Published online Nov 14, 2015. doi: 10.3748/wjg.v21.i42.12003
Peer-review started: July 4, 2015
First decision: July 20, 2015
Revised: August 3, 2015
Accepted: September 30, 2015
Article in press: September 30, 2015
Published online: November 14, 2015
Processing time: 134 Days and 7.1 Hours
Hepatocellular carcinoma (HCC) is an increasing health problem, representing the second cause of cancer-related mortality worldwide. The major risk factor for HCC is cirrhosis. In developing countries, viral hepatitis represent the major risk factor, whereas in developed countries, the epidemic of obesity, diabetes and nonalcoholic steatohepatitis contribute to the observed increase in HCC incidence. Cirrhotic patients are recommended to undergo HCC surveillance by abdominal ultrasounds at 6-mo intervals. The current diagnostic algorithms for HCC rely on typical radiological hallmarks in dynamic contrast-enhanced imaging, while the use of α-fetoprotein as an independent tool for HCC surveillance is not recommended by current guidelines due to its low sensitivity and specificity. Early diagnosis is crucial for curative treatments. Surgical resection, radiofrequency ablation and liver transplantation are considered the cornerstones of curative therapy, while for patients with more advanced HCC recommended options include sorafenib and trans-arterial chemo-embolization. A multidisciplinary team, consisting of hepatologists, surgeons, radiologists, oncologists and pathologists, is fundamental for a correct management. In this paper, we review the diagnostic and therapeutic management of HCC, with a focus on the most recent evidences and recommendations from guidelines.
Core tip: Hepatocellular carcinoma is an increasing health problem, representing the second cause of cancer-related mortality worldwide. The major risk factor for hepatocellular carcinoma (HCC) is cirrhosis. Early diagnosis is crucial for curative treatments. As a consequence, patients at risk of developing HCC should undergo surveillance programs in order to detect HCC in the initial stage. Surgical resection, radiofrequency ablation and liver transplantation are considered the cornerstones of curative therapy, while for patients with more advanced HCC recommended options include sorafenib and trans-arterial chemo-embolization.
- Citation: Bellissimo F, Pinzone MR, Cacopardo B, Nunnari G. Diagnostic and therapeutic management of hepatocellular carcinoma. World J Gastroenterol 2015; 21(42): 12003-12021
- URL: https://www.wjgnet.com/1007-9327/full/v21/i42/12003.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i42.12003
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths worldwide. More than 700000 new cases are diagnosed every year throughout the world and high incidence to mortality ratio (1.07) makes HCC the second most common cause of cancer-related deaths worldwide[1].
Although the majority of cases occur in Asia and Africa, the incidence has increased even in the developed world. The geographical variation in the incidence of HCC is mostly related with the different prevalence of major risk factors for HCC, such as hepatitis C virus (HCV) and hepatitis B virus (HBV) infection[2]. In developed countries, the epidemic of obesity, diabetes and nonalcoholic steatohepatitis (NASH) is also believed to contribute to the observed increase in HCC incidence[3]. However, the overriding risk factor for HCC, which is responsible for HCC in 80%-90% of cases regardless of etiology, is the presence of cirrhosis[4,5].
By recognizing the risk factors for HCC, high-risk groups can be identified and followed up with screening strategies. In fact, the management of high-risk patients with screening and surveillance has the real potential to detect HCC early and improve patient outcomes. When HCC is detected earlier, patients are candidates to receive curative treatments.
In this paper, we review the diagnostic and therapeutic management of HCC, with a focus on the most recent evidences and recommendations from guidelines.
Hepatic nodules can be detected on Ultrasounds (US), including contrast-enhanced US (CEUS), or on other noninvasive techniques, such as contrast-enhanced computerized tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET)-CT. The typical vascular profile of HCC on dynamic imaging is characterized by early arterial phase enhancement followed by loss of enhancement in the portal venous phase and delayed phase in comparison to the surrounding liver[6].
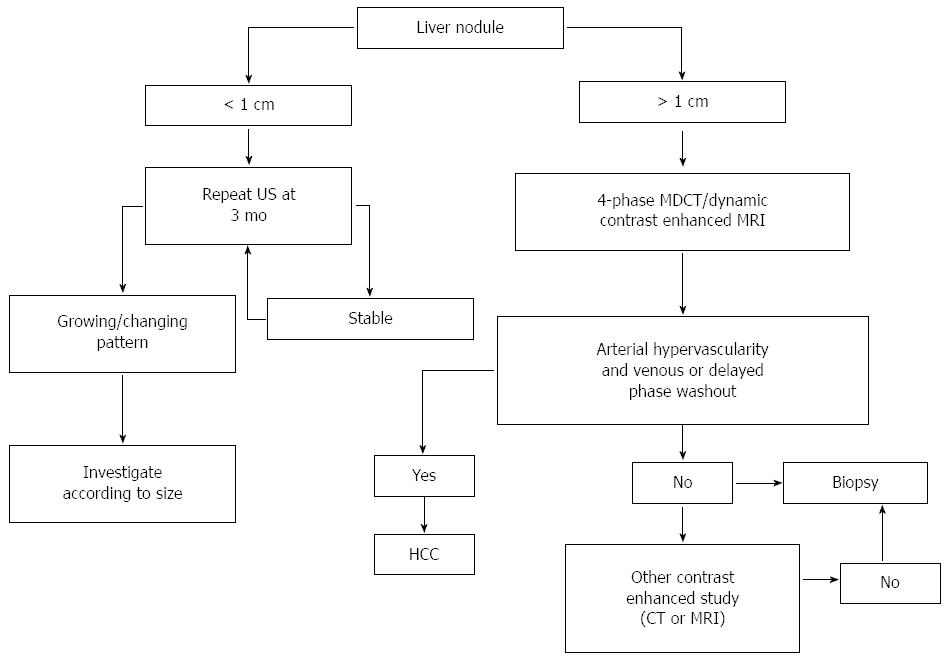
Moreover, molecular biomarkers could potentially be used for diagnosis, as well as prognostic evaluation and may help defining the individualized therapeutic approach to HCC. Figure 1 illustrates the diagnostic algorithms endorsed by the American Association for the Study of Liver Diseases (AASLD)[7].
Early diagnosis of HCC is important because, as expected, treatment is more effective when the tumor is small[8-10]. Dysplastic nodules (DNs) may develop into carcinoma[11]. Early detection of DNs with small areas of HCC is fundamental for effective treatment.
Cirrhotic patients are recommended to undergo HCC surveillance by abdominal US at 6-month intervals. However, the diagnosis of small HCC nodules may be challenging, as it is often difficult to differentiate benign from malignant lesions in the context of nodular cirrhosis; moreover, US depends on the operator and has limited sensitivity in obese patients. On the other hand, US is less expensive than other techniques and is radiation-free.
For HCC, a stepwise process of carcinogenesis has been proposed, involving a progression from regenerative nodules (RNs) to low-grade DNs or high-grade DNs to DNs with a focus of HCC and finally to HCC. This progression has been suggested to correlate with changes in the blood supply and perfusion of the nodules, which may be used to differentiate focal liver lesions[8,12-14]. Recently, SonoVue, a blood-pool marker used in CEUS, has been reported to help distinguishing RNs from small HCC based on the different enhancement pattern[15-18]. RN has an intranodular blood supply that is similar to the surrounding parenchyma. On the other hand, HCC usually exhibits an enhancement pattern in the arterial phase and washout in the late phase[19-26]. DN-HCC nodules have a mixed enhancement behavior, as they are composed of two different cells, high-grade HCC and atypical hepatic cells and their enhancement features are partially similar to HCC and partially similar to RNs[27]. Of interest, CEUS has been suggested to promote the diagnostic accuracy of biopsy, decreasing the false-negative rate for malignant lesions. In fact, CEUS may be used to identify the areas of viable tumor[27]. A biopsy of DN-HCC without CEUS guidance is more likely to give false-negative results, significantly affecting the possibility to early detect and treat HCC.
CT is largely used in most centers to make the radiological diagnosis of HCC after a liver nodule is detected on US. Most centers use a four-phase multidetector CT (MDCT) scan, which consists of a non-enhanced phase, an arterial phase (which occurs 20-30 s after contrast injection), a portal venous phase (6580 s after contrast injection) and a delayed phase. On the four-phase CT, HCC classically appears as a hyper-attenuated lesion in the arterial phase, with loss of enhancement termed rapid washout in the portal venous and/or delayed phase. CT has high specificity but variable sensitivity for detecting HCC. In a systematic review, traditional spiral CT was reported to have a specificity of 93% but a sensitivity of only 68% in diagnosing HCC. A more recent review assessing the diagnostic accuracy of the 64-slice MDCT technology vs spiral CT found improved sensitivity (65%-79% compared to 37%-54%), with similar specificity (above 90%)[28]. However, sensitivity dropped to 33.45% for nodules smaller than 1 cm.
MRI is an appealing imaging technique, since it does not use ionizing radiation. MRI allows the differentiation between tumoral and normal liver parenchyma using magnetic fields, even without a contrast media[29]. Traditional dynamic contrast-enhanced MRI of the liver is performed using gadolinium chelates. In gadolinium-enhanced MRI, the typical HCC lesion is hyper-intense on T1-weighted images during the arterial phase and exhibits rapid washout during portal venous and delayed phases[12,30,31]. The sensitivity of standard gadolinium-enhanced MRI is around 90%, with a specificity of at least 95% for the detection of HCC greater than 2 cm in diameter[32]. Dynamic MRI appears superior to CT for the detection of HCC nodules[33,34], but its sensitivity is highly affected by the lesion size, being as low as 30% in the case of lesions smaller than 2 cm[35,36].
Specific contrast agents have been developed to improve the sensitivity of MRI for HCC, including the “dual contrast” agents (gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid and gadobenate dimeglumine), which work both as markers of hepatobiliary excretion and vascularization. HCC nodules imaged with these contrast agents do not exhibit uptake, unlike benign nodules on the delayed phase[37]. While it appears performing similarly to MDCT for lesions larger than 2 cm, enhanced MRI might be more sensitive for lesions smaller than 1 cm[38].
Both 18F-FDG and 11C-acetate PET imaging have been used for HCC detection and staging[39-41]. However, up to 40%-50% of HCC are not sensitive to 18F-FDG PET, because of their high expression of the glucose-6-phosphatase enzyme, which prevents intracellular accumulation of 18F-FDG[39]. On the other hand, 11C-acetate, which is believed to mainly participate in fatty acid synthesis in the liver, has been suggested to have increased sensitivity and specificity in comparison to 18F-FDG[39,41,42]. However, several studies have reported that 11C-acetate PET does not properly differentiate HCC from benign lesions[41,43-45], because the latter also accumulate 11C-acetate. Some recent studies[41,44-47] have suggested dynamic PET with kinetic modeling to be a promising tool to differentiate benign hepatic tumors from HCC.
Α-fetoprotein (AFP) is the most widely used and broadly known biomarker for HCC, but its use as an independent tool for HCC surveillance is not recommended by current guidelines due to its low sensitivity and specificity. In the past, a significant concentration of AFP in the serum of a patient with liver cirrhosis and a suspicious mass in the liver larger than 2 cm was sufficient to diagnose HCC[48]. However, the current diagnostic algorithms endorsed by the AASLD and the European Association for the Study of the Liver strictly rely on typical radiological hallmarks in dynamic contrast-enhanced imaging apart from biomarkers[7,48,49].
Three serum biomarkers have been suggested as tools to determine the risk of liver cancer in high-risk populations worldwide: AFP, the ratio of lecithin-bound AFP to total AFP (AFP-L3), and des-gamma-carboxyprothrombin (DCP). However, most studies on the performance of biomarkers in HCC detection have not been performed in a surveillance setting but compared levels of predefined biomarkers in patients with HCC with a control group, in most cases represented by patients with chronic liver diseases. A randomized controlled study performed in a high-risk population in China showed that screening by AFP measurement led to earlier diagnosis of HCC but had no impact on mortality[50]. On the other hand, semiannual screening for HCC by AFP measurement in a population-based study in Alaska was effective in detecting HCC at early stages and significantly prolonged survival rates[51].
A meta-analysis on the performance of AFP in diagnosing HCC included seven studies and revealed a pooled sensitivity of 66% with a specificity of 86% [area under curve (AUC) = 0.87][52]. In a further meta-analysis including ten studies the pooled sensitivity of AFP for the diagnosis of HCC was 51.9%, with a specificity of 94% (AUC = 0.81)[53]. A major drawback of AFP as a surveillance tool is that serum levels are influenced by the activity of the underlying liver disease and therefore increased in patients with elevated alanine aminotransferase (ALT) levels, even in the absence of HCC, as shown in the HALT-C trial[54]. Furthermore, HCC biology is quite heterogeneous, with only a proportion of patients with HCC having elevated AFP serum levels, leading to low sensitivity of the marker. As a consequence, new complementary markers have been studied. The clinical utility of high-sensitivity AFP-L3 (hs-AFP-L3) in early prediction of HCC development in patients with chronic HBV or HCV infection was recently evaluated in a large Japanese study. Even in subjects with low AFP levels and without suspicious ultrasound findings, an elevation of hs-AFP-L3 was an early predictor of HCC development: in fact, hs-AFP-L3 increased in 34.3% of patients one year prior to diagnosis of HCC[55,56]. Numerous studies have investigated the performance of other markers, including α-l-fucosidase[57], glypican-3 (GPC-3), insulin-like growth factor[58], vascular endothelial growth factor (VEGF), or Dickkopf-1[59], Golgi protein 73 (GP73), interleukin-6 (IL-6) and squamous cell carcinoma antigen (SCCA)[60]. In a study comparing 144 patients with HCC to 152 patients with cirrhosis and 56 healthy controls, GP73 had a sensitivity of 62% and a specificity of 88% at a cut-off of 10 relative units[61]. Another study, including 4217 subjects (789 with HCC), revealed a sensitivity of 74.6% and a specificity of 97.4% at a cut-off of 8.5 relative units[62]. Using different cut-off values, IL-6 sensitivity ranged from 46% to 73% with a specificity of 87% to 95%[60,63,64]; in a large study including 961 patients, SCCA had a sensitivity of 42% and specificity of 83% using a cut-off of 3.8 ng/mL[60,65].
Serum IL-17 levels have been reported to be elevated in HCC patients[66]. In a retrospective study, Liu et al[67] found that plasma IL-17 concentration had a sensitivity of 74.3% and specificity of 75.6% (AUC = 0.86) at the cut-off value of 4.23 ng/L; however, the diagnostic accuracy of IL-17 was lower than AFP, which had a sensitivity and specificity of 100% and 66% respectively, at the cut-off value of 10.25 mg/L (AUC = 0.96).
Osteopontin, an integrin-binding glycol-phosphoprotein, was investigated in seven studies summarized in a meta-analysis[52]. The pooled sensitivity of osteopontin for HCC was 86% with a specificity of 86%, showing a diagnostic accuracy similar to that of AFP; however, further validation studies are needed before recommending the use of this biomarker in clinical practice.
Some studies have investigated the combined diagnostic performance of the three more validated non-invasive biomarkers used in HCC, namely AFP, AFP-L3 and DCP. By comparing 164 European patients with HCC to 422 subjects with chronic liver disease, a significant increase in AFP serum levels was shown in those with advanced HCC and viral hepatitis, while DCP was more frequently elevated in those with early-stage and NASH-associated HCC. Neither of the two parameters, if taken alone, could independently identify more than 30% of patients with HCC but combination of AFP (cut-off 10 ng/mL) and DCP (cut-off 5 ng/mL) showed a sensitivity of 55% for early stage HCC and 78% for all stages[68]. A further increase in sensitivity (up to 84%) was observed by adding AFP-L3[69]. The additional use of clinical variables, like age and gender, further improved the performance of the model[70,71].
A number of signal transduction pathways have been recognized as critical players in the pathophysiology of hepatocarcinogenesis, including the Wnt/β-Catenin pathway, the p53 pathway, the tumor suppressor retinoblastoma protein pRb1 pathway, the mitogen-activated protein kinase pathway, the Ras pathway, JAK/STAT signaling, mechanisms of cellular stress response, like heat shock proteins, epidermal growth factor receptor and transforming growth factor-β signaling[72,73].
Gene expression profiling of peripheral blood mononuclear cells using microarrays and bioinformatics-driven data analysis identified a blood-based signature of three genes, namely Chemokine (C-X-C motif) receptor 2 (CXCR2), C-C chemokine receptor type 2 (CCR2) and E1A Binding Protein P400 (EP400), able to predict HCC with a sensitivity of 93% and a specificity of 89%[74]. High-throughput metabolomics technology with the comprehensive analysis of small molecular metabolites may identify serum metabolic profiles to be used as biomarkers in HCC diagnosis. Molecular signatures may help to distinguish dysplastic nodules from well-differentiated HCC. In Asian and Western patients with HCV infection, specific gene signatures have been reported to accurately reflect the pathological progression of disease from cirrhosis to dysplasia to early and advanced HCC[75,76]. Moreover, a three-gene set including glypican3 (GPC3; 18-fold increase in HCC, P = 0.01), LYVE1 (12-fold decrease in HCC, P = 0.0001) and surviving (2.2-fold increase in HCC, P = 0.02) had an accuracy of 94% to distinguish DNs from early HCC in HCV-related cirrhosis[77]. Heat shock protein 70 and cyclase-associated protein 2 are other tissue biomarkers potentially useful to in the diagnosis of HCC[78,79].
As for novel biomarkers, microRNA (miRNA) have received particular attention[80]. miRNAs are small non-coding and evolutionary conserved RNA molecules that serve as posttranscriptional regulators of mRNA expression and interfere with mRNA translation to protein[81]. miRNAs are able to conserve their function into the cell by regulating the expression of a target population of molecules; moreover, they can be released from the cell both in combination with other proteins or as a free molecule[82-88].
Differences in miRNA expression patterns in several malignant conditions, including HCC, have been found[89-91]. In particular, three miRNAs, miR-122, miR-192 and miR-199a/b-3p, account for more than 70% of total miRNA released by normal liver tissue[91]. In HCC, a broad spectrum of changes in microRNAoma has been reported[91-94], suggesting that miRNAs may potentially become valid biomarkers in HCC. To improve the diagnostic utility of miRNAs in HCC, Li et al[95] performed deep sequencing in pooled samples from patients with chronic HBV patients, HCC and controls with and without cancer. They recognized a pattern of 6 miRNA differentially expressed in patients with HCC. The use of three miRNAs (miR-25, miR-375 and let7f) had a sensitivity of 97.9% and a specificity of 99.1% to discriminate between controls and HCC patients. Of interest, the use of two miRNAs (miR-10a and miR-125b) could adequately discriminate the cohort with chronic HBV and HBV-associated HCC with an AUC of 99.2% (sensitivity 98.5% and specificity 98.5%)[95]. In another study, a panel of 7 miRNAs (miR-122, miR-192, miR-21, miR-223, miR-26a, miR-27a and miR-801) provided a high diagnostic accuracy for the identification of HBV-related HCC[96], with a sensitivity of 81.8% and a specificity of 83.5%, independently of disease stage. However, the expression of selected miRNA was analyzed using RT-PCR, which may be critical for clinical translation of these findings[96]. miR-21, which is the most frequently deregulated miRNA in cancer, was found at higher level both in sera and plasma of HCC patients[97,98], while other studies showed no significant differences[99,100]. Similarly, miR-122, the most abundant miRNA in the liver, was also found at high level in sera of HCC patients[98,99]. Other inflammatory conditions of the liver, such as acute and chronic hepatitis and NASH may strongly influence miR-122 levels[101,102]. In this setting, further studies are required to establish the capability of these biomarkers to discriminate between chronic liver diseases and HCC.
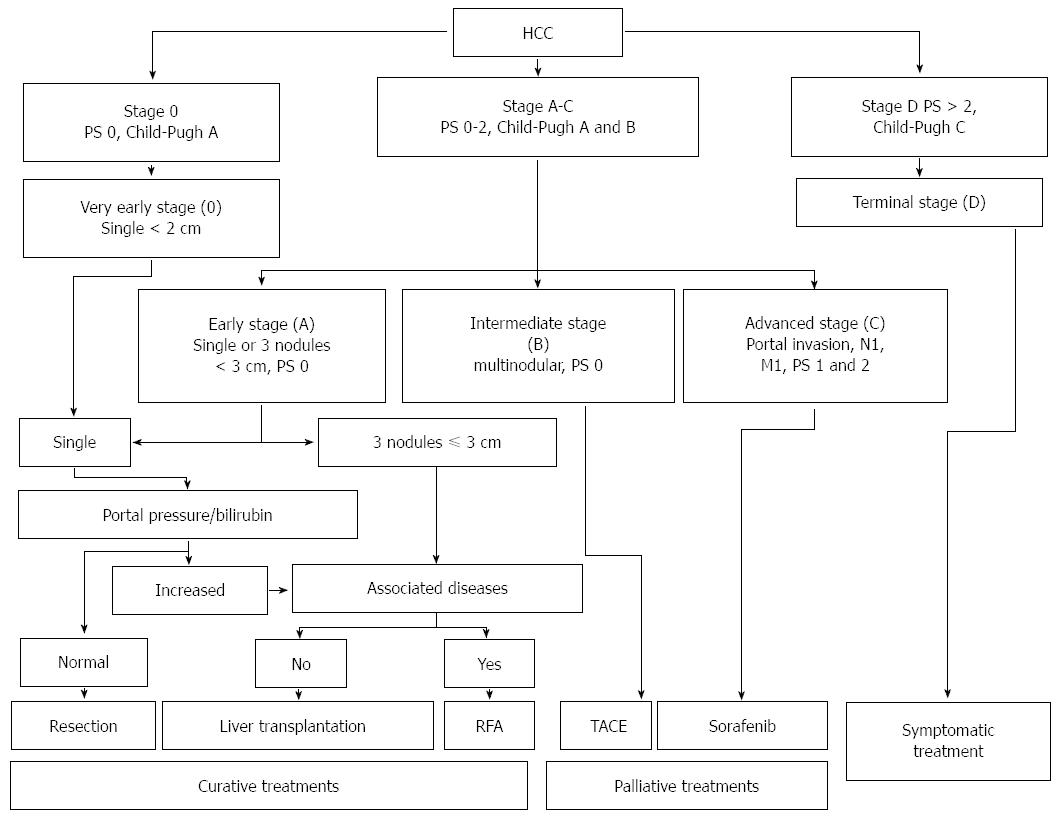
A number of staging systems have been used for HCC, even if the Barcelona Clinic Liver Cancer (BCLC) staging system is the most extensively used in clinical practice. BCLC staging system includes the evaluation of tumor stage, cirrhosis stage, functional performance status (PS) and it links staging with a treatment algorithm[103]. Moreover, the BCLC staging system was endorsed by both the American and European liver society and validated in European and American cohorts[104,105].
Early stage HCC (stage 0) has the best prognosis and is characterized by the presence of one lesion smaller than 2 cm in diameter, with no evidence of vascular invasion, in patients with stable cirrhosis (Child-Pugh class A).
Patients with stage A HCC could present with either a solitary lesion or up to three lesions of less than 3 cm in diameter. These patients have relatively preserved liver function (Child-Pugh class A or B) and good functional status (PS 0-2). The 5-year survival rate is 50%-75%; as reported in Figure 2, treatment may be different based on the presence of portal hypertension, the degree of liver dysfunction and other comorbidities.
Patients with intermediate stage HCC (stage B) have Child-Pugh class A or B cirrhosis, good functional status (PS 0) and multinodular HCC, with no evidence of vascular invasion. Patients with evidence of vascular invasion or extra-hepatic spread have advanced stage HCC (stage C). These patients typically have worse functional status (PS 1 or 2).
Patients with terminal stage HCC (stage D) present with decompensated cirrhosis (Child-Pugh class C), poor functional status (PS > 2), and advanced tumor growth (vascular invasion and/or extra-hepatic spread). Unfortunately, these patients receive no benefit from the currently available therapies, and survival is usually around 3 mo. Figure 2 illustrate the BCLC staging system and treatment strategies for HCC.
Several therapeutic options are available for HCC, depending on HCC stage, liver function, comorbidities, and local clinical expertise. A multidisciplinary team, consisting of hepatologists, surgeons, radiologists, oncologists and pathologists, is fundamental for a correct management.
The evaluation of liver functional reserve before hepatectomy is fundamental the maximum amount of liver mass that can be safely removed: on the one hand, liver functional overestimation may lead to hepatic failure; on the other hand, poor resection may significantly increase the risk of early recurrence of HCC.
The most important methods to assess liver function before surgery are the galactose tolerance test, 99mTc-galactosyl human serum albumin liver scintigraphy and the indocyanine green (ICG) test. Makuuchi’s selection criteria for hepatectomy rely on three factors, ascites, serum bilirubin and ICG retention rate at 15 min (ICGR15)[106]. Patients are considered eligible for liver resection if they have no ascites and if serum bilirubin is ≤ 2 mg/dL. Patients with total bilirubin of 1.1-1.9 mg/dL can undergo partial liver resection; for patients with serum bilirubin ≤ 1 mg/dL, the extent of resection is based on ICGR15: (1) resection of 2/3 of the total liver volume (TLV) (e.g., right lobectomy) in patients with normal ICGR15 of < 10%; (2) resection of 1/3 of the TLV (e.g., left lobectomy) in patients with ICGR15 of 10%-19%; (3) resection of 1/6 of the TLV in patients with ICGR15 of 20%-29%; and (4) limited resection or enucleation in patients whit ICGR15 ≥ 30%.
A surgical mortality rate of 0% has been reported in 1056 consecutive hepatic resections performed in accordance with these criteria[107].
In patients with portal venous invasion[108], the area supplied by the portal vein branches should be systemically removed as much as possible within the acceptable range of liver function. In this contest, systematic subsegmentectomy has been developed to overcome the potential incompatibility between the attempt to remove cancer and the need to preserve liver function[109]. Tumor stage, tumor size, number of tumors and capsule formation predict recurrence-free survival. Moreover, vascular invasion is a poor indicator of long-term survival[110]. In one study, risk factors for early recurrence (within 2 years after surgery) were non-anatomical resection, microscopic vascular invasion, and AFP ≥ 32 ng/mL[111]. Another retrospective study confirmed the association between the type of surgical approach and the outcome, showing that the cumulative survival rate was significantly higher after anatomical resection compared to non-anatomical resection[112].
As reported above, one crucial issue is the determination of the adequate liver remnant volume after hepatectomy. In normal livers, it is important to preserve the 20%-40% of the TLV or the standard liver volume (SLV)[113-120]. Anderson et al suggested that the smallest adequate liver remnant volume should be ≥ 20% of the SLV in patients with no underlying chronic liver disease[114,121]. However, HCC usually develops in patients with chronic hepatitis or cirrhosis, who are at risk of hepatic failure in case of insufficient liver remnant volume after hepatectomy. In this setting, portal vein embolization (PE) may prevent hepatic failure, because the portal vein branches are blocked to induce compensatory hypertrophy in the remnant liver area[122]. Three-dimensional CT scan allows an accurate determination of the position of major blood vessels and the tumor, as well as resection margins, and liver remnant volume[123].
Perioperative complications include bile leakage, hemorrhage and intra-abdominal abscesses[124,125]. Intraperitoneal drainage is necessary for monitoring and treatment of these complications, even if the Center for Disease Control and Prevention guidelines do not recommend routine drainage in elective hepatectomy. If drainage is required, a closed suction drain should be used and placed through a separate incision distant from the operative one. Moreover, the drainage should be removed as soon as possible[126]. These recommendations have been validated in several studies[127-133]. Moreover, in a randomized clinical trial, subcutaneous drainage was not effective in preventing surgical site infections[134]. Hepatic failure and disseminated intravascular coagulation are other postoperative complications. In one study, the authors evaluated the efficacy of steroids to improve liver function after hepatectomy[135]. They found that serum bilirubin levels were significantly lower in the steroid group on post-operative day (POD) 2 compared with the non-steroid group. The postoperative time courses of bilirubin, IL-6 and the C-reactive protein level were significantly lower whereas the prothrombin level was significantly higher in the steroid arm. No differences in the proportion of patients with complications and the length of hospital stay were reported between the two groups. To unify the definition of post-hepatectomy liver failure (PHLF), the International Study Group of Liver Surgery proposed defining PHLF as an increased international normalized ratio and concomitant hyperbilirubinemia on or after POD 5[136]. PHLF seems to predict the incidence of complications and mortality better than the 50-50 criteria [i.e. prothrombin time (PT) < 50% and serum bilirubin > 50 mmol/L][137] and MELD score[138].
Liver transplantation has become a feasible alternative for many patients with HCC, given the advances in surgical techniques and immunosuppression.
In 1996, Mazzaferro et al[139] defined the so-called Milan criteria, which identified as eligible for transplantation patients with solitary lesions < 5 cm in diameter and those with up to 3 lesions, each one < 3 cm in diameter. Similar survival rates in patients with tumors < 3 cm have been reported by the Bismuth group[140]. The Milan criteria have been accepted worldwide to identify patients which can be safely tranplanted. The limited number of available organs is the main limitation for this procedure. Yao et al[141,142] demonstrated that patients with a single lesions ≤ 6.5 cm, or up to three lesions each one ≤ 4 cm with a cumulative diameter ≤ 8 cm had surgical outcomes similar to those transplanted on the basis of Milan criteria. Tumor histology has an important impact on post-transplantation survival, with better outcome in patients with well-differentiated tumors[143,144]. The availability of transplantable grafts remains the critical issue for all patients awaiting liver transplantation, considering that time is a major determinant of overall survival[145-153]. Living donation can be a good choice for transplantation in patients with HCC because the transplant can be planned with an optimal timing to both assess the tumor aggressiveness and minimize the risk of recurrence[154-160]. Another factor that can affect the risk of recurrence after transplantation is the use of immunosuppressive agents. Sirolimus, a bacterial macrolide with immunosuppressive and antineoplastic properties, which inhibits IL-2-mediated lymphocyte proliferation, seems to decrease metastatic tumor growth and angiogenesis in the liver. It was demonstrated that the administration of post-transplant sirolimus, within a steroid-free protocol and a low tacrolimus target, was associated with decreased risk of tumor recurrence and no significant increase in the risk of infection and hepatic artery thrombosis[161-165].
Among non-surgical approaches, percutaneous ethanol injection (PEI), microwave ablation (MWA) and percutaneous radiofrequency ablation (RFA) represent the three most widely used techniques for the treatment of HCC less than 5 cm in diameter and/or with less than 3 tumoral lesions.
In RFA, electrical current is applied via an electrode resulting in resistive heating and tissue hyperthermia[166]. Tissues adjacent to the electrode are the most effectively heated[167-169]. The mechanism of cytotoxicity in RFA depends on tissue impedance, with power deposition hindered in regions of high tissue impedance, such as the surrounding lung or tissue adjacent to the electrode, that has undergone water vaporization due to rapid heating[166,169]. Multiple engineering designs have been developed to overcome the limitations caused by tissue impedance, including multi-tined electrodes to expand the contact surface area, saline injection, and internal cooling. Moreover, RFA requires the placement of grounding pads on the patient to close the electrical circuit, and skin burns related to the pads have been reported[170,171]. However, in clinical practice skin burns are rare, considering that larger grounding pads are usually used to improve the dispersion of thermal energy[172]. RFA efficacy may be limited by the “heat sink” effect, consisting in heat dissipation resulting from blood flow. This effect is more marked for lesions close to the liver hilum[173]. There are several reports on RFA use in both primary and metastatic liver tumors. In a Cochrane database analysis, including 11 randomized clinical trials, Weis et al[174] analyzed a total of 1819 participants with HCC with the primary outcome of overall survival, comparing RFA to hepatic resection[175-177], PEI[178-183], MWA[184], and percutaneous laser ablation (PLA)[185]. The authors concluded that hepatic resection was superior to RFA in terms of survival, even if RFA might be associated with fewer complications and shorter hospital stay. Moreover, RFA was associated with better survival than PEI, whereas there was no evidence of significant differences between RFA and MWA or PLA.
In the study by Lee et al[186], patients undergoing surgical resection were younger and had better liver function reserve and PS than those receiving RFA. When accounting for these differences using propensity score analysis, RFA was superior to surgery for patients with small HCC and Child-Pugh Turcotte score of 5.
MWA relies on the direct application of an electromagnetic field, which causes dielectric hysteresis, leading to local tissue hyperthermia[187]. MWA is able to penetrate through several tissues, including those with high impedance[166,187]. High tissue temperatures can be achieved with MWA, with increased efficacy as compared to RFA[188]. Given the efficacy profile and the shorter time required to achieve ablation, the use of MWA has gradually increased for the treatment of both primary and metastatic tumors of the liver. Ding et al[189,190] studied 198 patients with HCC, all in BCLC Stage A meeting Milan criteria and did not find any difference between RFA and MWA in terms of disease-free survival, cumulative survival, and complication rates. Similar results have been reported in other cohorts[184,191].
PEI involves the direct instillation of ethanol into tumors, which results in coagulative necrosis. The technique is relatively simple and inexpensive. However, in clinical practice PEI is limited by poor and irregular distribution of ethanol within the tumor and diffusion into the adjacent normal tissues. Even if some studies with PEI reported favorable outcomes after a long-term follow up (greater than 15 years), most evidences suggest that RFA is associated with better overall survival than PEI[192-194].
The use of external beam radiation therapy (SBRT) in treatment of liver tumors has been traditionally limited by the overall low tolerance of liver tissue to radiation[195]. In fact, radiation produces tumoral killing by transferring energy within atoms, determining the generation of reactive oxygen species with subsequent direct and indirect DNA and cellular damage. The final step is the generation of double-strand DNA breaks, leading to tumor cell death. Radiation can achieve excellent tumor control when delivered to ablative doses[196]. Maximum dose is limited by the radiation tolerance of the surrounding normal liver tissue and adjacent organs. Particularly, radiation-induced liver disease is a complication typically manifesting with the triad of anicteric hepatomegaly, ascites, and elevation of alkaline phosphatase. Imaging techniques, breathing motion control and advances in radiation machines technology permit accurate localization of hepatic tumors and help directing radiation to the tumor while minimizing exposure of surrounding normal liver[197,198]. The size and number of lesions that can be targeted, as well as the radiation dose that can be delivered, depends on normal liver reserve and estimated risk of liver complications. As expected, patients with reduced liver function require dose reduction[199]. Similarly, patients with Child Pugh class B cirrhosis may require dose reduction, while those with Child Pugh class C cirrhosis are not usually eligible for this type of treatment.
Another radiation-based technique is high-dose rate (HDR) CT-guided interstitial brachytherapy[200-202]. Radiation is delivered using an iridium-192 source as a single fraction. The advantage of this technique is a greater protection of the surrounding healthy liver compared to external radiation techniques. A prospective phase II trial[203] showed encouraging results for patients with large tumors near the hilum, using average dose of 17 Gy. Mearini et al[204] reported favorable outcomes of 35 patients with HCC (tumor size 5-12 cm), treated with HDR brachytherapy. At 12 mo, local control was 93% and no major toxicity was reported.
High-intensity focused ultrasound (HIFU) incorporates multiple ultrasound beams produced by piezoelectric or piezoceramic transducers directed into a three-dimensional focal point[205]. Ultrasound beams are both thermally ablative and cause cavitations to the underlying tissues. Coupling of the ultrasound source and the patient is achieved through a degassed water bath in order to have minimal reflection or absorption of the soundwaves prior to reaching the focal point. The patient is required to minimize movements during the procedure and the focal zone is shifted step by step to cover the area of interest for ablation. The safety and efficacy of HIFU was evaluated in several studies[206-212]. Ng et al[208] reported on a series of 49 patients with HCC (median tumor size 2.2 cm, range, 0.9-8 cm) and concluded that HIFU was effective for those who were not surgical candidates. He reported 1- and 3-year overall survival rates of 87.7% and 62.4%, respectively[209]. Similar data were published by Wu et al[210], with overall survival rates of 86.1%, 61.5%,and 35.3% at 6, 12, and 18 mo, respectively. Cheung et al[205] reported on the outcomes of HIFU for the treatment of HCC before liver transplantation in 10 patients as compared to 29 patients who received transarterial chemoembolization and found that HIFU was effective (90% had complete response, 10% partial response), with none of the patients on the liver transplant list (n = 5) dropping out[206].
Irreversible electroporation (IRE) is an apparently non-thermal technique in which the direct placement of electrodes creates a pulsed direct current, inducing cytotoxicity in tumor cells by altering transmembrane potentials, which irreversibly disrupt cell membrane integrity[213]. IRE requires the position of at least two applicators in parallel to create ablation zones in the range of 1.5-2 cm per electrode pair[214]. The zone of ablation created by IRE is dependent on multiple factors[213,215], such as electrode spacing and relative position, active tip length, pulse number and duration, and applied voltage. Because of these factors, IRE results more technically challenging than other locally ablative techniques. Moreover, the current generated by IRE causes whole-body muscle contractions and general anesthesia, requiring the use of neuromuscular blockage. In addition, IRE can induce cardiac arrhythmias, though this complication can be avoided with the use of cardiac synchronization of the administered pulses to the complete refractory period of the cardiac cycle[216]. IRE has a theoretical safety advantage as compared to other locally ablative techniques in the treatment of tumors close to structures susceptible to thermal injury, such as major bile ducts. In addition, because of the reduction in the “heat-sink” effect, IRE is potentially more effective for tumors next to major vessels, especially for smaller lesions, and showed excellent local tumor control at 3-6 mo, but high recurrence rates after 12-18 mo[215,217-220].
Cryoablation involves the direct application of a cryoprobe into the tumor. The thermal contact with the tumor results in ice-crystal development and osmotic shock. One recognized advantage of cryoablation is that the zone of ablation is readily visible (“iceball”) using CT scan, US, or MRI monitoring, allowing for precise targeting of the ablation area[221]. Moreover, multiple probes can be used simultaneously to create larger ablation zones and shorten procedural times. Despite the technical advantages of cryoablation, its use has been limited by the safety profile. Cryo-shock is an uncommon but potentially life-threatening complication, characterized by thrombocytopenia, acute renal failure, adult respiratory distress syndrome and disseminated intravascular coagulopathy[221]. In a meta-analysis comparing cryoablation to RFA in the treatment of unresectable HCC[222], RFA was superior, particularly in terms of complication rates and local tumor recurrence.
Percutaneous laser ablation (PLA) involves the direct deposition of laser light via fiber-optic applicators to induce tissue hyperthermia in tumors. The thin flexible fiber-optic delivery fibers allow for safer and technically easier approaches to tumors[223]. Moreover, feedback and dose-planning systems allow a good control of ablative zones and consequently low complication rates. However, it has been suggested that PLA has some limitations in achieving complete tumor ablation as compared to other locally ablative therapies[185,224,225].
HCC is preferentially supplied by the hepatic arterial inflow, while the normal parenchyma is largely supplied by the portal vein. The trans-arterial chemo-embolization (TACE) procedure is based on these blood supply dynamics. TACE consists in the placement of an intra-arterial catheter in the vessels supplying the tumor, to deliver high concentrations of a chemotherapeutic agent (e.g., doxorubicin, cisplatin or mitomycin) along with an embolic agent, such as lipiodol gelatin sponge or polyvinyl alcohol particles, in order to achieve both targeted chemotherapy and reduction in arterial supply to the tumor.
Drug eluting beads TACE (DEB-TACE), are becoming largely popular because of the favorable safety profile. DEB-TACE delivers small beads, which have been saturated for several hours with chemotherapeutic drugs. The beads occlude the feeding vessels of HCC, while doxorubicin is progressively released, increasing chemotherapeutic concentrations locally and creating tumor necrosis. The choice of bead size, from 75 to 700 μm, depends on tumor size and the preferred level of concentration within the treated volume. The best results are achieved when chemoembolization is performed selectively to segmental or subsegmental arteries feeding the tumor[226]. TACE is considered the standard of care for intermediate stage HCC without vascular invasion or metastases. In several randomized controlled trials, TACE was associated with partial response in 15%-62% of patients, and improved survival[227-233]. Some studies have suggested that complete tumor ischemia may stimulate angiogenesis, resulting in an increased susceptibility to tumor growth rather than suppression. It has been therefore suggested to maintain arterial patency both to prevent this pro-angiogenic effect and to permit repeated treatments[234-237]. Side effects associated with both DEB-TACE and TACE include nausea, vomiting and right upper quadrant pain (post-embolization syndrome), doxorubicin-related cardiac toxicity, bone marrow aplasia, hepatic abscesses, cholecystitis[229,231,238]. Two randomized controlled trials demonstrated improved side effect profiles[239,240] with equivalent survival rates[235,239] and longer time to progression for DEB-TACE in comparison with conventional TACE[240]. A meta-analysis showed comparable tumor response rates[241].
Radioembolization is a modestly invasive, fluoroscopically guided and microcatheter-based technique, using either yttrium-90 (Y-90) embedded non-biodegradable glass microspheres (25 ± 10 μm) or Y-90 embedded non-biodegradable glass resin-based microspheres (29-35 μm). Radioembolization exploits the preferential arterial blood supply of HCC by delivering radiotherapy directly to the tumor and preserving the normal liver parenchyma. In the target lesion, Y-90 delivers tumoricidal doses of a pure high-energy beta emitter. Because of the short tissue penetration and half-life, Y-90 is an ideal radioisotope for intra-arterial radiotherapy. Patients who have intermediate/advanced BCLC stage HCC and who are not candidates for TACE due to portal vein invasion are ideally candidate to radioembolization with Y-90[242-244]. Radioembolization represents a suitable alternative to chemotherapy for patients with advanced HCC[245]. Moreover, Y-90 radioembolization can be proposed as a bridge to liver transplantation[139,246,247].
As for combinations therapies, one of the most studied approaches is represented by the association of RFA plus TACE. In fact, the decreased blood flow due to TACE reduces heat loss and improves the RFA margins. On the other hand, TACE enhances nearby control of satellite lesions[248]. Several meta-analyses have found that the combination of RFA and TACE is associated with improved survival in comparison with RFA alone, particularly for tumors larger than 3 cm in diameter[249-252]. Hyperthermia is able to potentiate the cytotoxic effect of radiation[253,254]. Additionally, in animal studies, the combined use of radiation and RFA resulted in improved tumor growth control compared with RFA alone[255,256]. The combination of thermal ablation with SBRT represents another encouraging option, even if more research is required to establish the most appropriate dosing and timing regimen[257].
Sorafenib is a small-molecule multikinase inhibitor, which blocks Raf kinase, VEGF receptor and platelet derived growth factor receptor (PDGFR). In two randomized, double-blinded, controlled, phase III clinical trials, the SHARP (Sorafenib HCC Assessment Randomized Protocol trial) and the Asia-Pacific (conducted in the Asia-Pacific region), sorafenib was associated with improved progression-free and overall survival in patients with advanced unresectable HCC[258] and currently represents a therapeutic option for patients who are not candidates for curative treatment or TACE.
HCC is a major global public health problem due to the rising incidence and high mortality in both developing and developed countries. An important point to be addressed is the promotion of preventive strategies, such as hepatitis B vaccination, and chronic hepatitis B and C treatment, in order to cut down the number of patients who may develop cirrhosis and potentially progress to HCC. Early diagnosis is crucial for curative treatments. As a consequence, patients at risk of developing HCC should be regularly followed up to diagnose HCC in the initial stage. Surgical resection, RFA and liver transplantation are considered the cornerstones of curative therapy, while for patients with more advanced HCC recommended options include sorafenib and TACE.
Unfortunately, most evidence comes from case series and retrospective studies. There is a need for larger, multicenter, randomized studies in order to define the most appropriate, evidence-based therapeutic approach to patients with HCC.
| 1. | National Cancer Institute. Surveillance Research Program, National Cancer Institute. Fast stats: an interactive tool for access to SEER cancer statistics. Available from: http://surveillance.cancer.gov/. |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 3. | El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227-3230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 279] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 799] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 685] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 6. | Marrero JA, Hussain HK, Nghiem HV, Umar R, Fontana RJ, Lok AS. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transpl. 2005;11:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6629] [Article Influence: 441.9] [Reference Citation Analysis (1)] |
| 8. | Choi BI. Hepatocarcinogenesis in liver cirrhosis: imaging diagnosis. J Korean Med Sci. 1998;13:103-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Eskesen AN, Bjøro K, Aandahl EM, Line PD, Melum E. Low use of surveillance and early diagnosis of hepatocellular carcinoma in Norway--a population-based cohort study. Cancer Epidemiol. 2014;38:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Chen MH, Yan K, Yang W, Gao W, Dai Y, Huo L, Zhang H, Huang XF. [Long term (5 years) outcome of radiofrequency ablation for hepatocellular carcinoma in 256 cases]. Beijing Daxue Xuebao. 2005;37:671-672. [PubMed] |
| 11. | Matsui O, Kobayashi S, Sanada J, Kouda W, Ryu Y, Kozaka K, Kitao A, Nakamura K, Gabata T. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging. 2011;36:264-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 368] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Matsui O, Ueda K, Kobayashi S, Sanada J, Terayama N, Gabata T, Minami M, Kawamori Y, Nakanuma Y. Intra- and perinodular hemodynamics of hepatocellular carcinoma: CT observation during intra-arterial contrast injection. Abdom Imaging. 2002;27:147-156. [PubMed] |
| 14. | Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology. 2002;225:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Wu W, Chen MH, Sun M, Yan K, Yang W, Li JY. Contrast-enhanced ultrasound of hepatocarcinogenesis in liver cirrhosis. Chin Med J (Engl). 2012;125:3104-3109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Kim TK, Jang HJ. Contrast-enhanced ultrasound in the diagnosis of nodules in liver cirrhosis. World J Gastroenterol. 2014;20:3590-3596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Isozaki T, Numata K, Kiba T, Hara K, Morimoto M, Sakaguchi T, Sekihara H, Kubota T, Shimada H, Morizane T. Differential diagnosis of hepatic tumors by using contrast enhancement patterns at US. Radiology. 2003;229:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Numata K, Luo W, Morimoto M, Kondo M, Kunishi Y, Sasaki T, Nozaki A, Tanaka K. Contrast enhanced ultrasound of hepatocellular carcinoma. World J Radiol. 2010;2:68-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 686] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 20. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 337] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Wen YL, Kudo M, Zheng RQ, Ding H, Zhou P, Minami Y, Chung H, Kitano M, Kawasaki T, Maekawa K. Characterization of hepatic tumors: value of contrast-enhanced coded phase-inversion harmonic angio. AJR Am J Roentgenol. 2004;182:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Loria F, Loria G, Basile S, Crea G, Frosina L, Di Carlo I. Contrast-enhanced ultrasound appearances of enhancement patterns of intrahepatic cholangiocarcinoma: correlation with pathological findings. Updates Surg. 2014;66:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Kim TK, Choi BI, Han JK, Hong HS, Park SH, Moon SG. Hepatic tumors: contrast agent-enhancement patterns with pulse-inversion harmonic US. Radiology. 2000;216:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 169] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Wilson SR, Burns PN, Muradali D, Wilson JA, Lai X. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology. 2000;215:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 214] [Article Influence: 8.2] [Reference Citation Analysis (10)] |
| 25. | Quaia E, Degobbis F, Tona G, Mosconi E, Bertolotto M, Pozzi Mucelli R. [Differential patterns of contrast enhancement in different focal liver lesions after injection of the microbubble US contrast agent SonoVue]. Radiol Med. 2004;107:155-165. [PubMed] |
| 26. | Catalano O, Lobianco R, Cusati B, Siani A. Hepatocellular carcinoma: spectrum of contrast-enhanced gray-scale harmonic sonography findings. Abdom Imaging. 2004;29:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Wu W, Chen M, Yan K, Dai Y, Yin S, Yang W, Fan Z. Evaluation of contrast-enhanced ultrasound for diagnosis of dysplastic nodules with a focus of hepatocellular carcinoma in liver cirrhosis patients. Chin J Cancer Res. 2015;27:83-89. [PubMed] |
| 28. | Kim SH, Choi BI, Lee JY, Kim SJ, So YH, Eun HW, Lee JM, Han JK. Diagnostic accuracy of multi-/single-detector row CT and contrast-enhanced MRI in the detection of hepatocellular carcinomas meeting the milan criteria before liver transplantation. Intervirology. 2008;51 Suppl 1:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Ariff B, Lloyd CR, Khan S, Shariff M, Thillainayagam AV, Bansi DS, Khan SA, Taylor-Robinson SD, Lim AK. Imaging of liver cancer. World J Gastroenterol. 2009;15:1289-1300. [PubMed] |
| 30. | Ito K. Hepatocellular carcinoma: conventional MRI findings including gadolinium-enhanced dynamic imaging. Eur J Radiol. 2006;58:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Chanyaputhipong J, Low SC, Chow PK. Gadoxetate Acid-Enhanced MR Imaging for HCC: A Review for Clinicians. Int J Hepatol. 2011;2011:489342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Willatt JM, Hussain HK, Adusumilli S, Marrero JA. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247:311-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M, Brú C, Bruix J; Barcelona Clínic Liver Cancer Group. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Kim YK, Kim CS, Chung GH, Han YM, Lee SY, Chon SB, Lee JM. Comparison of gadobenate dimeglumine-enhanced dynamic MRI and 16-MDCT for the detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2006;186:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Ebara M, Ohto M, Watanabe Y, Kimura K, Saisho H, Tsuchiya Y, Okuda K, Arimizu N, Kondo F, Ikehira H. Diagnosis of small hepatocellular carcinoma: correlation of MR imaging and tumor histologic studies. Radiology. 1986;159:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 107] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Krinsky GA, Lee VS, Theise ND, Weinreb JC, Morgan GR, Diflo T, John D, Teperman LW, Goldenberg AS. Transplantation for hepatocellular carcinoma and cirrhosis: sensitivity of magnetic resonance imaging. Liver Transpl. 2002;8:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Kim JI, Lee JM, Choi JY, Kim YK, Kim SH, Lee JY, Han JK, Choi BI. The value of gadobenate dimeglumine-enhanced delayed phase MR imaging for characterization of hepatocellular nodules in the cirrhotic liver. Invest Radiol. 2008;43:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, Choi D, Lee WJ, Lim HK. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:1675-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 262] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 39. | Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213-221. [PubMed] |
| 40. | Delbeke D, Pinson CW. 11C-acetate: a new tracer for the evaluation of hepatocellular carcinoma. J Nucl Med. 2003;44:222-223. [PubMed] |
| 41. | Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, Lee WJ, Kim CM, Nam BH. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 42. | Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of primary hepatobiliary tumors, part 2. AJR Am J Roentgenol. 2011;197:W260-W265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 221] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK, Pinson CW. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg. 1998;133:510-515; discussion 515-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 199] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 45. | Gibbs JF, Litwin AM, Kahlenberg MS. Contemporary management of benign liver tumors. Surg Clin North Am. 2004;84:463-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Chen S, Ho C, Feng D, Chi Z. Tracer kinetic modeling of 11C-acetate applied in the liver with positron emission tomography. IEEE Trans Med Imaging. 2004;23:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Huo L, Guo J, Dang Y, Lv J, Zheng Y, Li F, Xie Q, Chen X. Kinetic analysis of dynamic (11)C-acetate PET/CT imaging as a potential method for differentiation of hepatocellular carcinoma and benign liver lesions. Theranostics. 2015;5:371-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4519] [Article Influence: 215.2] [Reference Citation Analysis (4)] |
| 49. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (4)] |
| 50. | Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, Zhu YR. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 51. | McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, Dunaway E, Williams J. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 242] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 52. | Wan HG, Xu H, Gu YM, Wang H, Xu W, Zu MH. Comparison osteopontin vs AFP for the diagnosis of HCC: a meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Xu C, Yan Z, Zhou L, Wang Y. A comparison of glypican-3 with alpha-fetoprotein as a serum marker for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Richardson P, Duan Z, Kramer J, Davila JA, Tyson GL, El-Serag HB. Determinants of serum alpha-fetoprotein levels in hepatitis C-infected patients. Clin Gastroenterol Hepatol. 2012;10:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Mossad NA, Mahmoud EH, Osman EA, Mahmoud SH, Shousha HI. Evaluation of squamous cell carcinoma antigen-immunoglobulin M complex (SCCA-IGM) and alpha-L-fucosidase (AFU) as novel diagnostic biomarkers for hepatocellular carcinoma. Tumour Biol. 2014;35:11559-11564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 56. | Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS. Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39:410-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 57. | Zhang J, Zhao Y, Yang Q. Sensitivity and specificity of Dickkopf-1 protein in serum for diagnosing hepatocellular carcinoma: a meta-analysis. Int J Biol Markers. 2014;29:e403-e410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Tanaka J, Kagebayashi C, Satomura S. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol. 2014;49:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 59. | Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A, Kanke F, Satomura S. Clinical utility of highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in hepatocellular carcinoma patients with alpha-fetoprotein & lt; 20 ng/mL. Cancer Sci. 2011;102:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Witjes CD, van Aalten SM, Steyerberg EW, Borsboom GJ, de Man RA, Verhoef C, Ijzermans JN. Recently introduced biomarkers for screening of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int. 2013;7:59-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D’Amelio A, Lok AS, Block TM. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 62. | Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 63. | Porta C, De Amici M, Quaglini S, Paglino C, Tagliani F, Boncimino A, Moratti R, Corazza GR. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | Hsia CY, Huo TI, Chiang SY, Lu MF, Sun CL, Wu JC, Lee PC, Chi CW, Lui WY, Lee SD. Evaluation of interleukin-6, interleukin-10 and human hepatocyte growth factor as tumor markers for hepatocellular carcinoma. Eur J Surg Oncol. 2007;33:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Giannelli G, Fransvea E, Trerotoli P, Beaugrand M, Marinosci F, Lupo L, Nkontchou G, Dentico P, Antonaci S. Clinical validation of combined serological biomarkers for improved hepatocellular carcinoma diagnosis in 961 patients. Clin Chim Acta. 2007;383:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 66. | Sha Sha F, Ai min LI, Rong LI, Feng sheng C, Jun yi Z, Rong cheng L. IL 17 expression increased as a diagnostic marker in the serum of hepatocellular carcinoma patients. J Nat Sci Hunan Norm Univ. 2014;37:19-23. |
| 67. | Liu J, Zhou G, Lu W. Plasma interleukin 17 in the diagnosis of hepatocellular carcinoma: a retrospective study of 39 cases. J Cancer Res Ther. 2014;10 Suppl:304-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, Gerken G, Schlaak JF. A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 69. | Hadziyannis E, Sialevris K, Georgiou A, Koskinas J. Analysis of serum α-fetoprotein-L3% and des-γ carboxyprothrombin markers in cases with misleading hepatocellular carcinoma total α-fetoprotein levels. Oncol Rep. 2013;29:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Wang M, Mehta A, Block TM, Marrero J, Di Bisceglie AM, Devarajan K. A comparison of statistical methods for the detection of hepatocellular carcinoma based on serum biomarkers and clinical variables. BMC Med Genomics. 2013;6 Suppl 3:S9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 72. | Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 513] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 73. | Liu CC, Wang YH, Chuang EY, Tsai MH, Chuang YH, Lin CL, Liu CJ, Hsiao BY, Lin SM, Liu LY. Identification of a liver cirrhosis signature in plasma for predicting hepatocellular carcinoma risk in a population-based cohort of hepatitis B carriers. Mol Carcinog. 2014;53:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Shi M, Chen MS, Sekar K, Tan CK, Ooi LL, Hui KM. A blood-based three-gene signature for the non-invasive detection of early human hepatocellular carcinoma. Eur J Cancer. 2014;50:928-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Wurmbach E, Chen YB, Khitrov G, Zhang W, Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 561] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 76. | Nam SW, Park JY, Ramasamy A, Shevade S, Islam A, Long PM, Park CK, Park SE, Kim SY, Lee SH. Molecular changes from dysplastic nodule to hepatocellular carcinoma through gene expression profiling. Hepatology. 2005;42:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 77. | Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, Thung SN, Khitrov G, Zhang W, Villanueva A. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 78. | Sakamoto M, Mori T, Masugi Y, Effendi K, Rie I, Du W. Candidate molecular markers for histological diagnosis of early hepatocellular carcinoma. Intervirology. 2008;51 Suppl 1:42-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Mínguez B, Lachenmayer A. Diagnostic and prognostic molecular markers in hepatocellular carcinoma. Dis Markers. 2011;31:181-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (1)] |
| 80. | Berindan-Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64:311-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 413] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 81. | Link A, Kupcinskas J, Wex T, Malfertheiner P. Macro-role of microRNA in gastric cancer. Dig Dis. 2012;30:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 82. | Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003-5008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2345] [Cited by in RCA: 2698] [Article Influence: 179.9] [Reference Citation Analysis (0)] |
| 83. | Link A, Goel A. MicroRNA in gastrointestinal cancer: a step closer to reality. Adv Clin Chem. 2013;62:221-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Redis RS, Calin S, Yang Y, You MJ, Calin GA. Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacol Ther. 2012;136:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 85. | Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513-10518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5636] [Cited by in RCA: 6414] [Article Influence: 356.3] [Reference Citation Analysis (0)] |
| 86. | Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3218] [Cited by in RCA: 3603] [Article Influence: 200.2] [Reference Citation Analysis (0)] |
| 87. | Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 2127] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 88. | Link A, Balaguer F, Shen Y, Nagasaka T, Lozano JJ, Boland CR, Goel A. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol Biomarkers Prev. 2010;19:1766-1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 89. | Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7723] [Cited by in RCA: 7422] [Article Influence: 353.4] [Reference Citation Analysis (5)] |
| 90. | Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4471] [Cited by in RCA: 4556] [Article Influence: 227.8] [Reference Citation Analysis (0)] |
| 91. | Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 600] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 92. | Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 898] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 93. | Toffanin S, Hoshida Y, Lachenmayer A, Villanueva A, Cabellos L, Minguez B, Savic R, Ward SC, Thung S, Chiang DY. MicroRNA-based classification of hepatocellular carcinoma and oncogenic role of miR-517a. Gastroenterology. 2011;140:1618-1628.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 94. | Giordano S, Columbano A. MicroRNAs: new tools for diagnosis, prognosis, and therapy in hepatocellular carcinoma? Hepatology. 2013;57:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 298] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 95. | Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, Zhang CY, Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798-9807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 380] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 96. | Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, Wang JF, Zhang Z, Lu S, Huang X. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781-4788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 505] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 97. | Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, Tanemura M, Tomokuni A, Takemasa I, Umeshita K. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012;56:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 98. | Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, Huang L, Li H, Tan W, Wang C. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 453] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 99. | Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011;6:e28486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 100. | Bihrer V, Waidmann O, Friedrich-Rust M, Forestier N, Susser S, Haupenthal J, Welker M, Shi Y, Peveling-Oberhag J, Polta A. Serum microRNA-21 as marker for necroinflammation in hepatitis C patients with and without hepatocellular carcinoma. PLoS One. 2011;6:e26971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 101. | Tan Y, Ge G, Pan T, Wen D, Gan J. A pilot study of serum microRNAs panel as potential biomarkers for diagnosis of nonalcoholic fatty liver disease. PLoS One. 2014;9:e105192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 102. | Schütte K, Schulz C, Link A, Malfertheiner P. Current biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosis. World J Hepatol. 2015;7:139-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 103. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2915] [Article Influence: 108.0] [Reference Citation Analysis (1)] |
| 104. | Grieco A, Pompili M, Caminiti G, Miele L, Covino M, Alfei B, Rapaccini GL, Gasbarrini G. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 105. | Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lok AS. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 476] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 106. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298-304. [PubMed] |
| 107. | Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206; discussion 1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 616] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 108. | Nakayama H, Takayama T, Okubo T, Higaki T, Midorikawa Y, Moriguchi M, Itoh A. Proposal of objective morphological classification system for hepatocellular carcinoma using preoperative multiphase computed tomography. J Gastroenterol. 2014;49:1430-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346-350. [PubMed] |
| 110. | Arii S, Tanaka J, Yamazoe Y, Minematsu S, Morino T, Fujita K, Maetani S, Tobe T. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer. 1992;69:913-919. [PubMed] |
| 111. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1263] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 112. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 509] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 113. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 199] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 114. | Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-680; discussion 680-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 338] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 115. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 420] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 116. | Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 419] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 117. | Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 118. | Ferrero A, Viganò L, Polastri R, Muratore A, Eminefendic H, Regge D, Capussotti L. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 119. | Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery. 2008;143:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 120. | Shimada K, Nara S, Esaki M, Sakamoto Y, Kosuge T, Hiraoka N. Extended right hemihepatectomy for gallbladder carcinoma involving the hepatic hilum. Br J Surg. 2011;98:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 121. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 122. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. [PubMed] |
| 123. | Saito S, Yamanaka J, Miura K, Nakao N, Nagao T, Sugimoto T, Hirano T, Kuroda N, Iimuro Y, Fujimoto J. A novel 3D hepatectomy simulation based on liver circulation: application to liver resection and transplantation. Hepatology. 2005;41:1297-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 124. | Ijichi M, Takayama T, Toyoda H, Sano K, Kubota K, Makuuchi M. Randomized trial of the usefulness of a bile leakage test during hepatic resection. Arch Surg. 2000;135:1395-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 125. | Liu Z, Jin H, Li Y, Gu Y, Zhai C. Randomized controlled trial of the intraoperative bile leakage test in preventing bile leakage after hepatic resection. Dig Surg. 2012;29:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 126. | Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250-278; quiz 279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2944] [Cited by in RCA: 2822] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 127. | Liu CL, Fan ST, Lo CM, Wong Y, Ng IO, Lam CM, Poon RT, Wong J. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 128. | Sun HC, Qin LX, Lu L, Wang L, Ye QH, Ren N, Fan J, Tang ZY. Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg. 2006;93:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 129. | Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Ann Surg. 1993;218:748-753. [PubMed] |
| 130. | Franco D, Karaa A, Meakins JL, Borgonovo G, Smadja C, Grange D. Hepatectomy without abdominal drainage. Results of a prospective study in 61 patients. Ann Surg. 1989;210:748-750. [PubMed] |
| 131. | Burt BM, Brown K, Jarnagin W, DeMatteo R, Blumgart LH, Fong Y. An audit of results of a no-drainage practice policy after hepatectomy. Am J Surg. 2002;184:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 132. | Lu L, Sun HC, Qin LX, Wang L, Ye QH, Ren N, Fan J, Tang ZY. Abdominal drainage was unnecessary after hepatectomy using the conventional clamp crushing technique. J Gastrointest Surg. 2006;10:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 133. | Yamazaki S, Takayama T, Moriguchi M, Mitsuka Y, Okada S, Midorikawa Y, Nakayama H, Higaki T. Criteria for drain removal following liver resection. Br J Surg. 2012;99:1584-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 134. | Nakayama H, Takayama T, Okubo T, Higaki T, Midorikawa Y, Moriguchi M, Aramaki O, Yamazaki S. Subcutaneous drainage to prevent wound infection in liver resection: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2014;21:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 135. | Hayashi Y, Takayama T, Yamazaki S, Moriguchi M, Ohkubo T, Nakayama H, Higaki T. Validation of perioperative steroids administration in liver resection: a randomized controlled trial. Ann Surg. 2011;253:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 136. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (1)] |
| 137. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 839] [Article Influence: 40.0] [Reference Citation Analysis (1)] |
| 138. | Rahbari NN, Reissfelder C, Koch M, Elbers H, Striebel F, Büchler MW, Weitz J. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol. 2011;18:3640-3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 139. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5388] [Article Influence: 179.6] [Reference Citation Analysis (7)] |
| 140. | Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 304] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 141. | Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 142. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1714] [Article Influence: 68.6] [Reference Citation Analysis (1)] |
| 143. | Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, Burra P, Fagiuoli S, Farinati F, Rugge M. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 144. | DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, McGilvray I, Ghanekar A, Selzner M, Greig PD. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 145. | Avolio AW, Siciliano M, Barone M, Lai Q, Caracciolo GL, Barbarino R, Nicolotti N, Lirosi MC, Gasbarrini A, Agnes S. Model for end-stage liver disease dynamic stratification of survival benefit. Transplant Proc. 2012;44:1851-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 146. | Lai JC, Feng S, Roberts JP. An examination of liver offers to candidates on the liver transplant wait-list. Gastroenterology. 2012;143:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 147. | Maggs JR, Suddle AR, Aluvihare V, Heneghan MA. Systematic review: the role of liver transplantation in the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:1113-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 148. | Washburn K, Pomfret E, Roberts J. Liver allocation and distribution: possible next steps. Liver Transpl. 2011;17:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 149. | Gordon Burroughs S, Busuttil RW. Optimal utilization of extended hepatic grafts. Surg Today. 2009;39:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 150. | Yaprak O, Akyildiz M, Dayangac M, Demirbas BT, Guler N, Dogusoy GB, Yuzer Y, Tokat Y. AFP level and histologic differentiation predict the survival of patients with liver transplantation for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 151. | Singal AG, Chan V, Getachew Y, Guerrero R, Reisch JS, Cuthbert JA. Predictors of liver transplant eligibility for patients with hepatocellular carcinoma in a safety net hospital. Dig Dis Sci. 2012;57:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 152. | Zarrinpar A, Kaldas F, Busuttil RW. Liver transplantation for hepatocellular carcinoma: an update. Hepatobiliary Pancreat Dis Int. 2011;10:234-242. [PubMed] |
| 153. | Vivarelli M, Risaliti A. Liver transplantation for hepatocellular carcinoma on cirrhosis: strategies to avoid tumor recurrence. World J Gastroenterol. 2011;17:4741-4746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 154. | Chok KS, Chan SC, Cheung TT, Chan AC, Fan ST, Lo CM. Late recurrence of hepatocellular carcinoma after liver transplantation. World J Surg. 2011;35:2058-2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 155. | Kulik LM, Fisher RA, Rodrigo DR, Brown RS, Freise CE, Shaked A, Everhart JE, Everson GT, Hong JC, Hayashi PH, Berg CL, Lok AS; A2ALL Study Group. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: results of the A2ALL cohort. Am J Transplant. 2012;12:2997-3007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 156. | Liang W, Wu L, Ling X, Schroder PM, Ju W, Wang D, Shang Y, Kong Y, Guo Z, He X. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:1226-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 157. | Moon JI, Kwon CH, Joh JW, Choi GS, Jung GO, Kim JM, Shin M, Choi SJ, Kim SJ, Lee SK. Primary versus salvage living donor liver transplantation for patients with hepatocellular carcinoma: impact of microvascular invasion on survival. Transplant Proc. 2012;44:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 158. | Sandhu L, Sandroussi C, Guba M, Selzner M, Ghanekar A, Cattral MS, McGilvray ID, Levy G, Greig PD, Renner EL. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl. 2012;18:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 159. | Earl TM, Chapman WC. Transplantation for hepatocellular carcinoma: the North American experience. Recent Results Cancer Res. 2013;190:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 160. | Lee SG, Moon DB. Living donor liver transplantation for hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:165-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 161. | Kneteman NM, Oberholzer J, Al Saghier M, Meeberg GA, Blitz M, Ma MM, Wong WW, Gutfreund K, Mason AL, Jewell LD. Sirolimus-based immunosuppression for liver transplantation in the presence of extended criteria for hepatocellular carcinoma. Liver Transpl. 2004;10:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 162. | Kawahara T, Asthana S, Kneteman NM. m-TOR inhibitors: what role in liver transplantation? J Hepatol. 2011;55:1441-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 163. | Schnitzbauer AA, Zuelke C, Graeb C, Rochon J, Bilbao I, Burra P, de Jong KP, Duvoux C, Kneteman NM, Adam R. A prospective randomised, open-labeled, trial comparing sirolimus-containing versus mTOR-inhibitor-free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Cancer. 2010;10:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 164. | Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 165. | Toso C, Meeberg GA, Bigam DL, Oberholzer J, Shapiro AM, Gutfreund K, Ma MM, Mason AL, Wong WW, Bain VG. De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: long-term outcomes and side effects. Transplantation. 2007;83:1162-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 166. | Ahmed M, Brace CL, Lee FT, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 579] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 167. | Haemmerich D, Pilcher TA. Convective cooling affects cardiac catheter cryoablation and radiofrequency ablation in opposite directions. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1499-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 168. | Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol. 2001;12:1135-1148. [PubMed] |
| 169. | Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13:129-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 277] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 170. | Rhim H, Dodd GD, Chintapalli KN, Wood BJ, Dupuy DE, Hvizda JL, Sewell PE, Goldberg SN. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics. 2004;24:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 221] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 171. | Huffman SD, Huffman NP, Lewandowski RJ, Brown DB. Radiofrequency ablation complicated by skin burn. Semin Intervent Radiol. 2011;28:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 172. | Kim KR, Thomas S. Complications of image-guided thermal ablation of liver and kidney neoplasms. Semin Intervent Radiol. 2014;31:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 173. | Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267-1274. [PubMed] |
| 174. | Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2013;12:CD003046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 175. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 653] [Article Influence: 40.8] [Reference Citation Analysis (1)] |
| 176. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1115] [Article Influence: 55.8] [Reference Citation Analysis (1)] |
| 177. | Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 178. | Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 179. | Giorgio A, Di Sarno A, De Stefano G, Scognamiglio U, Farella N, Mariniello A, Esposito V, Coppola C, Giorgio V. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res. 2011;31:2291-2295. [PubMed] |
| 180. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 674] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 181. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 438] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 182. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 432] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 183. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 635] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 184. | Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 393] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 185. | Ferrari FS, Megliola A, Scorzelli A, Stella A, Vigni F, Drudi FM, Venezia D. Treatment of small HCC through radiofrequency ablation and laser ablation. Comparison of techniques and long-term results. Radiol Med. 2007;112:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 186. | Lee YH, Hsu CY, Chu CW, Liu PH, Hsia CY, Huang YH, Su CW, Chiou YY, Lin HC, Huo TI. Radiofrequency ablation is better than surgical resection in patients with hepatocellular carcinoma within the Milan criteria and preserved liver function: a retrospective study using propensity score analyses. J Clin Gastroenterol. 2015;49:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 187. | Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 188. | Schramm W, Yang D, Wood BJ, Rattay F, Haemmerich D. Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. Open Biomed Eng J. 2007;1:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 189. | Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, Du Z. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013;82:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 190. | Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, Du Z. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol. 2013;68:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 191. | Zhang L, Wang N, Shen Q, Cheng W, Qian GJ. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One. 2013;8:e76119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 192. | McCarley JR, Soulen MC. Percutaneous ablation of hepatic tumors. Semin Intervent Radiol. 2010;27:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 193. | Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 194. | Taniguchi M, Kim SR, Imoto S, Ikawa H, Ando K, Mita K, Fuki S, Sasase N, Matsuoka T, Kudo M. Long-term outcome of percutaneous ethanol injection therapy for minimum-sized hepatocellular carcinoma. World J Gastroenterol. 2008;14:1997-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 195. | Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1352] [Cited by in RCA: 1222] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 196. | Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin W, Kane M. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 626] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 197. | Wagman R, Yorke E, Ford E, Giraud P, Mageras G, Minsky B, Rosenzweig K. Respiratory gating for liver tumors: use in dose escalation. Int J Radiat Oncol Biol Phys. 2003;55:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 198. | Wunderink W, Méndez Romero A, de Kruijf W, de Boer H, Levendag P, Heijmen B. Reduction of respiratory liver tumor motion by abdominal compression in stereotactic body frame, analyzed by tracking fiducial markers implanted in liver. Int J Radiat Oncol Biol Phys. 2008;71:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 199. | Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, Nuyttens JJ, Brandwijk RP, Verhoef C, Ijzermans JN. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006;45:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 356] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 200. | Denecke T, Lopez Hänninen E. Brachytherapy of liver metastases. Recent Results Cancer Res. 2008;177:95-104. [PubMed] |
| 201. | Ricke J, Mohnike K, Pech M, Seidensticker M, Rühl R, Wieners G, Gaffke G, Kropf S, Felix R, Wust P. Local response and impact on survival after local ablation of liver metastases from colorectal carcinoma by computed tomography-guided high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2010;78:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 202. | Collettini F, Lutter A, Schnapauff D, Hildebrandt B, Puhl G, Denecke T, Wust P, Gebauer B. Unresectable colorectal liver metastases: percutaneous ablation using CT-guided high-dose-rate brachytherapy (CT-HDBRT). Rofo. 2014;186:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 203. | Ricke J, Wust P, Wieners G, Beck A, Cho CH, Seidensticker M, Pech M, Werk M, Rosner C, Hänninen EL. Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavorable lesions for thermal ablation. J Vasc Interv Radiol. 2004;15:1279-1286. [PubMed] |
| 204. | Mearini L. High intensity focused ultrasound, liver disease and bridging therapy. World J Gastroenterol. 2013;19:7494-7499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 205. | Cheung TT, Fan ST, Chan SC, Chok KS, Chu FS, Jenkins CR, Lo RC, Fung JY, Chan AC, Sharr WW. High-intensity focused ultrasound ablation: an effective bridging therapy for hepatocellular carcinoma patients. World J Gastroenterol. 2013;19:3083-3089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 206. | Shen HP, Gong JP, Zuo GQ. Role of high-intensity focused ultrasound in treatment of hepatocellular carcinoma. Am Surg. 2011;77:1496-1501. [PubMed] |
| 207. | Xu G, Luo G, He L, Li J, Shan H, Zhang R, Li Y, Gao X, Lin S, Wang G. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol. 2011;37:1993-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 208. | Ng KK, Poon RT, Chan SC, Chok KS, Cheung TT, Tung H, Chu F, Tso WK, Yu WC, Lo CM. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 209. | Wu F, Wang ZB, Chen WZ, Zhu H, Bai J, Zou JZ, Li KQ, Jin CB, Xie FL, Su HB. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol. 2004;11:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 189] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 210. | Wu F, Wang ZB, Chen WZ, Wang W, Gui Y, Zhang M, Zheng G, Zhou Y, Xu G, Li M. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 220] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 211. | Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Jin CB, Xie FL, Su HB. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. 2005;235:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 212. | Lu DS, Kee ST, Lee EW. Irreversible electroporation: ready for prime time? Tech Vasc Interv Radiol. 2013;16:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 213. | Ben-David E, Appelbaum L, Sosna J, Nissenbaum I, Goldberg SN. Characterization of irreversible electroporation ablation in in vivo porcine liver. AJR Am J Roentgenol. 2012;198:W62-W68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 214. | Silk MT, Wimmer T, Lee KS, Srimathveeravalli G, Brown KT, Kingham PT, Fong Y, Durack JC, Sofocleous CT, Solomon SB. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 215. | Deodhar A, Dickfeld T, Single GW, Hamilton WC, Thornton RH, Sofocleous CT, Maybody M, Gónen M, Rubinsky B, Solomon SB. Irreversible electroporation near the heart: ventricular arrhythmias can be prevented with ECG synchronization. AJR Am J Roentgenol. 2011;196:W330-W335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 216. | Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 217. | Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat. 2013;12:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 218. | Kingham TP, Karkar AM, D’Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, Sofocleous CT, Solomon SB, Jarnagin WR, Fong Y. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg. 2012;215:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 219. | Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, Roberts S, Evans P, Ball C, Haydon A. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 220. | Smith MT, Ray CE. The treatment of primary and metastatic hepatic neoplasms using percutaneous cryotherapy. Semin Intervent Radiol. 2006;23:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 221. | Huang YZ, Zhou SC, Zhou H, Tong M. Radiofrequency ablation versus cryosurgery ablation for hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 222. | Pacella CM, Francica G, Di Costanzo GG. Laser ablation for small hepatocellular carcinoma. Radiol Res Pract. 2011;2011:595627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 223. | Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions). Radiology. 2002;225:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 224. | Pacella CM, Francica G, Di Lascio FM, Arienti V, Antico E, Caspani B, Magnolfi F, Megna AS, Pretolani S, Regine R. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol. 2009;27:2615-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 225. | Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, Ravaioli M, D’Errico-Grigioni A, Pinna AD, Bolondi L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (< 5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 226. | Geschwind JF. Chemoembolization for hepatocellular carcinoma: where does the truth lie? J Vasc Interv Radiol. 2002;13:991-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 227. | Geschwind JF, Ramsey DE, Choti MA, Thuluvath PJ, Huncharek MS. Chemoembolization of hepatocellular carcinoma: results of a metaanalysis. Am J Clin Oncol. 2003;26:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 228. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2008] [Article Influence: 83.7] [Reference Citation Analysis (2)] |
| 229. | Boily G, Villeneuve JP, Lacoursière L, Chaudhury P, Couture F, Ouellet JF, Lapointe R, Goulet S, Gervais N. Transarterial embolization therapies for the treatment of hepatocellular carcinoma: CEPO review and clinical recommendations. HPB (Oxford). 2015;17:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 230. | Forner A, Llovet JM, Bruix J. Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol. 2012;56:984-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (2)] |
| 231. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2654] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 232. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2301] [Article Influence: 100.0] [Reference Citation Analysis (1)] |
| 233. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1218] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 234. | Xiong ZP, Yang SR, Liang ZY, Xiao EH, Yu XP, Zhou SK, Zhang ZS. Association between vascular endothelial growth factor and metastasis after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2004;3:386-390. [PubMed] |
| 235. | Kobayashi N, Ishii M, Ueno Y, Kisara N, Chida N, Iwasaki T, Toyota T. Co-expression of Bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver. 1999;19:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 236. | Jaeger HJ, Mehring UM, Castañeda F, Hasse F, Blumhardt G, Loehlein D, Mathias KD. Sequential transarterial chemoembolization for unresectable advanced hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1996;19:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 237. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 721] [Article Influence: 37.9] [Reference Citation Analysis (1)] |
| 238. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 496] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 239. | Song MJ, Chun HJ, Song do S, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 240. | Gao S, Yang Z, Zheng Z, Yao J, Deng M, Xie H, Zheng S, Zhou L. Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 241. | Sherman M. Hepatocellular carcinoma: screening and staging. Clin Liver Dis. 2011;15:323-324, vii-x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 242. | Hong K, Georgiades CS, Geschwind JF. Technology insight: Image-guided therapies for hepatocellular carcinoma--intra-arterial and ablative techniques. Nat Clin Pract Oncol. 2006;3:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 243. | Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, Benson A, Espat J, Bilbao JI, Sharma RA. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 512] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 244. | Gramenzi A, Golfieri R, Mosconi C, Cappelli A, Granito A, Cucchetti A, Marinelli S, Pettinato C, Erroi V, Fiumana S. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int. 2015;35:1036-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 245. | Ettorre GM, Laurenzi A, Vennarecci G. Downstaging Hepatocellular Carcinoma with Yttrium-90 radioembolization: resection or transplantation? Eur J Surg Oncol. 2014;40:789-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 246. | Tohme S, Sukato D, Chen HW, Amesur N, Zajko AB, Humar A, Geller DA, Marsh JW, Tsung A. Yttrium-90 radioembolization as a bridge to liver transplantation: a single-institution experience. J Vasc Interv Radiol. 2013;24:1632-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 247. | Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 416] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 248. | Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 249. | Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872-3882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 250. | Jiang G, Xu X, Ren S, Wang L. Combining transarterial chemoembolization with radiofrequency ablation for hepatocellular carcinoma. Tumour Biol. 2014;35:3405-3408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 251. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 252. | Hurwitz MD, Hansen JL, Prokopios-Davos S, Manola J, Wang Q, Bornstein BA, Hynynen K, Kaplan ID. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: long-term results from Dana-Farber Cancer Institute study 94-153. Cancer. 2011;117:510-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 253. | Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR, Jones EL. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: a review of the randomised data. Int J Hyperthermia. 2010;26:612-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 254. | Goldberg SN. Science to practice: Can we expand focal interventional oncologic ablation treatments into an effective systemic therapy? Radiology. 2013;267:321-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 255. | Soundararajan A, Dodd GD, Bao A, Phillips WT, McManus LM, Prihoda TJ, Goins BA. Chemoradionuclide therapy with 186Re-labeled liposomal doxorubicin in combination with radiofrequency ablation for effective treatment of head and neck cancer in a nude rat tumor xenograft model. Radiology. 2011;261:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 256. | Li D, Kang J, Golas BJ, Yeung VW, Madoff DC. Minimally invasive local therapies for liver cancer. Cancer Biol Med. 2014;11:217-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 257. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10523] [Article Influence: 584.6] [Reference Citation Analysis (9)] |
| 258. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4736] [Article Influence: 263.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Yoshioka K S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM