INTRODUCTION
Irritable bowel syndrome (IBS) has become a critical issue worldwide, with the prevalence of 10%-20% of the population[1]. IBS is a heterogenous functional disorder, characterized by abdominal pain or discomfort and altered bowel habits. While there is no reliable biomarker, Rome III diagnostic criteria define IBS as a recurrent pain or discomfort for at least 3 d per month in the past 3 mo. In addition, the symptoms have to be associated with the two or more of the following: relief by defecation and the onset associated with the change of the frequency or form of the stool. IBS can be classified into four subtypes according to stool form, namely IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), mixed IBS (IBS-M) and unsubtyped IBS (IBS-U)[2]. Although not life-threatening, IBS has a negative economic effect on health service, among others due to the fact that the patient may need to undergo expensive tests and treatments before proper diagnosis. According to Maxion-Bergemann et al[3] total direct cost estimates per IBS patient range from USD 348-8750 per year. Moreover, IBS is an important reason for patients’ absenteeism from work; the average number of days off per year due to IBS was between 8.5 to 21.6. Finally, IBS provokes multiple comorbidities, such as chronic fatigue, headache, insomnia, psychiatric disturbances, dyspepsia and gastro-esophageal reflux which, taken together with direct symptoms of IBS lead to impaired quality of life[4].
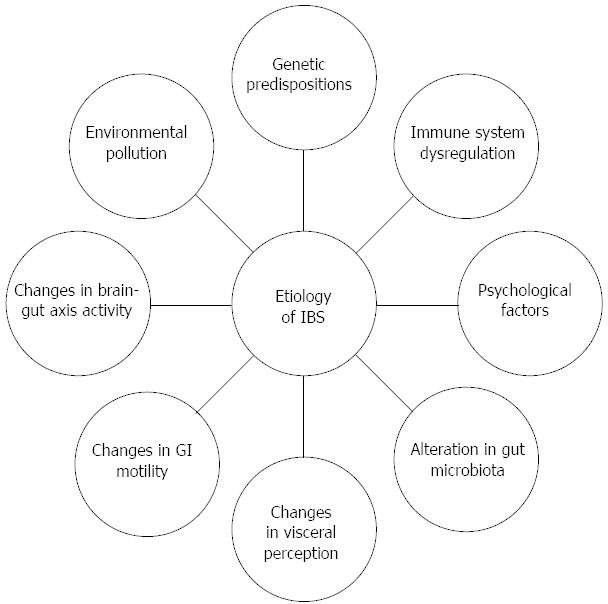
The etiology of IBS is undoubtedly multi-factorial, but its understanding is unsatisfactory due to lack of evident pathological abnormalities and infallible biomarkers. Currently, IBS is viewed as a disorder to which genetic, immune, and psychological factors, as well as alterations in microbiota, visceral perception and gastrointestinal (GI) motility contribute; diet and changes in brain-gut axis activity may also count (Figure 1). It still remains unclear which of these factors is the main trigger for the onset of IBS.
Figure 1 Multifactorial etiology of irritable bowel syndrome.
IBS: Irritable bowel syndrome; GI: Gastrointestinal.
Recently, the world has been seeing a dramatic rise in population growth in urban areas. As urban populations grow, the quality of the environment, and especially urban air pollution, will play an increasingly important role in public health. Consequently, the disease burden due to air pollution will be on the rise. While research on airborne pollutants has drawn attention mostly to respiratory and cardiovascular systems[5], emerging evidence suggests that these pollutants may also have adverse effects on the GI tract, being involved in the pathophysiology of inflammatory bowel disease (IBD)[6], appendicitis[7,8], and possibly irritable bowel syndrome[9]. While for instance smoking may affect the disease onset in IBD[10], the role of environmental pollution in IBS has not been fully elucidated.
In this review, we focus on the impact of environmental pollution - in its broadest sense - on development of IBS. We refer to current knowledge on the prevalence of IBS in regions with higher environmental pollution rate and discuss potential association between pollution and development of the disease.
MICROBIOLOGICAL POLLUTION
Postinfectious IBS
Postinfectious IBS (PI-IBS) is a particular case of IBS, which is caused by acute infectious gastroenteritis; in fact, it is considered as the most common cause of IBS[11]. It was shown in prospective studies that 4% to 36% patients suffer from PI-IBS because of previous infection[12]. Noteworthy, the first reports on the disease date back already to 50 years ago[13]. The pathogens that contribute to PI-IBS are Campylobacter jejuni, Salmonella enterica, Shigella sonnei, Escherichia coli O157:H7, noroviruses and Giardia lamblia. The disease symptoms are not immediate, it takes approximately 8-10 years to develop a full-blown PI-IBS[14]. The duration of infection is crucial; for example, a fortnight-long Shigella sonnei infection was considerably more associated with PI-IBS than a week-long one (RR = 4.6)[15].
The most common alterations during PI-IBS are found in mucosal cells. Contrary to healthy volunteers and control patients, in which Campylobacter jejuni infection had no consequences, rectal mucosal enterochromaffin cell (EC) levels are increased in PI-IBS patients[16]. Moreover, mucosal barrier is mutilated by Campylobacter jejuni infection, thus the transepithelial electrical resistance is decreased[17]. Of note, changes in mucosal barrier function are considered to be associated with the tumor necrosis factor α (TNF-α) pathway[18]. On the other hand, when post-Shigella IBS is concerned, rise in ileal mast cells and in nerve fibers immunoreactive for neuron-specific enolase, substance P, and 5-hydroxytryptamine can be observed[19]. Finally, postviral PI-IBS has also been described. However, its pathophysiology remains unclear; of note, it has been suggested that IBS after norovirus infection is rather temporary, as compared to post-bacterial[20,21].
Walkerton crisis as an example of bacterial pollution
In May 2000, an ecological crisis because of procedural mistakes took place in Walkerton, Ontario, Canada. Escherichia coli O157:H7 and Campylobacter jejuni entered the drinking water system, while chlorine level (which was not monitored regularly) was too low to counteract the pollution[22]. This event caused 7 fatalities and over 2300 victims among 4800 Walkerton residents. A cohort study conducted by Marshall et al[23] showed that the prevalence of PI-IBS in the local population increased significantly in comparison to healthy controls. Interestingly, 8 years after the incidence, the prevalence decreased from 28.3% (after about 3 years) to 15.4%; however, it was still higher than in unaffected subjects (OR = 3.12; 95%CI: 1.99-5.04).
Acquiring the cohort of 2069 adults after Walkerton outbreak was a great opportunity to investigate the long-term outcomes of water contamination on the GI tract function and more studies are expected. Moreover, it clearly showed that bacterial pollution resulting from human error, which is plausible, may have an effect that will be observed for several years after it occurred.
AIR POLLUTION
Air pollution is a mixture of a number of substances including gases, such as carbon dioxide, ozone, nitric oxide, volatile organic compounds (benzene) and particulate matter (PM), with the latter being the one mostly responsible for adverse health conditions. Of note, daily ingestion of PM on a typical Western diet is estimated at 1012-1014 particles per individual[24,25]. The GI tract is highly susceptible to PM (as well as smoking) and exposure to air pollution may exacerbate systemic inflammation or lead to oxidative damage of colonic mucosa[10]. For example, a study performed by Dybdahl et al[26] indicated that the exposure to diesel exhaust particles elaborated DNA adducts and oxidative stress, resulting in DNA strand breaks, apoptosis and protein oxidation in colon mucosa. Another study demonstrated that mice deficient in apolipoprotein E -/- exposed to low concentration of PM2.5 developed systemic inflammation, which was expressed mainly by vascular inflammation and increased atherosclerosis[27].
Air pollution and gut microbiome
Despite the well-studied effects of environmental pollutants on several health conditions[28,29], little is known on how air pollution impacts the gut microbiome. Kish et al[30] showed that pollutant particles ingested with chow altered gut microbiota composition by significant changes in the relative amounts of Bacteroidetes, Firmicutes and Verrucomicrobia. Moreover, mice exposed to polychlorinated biphenyls from contaminated food had decreased levels of Proteobacteria and increased levels of Bacteroidetes[31]. These results suggest that environmental pollutants may alter significantly the microbiome composition.
Air pollution and IBS-related pain
Non-specific abdominal pain is one of the crucial symptoms of IBS. In the years 1992-2002 and 1997-2002, Kaplan et al[9] performed two studies (in Edmonton and Montreal, respectively) focusing on the possible association between nonspecific abdominal pain and air pollution. The report published in 2012 showed that the young individuals aged 15 to 24 years, with preponderance of women, had the highest prevalence of non-specific abdominal pain and were significantly more likely to visit emergency department when the indicators of air pollution, such as CO, particles < 2.5 (PM2.5) μm, SO2, and NO2 were elevated[9]. Until today, the mechanism by which the air pollutants exacerbate abdominal pain has not been clarified. It has been suggested that increased IL-8 secretion from small bowel and changes in composition of colonic microflora[32] or alterations in colonic motility[9] may be the key factors. Moreover, in the same study it has been demonstrated that the exposure of mice to air pollutant EHC-6802 particles, recovered from filters of the single-pass air purification led to increased pain response. It is likely that air pollution may exacerbate systemic inflammation[27] and cause oxidative damage of colonic mucosa[26], what contributes to the occurrence of IBS symptoms.
RADIOACTIVE POLLUTION
Nuclear energy is a potent source of electric power in contemporary world. As the statistics show, it contributed to 10.8% of total world production of electricity in 2013[33]. On the one hand nuclear power is considered to be less harmful to the natural environment than conventional sources of energy since it does not produce common pollutants, such as greenhouse gases. On the other hand the humanity witnessed several nuclear reactor accidents in the past few years, which contaminated surrounding areas with radiation for several years. One of the most damaging was the explosion of nuclear power plant in Chernobyl (currently Ukraine) in 1986. As a result, about 14 EBq of radioactive substances were released to the atmosphere, contaminating the area of more than 200000 km2 in Europe. Up to 71% of radioactive caesium (137Cs), which affects inhabited areas to this day (t1/2 = 30.17 y), deposited in Belarus, the Russian Federation and Ukraine[34].
Several studies have been conducted in order to find the potential influence of post-Chernobyl radiation on the health of Ukrainians. For example, Chernobyl Childhood Illness Program (CCIP) examined 116 655 adolescents for thyroid gland disorders and found an increased prevalence of thyroid tumors, including thyroid cancer in this cohort[35], which may result from accumulation of radioactive iodine-131 in the thyroid gland. As regards GI tract diseases, Reshetnikov et al[36] reported that the prevalence of IBS in Russian children is as high as 38%, vs approximately 10%-15%[37]. Considering the proximity of Chernobyl and the Russian territory, it can be speculated that the post-Chernobyl radiation may be the main trigger. Furthermore, a series of cross-sectional studies on population of Ukrainian children and adolescents with IBS symptoms who live in the area contaminated with radioactive nuclides (60-90 km from Chernobyl) revealed significant abnormalities[38-41]. Namely, the subjects in the study were characterized by a higher level of internal whole body radiation due to 137Cs comparing to control group and exhibited differences in blood parameters, which indicated changes in innate and humoral immune status. In each age group an elevated leukocyte count was detected, with the difference reaching statistical significance in the group of mean age 14. Moreover, a decrease in total T-lymphocyte number, including CD4+ cell population was also observed. The changes were accompanied by an increase in CD8+ level, resulting in significantly lower proportions of CD4+: CD8+ in each group[38]. These observations remain consistent with the theory of low-grade inflammation occurring in some IBS patients[42]. Consequently, Ohman et al[43] confirmed the importance of alterations in blood T-cells for generation of symptoms in IBS.
High prevalence of functional GI diseases observed in Ukrainian children and adolescents could also be associated with changes in the non-specific immune system response, resulting from a significantly lower level of neutrophils, but higher level of CD16+ cells in peripheral blood compared with controls. Moreover, a decrease in phagocytic activity and phagocytic index were observed[39]. Altogether, these changes could trigger disturbances in the interaction between host and intestinal microorganisms, resulting in the overgrowth of the latter and/or inflammation, leading to IBS[44]. This hypothesis is supported by the results of a meta-analysis demonstrating the coexistence of intestinal inflammation and IBS in 39% of 1703 studied patients[45]. The impact of the radiation on the development of inflammation-based functional GI diseases in the Ukrainian children can be more complex, since they also present elevated plasma levels of proinflammatory cytokines, such as IL-4 and interferon γ (IFN-γ)[41], what signifies the involvement of several molecular pathways.
The humoral component of the immune response seemed to be altered in the studied population, since the CD22+ B lymphocyte level in peripheral blood was generally increased regardless of the group. In addition, the analysis of the serum immunoglobulin status revealed an increase in IgA, IgG and IgM levels, although the statistical significance was only reached for IgM[40]. Interestingly, several similarities were found between the Ukrainian cohort and a large group of IBS patients from another study[46]. The humoral status of the latter group seemed to be activated, with B lymphocyte and plasma cell density increased in intestinal mucosa compared with that in healthy controls. Moreover, the number of mucosal IgG+ cells and the luminal concentration of IgG were also higher. Additionally, the density of IgG+ cells in jejunal mucosa was positively correlated with the number of bowel movements per day and stool form. Whether the activation of humoral immunity is the principal factor in pathogenesis of IBS, remains to be elucidated.
STRESS POLLUTION
The association between stress and physical disorders was observed as early as in the 12th century by Maimonides, a medieval philosopher. He described emotional upset to be an important factor in asthma[47]. Today, we recognize a wide range of diseases with etiology linking mental and somatic disturbances and we call them “psychosomatic disorders”. The definition proposed by the World Health Organization states that the psychosomatic disorders are caused by events in the external environment which evoke responsive brain processes that activate neuro-endocrine systems and thereby induce changes in the functional state of “target” organs and motor systems. The events may play a dominant or only an additional role in the etiology of diseases, along with other factors, such as genetic and nutritional[48]. Such “external” events are often associated with stress and stressful stimuli, which - if in a high number - weaken the organism instead of making it prepared for a challenging situation. Here we propose that exposure to harmful stress can be compared to conventional pollution with regard to its negative impact on human health. Consequently, term “stress pollution” can be used.
Stress is an important contributor to anxiety disorders and thus their prevalence is currently high. For example, a report published in 2001 showed that 5.5% of Australians met the criteria for any anxiety disorder in the past 1 mo and 9.5% in the past 12 mo[49]. A survey conducted between 2001 and 2003 on 9282 Americans demonstrated the lifetime prevalence of anxiety disorders to be as high as 30%[50]. Analysis of 87 studies executed in different countries allows to estimate the current global prevalence of anxiety disorders at about 7.3%[51].
It has been hypothesized that IBS possesses psychosomatic basis and its association with anxiety disorders was therefore investigated. In a group of 94516 participants, in which IBS prevalence rate was 9.7% significantly more anxiety disorders were noted in IBS patients than in individuals free of any functional somatic syndromes[52]. Since median age of onset of anxiety disorders in Americans is 11 years[50], stress during childhood must be one of the most important underlying factors for IBS.
Emotional stress has been proven to induce IBS symptoms also in the adulthood. Lee et al[53] studied a group of 23698 subjects (mean age = 48 years) who underwent upper and lower endoscopy; Rome III criteria were used to diagnose IBS and The Brief Encounter Psychosical Instrument-Korean version (BEPSI-K) measured severity of stress in patients. More than 26% of participants fulfilled criteria for IBS and the disorder was more common amongst subjects with high stress score. Of note, stress was identified as an independent risk factor for IBS and the disease incidence rate increased along with the stress level. This association could be explained by the analysis of brain-gut interactions elicited by stress. The main culprit, corticotropin-releasing hormone (CRH) secreted by hypothalamus during stressful events can increase intestinal permeability leading to IBS development[54].
The disturbance of the circadian rhythm is another stressor which can determine IBS; furthermore, as such it can be regarded as occupational hazard. Professions particularly exposed to this type of stressor are those with shift work, for example the nurses. Nojkov et al[55] studied the prevalence of IBS in nurses based on their working hours. By comparing groups with day, night and rotating shifts it was found that the prevalence in the last group is significantly higher than that in the first one. The association was still significant after adjustment for sleep quality. Since circadian rhythm has been suggested to influence colonic motility in healthy subjects[56], the disruption of the process could have led to IBS development in the studied groups. The association seems to be supported by a clinical trial, which used melatonin, a hormone regulating circadian clock, as a potential new therapeutic in IBS management. Treatment with melatonin improved abdominal pain and distension compared with the placebo-treated group. Noteworthy, the observed effect was not due to the effect of melatonin on sleep patterns, meaning that the improvement in IBS symptoms could have resulted exclusively from the changes in circadian clock[57].
Stress could also influence the development and progression of IBS in an indirect manner. Namely, chronic psychological stress enhances vulnerability to some chemical exposures and in consequence increases the odds for some diseases to develop. The phenomenon has been extensively reviewed by Cooney[58], thus we limit our discussion to the link with IBS. A study investigating the influence of the exposure of rats to urban particles on visceral nociception has revealed an increased vulnerability to abdominal pain[9]. Another study revealed that the exposure to concentrated ambient particles (CAP), which represent modified urban air pollutants, had a more deleterious impact on the respiratory system in stressed rats comparing with non-stressed animals[59]. This means that not only air pollution is able to affect digestive system, but also the grade of its impact would depend on mental status of the animal. In line, epidemiological data collected by Kaplan et al[9] have shown that the number of admissions to emergency departments in Edmonton (Canada) due to non-specific abdominal pain was the highest on days when the concentration of polluting gases and solid particles in the air was elevated. Consequently, it can be hypothesized that there is interplay between stress and “conventional” pollution as regards development of functional GI diseases.
Finally, stress related to alteration in nutritional pattern should also be considered as a trigger for IBS. One of the most striking pieces of evidence comes from a study on the post war Dutch population. World War II in the Netherlands caused famine which persisted six months till the country was liberated. Daily ratios at that time were about 400-800 cal. Klooker et al[60] have investigated the prevalence of IBS in Dutch cohort exposed at the age of 0.5 to 1,5 years to the wartime condition described above and compared it with the prevalence estimated for population conceived after the war had ended; the result was 11% vs 8.5% in the post-war generation. It is not clear what caused IBS more prevalent in the patients exposed; however, animal models point at undernutrition as a probable candidate. In rats subjected to postnatal protein restriction, the growth and mucosal enzyme activity of the GI tract were impaired, what subsequently could have determined functional abnormalities[61].
CONCLUSION
To summarize, the evidence of the involvement of environmental pollution in the development and progression of IBS is very scarce. However, although the role of environmental pollution has not been fully elucidated, available data suggests that it is one of the key factors in IBS pathophysiology. Future research is thus warranted to provide reliable overview of this subject.
UEG scenarios and implication for digestive and liver disease
In October 2014, United European Gastroenterology published a document on Healthcare in Europe: Scenarios and Implication for digestive and liver disease. The main purpose of this release was to draw public attention to digestive diseases, which have become a heavy burden for primary care. Also, the aim of the publication was to raise awareness of current medical school students, who will soon come across this problem in their practice.
Three scenarios were proposed in the publication, namely Ice Age, Golden Age and Silicon Age; each draws a different condition, in which basic and clinical gastroenterology will be in 2040. Here our purpose is to comment on the relation between environmental pollution and functional GI diseases, including IBS according to each scenario.
Ice age
This scenario assumes nature devastation. Economic crisis will lead to negligence in care for environment, which means a higher risk for environmental catastrophes, such as microbiological pollution in Walkerton. In the Ice Age era a significant increase in IBS incidence may be expected. Moreover, unprocessed, healthy and non-polluted alimentation is out of range for middle class, and a widespread antibiotic resistance expands post-infectious IBS toll. An increasing economic gap creates an opportunity for the growth of private healthcare: only the richest receive accurate prevention and only in this group IBS is adequately diagnosed and treated. The underprivileged majority uses public healthcare. Finally, ubiquitous crisis leads to stress pollution, which increases IBS prevalence.
Golden age
In this idealistic scenario, better treatment is provided through better understanding of diseases. The gap between developing and developed countries still exists, but it is diminishing. Environmental protection is thriving, what provides healthy, high-quality and non-polluted food affordable for the majority of human population. Moreover, Europe-wide antibiotic resistance surveillance programs help avoid post-infectious IBS and reduce symptoms from the very beginning, in this way improving general quality of life. Additionally, local food consumption is promoted and chemical and pesticide use is regulated; severity of IBS symptoms is thus lowered. Level-handed politics minimizes everyday-life stress and thus stress-pollution, which also underlie IBS.
Silicon age
It is the most promising scenario. Better tools and procedures ensure better IBS diagnosis and in consequence more IBS cases; however, alleviation of symptoms is more plausible as well, owing to new generation of medications. Finally, more sophisticated technology helps avoid antimicrobial resistance. In this scheme, developing countries overhaul developed countries because of numerous e-medicine solutions. Noteworthy, in this scenario an increase in atomic power usage results in a greater risk of catastrophe; consequently, radioactive caesium (137Cs) leakage, which is a pollutant contributing to IBS development, is likely. On the other hand, automation and overall progress in genetic engineering allow less frequent use of pesticides, what lowers soil pollution with chemicals.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Plaza MA S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN