Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1148
Peer-review started: July 15, 2014
First decision: August 15, 2014
Revised: September 1, 2014
Accepted: September 29, 2014
Article in press: September 30, 2014
Published online: January 28, 2015
Processing time: 196 Days and 20.9 Hours
AIM: To develop a safe and effective agent for cholangiocarcinoma (CCA) chemotherapy.
METHODS: A drug combination experiment was conducted to determine the effects of β-escin in combination with chemotherapy on CCA cells. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay was performed to determine the effects of β-escin and common chemotherapeutics on the proliferation of human CCA cells (QBC939, Sk-ChA-1, and MZ-ChA-1). Immunocytochemistry was used to detect the expression of P-glycoprotein (P-gp) protein. Luciferase reporter assay was used to detect the activation of the Wnt/β-catenin pathway. The protein levels of P-gp, pS9-GSK3β, pT216-GSK3β, GSK3β, β-catenin, and p-β-catenin were further confirmed by western blotting.
RESULTS: The drug sensitivity of QBC939 and QBC939/5-fluorouracil (5-FU) cells to 5-FU, vincristine sulfate (VCR), or mitomycin C was significantly enhanced by β-escin compared with either agent alone (P < 0.05). In addition, the combination of β-escin (20 μmol/L) with 5-FU and VCR was synergic with a combination index < 1. Further investigation found that the mRNA and protein expression of P-gp was down-regulated by β-escin. Moreover, β-escin induced GSK3β phosphorylation at Tyr-216 and dephosphorylation at Ser-9, resulting in phosphorylation and degradation of β-catenin. Interestingly, activation of the GSK3β/β-catenin pathway induced by Wnt3a resulted in up-regulation of P-gp, which was effectively abolished by β-escin, indicating that β-escin down-regulated P-gp expression in a GSK3β-dependent manner.
CONCLUSION: β-escin was a potent reverser of P-gp-dependent multidrug resistance, with said effect likely being achieved via inhibition of the GSK3β/β-catenin pathway and thus suggesting a promising strategy of developing combination drugs for CCA.
Core tip: In our study, we received interesting and challenging results concerning the role of β-escin in reversing the multidrug resistance of cholangiocarcinoma (CCA). β-escin could enhance drug sensitivity of cholangiocarcinoma cells to common chemotherapeutics. 5-Fluorouracil, vincristine sulfate, or mitomycin C significantly reduced cell proliferation when combined with β-escin. In the molecular study, we found that β-escin could down-regulate P-gp expression via inhibiting the activation of GSK3β/β-catenin pathways. This study might offer a possible molecular basis for the further development of combinations of β-escin with common agents as a novel therapeutic approach for multidrug resistant CCA patients.
- Citation: Huang GL, Shen DY, Cai CF, Zhang QY, Ren HY, Chen QX. β-escin reverses multidrug resistance through inhibition of the GSK3β/β-catenin pathway in cholangiocarcinoma. World J Gastroenterol 2015; 21(4): 1148-1157
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1148.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1148
Cholangiocarcinoma (CCA) is the second most common primary hepatobiliary cancer, and originates from the biliary epithelium[1]. Incidence and mortality rates of CCA have been increasing worldwide over time[2], with the incidence rising by 22% between 1979 and 2004, accompanied with a 39% increase in mortality[3]. Surgical resection is the best available and potentially curative therapy for CCA[4]. However, it is difficult to make an early diagnosis of CCA patients[5], as most patients with CCA are not resectable at the time of diagnosis[6], and the results of surgical resection tend to be disappointing due to recurrence[7]. Hence, there is an urgent need for effective therapeutic strategies and drugs to combat this lethal tumor.
The problem of multidrug resistance (MDR), which affects a superfamily of ATP-dependent transporters, is a major obstacle to successful cancer therapy. One of the main reasons for MDR is the over-expression of the membrane pump, an active efflux pump affecting the pharmacokinetic profiles of drugs in cancer cells[8]. P-glycoprotein (P-gp) has been implicated in MDR in the pathogenesis of CCA[9]. Recent evidence that inhibition of P-gp could lead to an avoidance of drug resistance and the elimination of tumor cells[10] underscores the importance of the identification of different potential P-gp-mediated MDR reversal agents (or chemosensitizers) which may lead to strategies for developing improved target-based drugs for CCA therapy. P-gp modulators that have thus been intensively studied as prospective MDR reversers.
The ongoing search for P-gp inhibitors, applied in combination with anticancer drugs, is urgent. Plant-based agents capable of inhibiting P-gp with minimal adverse side effects are being increasingly utilized in drug discovery and development programs[11]. β-escin, the major active compound in extracts from the seeds of the horse chestnut (Aesculus hippocastanum), has shown clinically significant anti-inflammatory activity[12], as well as inhibitory effects on colon cancer, lung cancer, leukemia, CCA, and hepatocellular carcinoma[13-17]. β-escin has received extensive attention because of its potent induction of apoptosis and inhibition of cancer cell growth[13]. Several studies have elucidated that β-escin has a synergistic interaction with chemotherapeutics on cancer cells, such as the synergistic effects of β-escin and 5-FU on human hepatocellular carcinoma[18], β-escin potentiating the antitumor activity of gemcitabine on pancreatic cancer[19], and the synergistic effect of combinations of β-escin and the monoterpenes alpha-pinene, thymol, menthol in HeLa, and Cos7 cells[20]. Compelling evidence has spurred on efforts to search for the mechanisms by which β-escin regulates diverse biological functions.
In this study, we sought to investigate the mechanisms of β-escin that improve the sensitivity of CCA cells to common chemotherapeutics. We found that β-escin suppressed P-gp expression via the inhibition of the GSK3β/β-catenin pathways. Our results may provide a reference for gaining additional insight into the potential synergistic effects of β-escin in combination with chemotherapeutics on CCA cells, and to find a better method for the further development of anticancer drugs.
Vincristine sulfate (VCR), 5-fluorouracil (5-FU), mitomycin C (MMC), cisplatin (CDDP), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and Wnt3a were purchased from Sigma-Aldrich (Indianapolis, IN, United States). β-escin was purchased from Aladdin Chemistry Co (Shanghai, China). RPMI-1640 medium and fetal calf serum were purchased from Gibco (Grand Island, NY, United States). Monoclonal antibodies against β-catenin, p-β-catenin, P-gp, and β-actin were purchased from Santa Cruz Biotechnology (San Jose, CA, United States). Polyclonal GSK3β, pS9-GSK3β, and pT216- GSK3β antibodies were from Abcam Ltd (Cambridge, United Kingdom). Goat anti-rabbit and anti-mouse secondary antibodies conjugated to horseradish peroxidase were purchased from Thermo Scientific Pierce Co. Ltd (Rockford, IL, United States). Polyvinylidene difluoride (PVDF) membranes were from Millipore (Billerica, MA, United States). EliVision Plus Kit was from Maixin Bio (Fuzhou, China). Dual-Luciferase Reporter Assay Kit was from Promega (Madison, WI, United States).
The human CCA cell line QBC939 was kindly provided by Professor Shu-Guang Wang from Southwest Hospital, Third Military Medical University, Chongqing, China. The human cholangiocarcinoma MDR cell line QBC939/5-FU was established in our lab. The cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/mL of ampicillin, and 100 U/mL of streptomycin sulfate at 37 °C in a humidified atmosphere under 5% CO2. The cells were treated with culture medium containing various concentrations of drugs after 24 h seeding.
Cell proliferation was analyzed by MTT method. The cells were seeded at 5 × 103 per well into 96-well plates overnight. After treatment of the cells with a series of concentrations of 5-FU, CDDP, VCR, MMC, and β-escin, alone or in combination for 24 h, 20 μL MTT (5 mg/mL) was added to each well, and the cells were cultured for another 4 h at 37 °C. Formazan crystals were then were dissolved in DMSO. The absorbance was measured at 490 nm using an ELISA microplate reader. The experiment was performed in triplicate.
Combination effect was analyzed using the combination index (CI) method. The CI was defined by the following equation: CI= (OD490)AB/[(OD490)A + (OD490)B], where (OD490)AB was the absorbance of the drug A and B combined treated group, whereas (OD490)A and (OD490)B were that of the drug A or B alone treated group. Parameter CI values > 1 indicated antagonism, CI values = 1 indicated additivity, CI values < 1 indicated synergy, and CI values < 0.7 indicated significant synergy. Each represented CI ratio was the mean value derived from at least three independent experiments.
Total RNA was extracted and reverse transcribed. The PCR primers are as follows:
MRP1, forward primer ATCTCTCCCGACATGACCGA, reverse primer CACACACTAGGGCTACCAGC; MRP2, forward primer CTGCCACTTTGTTTTGAGCA, reverse primer TACAAGGGCCAGCTCTATGG; MRP3, forward primer CTCCAAGTTCTGGGACTCCA, reverse primer CAGGTGGGAGAGGATGATGT; P-gp, forward primer GACATCCCAGTGCTTCAGG, reverse primer GCCACTGAACATTCAGTCG; GAPDH, forward primer CACATGGCCTCCAAGGAGTAAG, reverse primer TGAGGGTCTCTCTCTTCCTCTTGT. Real-time PCR was performed using a fluorescent temperature cycler (LC480 Real Time PCR System, Roche Co., Ltd). GAPDH was used as an internal standard.
Cell lysates containing equal amounts of proteins were separated on 10% SDS-polyacrylamide gels and electrotransferred onto PVDF membranes, which were blocked in 5% milk in PBST (NaCl 137 mmol/L, KCl 2.7 mmol/L, Na2HPO4 10 mmol/L, KH2PO4 2 mmol/L, and 0.05% Tween-20) for 1 h, with the primary indicated antibody then being incubated overnight at 4 °C. After three washes in PBST, blots were incubated with horseradish peroxidase-conjugated secondary antibody and visualized by chemiluminescence. β-Actin was used as an internal control.
QBC939 cells and QBC939/5-FU cells (1.0 × 105 cells/well) were seeded in 24-well plates and incubated overnight before transfection. The cells were co-transfected with reporter plasmid 200 ng pTOP-Flash or pFOP-Flash and 200 ng β-galactosidase (β-gal) using Lipofectamine 2000. TCF-responsive TOP-Flash reporter contains three TCF binding sites and the corresponding FOP-Flash contains three mutated TCF sites[21]. The indicated cells treated with β-escin (20 μmol/L) and Wnt3a (50 ng/mL) alone or together for 4 h were analyzed for luciferase activity using a Dual-Luciferase Reporter Assay System according to the manufacturer’s instructions. Luciferase activity was normalized for transfection efficiency using the corresponding β-gal activity. The experiment was performed in triplicate.
After overnight culture, QBC939 and QBC939/5-FU cells were treated with 20 μmol/L β-escin for 0.5 h, fixed in 4% paraformaldehyde for 15 min, permeabilized with 0.5% Triton X-100 for 20 min, and then incubated with primary P-gp antibody overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated secondary antibody for 30 min at room temperature. Images were collected and analyzed using an inverted fluorescence microscope (Leica, Barcelona, Spain). The experiment was performed in triplicate.
SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. The data were expressed as the mean ± SEM for samples and evaluated by one-way analysis of variance or Kruskal-Wallis tests to compare mean values. Each assay was repeated in triplicate. The level of significance was set at P < 0.05.
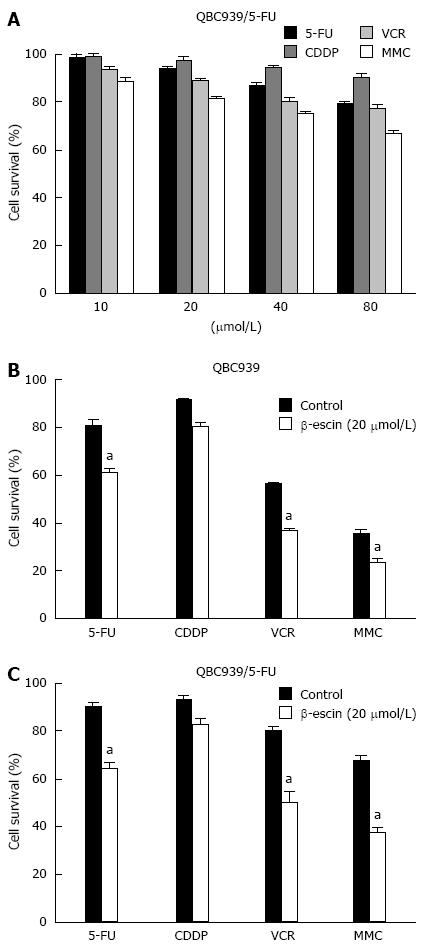
To explore the mechanisms of MDR in CCA, we established a human MDR CCA cell line QBC939/5-FU[22]. The MTT assay was performed after exposure of QBC939/5-FU cells to a series of concentrations of 5-FU, CDDP, VCR, and MMC. MDR tumor cells QBC939/5-FU presented resistant to all chemotherapeutics (Figure 1A). To identify effective treatment for human CCA, our findings showed that β-escin at a concentration of 20 μmol/L showed a significant synergistic effect in combination with 5-FU, VCR, and MMC on CCA cells (P < 0.05) (Figures 1B and C). The effects of β-escin in combination with 5-FU and VCR on QBC939 and QBC939/5-FU cells are further detailed in Tables 1 and 2. The 50% inhibition concentrations (IC50) of QBC939 cells to 5-FU in combination with β-escin (0, 5, 10, and 20 μmol/L) were 114 ± 2.17, 101 ± 1.14, 96 ± 1.54, and 31 ± 1.89 μmol/L, respectively (Table 1). Similarly, the IC50 of QBC939/5-FU cells to 5-FU were 135 ± 2.59, 130 ± 2.75, 99 ± 2.96, and 37 ± 1.32 μmol/L, respectively (Table 1). Significantly, the IC50 of 5-FU in QBC939 and QBC939/5-FU cells was decreased, especially when combined with β-escin (20 μmol/L). Similar results for VCR were showed in Table 2. The IC50 of QBC939 and QBC939/5-FU cells to VCR were 102 ± 1.83, 88 ± 2.47, 72 ± 1.53, 18 ± 1.25 μmol/L, 125 ± 2.37, 104 ± 1.59, 82 ± 2.57, and 35 ± 1.36 μmol/L, respectively. These results indicate that drug sensitivity of QBC939 and QBC939/5-FU cells to 5-FU and VCR was enhanced by β-escin.
| β-escin(μmol/L) | 5-FU(μmol/L) | QBC939 | QBC939/5-FU | ||||
| A490 | CI | IC50 | A490 | CI | IC50 | ||
| 0 | 0 | 1.058 ± 0.073 | 0.998 ± 0.063 | ||||
| 5 | 0 | 1.003 ± 0.112 | 0.992 ± 0.091 | ||||
| 10 | 0 | 0.943 ± 0.088 | 0.894 ± 0.072 | ||||
| 20 | 0 | 0.658 ± 0.033 | 0.653 ± 0.027 | ||||
| 0 | 20 | 0.964 ± 0.023 | 0.997 ± 0.051 | ||||
| 0 | 40 | 0.872 ± 0.067 | 114 ± 2.17 | 0.952 ± 0.071 | 135 ± 2.59 | ||
| 0 | 80 | 0.812 ± 0.089 | 0.882 ± 0.099 | ||||
| 5 | 20 | 0.932 ± 0.014 | 1.0191 | 0.989 ± 0.031 | 1.0002 | ||
| 5 | 40 | 0.852 ± 0.082 | 1.0311 | 101 ± 1.14 | 0.901 ± 0.062 | 0.9523 | 130 ± 2.75 |
| 5 | 80 | 0.798 ± 0.041 | 1.0371 | 0.797 ± 0.026 | 0.9093 | ||
| 10 | 20 | 0.892 ± 0.066 | 1.0381 | 0.851 ± 0.045 | 0.9533 | ||
| 10 | 40 | 0.802 ± 0.072 | 1.0311 | 96 ± 1.54 | 0.747 ± 0.036 | 0.8763 | 99 ± 2.96 |
| 10 | 80 | 0.724 ± 0.018 | 1.0002 | 0.657 ± 0.031 | 0.8323 | ||
| 20 | 20 | 0.595 ± 0.062 | 0.9923 | 0.517 ± 0.041 | 0.7933 | ||
| 20 | 40 | 0.492 ± 0.041 | 0.9073 | 31 ± 1.89 | 0.452 ± 0.020 | 0.6934 | 37 ± 1.32 |
| 20 | 80 | 0.388 ± 0.024 | 0.7683 | 0.331 ± 0.015 | 0.5074 | ||
| β-escin | VCR | QBC939 | QBC939/5-FU | ||||
| (μmol/L) | (μmol/L) | A490 | CI | IC50 | A490 | CI | IC50 |
| 0 | 0 | 1.112 ± 0.093 | 1.005 ± 0.042 | ||||
| 5 | 0 | 0.989 ± 0.089 | 0.988 ± 0.086 | ||||
| 10 | 0 | 0.902 ± 0.064 | 0.933 ± 0.067 | ||||
| 20 | 0 | 0.674 ± 0.044 | 0.712 ± 0.044 | ||||
| 0 | 20 | 0.922 ± 0.071 | 0.992 ± 0.052 | ||||
| 0 | 40 | 0.752 ± 0.053 | 102 ± 1.83 | 0.892 ± 0.035 | 125 ± 2.37 | ||
| 0 | 80 | 0.689 ± 0.032 | 0.743 ± 0.028 | ||||
| 5 | 20 | 0.901 ± 0.045 | 1.0981 | 0.985 ± 0.035 | 1.0101 | ||
| 5 | 40 | 0.687 ± 0.052 | 1.0271 | 88 ± 2.47 | 0.851 ± 0.022 | 0.9613 | 104 ± 1.59 |
| 5 | 80 | 0.614 ± 0.042 | 1.0021 | 0.698 ± 0.017 | 0.9463 | ||
| 10 | 20 | 0.815 ± 0.036 | 1.0891 | 0.901 ± 0.043 | 0.9783 | ||
| 10 | 40 | 0.631 ± 0.026 | 1.0341 | 72 ± 1.53 | 0.784 ± 0.035 | 0.9463 | 82 ± 2.57 |
| 10 | 80 | 0.556 ± 0.024 | 0.9953 | 0.602 ± 0.011 | 0.8723 | ||
| 20 | 20 | 0.559 ± 0.025 | 1.0002 | 0.605 ± 0.021 | 0.8613 | ||
| 20 | 40 | 0.368 ± 0.011 | 0.8073 | 18 ± 1.25 | 0.499 ± 0.009 | 0.7893 | 35 ± 1.36 |
| 20 | 80 | 0.289 ± 0.008 | 0.6924 | 0.326 ± 0.010 | 0.6194 | ||
CI analysis was performed to determine the effect of β-escin in combination with 5-FU or VCR. The results of β-escin in combination with 5-FU or VCR on QBC939 and QBC939/5-FU cells are showed in Tables 1 and 2. The CIs of β-escin (20 μmol/L) in combination with 5-FU (20, 40, 80 μmol/L) on QBC939 and QBC939/5-FU cells were 0.992, 0.907, 0.768, 0.793, 0.693, and 0.507, respectively, indicating synergistic interactions (0.7 < CI < 1) and significantly synergistic interactions (CI < 0.7). However, the CIs of β-escin (5, 10 μmol/L) were mostly greater than 1, which showed an antagonistic interaction (Table 1). Likewise, the effects of β-escin (20 μmol/L) in combination with VCR were also synergistic interactions and significantly synergistic interactions, which were better than that of β-escin (5, 10 μmol/L) (Table 2). Together, these results demonstrated that a combination of β-escin with chemotherapeutics might be an effective therapy for CCA.
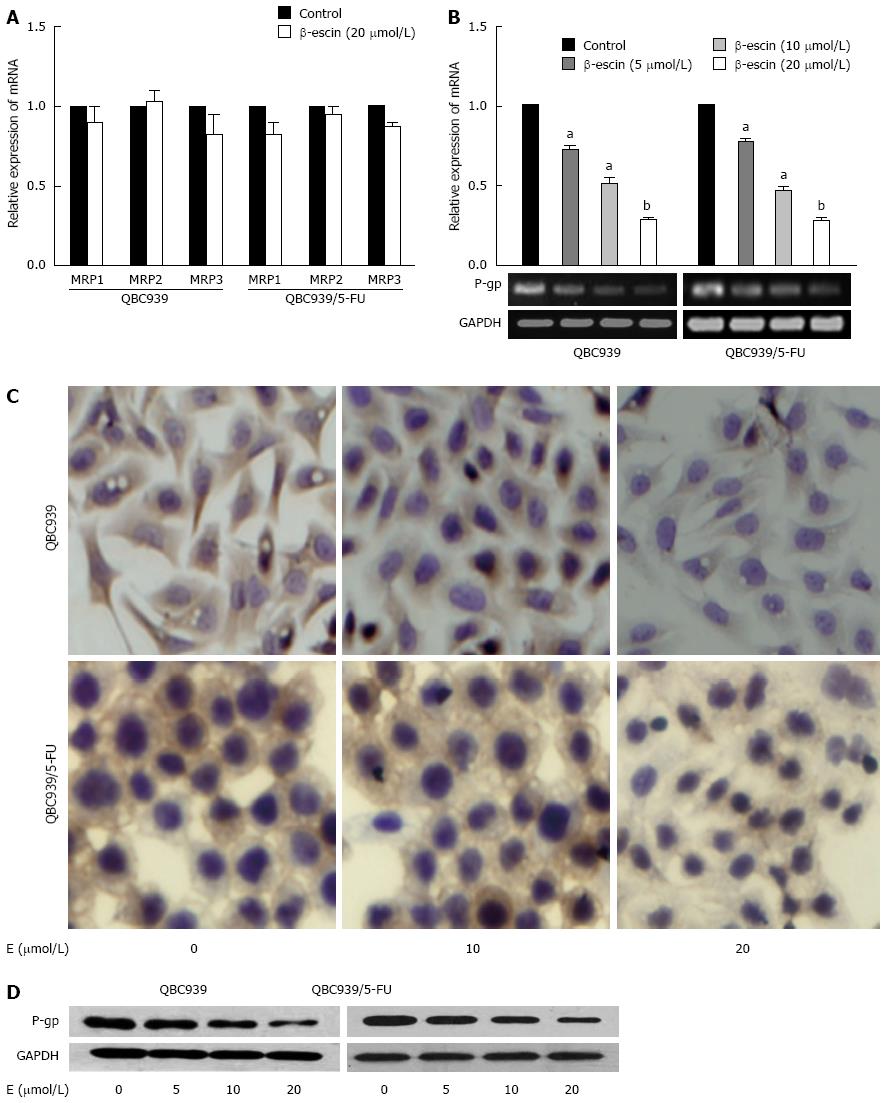
To investigate the mechanisms that cause β-escin to enhance the drug sensitivity of CCA cells to common chemotherapeutics, real-time PCR, immunocytochemistry (ICC), and western blot were performed to assess the expression of MDR-associated genes in QBC939 and QBC939/5-FU cells after treatment with various concentrations of β-escin (0, 5, 10, and 20 μmol/L) for 24 h. As shown in Figure 2A, there was no significant change in MRP1, MRP2, or MRP3 mRNA expression after β-escin treatment, but a significant decrease was found in P-gp mRNA expression (Figure 2B). In addition, results of ICC show that β-escin reduced the expression of P-gp in a dose-dependent manner (Figure 2C) consistent with the suppressed protein expression detected by western blot (Figure 2D). These results indicated that β-escin could down-regulate the expression of P-gp in QBC939 and QBC939/5-FU cells.
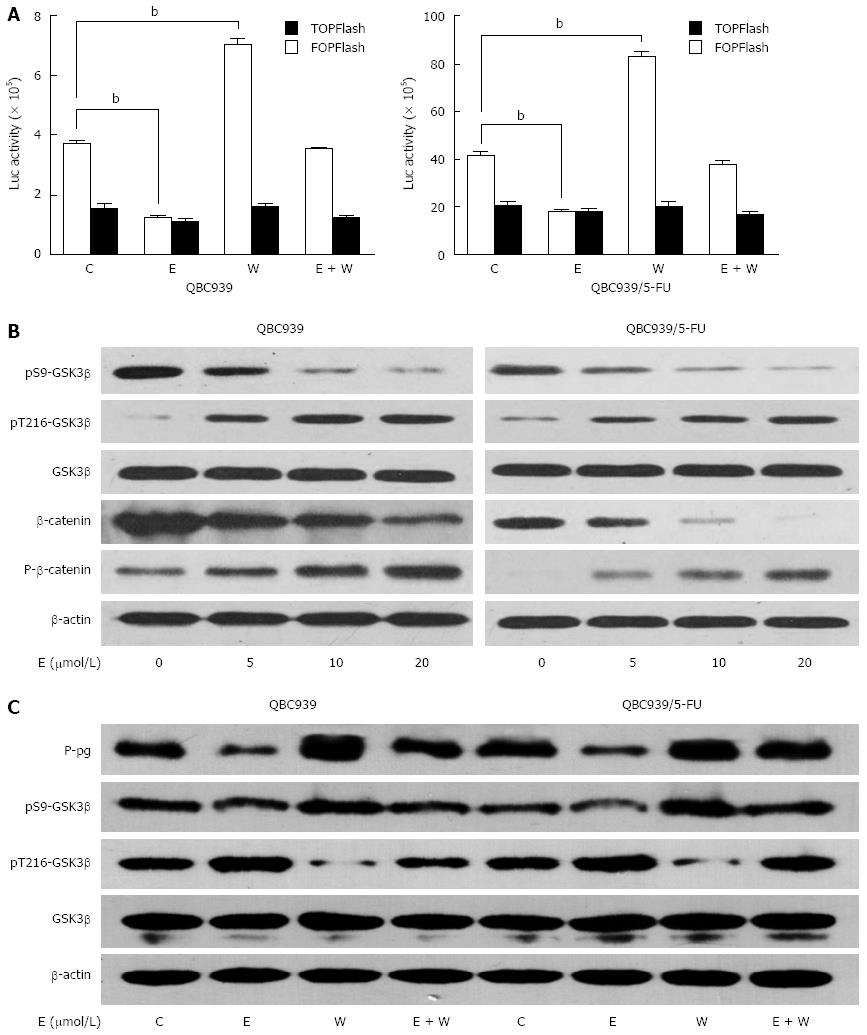
We recently reported that P-gp might be a downstream target gene of the Wnt/β-catenin pathway in CCA cells[23], so we evaluated whether down-regulation of P-gp induced by β-escin was associated with the inhibition of said pathway. Luciferase reporter assays showed that activation of the Wnt/β-catenin pathway was remarkably enhanced by Wnt3a treatment and significantly reduced by β-escin on both QBC939 and QBC939/5-FU cells. Moreover, Wnt3a-induced activation of the Wnt/β-catenin pathway was almost completely prevented by β-escin (Figure 3A). In an effort to study the precise regulation of β-escin on Wnt/β-catenin signaling, we examined the expression levels of related proteins in this pathway. As shown in Figure 3B, the levels of β-catenin phosphorylation and degradation were increased after β-escin treatment. In addition, the level of phosphorylation at Ser-9 was decreased and dephosphorylation at Tyr-216 was increased, indicating that β-escin inhibited the activation of the GSK3β/β-catenin pathway in both QBC939 and QBC939/5-FU cells.
We further studied whether β-escin-induced P-gp down-regulation was GSK3β dependent. Western blot analysis showed that β-escin led to phosphorylation in Tyr-216 of GSK3β and dephosphorylation in Ser-9 of GSK3β, as well as down-regulation of P-gp (Figure 3C). In contrast, Wnt3a led to phosphorylation in Ser-9, dephosphorylation in Tyr-216 of GSK3β, and up-regulation of P-gp. Interestingly, co-treatment of cells with β-escin almost completely suppressed the increase of P-gp induced by Wnt3a. Similar changes were seen in the protein expression of pS9-GSK3β with β-escin and Wnt3a treatment. These data suggested that β-escin-induced P-gp down-regulation was GSK3β dependent.
An increase in basic research on CCA has been seen in recent years. However, there is still a notable lack of an effective targeted therapy for CCA patients because of MDR[7,22,24]. Overexpression of ATP-binding cassette transporters of the MDR protein and MDR-related protein (MRP) family in cancer cells is a major cause of multidrug resistance[24]. P-gp, intrinsically over-expressed in many neoplasms, including the majority of carcinomas arising in the colon, rectum, pancreas, liver, kidneys, and bile duct[22,24], is known to play a pivotal role in the development of MDR, which leads to the failure of chemotherapy in numerous cancers, including CCA[10]. Due to the complex pathological mechanisms of CCA, drug combination treatments have received extensive attention. In an effort to research a series of potent and efficacious P-gp-dependent MDR reversers, we have undertaken the screening of common chemotherapeutics, and found that β-escin combined with 5-FU, VCR, or MMC produced remarkable inhibitory effects in CCA cells. The IC50 of 5-FU and VCR in combination with β-escin were reduced significantly, showing a synergistic effect. Due to the combination of β-escin and other drugs (such as 5-FU and VCR) being particularly efficacious on CCA cells, we speculated that β-escin might decrease the expression of the tumor’s drug-resistant protein P-gp, thus allowing another kind of drug to enter the cell and prevent the tumor from adapting its microenvironment. Furthermore, in agreement with our previous studies that P-gp expression was up-regulated in MDR CCA cell line QBC939/5-FU[22], we demonstrated that β-escin reversed MDR of human CCA cell line QBC939 and QBC939/5-FU at least partly via the down-regulation of P-gp.
Overcoming MDR is broadly known to be effective in cancer therapy. β-escin, obtained via its isolation from the seed of the horse chestnut[25], played an important role in circumventing drug resistance in drug combination on CCA cells. However, the molecular mechanisms of β-escin on down-regulating P-gp expression remain unclear. It has been confirmed that the PI3K/Akt, NF-κB, Wnt/β-catenin, and ERK signal pathways are associated with P-gp expression in cancer[26,27]. In our previous study, we found that the role of the Wnt/β-catenin pathway has been implicated in chemoresistance in CCA[22]. An important finding of the present study is that the Wnt/β-catenin pathway was involved in the β-escin-induced down-regulation of P-gp on CCA cells. Aberrant regulation of Wnt/β-catenin signaling has been shown to cause a wide spectrum of diseases, and especially tumors[28-31]. It is very clear that the activation of β-catenin through the canonical Wnt pathway plays a role in a number of human cancers, including CCA[23]. When stable, non-phosphorylated β-catenin migrates from the cytoplasm to the nucleus, where it interacts with T-cell factor/lymphoid enhancer factor that binds to the promoters of downstream target genes, recruiting transcriptional activators[30,32,33]. Our results showed that β-escin suppressed Wnt3a-induced β-catenin-mediated expression of P-gp. Therefore, β-escin might represent a lead for classes of anticancer agents targeting the Wnt/β-catenin pathway for CCA therapy.
GSK3β, a “destruction complex”[34] and key mediator of the Wnt/β-catenin pathway, suppresses tumor progression by down-regulating the Wnt survival pathway through the phosphorylation and degradation of β-catenin[35]. The phosphorylation in Ser-9 of GSK3β inhibits its activity[36]. In contrast to ser-9 phosphorylation, phosphorylation of GSK3β on Tyr-216 increases its activity[37]. Activated GSK3β phosphorylates and degrades β-catenin[35]. We found that β-escin activated GSK3βvia phosphorylation of Tyr-216 and simultaneous dephosphorylation of Ser-9 and, paralleled with a decreasing accumulation of free cytoplasmic β-catenin, consequently led to the down-regulation of P-gp expression. Additionally, expression of P-gp was up-regulated after Wnt3a treatment, which is an activator of the GSK3β/β-catenin pathway. Conversely, down-regulation of P-gp expression induced by β-escin was consistent with the inhibition of the GSK3β/β-catenin pathway. Upregulation of P-gp expression induced by Wnt3a could be suppressed by β-escin, providing insight into the mechanism of GSK3β-mediated P-gp regulation.
Taken together, our study has demonstrated that β-escin can sensitize CCA cells to common chemotherapeutics, and also clarified that β-escin could inhibit P-gp expression through the inhibition of the GSK3β/β-catenin pathways. This study might offer a possible molecular basis for the further development of combinations of β-escin and common agents as a novel therapeutic approach for CCA patients.
Cholangiocarcinoma (CCA), a chemoresistant bile duct carcinoma which results in a poor prognosis, is resistant to all currently available chemotherapeutics because of its multidrug resistance (MDR). A safe and effective agent for CCA chemotherapy is therefore urgently needed.
β-escin could enhance the drug sensitivity of cholangiocarcinoma cells to common chemotherapeutics. Furthermore, β-escin could down-regulate P-gp expression via inhibiting the activation of GSK3β/β-catenin pathways. The results may provide a reference for additional insight into the potential synergistic effects of β-escin in combination with chemotherapeutics on CCA cells.
Overcoming MDR is broadly known to be effective in cancer therapy. β-escin, isolated from the seed of the horse chestnut, plays an important role in circumventing drug resistance in drug combination on CCA cells. An important finding of the present study is that the Wnt/β-catenin pathway was involved in the β-escin-induced down-regulation of P-gp on CCA cells. This study has demonstrated that β-escin can sensitize CCA cells to common chemotherapeutics, and also clarified that β-escin could inhibit P-gp expression through the inhibition of the GSK3β/β-catenin pathways.
β-escin was a potent reverser of P-gp-dependent MDR, and this effect was likely through the inhibition of the GSK3β/β-catenin pathway, suggesting a promising strategy of developing combination drugs for CCA.
β-escin, the major active compound in extracts of the horse chestnut (Aesculus hippocastanum) seed, has shown clinically significant anti-inflammatory activity, as well as inhibitory effects on colon cancer. MDR belonging to a superfamily of ATP-dependent transporters, is the major obstacle to a successful cancer therapy.
This is a comprehensive manuscript with fine structure which adequately highlights the role of β-escin in cholangiocarcinoma. To date, there is no sufficiently adequate study concerning this subject. Usually this disease is already at the advanced stage by the time of diagnosis, and thus responds poorly to chemotherapy. Genetic study of the tumor and research into inhibitors of pathways make up the future of targeted therapy.
| 1. | Ieta K, Tanaka F, Utsunomiya T, Kuwano H, Mori M. CEACAM6 gene expression in intrahepatic cholangiocarcinoma. Br J Cancer. 2006;95:532-540. [PubMed] |
| 2. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 855] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 3. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655-1667. [PubMed] |
| 5. | Vickers SM, Jhala NC, Ahn EY, McDonald JM, Pan G, Bland KI. Tamoxifen (TMX)/Fas induced growth inhibition of human cholangiocarcinoma (HCC) by gamma interferon (IFN-gamma). Ann Surg. 2002;235:872-878. [PubMed] |
| 6. | Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Liu ZH, Ma YL, He YP, Zhang P, Zhou YK, Qin H. Tamoxifen reverses the multi-drug-resistance of an established human cholangiocarcinoma cell line in combined chemotherapeutics. Mol Biol Rep. 2011;38:1769-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Takara K, Sakaeda T, Okumura K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr Pharm Des. 2006;12:273-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Liu ZH, He YP, Zhou Y, Zhang P, Qin H. Establishment and identification of the human multi-drug-resistant cholangiocarcinoma cell line QBC939/ADM. Mol Biol Rep. 2011;38:3075-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Li X, Li JP, Yuan HY, Gao X, Qu XJ, Xu WF, Tang W. Recent advances in P-glycoprotein-mediated multidrug resistance reversal mechanisms. Methods Find Exp Clin Pharmacol. 2007;29:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Curr Med Chem. 2012;19:1946-2025. [PubMed] |
| 12. | Sirtori CR. Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol Res. 2001;44:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Shen DY, Kang JH, Song W, Zhang WQ, Li WG, Zhao Y, Chen QX. Apoptosis of human cholangiocarcinoma cell lines induced by β-escin through mitochondrial caspase-dependent pathway. Phytother Res. 2011;25:1519-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Patlolla JM, Raju J, Swamy MV, Rao CV. Beta-escin inhibits colonic aberrant crypt foci formation in rats and regulates the cell cycle growth by inducing p21(waf1/cip1) in colon cancer cells. Mol Cancer Ther. 2006;5:1459-1466. [PubMed] |
| 15. | Ji DB, Xu B, Liu JT, Ran FX, Cui JR. β-Escin sodium inhibits inducible nitric oxide synthase expression via downregulation of the JAK/STAT pathway in A549 cells. Mol Carcinog. 2011;50:945-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Tan SM, Li F, Rajendran P, Kumar AP, Hui KM, Sethi G. Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2010;334:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Niu YP, Wu LM, Jiang YL, Wang WX, Li LD. Beta-escin, a natural triterpenoid saponin from Chinese horse chestnut seeds, depresses HL-60 human leukaemia cell proliferation and induces apoptosis. J Pharm Pharmacol. 2008;60:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Ming ZJ, Hu Y, Qiu YH, Cao L, Zhang XG. Synergistic effects of beta-aescin and 5-fluorouracil in human hepatocellular carcinoma SMMC-7721 cells. Phytomedicine. 2010;17:575-580. [PubMed] [DOI] [Full Text] |
| 19. | Wang YW, Wang SJ, Zhou YN, Pan SH, Sun B. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-κB and nuclear factor-κB-regulated gene products in pancreatic cancer both in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138:785-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Herrmann F, Wink M. Synergistic interactions of saponins and monoterpenes in HeLa cells, Cos7 cells and in erythrocytes. Phytomedicine. 2011;18:1191-1196. [PubMed] [DOI] [Full Text] |
| 21. | Xu S, Gotlieb AI. Wnt3a/β-catenin increases proliferation in heart valve interstitial cells. Cardiovasc Pathol. 2013;22:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Shen DY, Zhang W, Zeng X, Liu CQ. Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013;104:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Huang GL, Luo Q, Rui G, Zhang W, Zhang QY, Chen QX, Shen DY. Oncogenic activity of retinoic acid receptor γ is exhibited through activation of the Akt/NF-κB and Wnt/β-catenin pathways in cholangiocarcinoma. Mol Cell Biol. 2013;33:3416-3425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Molnár J, Engi H, Hohmann J, Molnár P, Deli J, Wesolowska O, Michalak K, Wang Q. Reversal of multidrug resitance by natural substances from plants. Curr Top Med Chem. 2010;10:1757-1768. [PubMed] |
| 25. | Xin W, Zhang L, Fan H, Jiang N, Wang T, Fu F. Escin attenuates acute lung injury induced by endotoxin in mice. Eur J Pharm Sci. 2011;42:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe S, Mills GB, Unate H. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene. 2002;21:1945-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Zhang H, Zhang X, Wu X, Li W, Su P, Cheng H, Xiang L, Gao P, Zhou G. Interference of Frizzled 1 (FZD1) reverses multidrug resistance in breast cancer cells through the Wnt/β-catenin pathway. Cancer Lett. 2012;323:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Monga SP. Role of Wnt/β-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2011;43:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006;7:41-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469-480. [PubMed] |
| 33. | Rao TP, Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798-1806. [PubMed] |
| 34. | Gwak J, Hwang SG, Park HS, Choi SR, Park SH, Kim H, Ha NC, Bae SJ, Han JK, Kim DE. Small molecule-based disruption of the Axin/β-catenin protein complex regulates mesenchymal stem cell differentiation. Cell Res. 2012;22:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Lang UE, Kocabayoglu P, Cheng GZ, Ghiassi-Nejad Z, Muñoz U, Vetter D, Eckstein DA, Hannivoort RA, Walsh MJ, Friedman SL. GSK3β phosphorylation of the KLF6 tumor suppressor promotes its transactivation of p21. Oncogene. 2013;32:4557-4564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3826] [Cited by in RCA: 4087] [Article Influence: 131.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Vasilieva LE S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Wang CH