fundamental changes in their lifestyle and habits during the 2 mo of the randomized clinical trial. This report will contribute to develop potent dietary methods for improving liver health and function.
BS extract powder, which is industrially produced by Kagome Co., Ltd., was used in the present study. BS was grown from specially selected seeds (Caudill Seed Co. Inc., Louisville, KY) for 1 d after the germination. Then the 1-d-old BS was added into boiling water and maintained at 95 °C for 30 min, and the sprout residues were removed by filtration. The boiling water extract was mixed with a dextrin and then spray dried to yield the BS extract powder containing 135 mg (approximately 310 μmol) of GR per gram, which was confirmed by a high performance liquid chromatograph analysis as previously described[24-26]. For the clinical trial, the BS extract powder was blended with waxy corn starch, crystalline cellulose, and calcium stearate, and then encapsulated in hydroxypropyl methylcellulose capsules to yield 10 mg (approximately 23 μmol) of GR per a capsule (BS capsule). Placebo capsules were prepared similarly but without the BS extract powder. These were prepared by a Good manufacturing practices facility (Sansho Pharmaceutical Co., Ltd., Shizuoka, Japan).
We conducted a randomized, placebo-controlled, double blind trial from January through May 2014 at a single institute, Tokai University Tokyo Hospital (Tokyo, Japan) in accordance with the International Ethical Guidelines and Declaration of Helsinki. The trial has been registered with UMIN-CTR (#UMIN000012855). The protocol was approved by the Institutional Review Board for Clinical Research of Tokai University School of Medicine (#13R-169) and the Ethics Committee of Kagome Co. Ltd (#2013-R05).
Participants were recruited from among male outpatients, aged between 30 and 69, who had higher activity of at least one of three liver function markers, alanine aminotransferase (ALT) ≥ 40 IU/L, aspartate aminotransferase (AST) ≥ 35 IU/L, or γ-glutamyl transpeptidase (γ-GTP) ≥ 80 IU/L for at least the last 2 consecutive months, and who were diagnosed with fatty liver using ultrasonography. Patients were excluded if they had serious liver diseases, were suspected of acute liver failure, viral hepatitis, or other serious diseases including cardiac disease, renal dysfunction (serum creatinine > 2.0 mg/dL), and bile duct cancer, or if they habitually consumed higher amounts of alcohol (more than 60 g of alcohol per day). Written informed consent was obtained from all participants.
Randomization was performed in a 1:1 ratio using a random number table. Study treatment was randomly assigned and labeled with the participant numbers before the study. Participants were numbered consecutively in the order in which they entered the study. They received either 3 BS capsules containing 30 mg of GR, the precursor of SF, or the placebo for 2 mo. The participants as well as investigators were blinded to the treatment until the study completion.
Primary outcome measures were decreased levels of serum ALT, AST, and γ-GTP. Secondary outcome measures were improvement of the following physical parameters: body weight, body mass index (BMI), and waist circumference; blood biochemical markers: albumin, total bilirubin, alkali phosphatase (ALP), choline esterase (ChE), ferritin, urinary acid (UA), triglyceride (TG), total-cholesterol, high-density lipoprotein (HDL)-cholesterol, and low-density lipoprotein (LDL)-cholesterol, fasting blood sugar (FBS), hemoglobin A1c (HbA1c), high-sensitivity C-reactive protein (hs-CRP), serum adipokines (leptin and adiponectin), urinary oxidative marker 8-hydroxydeoxyguanosine (8-OHdG); and diagnosis of fatty liver on ultrasonography.
Animal experiments were approved by the Animal Care and Use Committee of Kagome Co., Ltd. in accordance with the guidelines established by the Japanese Society of Nutrition and Food Science (Law and Notification 6 of the Japanese Government). The animal experiment was designed to minimize pain or discomfort to the animals.
Male Sprague Dawley rats (7-wk-old) were acclimatized on an AIN-76 diet (CLEA Japan, Tokyo, Japan) in individual stainless steel cages in a room maintained at 20 ± 2 °C, and a relative humidity of 65% ± 6%, with a 12/12-h light cycle. Rats were divided into five groups: sham, control, BS-low, BS-middle, and BS-high groups (sham: n = 6, others: n = 8 each). Sham and control rats received the AIN-76 diet for 4 wk. BS-low, BS-middle, and BS-high rats received 62.5, 125, and 250 mg of GR per 100 g of the AIN-76 diet, respectively (Table 1). Except for sham, all rats were intraperitoneally injected with N-nitrosodimethylamine (NDMA) at a dose of 5 mg/kg body weight on 3 consecutive days (every Tuesday to Thursday) of the week for 4 wk. Sham rats were injected with the vehicle (saline) in the same manner. During this period, their body weights and food intakes were monitored. After 4 wk, rats were sacrificed with no pain, and their blood and livers were harvested, frozen in liquid N2, and stored at -80 °C until analyzed.
| Contents, g/100 g | Normal diet | BS low | BS middle | BS high |
| Casein | 25.00 | 25.00 | 25.00 | 25.00 |
| Corn starch | 40.25 | 39.83 | 39.41 | 38.56 |
| Sucrose | 20.00 | 20.00 | 20.00 | 20.00 |
| Corn oil | 5.00 | 5.00 | 5.00 | 5.00 |
| Mineral mix1 | 3.50 | 3.50 | 3.50 | 3.50 |
| Vitamin mix1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 | 0.25 |
| Cellulose | 5.00 | 5.00 | 5.00 | 5.00 |
| BS extract | 0.00 | 0.42 | 0.84 | 1.69 |
| Glucoraphanin | 0.00 | 0.063 | 0.13 | 0.25 |
Parameters of blood biochemistry of participants were automatically measured using an Auto Blood Biochemistry Analyzer. Serum levels of adiponectin and leptin were determined with an ELISA kit (Human adiponectin ELISA kit, Otsuka Pharmaceutical Co., Ltd, Tokushima, Japan) and an enzyme immune assay kit (Human leptin highly sensitive assay kit, Immuno-Biological Laboratories Co., Ltd., Gunma, Japan), respectively. Urinary levels of 8-OHdG were measured with a commercial ELISA kit (New 8-OHdG check, Japan Institute for the Control of Aging, Shizuoka, Japan), and standardized to creatinine concentrations that were measured using a creatinine urinary assay kit (Cayman Chemical Company, MI).
Activities of AST and ALT in rat sera were determined using a transaminase CII-test kit (Wako Pure Chemical Institute, Osaka, Japan). Levels of albumin and total bilirubin in rat sera were measured by using a modified bromocresol purple method and a chemical oxidation method, respectively (SRL Inc., Tokyo, Japan). Hepatic levels of thiobarbituric acid reactive substances (TBARS), byproducts of lipid peroxidation, were measured according to the method by Kikugawa et al[24]. In brief, a portion of rat liver (0.5 g) was homogenized in 4.5 mL of ice-cold 10 mmol/L Tris-HCl (pH 7.4) with a polytron homogenizer. The homogenate was centrifuged at 12000 ×g, for 20 min at 4 °C, and the supernatant (0.2 mL) was added to a glass tube containing 0.65 mL of a reaction mixture, 0.04 mL of 5.0% sodium dodecyl sulfate, and 0.01 mL of 0.8% butylhydroxytoluene, 0.28 mL of 0.8% thiobarbituric acid, and 0.32 mL of distilled water. After the addition of 0.15 mL of 20% acetate buffer (pH 3.5), the test tube was carefully sealed with a plastic paraffin film and then incubated at 100 °C for 1 h. TBARS was extracted with 1 mL of butanol/pyridine (15/1) and then measured by spectrophotometric assay (OD 532 nm). Levels of TBARS in the liver were calculated using a standard curve of 1,1,3,3-tetramethoxypropane dissolved in ethanol. The enzyme activity of glutathione S-transferase (GST) was determined with 1-chloro-2,4-dinitrobenzene as the substrate as described previously[25].
Parametric data are represented as the mean ± SD and non-parametric data as the median with percentile distribution. In the clinical trial, differences between the two groups were analyzed using the non-parametric Mann-Whitney U test, and compared before and after intervention by using the Wilcoxon single rank tests. Correlations between variables were examined by using the Spearman rank correlation test. In the animal experiment, differences among five groups were analyzed with the Kruskal-Wallis test followed by the Steel-Dwass test for non-parametric data or with the ANOVA followed by the Tukey Kramer test or the Dunnett test for parametric data. P values less than 0.05 were considered significant.
The sample size in the clinical study was calculated using an estimated 10% loss in the intervention period, a confidence level of 95%, and a power of 80% (at least 20 in each group). Statistical significance was inferred at P < 0.05.
Statistical analyses were carried out using JMP (SAS institute, Cary, NC).
A total of 55 male outpatients were enrolled in this clinical study, and assigned to one of the following two groups: SF group (n = 27) and placebo group (n = 28). Of those, 24 and 28 participants, respectively, completed the study protocol and were available for efficacy analyses. Three participants in the SF group were excluded for following reasons: 2 had poor compliance and 1 had gallstone pancreatitis during the intervention period. There were no adverse events due to the treatment or to the study protocol. Table 2 shows the characteristics and baseline parameters of primary outcome measures of participants in both groups. No significant differences were observed in the parameters including liver function markers, ALT, AST, and γ-GTP.
| Median (IQR) | ||
| SF group | Placebo group | |
| n | 24 | 28 |
| Age, yr | 51.5 (42.0-57.5) | 56.0 (49.8-63.3) |
| BMI, kg/cm2 | 25.7 (24.2-27.6) | 25.9 (24.5-27.2) |
| Waist circumference, cm | 86.5 (81.0-95.0) | 92.3 (88.3-95.3) |
| AST, IU/L | 37.5 (24.8-48.3) | 30.0 (27.5-39.5) |
| ALT, IU/L | 54.0 (34.5-79.0) | 41.5 (34.0-64.3) |
| γ-GTP, IU/L | 51.5 (40.8-91.3) | 52.0 (39.5-91.5) |
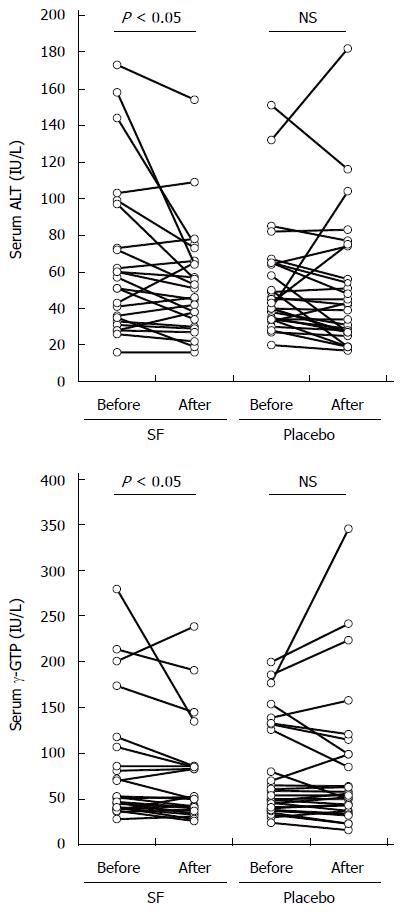
Primary outcome measures of the present study were significant decreases in serum levels of ALT, AST, and γ-GTP by dietary supplementation with 30 mg of SF precursor GR per day. Serum levels of those markers before and after 2 mo of intervention are displayed in Figure 1 and Table 3. A non-parametric Wilcoxon single rank test revealed that serum levels of ALT and γ-GTP, but not AST, were significantly decreased comparing levels of ALT and γ-GTP before and after intervention in the SF group, whereas, there were no significant differences in the placebo group. The mean percent changes in ALT and γ-GTP levels from before intervention were -10.7% and -8.9% in the SF group, which seemed to be larger than those (-4.3% and -1.1%, respectively) in the placebo group (no significant differences).
| SF group (n = 24) | Placebo group (n = 28) | |||
| Before | After | Before | After | |
| BMI, kg/m2 | 25.7 (24.2-27.6) | 25.8 (24.2-27.3) | 25.9 (24.6-26.8) | 25.6 (24.5-26.8)a |
| Waist Circumference, cm | 86.5 (81.0-95.0) | 88.8 (82.5-95.0) | 92.3 (88.4-95.3) | 91.4 (88.5-97.1) |
| AST, IU/L | 37.5 (24.8-48.3) | 37.5 (27.0-39.8) | 30.0 (27.5-39.5) | 26.0 (22.5-42.8) |
| ALT, IU/L | 54.0 (34.5-79.0) | 48.5 (33.0-65.3)a | 41.5 (34.0-64.3) | 40.5 (27.0-60.5) |
| γ-GTP, IU/L | 51.5 (40.8-91.3) | 50.0 (37.8-85.3)a | 52.0 (39.5-127.5) | 53.0 (37-99) |
| Alb, g/dL | 4.6 (4.4-4.9) | 4.5 (4.3-4.7)a | 4.6 (4.5-4.7) | 4.6 (4.5-4.8) |
| TB, mg/dL | 0.9 (0.6-1.1) | 0.9 (0.7-1.2) | 0.9 (0.6-1.1) | 0.8 (0.7-1.0) |
| ALP, IU/L | 210 (168-248) | 182 (166-140)a | 230 (206-274) | 231 (214-281) |
| ChE, IU/L | 390 (359-436) | 388 (358-421)a | 398 (369-458) | 406 (370-424) |
| Ferritin, ng/mL | 281 (131-381) | 216 (136-365) | 231 (162-322) | 215 (168-321) |
| UA, mg/dL | 6.1 (5.5-7.0) | 6.0 (5.4-6.8) | 6.4 (5.4-6.8) | 6.1 (5.6-6.7) |
| TG, mg/dL | 146 (118-193) | 135 (95.5-208) | 133 (99.3-166) | 113 (83.0-160) |
| TC, mg/dL | 201 (182-219) | 194 (184-211)a | 196 (183-224) | 207 (184-225) |
| HDL-C, mg/dL | 50.0 (44.0-59.0) | 48.5 (44.8-54.0) | 47.5 (44.8-58.0) | 49.5 (45.0-57.0) |
| LDL-C, mg/dL | 123 (107-138) | 123 (111-141) | 125 (116-142) | 137 (118-146) |
| HbA1c, % | 5.7 (5.4-6.1) | 5.7 (5.5-6.0) | 5.8 (5.5-6.1) | 5.8 (5.5-6.1) |
| FBS, mg/dL | 105 (99.8-113) | 106 (101-115) | 105 (99-115) | 106 (97.5-115) |
| hs-CRP, mg/dL | 0.067 (0.036-0.13) | 0.059 (0.031-0.13) | 0.056 (0.037-0.14) | 0.068 (0.028-0.15) |
| 8-OHdG, ng/mg-CRE | 6.8 (5.5-9.0) | 5.5 (4.9-6.7)a | 6.6 (5.3-8.4) | 6.1 (4.7-7.6) |
| Adiponectin, μg/mL | 6.1 (4.8-7.4) | 4.8 (4.1-7.7)a | 6.3 (4.6-9.5) | 6.1 (4.6-7.9)a |
| Leptin, ng/mL | 5.9 (4.2-7.5) | 5.8 (3.0-8.7) | 7.3 (5.2-11.4) | 6.6 (4.1-10.1) |
As shown in Table 3, serum levels of ALP and albumin, relevant markers of liver function, were significantly changed following treatment with capsules containing GR. The median change in ALP levels (IU/L) before and after the trial was -6.0 with an interquartile range (IQR) of 17.8 in the SF group, and 3.5 with an IQR of 25.5 in the placebo group, respectively (P < 0.05). Serum albumin levels were significantly lowered in the SF group, but the change was very slightly, and was within the reference level range.
Dietary supplementation with 30 mg of GR in BS capsules did not show any remarkable impact on physical parameters (BMI and waist circumference) and markers of sugar metabolism (FBS and HbA1c). Only BMI significantly improved in the placebo group, but the reason was unknown. However, relevant markers of lipid metabolism were partly improved in the SF group. At baseline, the median level of serum total cholesterol was more than 200 mg/dL, which is considered a borderline-to-high range according to the American Heart Association. The level was lowered to less than 200 mg/dL after supplementation with BS capsules containing GR. Conversely, the level was elevated in the placebo group. Neither HDL- nor LDL-cholesterol levels were significantly changed in the study. In association with the improvement of total cholesterol level, serum activity of ChE, which is known to be higher in those developing obesity and fatty liver, was significantly decreased in the SF group. Furthermore, a representative marker of oxidative stress, urinary 8-OHdG level was significantly reduced by approximately 20% by dietary supplementation with GR.
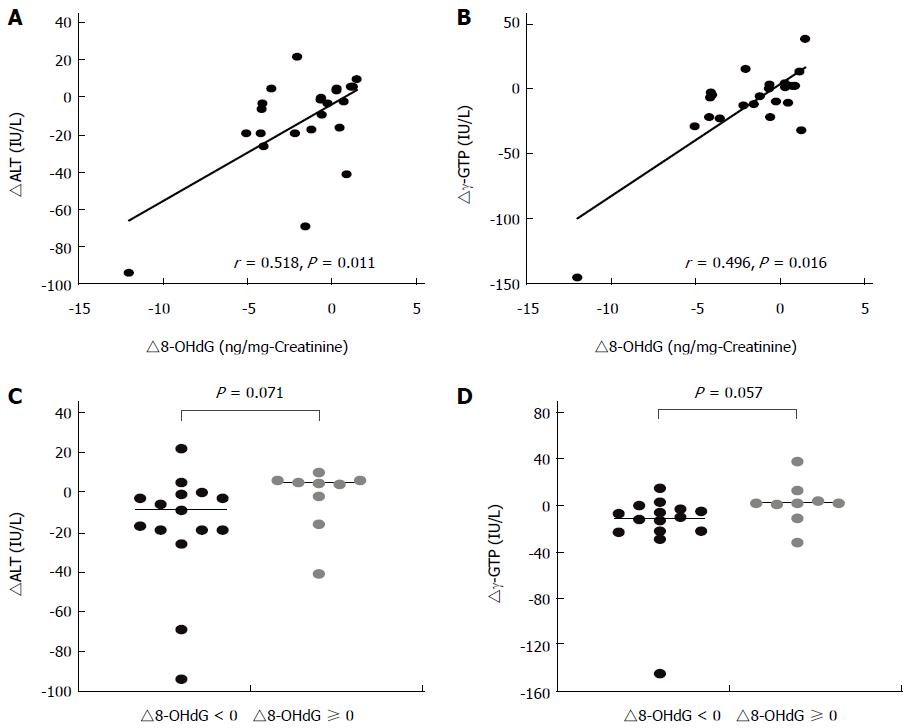
The Spearman rank correlation test identified significant positive relationships between changes in levels of urinary 8-OHdG (∆8-OHdG) and in liver function markers (∆ALT and ∆γ-GTP) in participants in the SF group (Figure 2A and B). No significant relationships between in these levels were observed in the placebo group. To further understand the contribution of reduced oxidative stress in the improvements of liver function markers, ∆ALT and ∆γ-GTP were compared in participants with or without reduction in 8-OHdG levels (∆8-OHdG < 0 and ≥ 0, respectively). In the SF group, 15 participants showed a reduction in 8-OHdG levels, but the other 9 participants did not. In participants with ∆8-OHdG < 0 in the SF group, stratified analysis showed clear trends of lowering both levels of ∆ALT and ∆γ-GTP, where the median levels were lowered by 17 and 11.5 IU/L, respectively, compared with those participants with ∆8-OHdG ≥ 0.
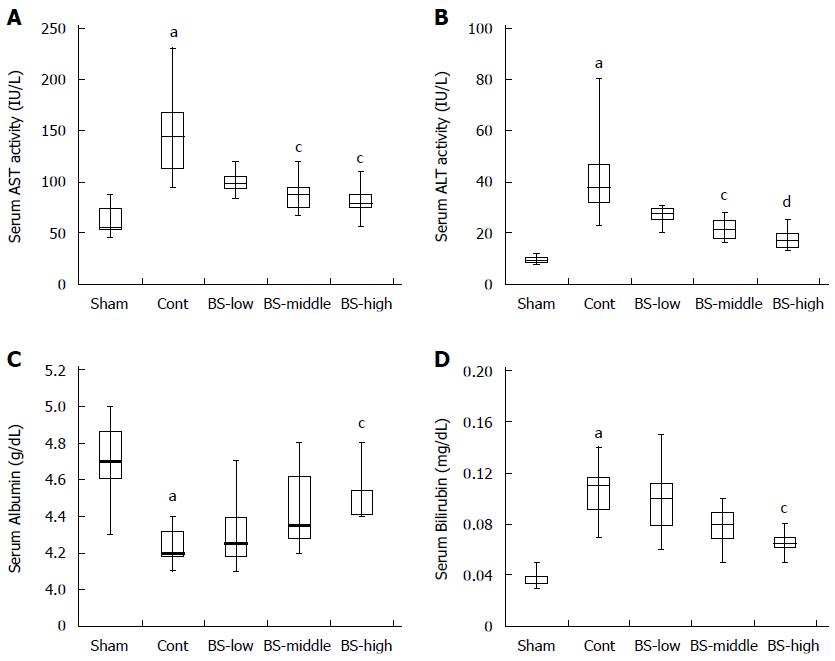
Repeated intraperitoneal injection of NDMA resulted in a body weight loss of approximately 17% and a significant reduction of liver weight (29%) in control rats compared with sham rats (Table 4). The NDMA-induced hepatotoxicities were prevented by the intake of BS extract in a dose-dependent manner. A significant protective effect on reducing liver weight was observed in rats belonging to the BS-high group. There was no significant difference in food intake among groups. Serum levels of representative liver function markers, AST and ALT, were dramatically elevated in control rats by more than 2-fold compared to sham rats, which clearly showed that NDMA toxicity induced chronic liver failure in the control rats (Figure 3A and B). On the other hand, elevations of those levels were dose-dependently prevented in rats that had been fed diets containing serial doses of BS. Significant differences from control were observed in BS-middle and BS-high group rats. Furthermore, the intake of BS resulted in the improvement of serum levels of albumin and bilirubin, which are also used to evaluate functions of the liver and bile duct (Figure 3C and D).
In connection with the elevation of serum AST and ALT activities, hepatic TBARS levels were significantly increased in control rats, which indicated that NDMA toxicity resulted in excess oxidative stress in rat livers (Figure 4A). However, lower levels of hepatic TBARS were observed in BS-fed rats. In particular, these levels in the BS-high group rats improved almost completely. Figure 4B shows the enzyme activities of hepatic GST, a typical cytoprotective phase 2 enzyme that plays an important role in the detoxification of NDMA and in antioxidant activity. GST activity was very slightly increased by repeated injection of NDMA, whereas it increased significantly by continuous intake of BS.
The liver is so called a silent organ; symptoms tend not to appear until a disease is well advanced. Therefore, for the prevention of chronic liver failure, it is quite important to manage liver condition on the basis of serum levels of liver function markers, which can be easily measured during routine health checkups. We describe herein the first demonstration that dietary supplementation with BS extract containing a low dose of GR (30 mg or approximately 69 μmol), a precursor of SF, is much likely to be effective in the improvement of liver function by reducing oxidative stress in male participants with abnormally higher levels of liver function markers.
The improvement on liver function was judged from the results of the present randomized, placebo-controlled, double blind trial, where serum levels of representative liver function markers, ALT and γ-GTP, as well as the relevant marker ALP were significantly lowered in male participants with fatty liver in SF group who had taken BS capsules containing 30 mg of GR per day for 2 mo, but not in placebo group participants. Unfortunately, no significant differences were observed in the decreased levels of the markers between the SF and placebo groups, probably due to the design of the present trial such as a small sample size and a no limit on criteria for subject inclusion: at least one higher activity of three liver function markers, ALT ≥ 40 IU/L, AST ≥ 35 IU/L, or γ-GTP ≥ 80 IU/L. In the present trial, serum levels of liver function markers were decreased in some participants even in the placebo group possibly because of the so-called placebo effect or subtle lifestyle related bias, although actual reasons are unclear. However, the lowering activities of the markers were not associated with reduction of urinary levels of 8-OHdG as a biological marker of oxidative stress in the placebo group, which was distinctly different from that in the SF group participants. This suggested that continuous intake of GR for 2 mo surely affected physiologies of participants beyond the placebo effect, and the reduction of oxidative stress was much likely to be involved in the improvement of liver function. The urinary level of 8-OHdG has been established to reflect oxidative damage of DNA occurring in various cells and tissues in the body[26,27], and thereby widely used for evaluating in vivo oxidative stress[28]. As for the liver, a previous clinical study showed significant correlations between hepatic expression levels and urinary levels of 8-OHdG in patients with chronic hepatitis C[29]. In the present clinical trial, although a biopsy test was not conducted, hepatic oxidative stress might also be reduced in the SF group participants.
As previously mentioned, there is no report describing the efficacy of SF or BS preparations on the improvement of hepatic abnormalities in humans to date, whereas many animal experiments have been carried out to demonstrate the hepatoprotective effects of SF and clarify the underlying mechanism involved. Traditional experimental models in toxicology have demonstrated that intake of SF or the precursor GR can markedly prevent liver damage induced by a wide variety of hepatotoxic chemicals such as cisplatin[12], mycocystin[13], carbon tetrachloride[14], acetaminophen (unpublished our preliminary data), and D-galactosamine and lipopolysaccharide[15]. Additionally, it has been suggested that SF is potent in preventing lifestyle-related liver diseases caused by excessive consumption of alcohol[16] and high energy diets[17,18]. These hepatoprotective effects have been suggested to be attributable to eminent inducer potency of SF for phase 2 cytoprotective proteins including antioxidant and detoxifying enzymes through activation of the transcriptional factor Nrf2. This speculation is strongly supported by numerous basic studies using Nrf2 knockout mice and cells, which revealed that Nrf2 plays an essential role in the prevention of liver diseases[30,31].
In the present study, we assessed the potency of the BS extract containing the SF precursor GR as an inducer of Nrf2 and its downstream phase 2 cytoprotective enzymes in animal models. Intake of the BS extract for 4 wk significantly increased the hepatic activity of GST, a typical phase 2 enzyme. Consequently, elevation of oxidative stress and resulting liver failure by successive exposure to NDMA was significantly prevented in rats fed the BS extract. This finding ascertained the inducer potency of the BS extract used in the present clinical trial, and inferred that Nrf2-regulated phase 2 enzymes might also be induced in participants by supplementation with BS capsules in lieu of biopsy data of the human liver.
Of special note is that the dose of SF precursor GR was set at 30 mg (approximately 69 μmol) per day in the present clinical trial, which was much lower than previous clinical studies evaluating cancer prevention effects of SF. The therapeutic effect on Helicobacter pylori infection was demonstrated by a continuous daily intake of 70 g of fresh BS containing 420 μmol GR[19]. Furthermore, in a large-scale randomized clinical study in China, a detoxification effect on airborne pollutants was clearly observed in participants given a BS beverage containing 800 μmol GR[20,21]. It is generally recognized that GR is a safer compound based on a long-term eating habit of broccoli and other cruciferous vegetables, which was also demonstrated by previous clinical trials including phase I studies[32,33] and the above-mentioned high-dose tests. However, we set our study to the lower GR dose, which was considered a sufficiently safe dose even for participants with hepatic abnormalities, because of the estimated range of daily intake of GSLs from cruciferous vegetables. A dose-dependent effect should be examined in future trials.
Our study has several limitations. First, in addition to the small sample size as mentioned above, only male participants were recruited for this randomized clinical trial. Thus, the efficacy of the BS extract on female participants is unclear. Second, the trial period was only 2 mo, which was shorter than the previously reported randomized controlled trials describing the efficacy of silymarin from milk thistle[34]. Although the longer term effects of BS extract is yet unknown, striking outcomes could be obtained in future intervention studies with a longer period of observation. Third, the BS extract but not the purified SF was used in the present study. Similar to a number of previous studies, our BS extract contains a rich SF precursor of GR; therefore, we consider SF as the predominant active compound in the BS extract responsible for the reduction in oxidative stress and the resulting improvement in liver function. Fourth, we did not perform a histological and pathological examination of fat deposition, inflammation, or fibrosis in the liver. In a future randomized clinical trial that is being planned, we will address the above-mentioned limitations to comprehend the efficacy of the BS extract containing the SF precursor in liver diseases.
The present study received no specific grant from any public funding agency, but was supported by Kagome Co., Ltd. We thank Toshihiro Matsumoto for technical assistance and enlightened discussion for animal experiments.
Instead of viral hepatitis, the growing incidence of lifestyle-related “obesity and hepatic abnormalities” has been of great concern to many researchers and clinicians involved in the care and management of liver diseases. The modification of lifestyle is preferentially chosen for preventing and improving the hepatic abnormalities. However, it is difficult to encourage patients to change their lifestyles and habits such as diet, alcohol consumption, and exercise.
A phytochemical sulforaphane (SF) from broccoli shows potent cytoprotective effects through activation of a transcription factor Nrf2 that has recently been suggested to play a critical role in protecting liver health not only from hepatotoxic chemicals but also from lifestyle-related factors. Hepatoprotective effects of SF have been demonstrated in various animal models.
The present randomized, placebo-controlled, double blind study demonstrates that supplementation with a low dose of glucoraphanin (GR), a precursor of SF for 2 mo significantly improved serum levels of alanine aminotransferase and γ-glutamyl transpeptidase, representative liver function markers in Japanese male subjects with hepatic abnormalities. The improvement effect is associated with a reduction of urinary 8-hydroxydeoxyguanosine (8-OHdG), an oxidative stress marker.
The daily dose of SF precursor GR (69 μmole) in the present study was within the estimated daily intake amount of glucosinolates (less than 100 μmol), and thus is applicable to dietary supplements. The present findings will contribute to develop potent dietary methods for improving liver health and function.
GR, a glucosinolate precursor of SF, is highly contained in cruciferous vegetables in particular broccoli sprout. SF was identified as a potent inducer for cytoprotective genes such as phase 2 detoxyfication and antioxidant enzymes. The induction is mediated by the transcriptional upregulation of the Kelch-like ECH-associated protein 1-NF-E2-related factor 2 pathway. Urinary level of 8-OHdG is widely used as an in vivo oxidative stress marker.
The purpose of the research was to determine whether SF will reduce/prevent hepatic abnormalities. The authors have selected well known markers to evaluate the state of the liver, and are able to draw a relatively reliable conclusion with regard to the effects of the treatment of the male participants in the study. The animal studies also support the conclusion that SF improves liver function through reduction of oxidative stress. The data obtained are interesting. In a follow-up project it would in particular be of interest to determine the effects of higher doses of SF-precursor and longer trial periods.
| 1. | Kikuchi M, Shiozawa H, Yamada C, Ogawa T, Matsushima M, Mine T, Watanabe N, Nishizaki Y. Nonalcoholic Steatohepatitis as Fatal Fatty Liver - The next main target in the field of liver disease. HEP. 2013;40:476-481. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 499] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 4. | Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399-2403. [PubMed] |
| 5. | Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol. 2012;64:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 6. | Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105-1127. [PubMed] |
| 7. | Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 806] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 8. | Gerhauser C. Cancer chemoprevention and nutriepigenetics: state of the art and future challenges. Top Curr Chem. 2013;329:73-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169-177. [PubMed] |
| 10. | Lamlé J, Marhenke S, Borlak J, von Wasielewski R, Eriksson CJ, Geffers R, Manns MP, Yamamoto M, Vogel A. Nuclear factor-eythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134:1159-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Shimozono R, Asaoka Y, Yoshizawa Y, Aoki T, Noda H, Yamada M, Kaino M, Mochizuki H. Nrf2 activators attenuate the progression of nonalcoholic steatohepatitis-related fibrosis in a dietary rat model. Mol Pharmacol. 2013;84:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Gaona-Gaona L, Molina-Jijón E, Tapia E, Zazueta C, Hernández-Pando R, Calderón-Oliver M, Zarco-Márquez G, Pinzón E, Pedraza-Chaverri J. Protective effect of sulforaphane pretreatment against cisplatin-induced liver and mitochondrial oxidant damage in rats. Toxicology. 2011;286:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Sun X, Mi L, Liu J, Song L, Chung FL, Gan N. Sulforaphane prevents microcystin-LR-induced oxidative damage and apoptosis in BALB/c mice. Toxicol Appl Pharmacol. 2011;255:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Baek SH, Park M, Suh JH, Choi HS. Protective effects of an extract of young radish (Raphanus sativus L) cultivated with sulfur (sulfur-radish extract) and of sulforaphane on carbon tetrachloride-induced hepatotoxicity. Biosci Biotechnol Biochem. 2008;72:1176-1182. [PubMed] |
| 15. | Sayed RH, Khalil WK, Salem HA, Kenawy SA, El-Sayeh BM. Sulforaphane increases the survival rate in rats with fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide. Nutr Res. 2014;34:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Zhou R, Lin J, Wu D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochim Biophys Acta. 2014;1840:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Choi KM, Lee YS, Kim W, Kim SJ, Shin KO, Yu JY, Lee MK, Lee YM, Hong JT, Yun YP. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J Nutr Biochem. 2014;25:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Kay HY, Kim WD, Hwang SJ, Choi HS, Gilroy RK, Wan YJ, Kim SG. Nrf2 inhibits LXRα-dependent hepatic lipogenesis by competing with FXR for acetylase binding. Antioxid Redox Signal. 2011;15:2135-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila). 2009;2:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Muñoz A, Egner PA, Chen JG, Qian GS, Chen TY. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2012;33:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Muñoz A, Johnson JL, Groopman JD. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila). 2014;7:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Agudo A, Ibáñez R, Amiano P, Ardanaz E, Barricarte A, Berenguer A, Dolores Chirlaque M, Dorronsoro M, Jakszyn P, Larrañaga N. Consumption of cruciferous vegetables and glucosinolates in a Spanish adult population. Eur J Clin Nutr. 2008;62:324-331. [PubMed] |
| 23. | Sones K, Heaney RK, Fenwick GR. An estimate of the mean daily intake of glucosinolates from cruciferous vegetables in the UK. J Sci Food Agric. 1984;35:712-720. [DOI] [Full Text] |
| 24. | Kikugawa K, Hiramoto K, Hirama A. Beta-carotene generates thiobarbituric acid-reactive substances by interaction with nitrogen dioxide in air. Free Radic Res. 1999;31:517-523. [PubMed] |
| 25. | Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130-7139. [PubMed] |
| 26. | Kasai H, Crain PF, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7:1849-1851. [PubMed] |
| 27. | Evans MD, Cooke MS, Podmore ID, Zheng Q, Herbert KE, Lunec J. Discrepancies in the measurement of UVC-induced 8-oxo-2’-deoxyguanosine: implications for the analysis of oxidative DNA damage. Biochem Biophys Res Commun. 1999;259:374-378. [PubMed] |
| 28. | Shi M, Takeshita H, Komatsu M, Xu B, Aoyama K, Takeuchi T. Generation of 8-hydroxydeoxyguanosine from DNA using rat liver homogenates. Cancer Sci. 2005;96:13-18. [PubMed] |
| 29. | Tachi Y, Katano Y, Honda T, Hayashi K, Ishigami M, Itoh A, Hirooka Y, Nakano I, Samejima Y, Goto H. Impact of amino acid substitutions in the hepatitis C virus genotype 1b core region on liver steatosis and hepatic oxidative stress in patients with chronic hepatitis C. Liver Int. 2010;30:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Bataille AM, Manautou JE. Nrf2: a potential target for new therapeutics in liver disease. Clin Pharmacol Ther. 2012;92:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 31. | Shin SM, Yang JH, Ki SH. Role of the Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev. 2013;2013:763257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53-62. [PubMed] |
| 33. | Murashima M, Watanabe S, Zhuo XG, Uehara M, Kurashige A. Phase 1 study of multiple biomarkers for metabolism and oxidative stress after one-week intake of broccoli sprouts. Biofactors. 2004;22:271-275. [PubMed] |
| 34. | Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, Hawke RL, Doo E, Meyers CM, Reddy KR. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA. 2012;308:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Berg T S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM