Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1125
Peer-review started: May 11, 2014
First decision: June 10, 2014
Revised: July 17, 2014
Accepted: August 13, 2014
Article in press: August 28, 2014
Published online: January 28, 2015
Processing time: 266 Days and 1.3 Hours
AIM: To examine the effect of Weichang’an (WCA) and 5-fluorouracil (5-FU) on colorectal tumor and hepatic metastasis in a mouse model.
METHODS: Quantitative determination of hesperidin, the effective component in WCA decoction, was performed using HPLC. In vitro cytotoxicity of WCA was determined by treating HCT-116 cells with WCA diluents or serum extracted from rats that received WCA by oral gavage for 1 wk and MTT assays. Forty-eight nude mice received cecum implantation with tumor blocks subcutaneously amplified from human colon cancer cell line HCT-116. Mice were randomly divided into four treatment groups: control (CON), WCA, 5-FU and combination (WCA + 5-FU). Pathological examination of tumors consisted of tissue sectioning and hematoxylin and eosin staining. Tumor weight and size were measured, and the number of metastatic lesions was counted. Serum carcinoembryonic antigen (CEA) level was determined by ELISA. The expression levels of tumor genesis and metastasis-related proteins β-catenin and matrix metalloproteinase (MMP)-7 were measured by real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR), immunohistochemistry and immunoblotting. Cell fractionation was used to investigate intracellular distribution of β-catenin.
RESULTS: Parenchymal tumors were palpable in the abdomens of nude mice 2 wk post-implantation and orthotopic tumor formation rate was 100% in all groups. 5-FU treatment alone significantly decreased tumor weight compared to the CON group (1.203 ± 0.284 g vs 1.804 ± 0.649 g, P < 0.01). WCA treatment alone reduced the rate of metastasis (50% vs 100%, P < 0.05). Combination treatment of WCA + 5-FU was the most effective, reducing the tumor weight (1.140 ± 0.464 g vs 1.804 ± 0.649 g, P < 0.01) and size (1493.438 ± 740.906 mm3vs 2180.259 ± 816.556 mm3, P < 0.05), the rate of metastases (40% vs 100%, P < 0.01), and serum CEA levels (31.263 ± 7.421 μg/L vs 43.040 ± 11.273 μg/L, P < 0.05). Expression of β-catenin and MMP-7 was decreased in drug-treated groups compared to controls, as detected using real-time quantitative RT-PCR, immunohistochemistry and immunoblotting, respectively. Cell fractionation assays revealed that nuclear translocation of β-catenin was reduced by WCA and/or 5-FU treatments.
CONCLUSION: Combination of WCA with 5-FU significantly inhibited colon tumor growth and hepatic metastases. Decreased expression of β-catenin and MMP-7 may be important.
Core tip: In this study, the anti-colon cancer activity of Weichang’an (WCA), a Chinese herbal medicine, was assessed in an orthotopic transplantation nude mouse model. Combination of WCA with 5-fluorouracil inhibited orthotopic tumor growth and hepatic metastases. Decreased expression of β-catenin and metalloproteinase-7, which are crucial proteins modulating tumor aggression, may be important for the anti-tumor properties of WCA.
- Citation: Tao L, Yang JK, Gu Y, Zhou X, Zhao AG, Zheng J, Zhu YJ. Weichang’an and 5-fluorouracil suppresses colorectal cancer in a mouse model. World J Gastroenterol 2015; 21(4): 1125-1139
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1125.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1125
Colorectal cancer is the third most commonly diagnosed cancer and the third most common cause of cancer-related death worldwide. Approximately one million new cases of colorectal cancer are diagnosed in both men and women around the world each year[1]. It is estimated that 50%-60% of patients diagnosed with colorectal cancer will develop hepatic metastases during the course of their disease[2-4], and unfortunately, 80%-90% of these metastases will be unresectable[5-7]. While metastatic lesions may be found throughout the body following locoregional treatments, the most frequent site of colorectal cancer metastasis is the liver[8]. The 5-year survival rate of patients with hepatic metastases and not undergoing surgical treatment is low[3,9]. Patients with unresectable hepatic metastases require multidisciplinary treatments, including surgery, chemotherapy, liver-targeting therapies, and traditional Chinese medicine (TCM). TCM has been used extensively in China for treatment of various diseases, including cancer. TCMs have a number of benefits, including decreasing the negative side effects associated with chemotherapy and radiotherapy, improving patients’ immune function, and enhancing the effects of conventional cancer treatments[10-12].
Weichang’an (WCA) is a traditional Chinese formula prescribed by practitioners of TCM, based on clinical experiences and pharmaceutical screening. The principal elements comprising WCA are invigorating spleen herbs, whereas heat-clearing and detoxicating herbs, hard lump-resolving herbs and blood stasis removing herbs are adjuvant components. Previous clinical studies have indicated that patients with gastric carcinoma benefit from WCA treatment[13,14]. In addition, WCA has been shown to increase the 5-year survival rate and reduce the 1- and 2-year metastatic rates in patients with colorectal cancer[15]. However, whether WCA is effective in colorectal cancer with hepatic metastasis is still unclear.
Many studies have shown that the Wnt/β-catenin signaling pathway regulates tumor cell invasion and metastasis. In oral squamous cell carcinoma cells, the accumulation of β-catenin in the cytoplasm induced T-cell factor/lymphoid enhancing factor transcriptional activity, and increased matrix metalloproteinase (MMP)-7 expression, thereby inducing the conversion of epithelial cells to mesenchymal cells, as well as enhancing invasion and metastasis[16]. The Wnt/β-catenin pathway also plays a critical role in colorectal cancer development[17]. We hypothesized that WCA may affect the Wnt/β-catenin pathway.
To elucidate the efficacy of WCA in inhibiting colorectal cancer cell growth and hepatic metastasis and underlying mechanisms, we administrated WCA combined with 5-fluorouracil (5-FU) in an orthotopic transplant nude mouse model grafted with HCT-116 human colon cancer cells. The pathological phenotype, tumor genesis, metastatic lesions, carcinoembryonic antigen (CEA) levels and β-catenin/MMP-7 expression were detected.
WCA was provided by the Dispensary of TCM, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China. The composition of WCA includes Pseudostellaria heterophylla (Miq.) Pax, Atractylodes macrocephala koidz., Poria cocos (Schw.) Wolf, Glycyrrhiza uralensis Fisch., Sargentodoxa cuneata, and Prunella vulgaris L. The preparation of the WCA decoction has been described previously[13,14]. The concentration of hesperidin (National Institutes for Food and Drug Control, Beijing, China) in the WCA formula was determined by HPLC (Agilent 1100; Palo Alto, CA, United States) using the following conditions: Agilent 1100 C18 column (250 mm × 4.6 mm, 5 μm); mobile phase, acetonitrile:water 8% HAC (18:82); wavelength, 284 nm; flow rate, 1 mL/min; and column temperature, 15 °C. 5-FU was purchased from Shanghai Xudong Haipu Pharmaceutical Co. Ltd. (Shanghai, China). In the present study, 5-FU was used at a concentration of 2.5 mg/mL.
Male BALB/C-nu/nu mice, aged 4-6 wk and weighing 18-20 g, were purchased from the Shanghai SLAC Laboratory Animal Co. Ltd. [Shanghai, China; certification NO. SCXK (Shanghai) 2012-0002]. Mice were housed under specific pathogen-free conditions and were given free access to food and water. All animal experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals, Ministry of Science and Technology, China. This study was approved by the Animal Care and Scientific Committee of the Shanghai University of traditional Chinese medicine.
The human colon cancer cell line HCT-116 was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in McCoy’s 5A modified medium (Gibco, Carlsbad, CA, United States) containing 10% heat-inactivated fetal bovine serum and supplemented with penicillin (100 U/mL) and streptomycin (100 g/mL). Cells were maintained at 37 °C and 5% CO2.
During the logarithmic growth phase, HCT-116 cells were conventionally digested, after which the cells were seeded into a 96-well plate at a density of 2 × 104 cells/mL. The control group was set, and the cells were cultured at 37 °C in a humidified atmosphere of 5% CO2 in air. The medium was discarded when cells had reached 70%-80% confluence. Medium containing 0, 3%, 6% and 9% WCA decoction or 5%, 10% and 20% of serum extracted from rats that received WCA by oral gavage (OG) (2 mL/d for 1 wk) was added to each well (100 μL/well). Each condition was tested in quadruplicate, and the cells were treated for 0, 24, 48 and 72 h. After treatment, 20 μL MTT reagent (Sigma, St Louis, MO, United States) was added to each well for 4 h. The culture medium was discarded, and 150 μL dimethyl sulfoxide (Sigma) was added. Formazan was dissolved by gentle agitation for 10 min. Optical density (OD) for each well was determined using a plate reader (Synergy2; Bio-tek, Winooski, VT, United States) at a wavelength of 490 nm. All experiments were performed three times.
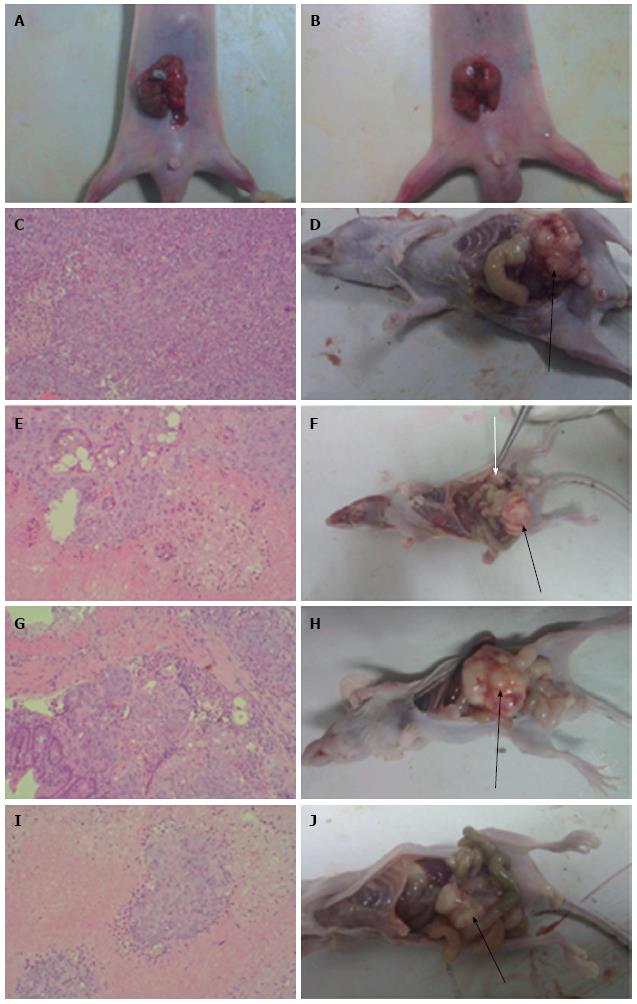
HCT-116 cells (2 × 107) were subcutaneously injected into the right axilla of nude mice. After 3 wk, tumors were excised and cut into 1-2 mm3 pieces using sterile techniques. Tumor blocks were transplanted into the cecum of 48 nude mice by a purse-string suture (Figure 1A and 1B). Drug administration started 7 d after tumor implantation. The 48 nude mice were randomly divided into four treatment groups (12 mice/group). Mice in the control (CON) group received saline by OG (0.5 mL/d) and by intraperitoneal (IP) injection (0.4 mL/time, once/week). Mice in the WCA group received WCA by OG (0.5 mL/d) and saline by IP injection (0.4 mL/time, once/week). Mice in the 5-FU group were given saline by OG (0.5 mL/d) and 5-FU by IP injection (50 mg/kg per time, once/week). Mice in the WCA + 5-FU group received WCA by OG (0.5 mL/d) and 5-FU by IP injection (50 mg/kg per time, once/week). All treatments lasted for 7 wk. Twenty-four hours after the final drug administration, laparotomy was performed and blood samples were collected from each animal. Tumors (both orthotopic and metastatic) and tumor-adjacent tissues were collected, and conventional pathological examination, immunohistochemistry (IHC), real-time polymerase chain reaction (PCR), and immunoblotting assays were performed.
Weights of the orthotopic tumors were measured using an electronic platform balance. The width (a) and length (b) of each orthotopic tumor were measured with a caliper, and tumor size was calculated according to the following formula: tumor size (mm3) = π×a2×b/6. Inhibition of tumor growth caused by drug treatment was estimated based on the following formula: inhibition rate of tumor (%) = (1 - mean tumor weight in treatment group/mean tumor weight in CON group) × 100%.
Orthotopic tumors, tumor-adjacent tissues, and metastatic tumors were embedded in paraffin and cut into sections. These sections were then stained with hematoxylin and eosin (HE; Sigma) and visualized under a BH2 optical microscope (Olympus, Tokyo, Japan).
Blood samples obtained from mice and were naturally coagulated for 20 min after collection. Samples were centrifuged at 1000 g for 10 min, and the supernatant was collected and stored at -20 °C. Serum CEA levels were determined using a CEA ELISA kit (Bio-Swamp, Wuhan, China) according to the manufacturer’s instructions. Absorbance was measured at 450 nm on an MK3 microplate reader (Thermo Fisher Scientific, Waltham, MA, United States).
Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA, United States), and the concentration and purity of RNA were determined using a UV spectrophotometer. Total RNA was reverse-transcribed into cDNA with reverse transcriptase reagents (Shanghai Jierdun Biotech Co. Ltd., Shanghai, China) according to the manufacturer’s protocol. An ABI Step One Plus Real-Time-PCR System (Applied Biosystems, Foster City, CA, United States) was used with SYBR Green Master Mix (ABI) and primers (Invitrogen, Shanghai, China) for measurement of β-catenin and MMP-7. Primers were: β-catenin sense (5’-GGT TTC CCA TTG GTT CAC-3’) and antisense (5’-CAT AAA TCC CGC CTA ACG-3’); and MMP-7 sense (5’-GAA CAG GCT CAG GAC TAT CTC-3’) and antisense (5’-ACA TCT GGC ACT CCA CAT C-3’). GAPDH was used as a reference to obtain the relative fold change for target genes using the comparative Ct method. Primer sequences for GAPDH were: sense (5’-CGG AGT CAA CGG ATT TGG TCG TAT-3’) and antisense (5’-AGC CTT CTC CAT GGT GGT GAA GAC-3’). Relative β-catenin and MMP-7 expression were estimated using the 2-ΔΔCT method.
We performed IHC on orthotopic and metastatic tumors to determine protein expression levels of both β-catenin and MMP-7. The following primary antibodies were used: rabbit monoclonal anti-β-catenin antibody (diluted 1:200, rabbit IgG1; ab79089, Abcam, Cambridge, United Kingdom) and anti-MMP-7 antibody (diluted 1:200, rabbit IgG1; ab4044, Abcam, Cambridge, United Kingdom). The secondary antibody used was a biotinylated goat anti-rabbit IgG (Beyotime Institute of Biotechnology, Shanghai, China). Sections were visualized with diaminobenzidine (DAB; Shanghai Jierdun Biotech Co. Ltd., Shanghai, China) and counterstained with HE. IHC images were captured with a digital camera (Nikon, Tokyo, Japan) and analyzed using the IMS imaging processing system (Shanghai Jierdun Biotech). Positively stained regions were counted and analyzed.
Cells were fractionated into cytosolic and nuclear fractions, with little modification[18]. Cells from both orthotopic tumor tissues and hepatic metastatic cancer tissues were collected, washed with PBS and centrifuged at 500 ×g. Pelleted cells were resuspended in ice-cold 0.1% NP40-PBS and lysed by pipetting up and down several times. A portion of the cell suspension was kept as a whole cell lysate. The cell lysates were centrifuged at 14000 ×g for 1 min and the supernatants were collected as a cytosolic fraction, while the pellets (nuclei) were washed twice with ice-cold 0.1% NP40-PBS. The harvested pellets were resuspended in Laemmli sample buffer (Bio-Rad), sonicated for 30 s, and collected as nuclear fractions. Equivalent proportions of two fractions were analyzed by SDS-PAGE and immunoblotting. The purity of the fractions was assessed by detecting specific subcellular marker proteins such as GAPDH as cytosolic protein and H3 as nuclear protein.
Total protein was extracted from both orthotopic tumor tissues and hepatic metastatic cancer tissues, and protein concentration was determined using the BCA protein assay kit (Thermo Fisher Scientific). Immunoblotting was performed with the following primary antibodies: rabbit monoclonal anti-β-catenin antibody (diluted 1:1000, rabbit IgG1; ab79089, Abcam, Cambridge, MA, United States) and anti-MMP-7 antibody (diluted 1:300, rabbit IgG1; ab4044, Abcam, Cambridge, MA, United States). Anti-rabbit horseradish-peroxidase-conjugated secondary antibody was used at a dilution of 1:2000. Detection of GAPDH [diluted 1:1500; Cell Signaling Technology (CST), Beverly, MA, United States] and H3 (diluted 1:1000, #4499S, CST) served as internal loading controls. All blots were scanned with the Labwork imaging processing system. Protein band pixels were calculated using Gel-pro Analyzer 4.0 (Media Cybernetics, Rockville, MD, United States).
Continuous data are expressed as mean ± standard deviation (SD) and comparison of the means among four groups was performed using one-way analysis of variance (ANOVA). If Levene’s test revealed homogeneity of variance, multiple comparisons were performed using Fisher’s least significant difference test. If the Levene’s test revealed heterogeneity of variance, the Welch and Brown-Forsythe tests were used. In this case, multiple comparisons were performed using Dunnett’s T3 and Dunnett’s C tests. Data with small sample size are expressed as mean ± SD and comparisons among four groups were performed using Kruskal-Wallis tests. Nemenyi tests were further used to perform multiple comparisons. Categorical data were analyzed using χ2 tests. R 3.1 software was used to perform Nemenyi tests and other statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, United States). P < 0.05 were considered significant.
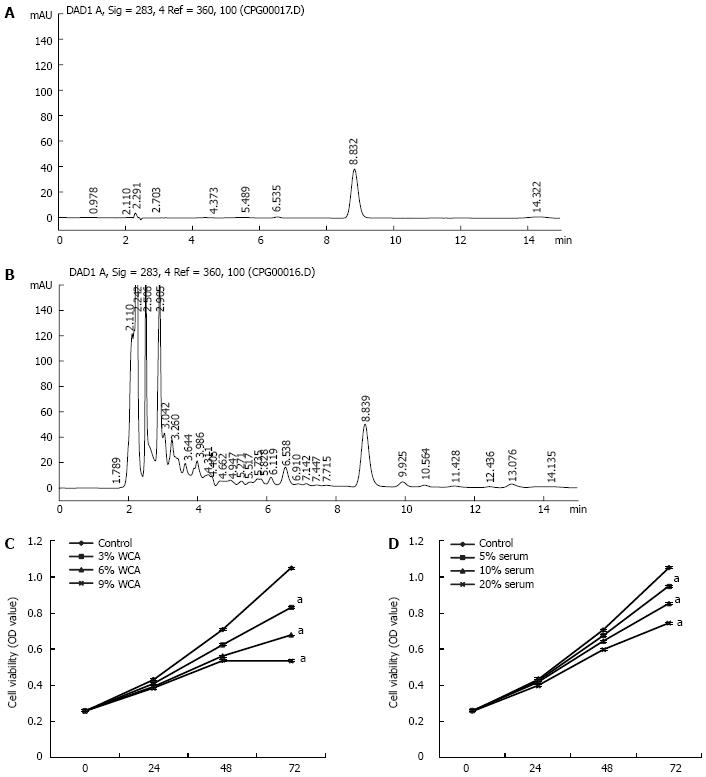
To normalize the concentration of WCA, the concentration of hesperidin in WCA was determined by HPLC and a standard curve (y = 1639.7x + 9.1343; correlation coefficient r = 0.9990) was obtained with the peak area as the Y axis and concentration of control hesperidin as the X axis. HPLC fingerprinting of control hesperidin was performed at 8.832 min (Figure 2A), and HPLC fingerprinting of WCA was done at 8.839 min (Figure 2B). Three batches of WCA had similar concentrations of hesperidin.
The human colon cancer cell line HCT-116 was treated with 0, 3%, 6% and 9% WCA decoction filtrate or with 5%, 10% and 20% of serum extracted from rats that received WCA by OG (2 mL/d for 1 wk). As shown in Figure 2C and 2D, 72 h after treatment, WCA exerted significant inhibition on HCT-116 cell proliferation (P < 0.05).
One mouse in each group died within 1 wk of orthotopic transplant surgery; in each instance, death may have been caused by the surgical procedure itself. Additionally, one mouse died 12 d after surgery in the WCA group, and one 10 d after surgery in the WCA + 5-FU group. Therefore, 42 mice survived the entire length of the procedure and drug administration. Parenchymal tumors were palpable in the abdomens of mice 2 wk post-surgery. Furthermore, the body weights of mice were reduced by 5 wk after surgery. At 7 wk post-surgery, marasmus and loss of appetite occurred. Additionally, feces appeared soft and red in color; mice also displayed skin casting and slow movement and activity.
All mice that underwent orthotopic transplantation surgery developed tumors. HE staining and gross anatomy of tumors are shown in Figure 1. The CON group showed obvious atypia of orthotopic cancer cells; large cancerous cells displaying karyokinesis could also be seen. Irregularly arranged tumor-giant cells were also present. Blood vessels were abundant, and there was little connective tissue found in the interstitium. Monocyte infiltration and necrosis were apparent in the tumor interstitium (Figure 1A). The WCA, 5-FU and WCA + 5FU groups showed reduced atypia of orthotopic cancer cells and the presence of large cancerous cells. Irregularly arranged tumor-giant cells were also seen. Blood vessels were abundant in the interstitium. Increased monocyte infiltration was observed in the tumor interstitium, and enlarged necrotic areas were apparent. A significant increase in the cavity-like structure could also be observed (Figure 1B, 1C and 1E). One-way ANOVA showed significant differences in the mean tumor weight among the four groups (P = 0.010). Moreover, the mean tumor weight in the CON group was significantly different from the mean tumor weight in both the 5-FU (P = 0.005) and WCA + 5-FU groups (P = 0.003). Treatment of mice with WCA, 5-FU and WCA + 5-FU inhibited tumor growth by 19.087%, 33.298% and 36.802%, respectively (Table 1). The average size of orthotopic tumors between the CON and the WCA + 5-FU groups was significantly different (P = 0.031); however, no other significant differences were identified (Table 1).
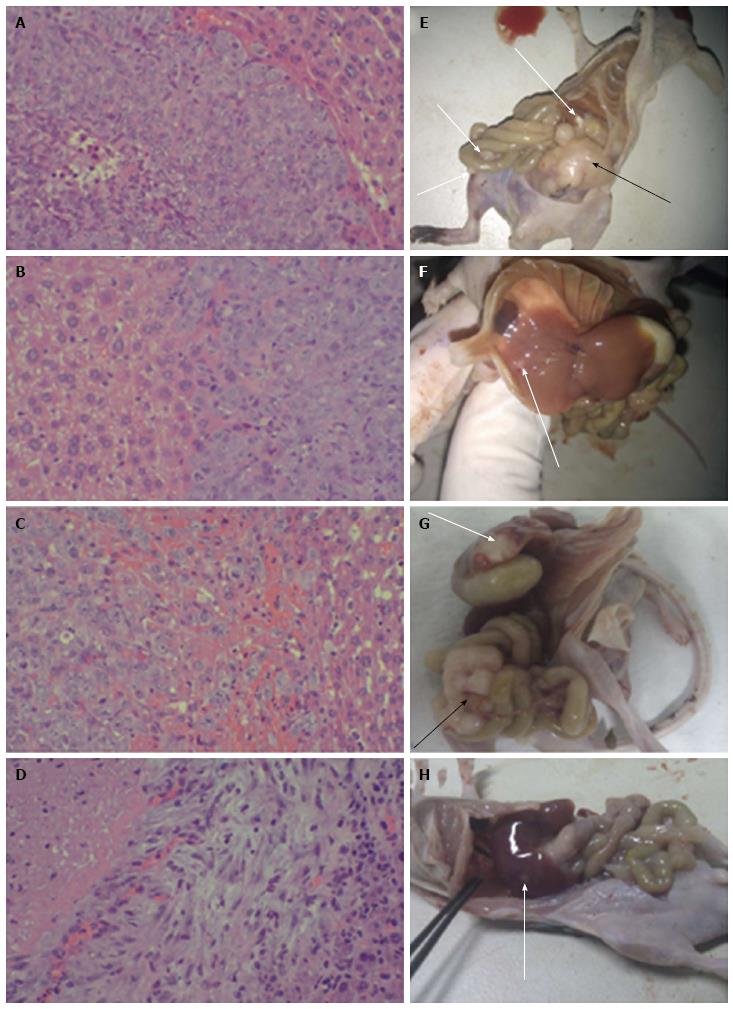
Compared with the CON group, mice in the WCA and the WCA + 5-FU groups had fewer liver, abdominal and intestinal metastases (Figure 3). Hepatic metastatic tumors appeared as nodules with massive atypia in mice belonging to the CON group. Cancer cells were large, and little connective tissue was observed in the interstitium. Infiltration and necrotic alteration of monocytes were found in the tumor interstitium. There were hepatic tissue necrosis and lymphocyte infiltration at the boundary of the tumor interstitium (Figure 3A). As for hepatic metastatic tumors in mice in the WCA group, no apparent boundary between the metastases was evident. There were obvious hepatic tissue necrosis and infiltration of both lymphocytes and monocytes (Figure 3B). In hepatic metastatic tumors in mice from the 5-FU and WCA + 5FU groups, cancer cells were irregularly arranged and an increase in the interstitium was observed. Massive bleeding has also occurred (Figure 3C and 3D). The gross metastatic rates in the WCA and the WCA + 5-FU groups were significantly lower than in the CON group (P = 0.012 and 0.004, respectively). However, there was no significant difference in hepatic metastases among any of the drug-treated groups (Table 2).
There was no significant difference in serum CEA levels among the CON (43.040 ± 11.273 μg/L), WCA (34.282 ± 14.731 μg/L), and 5-FU (35.462 ± 11.022 μg/L) groups (P = 0.122). However, serum CEA levels in the WCA + 5-FU group (31.263 ± 7.421 μg/L) were significantly lower than that in the CON group (P = 0.023).
To begin to investigate the molecular mechanisms underlying metastasis in our model, we measured mRNA expression of β-catenin, a Wnt pathway regulator of cell-cell adhesion, by real-time RT-PCR. One-way ANOVA revealed significant differences in β-catenin expression in orthotopic tumors among the four groups (P = 0.001). Specifically, mice treated with WCA, 5-FU and WCA + 5-FU showed decreased β-catenin mRNA expression in orthotopic tumors compared to control treated mice (P = 0, 0.021 and 0.006, respectively; Table 3).
| Group | β-catenin | MMP-7 | ||||||
| Orthotopic tumors | Hepatic metastatic tumors | Orthotopic tumors | Hepatic metastatic tumors | |||||
| n | Relative expression | n | Relative expression1 | n | Relative expression | n | Relative expression2 | |
| CON | 11 | 0.809 ± 0.354 | 5 | 20.588 ± 13.595 | 11 | 5.688 ± 3.255 | 5 | 1.211 ± 0.593 |
| WCA | 10 | 0.271 ± 0.173b | 3 | 3.679 ± 3.271 | 10 | 2.236 ± 1.528a | 3 | 0.030 ± 0.025b |
| 5-FU | 11 | 0.513 ± 0.261a | 4 | 4.559 ± 5.491 | 11 | 2.427 ± 2.111 | 4 | 0.061 ± 0.021 |
| WCA + 5-FU | 10 | 0.445 ± 0.325b | 3 | 5.788 ± 2.029 | 10 | 0.918 ± 1.011b | 3 | 0.044 ± 0.019a |
In liver metastases, as sample size was small (3-5 per group), Kruskal-Wallis nonparametric tests were performed. The results showed that there was no significant difference in β-catenin mRNA expression levels in the CON, WCA, 5-FU and WCA + 5-FU treatment groups (P = 0.155; Table 3).
MMP-7 is an important target gene in the Wnt/β-catenin signaling pathway and the main member of the MMP family, and it was also examined in our model. The Welch and Brown-Forsythe tests showed significant differences in MMP-7 mRNA expression in orthotopic tumors among the four groups (P = 0.001). Moreover, MMP-7 transcript levels were significantly decreased in both the WCA and WCA + 5-FU treatment groups (P = 0.038 and 0.003, respectively; Table 3).
In liver metastases, MMP-7 mRNA was significantly decreased (P = 0.012, Kruskal-Wallis tests; Table 3) and Nemenyi tests revealed that both WCA and WCA + 5-FU treatments decreased MMP-7 mRNA expression compared to the CON group (P = 0.001 and 0.013, respectively; Table 3).
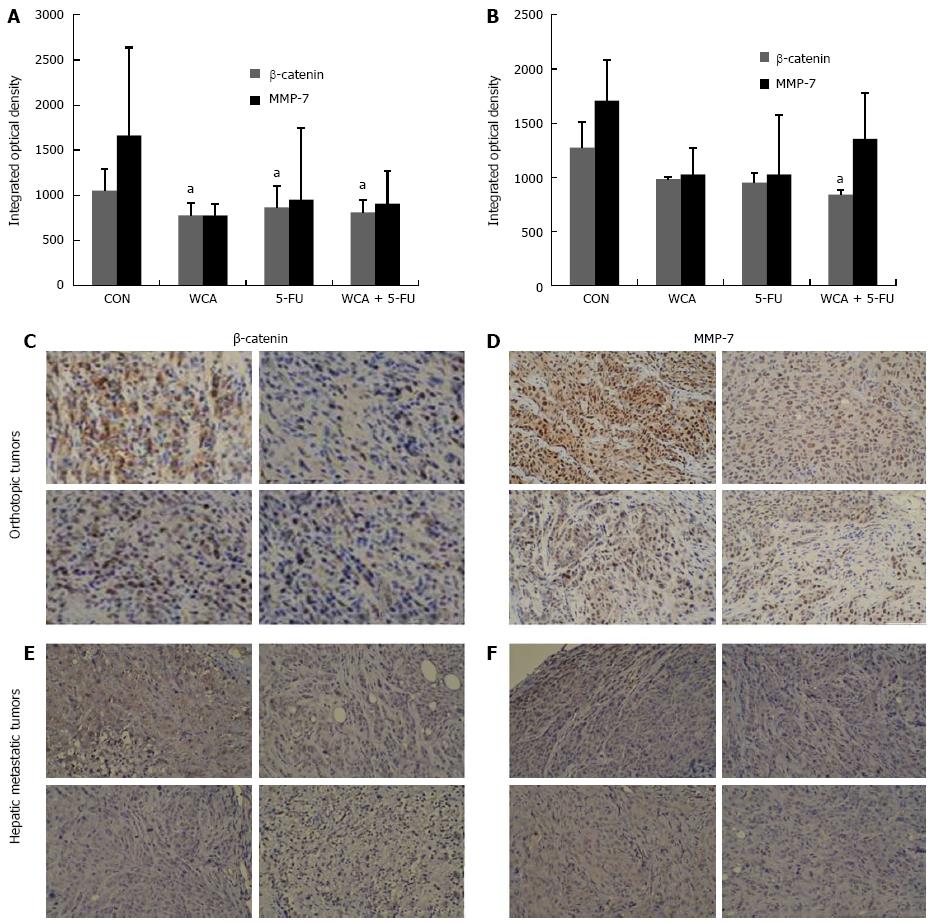
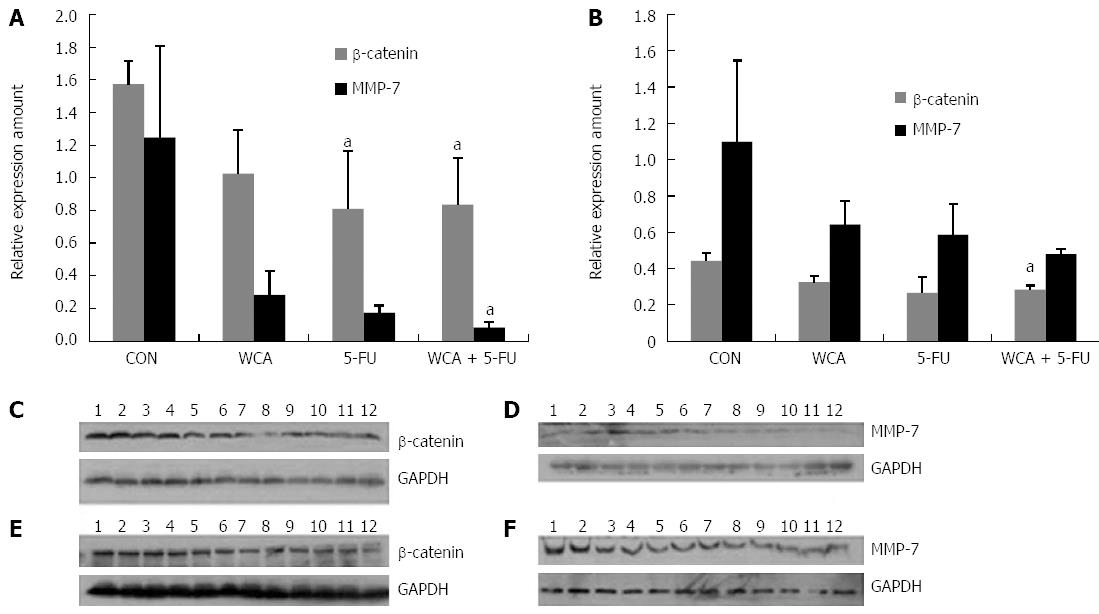
We measured protein expression of β-catenin and MMP-7 in both orthotopic tumors and liver metastases by IHC and immunoblotting. Figure 4A and 4B show integrated OD of β-catenin and MMP-7 protein detected by IHC in orthotopic tumors and liver metastases, respectively. As shown in Figure 4C and 4E, in both orthotopic tumors and liver metastases, β-catenin was expressed at high levels and was located in both the nucleus and cytoplasm in the CON group, and total expression of β-catenin was reduced in the WCA/5-FU/WCA + 5-FU groups compared with the CON group. As shown in Figure 4D and 4F, MMP-7 was highly expressed (brown staining) in the cytoplasm of orthotopic and liver metastatic tumors from the CON group. We also observed some membrane staining and some staining of the tumor interstitium. Expression of MMP-7 was reduced in the WCA, 5-FU and WCA + 5-FU groups compared with the CON group. The structure of the tumor tissue was also destroyed by massive necrotic areas.
One-way ANOVA analysis of IHC staining showed a significant difference in β-catenin protein expression in orthotopic tumors among the four groups (P = 0.012, 10 or 11 per group). Furthermore, β-catenin protein was decreased in all treatment groups compared with CON (WCA, P = 0.003; 5-FU, P = 0.033; WCA + 5-FU, P = 0.008; Figure 4A).
Levene’s test revealed heterogeneity of variance in MMP-7 protein levels in the orthotopic tumors, and the Brown-Forsythe test showed significant differences in MMP-7 protein expression in the orthotopic tumors among the four groups (P = 0.020, 10 or 11 per group). Pairwise comparisons showed no significant difference between groups (all P > 0.05). However, a decreasing trend was observed in both the WCA group (776.30 ± 124.030) and the WCA + 5-FU group (907.30 ± 359.492) compared with the CON group (1663.73 ± 975.557; Figure 4A).
β-catenin protein expression in hepatic metastases detected using IHC was significantly different among the four groups [CON group (1272.40 ± 234.658); WCA group (982.67 ± 17.039); 5-FU group (949.75 ± 86.083); and WCA + 5-FU group (838.00 ± 46.936); P = 0.025 using the Kruskal-Wallis test, Figure 4B, 3-5 per group]. Pairwise comparisons using Nemenyi tests revealed that the WCA + 5-FU treatment decreased β-catenin expression compared to the CON group (P = 0.002).
MMP-7 expression in hepatic metastases was not significantly different among the groups (P = 0.113 using Kruskal-Wallis test, 3-5 per group), while a trend toward drug-mediated decrease in MMP-7 protein expression was observed in hepatic metastases by IHC (1709.00 ± 371.485, 1026.67 ± 245.194, 1026.00 ± 546.233 and 1353.00± 421.138 in the CON, WCA, 5-FU and WCA + 5-FU groups and mean rank 11.40, 5.67, 4.75 and 9.0, respectively).
Figure 5A and B show gray scale scanning of β-catenin and MMP-7 protein detected by western blot in orthotopic tumors and liver metastases (3 per group), respectively. β-catenin expression was significantly different among the four groups in both orthotopic and hepatic metastatic tumors (P = 0.043 and P = 0.041 by Kruskal-Wallis test, respectively). Pairwise comparisons using Nemenyi tests showed decreased expression of β-catenin in the 5-FU and WCA + 5-FU groups compared with the CON group (P = 0.023 and P = 0.043, respectively) in orthotopic tumors, while in hepatic metastatic tumors, β-catenin expression in the WCA + 5-FU group was decreased compared with the CON group (P =0.012).
Using Kruskal-Wallis tests, MMP-7 showed a difference in orthotopic tumors among the four groups (P = 0.033) but not in hepatic metastatic tumors (P = 0.069). Pairwise comparisons using Nemenyi tests showed decreased expression of MMP-7 in the WCA + 5-FU group compared with the CON group (P = 0.001) in orthotopic tumors.
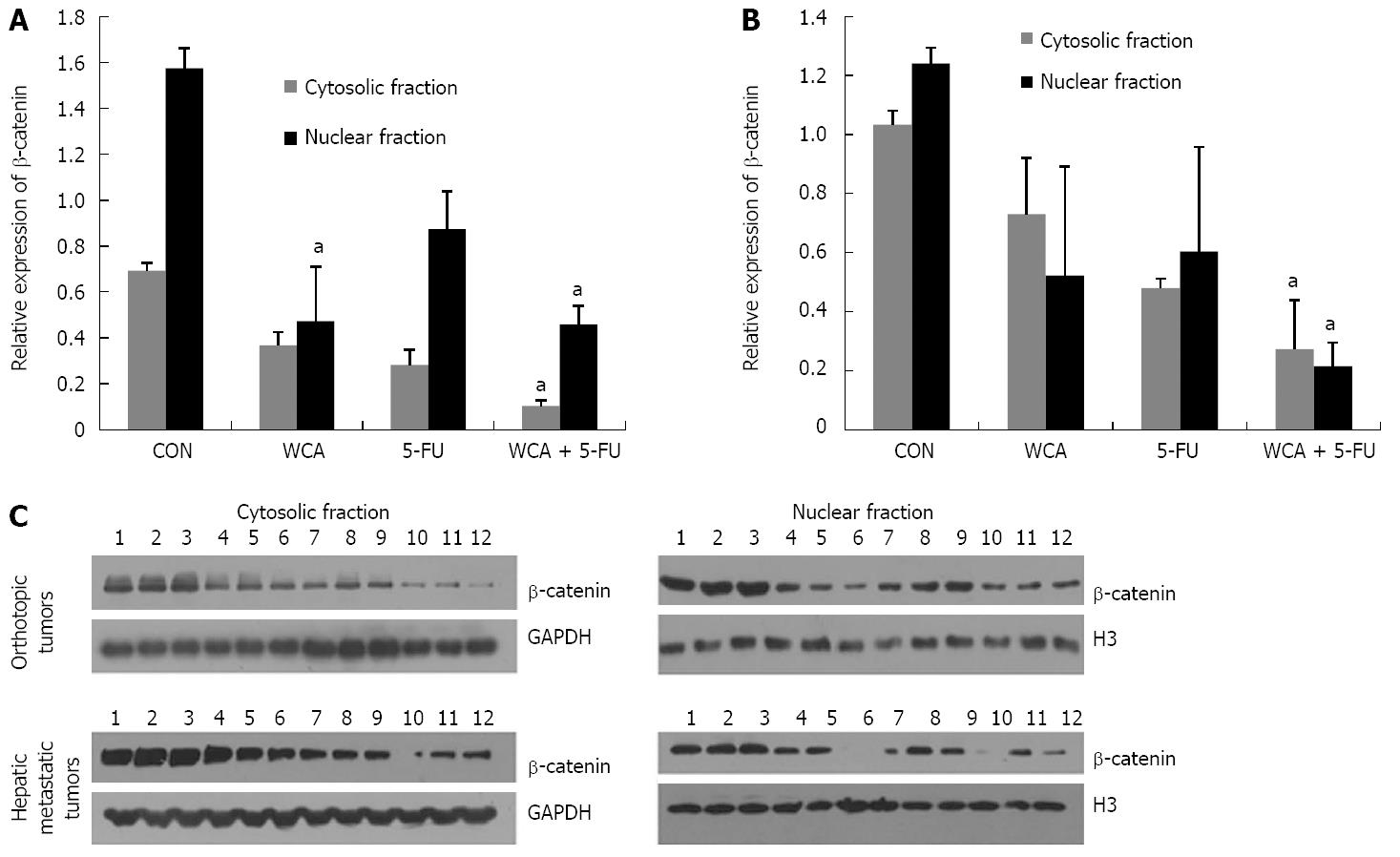
To clarify whether the intracellular distribution of β-catenin was affected by WCA, we performed cell fractionation and detected β-catenin in the cytosolic and nuclear fractions in orthotopic tumors and hepatic metastatic tumors, respectively. The immunoblotting images and quantitative diagrams are shown in Figure 6.
In orthotopic tumors (Figure 6A), Kruskal-Wallis tests showed significant cytosolic and nuclear β-catenin expression (P = 0.019 and P = 0.033, respectively). Pairwise comparisons using Nemenyi tests revealed that WCA + 5-FU treatment decreased cytosolic β-catenin expression compared to the CON group (P < 0.001), while WCA and WCA + 5-FU decreased nuclear β-catenin expression compared to the CON group (P = 0.043 and P = 0.012, respectively).
In hepatic metastatic tumors (Figure 6B), Kruskal-Wallis tests revealed that there was a significant difference in cytosolic (P = 0.016) or nuclear (P = 0.031) β-catenin expression among groups, and Nemenyi tests showed that WCA + 5-FU treatment significantly decreased cytosolic (P < 0.001) and nuclear (P = 0.001) β-catenin.
These data showed that WCA/5-FU treatments not only depressed β-catenin expression but also inhibited nuclear translocation both in orthotopic tumors and hepatic metastatic tumors.
Colorectal cancer most frequently metastasizes to the liver; an event that is the primary cause of death in patients with this disease[8,19]. Those with unresectable colorectal hepatic metastases have a median survival of 6.9 mo and a 5-year survival rate close to zero[20,21]. Therefore, it is critical to understand better the mechanisms underlying the process of metastasis development from colorectal cancer to the liver, in order to prolong patient survival and improve quality of life.
Quantitative determination of WCA was conducted using HPLC detection of hesperidin, which is a citrus flavonoid known to be active against some oxidative-stress-mediated diseases. Hesperidin found in orange peel is a flavanone glycoside consisting of the flavone hesperidin bound to the disaccharide rutinose. The sugar group makes hesperidin more water-soluble than hesperitin; another compound in orange peel[22]. Hesperidin may moderately stimulate the gastrointestinal tract, promote the secretion of digestive enzymes, remove intestinal pneumatosis, and invigorate the stomach to relieve phlegm. Exogenous hesperidin has been shown to influence a wide variety of biological functions, such as inducing apoptosis and suppressing proliferation of human cancer cells[23], as well as inhibiting tumor development in various tissues, including colon cancer[24,25]. In WCA, hesperidin cooperates with other components to exert spleen invigorating, heat clearing, detoxicating and hard lump resolving effects. Hesperidin is a stable and controllable component, and HPLC detection of hesperidin facilitates the monitoring of WCA concentration.
In our current study, orthotopic colon tumors grown in 100% of the mice. Importantly, tumors displayed pathogenic characteristics similar to those observed in clinical cases of advanced colorectal cancer[26]. The mean weights of the orthotopic tumors in the 5-FU and WCA + 5-FU groups were significantly lower than in the CON group (both P < 0.01). Additionally, the mean size of orthotopic tumors in the WCA + 5-FU group was significantly smaller than in the CON group (P < 0.05). Treatment with WCA, 5-FU, or their combination resulted in orthotopic tumor inhibition of 19.09%, 33.30% and 36.80%, respectively, compared with controls. We detected hepatic metastases in a third of the mice used in the study. Overall, the occurrence of hepatic metastasis in the CON group was 45.45% and 36.36% in the 5-FU group, 30% in the WCA group, and 30% in the WCA + 5-FU group. Although the percentage of hepatic metastasis was lower in the three treatment groups compared with the CON group, statistical significance was not reached. This should be followed up by additional studies with larger sample sizes to determine if the drug-mediated decrease in metastatic rate is significant. We also found a significantly lower gross metastatic rate in the WCA group (50%) and in the WCA + 5-FU group (40%) compared with the CON group (100%). Upon treatment by OG, WCA is primarily absorbed by the intestine, resulting in a high concentration of WCA in the liver. This likely reduces hepatic metastasis of colon cancer. Furthermore, administration of WCA combined with abdominal 5-FU chemotherapy reduces peritoneal metastasis of the tumor cells.
Previous research has established that deregulated Wnt signaling is involved in the development of tumors and closely correlates with the invasiveness and metastatic potential of colorectal cancer[27]. β-catenin is a key downstream mediator of the Wnt signaling pathway[28,29]. Nuclear accumulation of β-catenin at the invasive front of tumors and within blood vessels is strongly associated with hepatic metastasis and may be a useful predictor of hepatic metastasis in colorectal cancer[30-32]. MMP-7 is transcribed in response to active Wnt-β-catenin signaling. Once translated into a functional protein, MMP-7 assists in the degradation of extracellular matrix, which is a critical event in tumor invasion and metastasis. IHC expression of MMP-7 has been shown to correlate with Dukes’ classification in colorectal cancer specimens, and introduction of MMP-7 into colorectal cancer cells markedly upregulates their in vivo invasive and metastatic potential[33]. Furthermore, MMP-7 promotes hepatic metastasis in colorectal cancer[34]. Research has also shown that elevated MMP-7 expression is related to decreased survival, and it is considered as a predictor of disease recurrence and hepatic metastasis[35].
The present study showed that administration of WCA significantly reduced the expression of β-catenin and MMP-7 mRNA in orthotopic tumors and in hepatic metastatic tumors in mice. Similarly, WCA, alone and in combination with 5-FU, reduced β-catenin and MMP-7 protein expression in orthotopic and metastatic tumors. Some results detected using immunoblotting, IHC and real-time PCR did not show significant differences. The orthotopic transplant model used in this study is a relatively natural nude mouse model of hepatic metastases, and the metastasis rate is not very high. Only 3-5 animals in each group developed liver metastases, which is not statistically powerful. In a future study, we will increase the sample size and this problem may be resolved.
CEA is often expressed in colorectal cancer and mediates the metastasis of these cancerous cells from the colon to the liver[36]. CEA binds to receptors on the surface of Kupffer cells and promotes the release of interleukin (IL)-1, IL-6, and tumor necrosis factor-α[37]. In turn, these molecules increase the expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 by endothelial cells, thereby reinforcing the adhesion between the tumor cells and endothelial cells. Recent reports have shown that measurement of serum CEA levels, in combination with CA199 and CA125, can serve as an important predictor of hepatic metastasis of colorectal cancer[36]. Moreover, serum CEA levels in combination with imaging techniques may accurately predict tumor recurrence following radical surgery of colorectal hepatic metastases[38]. Importantly, our data showed that administration of WCA and 5-FU effectively reduced serum CEA levels in the orthotopic transplant nude mouse model, which may be associated with the decreased rate of hepatic metastasis that we observed.
Pathological analysis revealed that the degree of atypia in both orthotopic tumors and hepatic metastases was significantly lower in the WCA group compared with the CON group. The difference between the CON group and the WCA + 5-FU group was even more striking. Considering the relatively low rate of adverse effects of TCM, the combination of WCA with 5-FU may present a good strategy in colon cancer treatment.
Limitations of this study included the relatively small number of animals in each group, unclear exact components in WCA, and inadequate assessment of the immunological effects of WCA. In addition, different treatment strategies need to be investigated to optimize the effect of WCA. Furthermore, how WCA affects the angiogenesis of metastatic tumor, tumor microenvironment and immunological response requires further research.
In summary, combination of the TCM WCA with 5-FU inhibited colon tumor growth and hepatic metastasis in an orthotopic transplant nude mouse model. Decreased expression of β-catenin and MMP-7 may be important for the anti-tumor properties of WCA and 5-FU.
It is estimated that 50%-60% of patients diagnosed with colorectal cancer will develop hepatic metastases and the most frequent site of metastasis is the liver. Eighty to ninety percent of these metastases will be unresectable and require multidisciplinary treatments, including chemotherapy, liver-targeting therapies and traditional Chinese medicine (TCM). TCM has a number of benefits, including decreasing the negative side effects associated with chemotherapy and radiotherapy, improving patients’ immune function, and enhancing the effects of conventional cancer treatments.
A previous clinical study has indicated that patients with gastric carcinoma benefit from Weichang’an (WCA) treatment. In addition, WCA has been shown to increase the 5-year survival rate and reduce the 1- and 2-year metastatic rates in patients with colorectal cancer.
We demonstrated that combination of WCA with 5-fluorouracil (5-FU) suppressed colon tumor growth and hepatic metastases, reducing the tumor weight and size, the rate of metastases and serum carcinoembryonic antigen levels. The authors also confirmed that expression levels of β-catenin and matrix metalloproteinase (MMP)-7 are involved in the process of metastasis from colorectal cancer to the liver, as well as the inhibitory effect of WCA and 5-FU.
This study provided a new therapeutic strategy for colorectal cancer with hepatic metastasis and may greatly contribute to prolong patient survival and improve their quality of life.
Orthotopic transplant nude mouse model: HCT-116 cells were subcutaneously injected into the right axilla of nude mice. After 3 wk, tumors were excised and cut into 1-2-mm3 pieces using sterile techniques. Tumor blocks were transplanted into the cecum of nude mice by a purse-string suture.
The authors analyzed the effect of the TCM WCA on colorectal carcinoma in a clinically interesting orthotopic model. Their major finding was a reduction in tumor weight and size as well as the number of metastases when WCA was combined with 5-FU. They also speculate that decreased expression of β-catenin and MMP-7 may be involved in the anti-tumorigenic properties of WCA. The design, technical performance and some of the conclusions made in this study are comprehensible. The authors address a clinically very relevant topic. The manuscript is overall written in good English.
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9876] [Article Influence: 759.7] [Reference Citation Analysis (5)] |
| 2. | Lee WS, Yun SH, Chun HK, Lee WY, Yun HR, Kim J, Kim K, Shim YM. Pulmonary resection for metastases from colorectal cancer: prognostic factors and survival. Int J Colorectal Dis. 2007;22:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 4. | Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer. 2006;6:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, Levitt R, Rowland K, Nair S, Sargent DJ. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243-9249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 383] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 6. | Kemeny N. Management of liver metastases from colorectal cancer. Oncology (Williston Park). 2006;20:1161-176, 1179; discussion 1161-176, 1161-176. [PubMed] |
| 7. | Muratore A, Zorzi D, Bouzari H, Amisano M, Massucco P, Sperti E, Capussotti L. Asymptomatic colorectal cancer with un-resectable liver metastases: immediate colorectal resection or up-front systemic chemotherapy? Ann Surg Oncol. 2007;14:766-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938-946. [PubMed] |
| 9. | Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405-1410. [PubMed] |
| 10. | Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, Sun X, Zhou X, Guo Y, Xu Y. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in chinese. PLoS One. 2013;8:e60338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Liu J, Li X, Liu J, Ma L, Li X, Fønnebø V. Traditional Chinese medicine in cancer care: a review of case reports published in Chinese literature. Forsch Komplementmed. 2011;18:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Lin H, Liu J, Zhang Y. Developments in cancer prevention and treatment using traditional Chinese medicine. Front Med. 2011;5:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Zhao AG, Cao W, Xu Y, Zhao G, Liu BY, Cai Y, Yang JZ, Gu Y, Yuan W, Zhu YJ. [Survival benefit of an herbal formula for invigorating spleen for elderly patients with gastric cancer]. Zhong Xi Yi Jie He Xue Bao. 2010;8:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Xu Y, Zhao AG, Li ZY, Zhao G, Cai Y, Zhu XH, Cao ND, Yang JK, Zheng J, Gu Y. Survival benefit of traditional Chinese herbal medicine (a herbal formula for invigorating spleen) for patients with advanced gastric cancer. Integr Cancer Ther. 2013;12:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Gu Y, Han YY, Zheng J, Yang JK. Analysis of therapeutic effect of Wei Chang An in Colorectal Cancer. Liaoning Zhongyiyao Daxue Xuebao. 2006;8:5-6. |
| 16. | Iwai S, Yonekawa A, Harada C, Hamada M, Katagiri W, Nakazawa M, Yura Y. Involvement of the Wnt-β-catenin pathway in invasion and migration of oral squamous carcinoma cells. Int J Oncol. 2010;37:1095-1103. [PubMed] |
| 17. | Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 633] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 18. | Suzuki K, Bose P, Leong-Quong RY, Fujita DJ, Riabowol K. REAP: A two minute cell fractionation method. BMC Res Notes. 2010;3:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 385] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 19. | Foster JH. Treatment of metastatic disease of the liver: a skeptic’s view. Semin Liver Dis. 1984;4:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Sharma S, Camci C, Jabbour N. Management of hepatic metastasis from colorectal cancers: an update. J Hepatobiliary Pancreat Surg. 2008;15:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 21. | Elias D, Youssef O, Sideris L, Dromain C, Baton O, Boige V, Ducreux M. Evolution of missing colorectal liver metastases following inductive chemotherapy and hepatectomy. J Surg Oncol. 2004;86:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Vallejo F, Larrosa M, Escudero E, Zafrilla MP, Cerdá B, Boza J, García-Conesa MT, Espín JC, Tomás-Barberán FA. Concentration and solubility of flavanones in orange beverages affect their bioavailability in humans. J Agric Food Chem. 2010;58:6516-6524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Ghorbani A, Nazari M, Jeddi-Tehrani M, Zand H. The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: involvement of PPARγ-dependent mechanism. Eur J Nutr. 2012;51:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Aranganathan S, Nalini N. Antiproliferative efficacy of hesperetin (citrus flavanoid) in 1,2-dimethylhydrazine-induced colon cancer. Phytother Res. 2013;27:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Saiprasad G, Chitra P, Manikandan R, Sudhandiran G. Hesperidin alleviates oxidative stress and downregulates the expressions of proliferative and inflammatory markers in azoxymethane-induced experimental colon carcinogenesis in mice. Inflamm Res. 2013;62:425-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Cersosimo RJ. Management of advanced colorectal cancer, Part 2. Am J Health Syst Pharm. 2013;70:491-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311-320. [PubMed] |
| 28. | Bryja V, Cajánek L, Grahn A, Schulte G. Inhibition of endocytosis blocks Wnt signalling to beta-catenin by promoting dishevelled degradation. Acta Physiol (Oxf). 2007;190:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 348] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 30. | Suzuki H, Masuda N, Shimura T, Araki K, Kobayashi T, Tsutsumi S, Asao T, Kuwano H. Nuclear beta-catenin expression at the invasive front and in the vessels predicts liver metastasis in colorectal carcinoma. Anticancer Res. 2008;28:1821-1830. [PubMed] |
| 31. | Bandapalli OR, Dihlmann S, Helwa R, Macher-Goeppinger S, Weitz J, Schirmacher P, Brand K. Transcriptional activation of the beta-catenin gene at the invasion front of colorectal liver metastases. J Pathol. 2009;218:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Wang L, Cheng H, Liu Y, Wang L, Yu W, Zhang G, Chen B, Yu Z, Hu S. Prognostic value of nuclear β-catenin overexpression at invasive front in colorectal cancer for synchronous liver metastasis. Ann Surg Oncol. 2011;18:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Adachi Y, Yamamoto H, Itoh F, Hinoda Y, Okada Y, Imai K. Contribution of matrilysin (MMP-7) to the metastatic pathway of human colorectal cancers. Gut. 1999;45:252-258. [PubMed] |
| 34. | Zeng ZS, Shu WP, Cohen AM, Guillem JG. Matrix metalloproteinase-7 expression in colorectal cancer liver metastases: evidence for involvement of MMP-7 activation in human cancer metastases. Clin Cancer Res. 2002;8:144-148. [PubMed] |
| 35. | Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, Yun JP, Zhang MF, Wan DS. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Zhang D, Yu M, Xu T, Xiong B. Predictive value of serum CEA, CA19-9 and CA125 in diagnosis of colorectal liver metastasis in Chinese population. Hepatogastroenterology. 2013;60:1297-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 37. | Gangopadhyay A, Lazure DA, Thomas P. Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metastasis. 1998;16:703-712. [PubMed] |
| 38. | Verberne CJ, Wiggers T, Vermeulen KM, de Jong KP. Detection of recurrences during follow-up after liver surgery for colorectal metastases: both carcinoembryonic antigen (CEA) and imaging are important. Ann Surg Oncol. 2013;20:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Crea F, Linnebacher M, Martinez-Zorzano VS S- Editor: Ma YJ L- Editor: Kerr C E- Editor: Zhang DN